Abstract
Purpose
Local/systemic symptoms during cancer therapy may be exacerbated by dysregulated inflammation and its downstream toxic effects. Minocycline can suppress proinflammatory cytokine release; therefore, we investigated its potential to reduce patient-reported symptom severity during radiotherapy (RT) for head and neck cancer (HNC).
Methods
Eligible patients for this blinded, placebo–controlled trial were adults with T0–3, N-any, and M0 HNC receiving single-modality RT. Participants were randomized 1:1 to either minocycline (200 mg/day) or placebo during RT. The primary endpoint was the area under the curve (AUC) of 5 prespecified symptoms (pain, fatigue, disturbed sleep, poor appetite, difficulty swallowing/chewing) during RT, assessed with the MD Anderson Symptom Inventory for HNC (MDASI-HN).
Results
We analyzed data from 20 evaluable patients per arm. Overall, 75% had oropharyngeal cancer and 78% were male. No grade 3+ adverse events potentially related to study medication were observed. Two minocycline patients required a feeding tube during RT vs 5 placebo patients (P = 0.21). The average daily AUC during RT for the 5 MDASI-HN symptoms was 3.1 (SD =1.0) for minocycline and 3.7 (SD = 1.7) for placebo (P = 0.16); the 0.37 effect size was less than our 0.70 target. AUC comparisons for several individual symptoms and symptom interference favored minocycline but were not statistically significant. The greatest numerical differences occurred for systemic symptoms, larger toward treatment end, and in early post-RT recovery.
Conclusions
Minocycline was feasible, well tolerated, and achieved a positive signal toward reducing patient-reported symptom severity during RT for HNC, particularly for systemic symptoms. This justifies additional study and informs future trial design.
Keywords: Treatment-related symptoms, Minocycline, Inflammation, Radiation treatment, Head and neck cancer
Introduction
Radiation therapy (RT) is often a key treatment component for patients with head and neck cancer (HNC). Treatment-related symptoms, such as mucositis, difficulty swallowing, fatigue, and disrupted sleep, are known to cause significant distress during treatment and in early recovery. Although reported cancer control and long-term survival rates after RT-based treatment for many patients with HNC are encouraging [1], substantial treatment–related acute and long-term toxicities and associated bothersome symptoms can negatively affect patient quality of life and overall functioning [2, 3]. Given current epidemiological trends in HNC that point toward an overall younger patient profile and an accompanying favorable prognosis for those with viral-associated disease—and thus the potential to live for decades with the sequelae of treatment—toxicity reduction is now a primary emphasis in ongoing clinical investigations [4].
Beyond RT’s direct effects on normal tissues within the RT treatment volume, both local symptoms (those in the head and neck region) and systemic or general symptoms (such as fatigue) are often reported by patients. As has been shown in animal models, symptoms such as pain, fatigue, and behavioral analogues of a variety of sickness-related symptoms are associated with inflammatory response [5] and may be exacerbated by treatment-induced dysregulated inflammation and its accompanying downstream toxic effects, including those mediated centrally (i.e., by the central nervous system) [6].
As part of a larger goal to improve outcomes for patients with HNC through the reduction of treatment-related toxicities, and in order to inform future clinical study design, we conducted a phase II exploratory clinical trial to investigate the potential of minocycline to reduce patient-reported symptom severity during RT. Minocycline is a safe, readily available, enteral, third-generation tetracycline antibiotic that is widely used to treat acne and other infections [7]. Beyond its traditional indications in infectious diseases, minocycline has been shown to suppress proinflammatory cytokine release [7], and its use in inflammatory diseases, such as rheumatoid arthritis, is well established [8, 9]. Minocycline has also demonstrated tissue-protective effects [10]. Minocycline crosses the blood brain barrier, and its use as a neuroprotectant has been explored in several neurodegenerative conditions [11, 12] as well as in rheumatoid arthritis [9] and Huntington disease [13]. Minocycline has been used as a prophylactic agent for dermatological toxicities in patients with cancer being treated with epidermal growth factor inhibitors [14, 15] but has yet to be specifically tested in combination with RT. Especially when used for a limited time, minocycline is a relatively safe drug with minimal side effects and has serum half-life ranging from 11 to 17 h [16]. The hypothesis of this study, based in these anti-inflammatory and tissue-protective effects, was that minocycline can detectably reduce the patient-reported symptom burden during RT for HNC as measured by the MD Anderson Symptom Inventory Head and Neck module (MDASI-HN).
Materials and methods
Patients
Eligible patients for this single-institution, prospective, blinded, placebo-controlled randomized trial (ClinicalTrials.gov Identifier: NCT01173692) were (1) adults with oropha-ryngeal, nasopharyngeal, or laryngeal carcinoma (T1–T3, N-any, M0) who after multidisciplinary evaluation had been dispositioned to receive definitive intensity–modulated RT as a single modality, or (2) adults with unknown primary carcinoma of the head and neck and of suspected mucosal origin, for which radiation therapy to a mucosal target was planned. RT dose to gross primary tumor and involved lymph nodes were 66–70 Gy over 6–7 weeks and elective lymph node regions at risk were prescribed 54–63 Gy depending on the estimated risk and number of fractions. Patients with well-lateralized primaries of the tonsillar fossa were selected for ipsilateral (unilateral) neck RT, while all others were prescribed bilateral neck RT. Participants were randomized equally (1:1) using a permuted block design to receive either minocycline (100 mg twice daily enterally) or matching placebo during therapy (i.e., from day 1 to the final day of the 6– 7-week course of RT). We chose to initiate study drug in a prophylactic rather than a reactive way given that RT-related toxicity and associated symptoms are uniformly expected and known to occur early in the course of RT. Standard minocycline dosing for antibiotic and anti-inflammatory indications was used with goal to ensure expected tolerability and compliance across the drug period. All participants were required to have adequate renal and hepatic function and to be able to read and understand English. Exclusion criteria for this study included plans to receive concurrent chemotherapy (receipt of prior induction chemotherapy was allowed), prior resection of study cancer (diagnostic excisions such as tonsillectomy and lymph node biopsies were allowed), prior head and neck RT, hypersensitivity to or having taken a tetracycline within 15 days of registration, or pregnancy. Baseline patient demographic and clinical variables were collected. Patient performance status before radiation-based treatment was rated by the treating radiation oncologist according to the Eastern Cooperative Oncology Group scale. Tumor and lymph node status was recorded according to the 7th edition of the American Joint Committee on Cancer staging system. Other than the study drug, toxicity and symptom management was not specified by the protocol and left to the discretion of the treating radiation oncologist, including prescription of medications or consultation with available supportive services.
This study was approved by our Institutional Review Board. Informed consent was obtained from all individual participants included in the study.
Patient-reported symptoms
Patients rated symptoms using the MDASI-HN, a psychometrically validated, 28-item questionnaire containing 13 “core” items representing important symptoms common to all cancer types, 9 additional items related specifically to head and neck cancer (such as problems with mucus and difficulty swallowing), and 6 questions about the extent to which the symptom complex interferes with activities of daily life (“interference” items) [17]. The 22 symptom items are rated on numeric 0–10 scales from “not present” to “as bad as you can imagine.” The 6 interference items are rated on numeric 0– 10 scales from “did not interfere” to “interfered completely.”
Participants self-administered and completed the MDASI-HN questionnaires before starting RT (baseline, week 0), then twice weekly during the 6–7-week course of RT and for 1 month after, and then weekly for 1 additional month, yielding a total of 13 weeks (± 1 week) of MDASI-HN assessments. Participants being evaluated in the clinic areas completed the MDASI-HN on paper forms; otherwise, assessments were done via telephone interview or using a computerized, telephone-based interactive voice response system.
Other study assessments
Toxicities and any adverse events were prospectively assessed weekly by the study team and treating physicians during the study period and graded according to the Common Terminology Criteria for Adverse Events, version 3. Likewise, common and expected RT–related toxicities, such as dermatitis, mucositis, fatigue, and pain, were assessed weekly by the treating radiation oncologist. Attribution and severity of other observed toxicities were determined by the treating radiation oncologist and principal investigator and further categorized as potentially related to study medication versus radiation therapy. Safety and futility monitoring was overseen by our institutional Data Safety and Monitoring Board.
Statistical methods
The primary endpoint was the area under the curve (AUC) using trapezoidal approximation of 5 prespecified patient– reported symptoms (pain, fatigue, disturbed sleep, lack of appetite, and difficulty swallowing/chewing) as assessed by the MDASI-HN during the 6–7-week course of RT. These symptoms were selected a priori as a representative combination of problematic local and systemic symptoms in HNC [2]. Because the overall goal of this study was to generate estimates of the potential signal and benefit of minocycline to inform future clinical trial design, we selected a modest power (70%) to detect a relatively large standardized effect size of 0.70 (one-sided, 5% significance level) on the primary end-point, requiring 20 evaluable patients per arm. We prespecified that patients must provide at least 2 weeks of MDASI-HN data to be evaluable. Missing symptom data were imputed using the last observation carried forward method.
Clinical characteristics by treatment group were compared using independent t tests for continuous variables and chi-square tests for categorical variables. Adverse events and toxicities were tabulated.
Exploratory analyses
Effect sizes comparing treatment groups by all individual MDASI-HN item AUCs were tabulated. Similarly, AUC comparisons by treatment group for all 6 individual MDASI-HN symptom interference items also are presented.
The primary outcome involved examining symptom severity during cancer treatment. However, we also collected follow-up symptom data for up to 6 weeks after the end of treatment. In addition, we were interested in examining systemic symptoms (based on the composite of disturbed sleep, reduced appetite, fatigue, pain, and drowsiness) and local head and neck symptoms (based on dry mouth, taste change, mucus, mouth sores, and chewing problems). Further, to determine whether there were group differences in MDASI-HN core and head and neck-specific symptoms, we fitted piecewise linear mixed models over 84 days with a breakpoint at day 42. The breakpoint coincides with the traditional symptom peak and end of cancer treatment. A restricted model incorporated all potential covariates and main effects. These covariates include age, sex, cancer stage, diagnosis (tonsil vs non tonsil), and laterality (unilateral vs bilateral). Day was included as a continuous fixed-effect variable. The full model includes all predictors in the restricted model in addition to the group-by-time interaction terms (linear and quadratic). We tested for significance by using chi-square tests on the difference between the full and restricted models.
Results
Patient characteristics
From August 2010 to July 2013, 82 of 1744 screened patients were identified as eligible and were approached. Of these, 47 provided study-specific informed consent and were enrolled; 7 patients subsequently elected to withdraw or provided less than 2 weeks of symptom reports. Of these, 2 were from the intervention group and 5 were from the placebo group. Thus, 20 evaluable patients in each arm formed the cohort used for symptom analyses, although all 47 enrolled were monitored for safety (see Fig. 1). Patient and clinical characteristics by study arm are shown in Table 1.
Fig. 1.
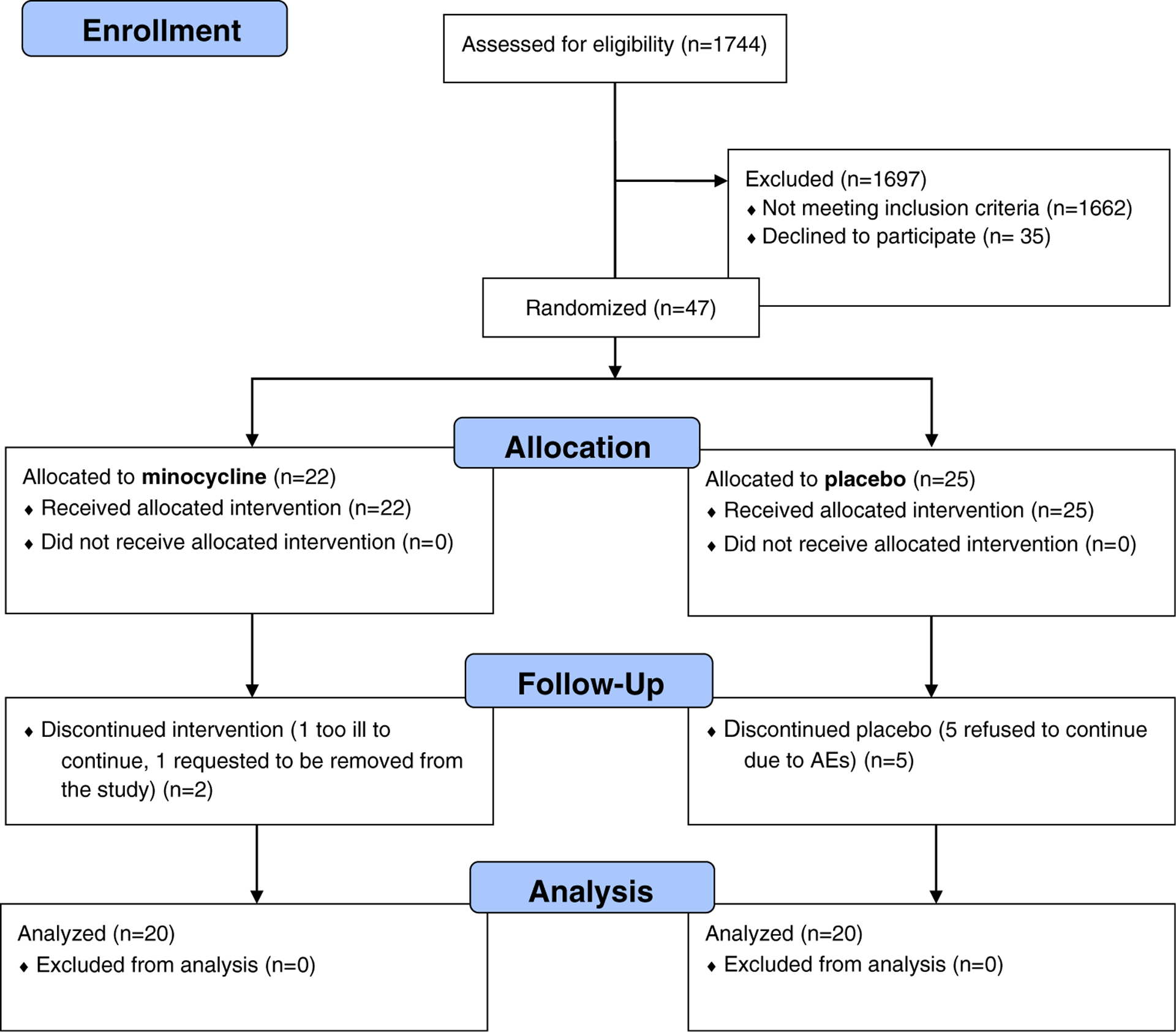
Minocycline study CONSORT flow diagram
Table 1.
Patient, tumor, and treatment characteristics of evaluable patients, by study group
| Minocycline (n = 20) | Placebo (n = 20) | P | |
|---|---|---|---|
| Age | 56.15 (SD 5.8) | 55.6 (SD 10.2) | 0.58 |
| Educational level | 0.63 | ||
| 12th grade and below | 3 | 2 | |
| Beyond 12th grade | 17 | 18 | |
| Race | 0.31 | ||
| White non-Hispanic | 19 | 20 | |
| Hispanic | 1 | 0 | |
| Sex (Male) | 14 | 17 | 0.45 |
| Feeding tube (yes) | 2 | 5 | 0.21 |
| Diagnosis | 0.76 | ||
| Tonsil | 10 | 8 | |
| Base of tongue | 5 | 7 | |
| Overlap/unknown | 5 | 5 | |
| ECOG performance status | 0.05 | ||
| Grade 0 | 16 | 10 | |
| Grade 1 | 4 | 10 | |
| Stage | 0.61 | ||
| I | 1 | 1 | |
| II | 2 | 4 | |
| III | 3 | 5 | |
| IV | 14 | 10 | |
| T category | 0.18 | ||
| 0 | 4 | 4 | |
| I | 13 | 7 | |
| 2 | 2 | 7 | |
| X | 1 | 2 | |
| N category | 0.43 | ||
| 0 | 2 | 2 | |
| 1 | 2 | 5 | |
| 2a | 5 | 3 | |
| 2b | 8 | 7 | |
| 2c | 1 | 3 | |
| X | 2 | 0 | |
| RT dose | 66.6 | 67.3 | 0.56 |
| Induction chemotherapy (yes) | 4 | 7 | 0.29 |
| Neck RT location | 0.74 | ||
| Ipsilateral | 14 | 13 | |
| Bilateral | 6 | 7 | |
| Charlson comorbidity index | 0.10 (SD 0.31) | 0.15 (SD 0.37) | 0.65 |
ECOG Eastern Cooperative Oncology Group performance status, RT radiation therapy, SD standard deviation
Compliance and toxicity
Symptom assessment compliance
Patients in the minocycline and placebo groups were equally compliant in responding about their symptoms. The overall compliance rate, calculated as the ratio of the actual number of MDASI-HN questionnaires completed by patients divided by the number of expected MDASI-HNs, was 90% from baseline to day 42 for both groups and 78% from baseline to day 87 for both groups.
Study medication compliance
Overall, study medication compliance based on pill count was 87% (standard deviation [SD] 16%). There were no significant differences in compliance by group (88% for minocycline vs 86% for placebo; P < 0.65).
No grade ≥ 3 adverse events that were potentially related to study medication were reported. Seven grade 1–2 toxicities reported in the minocycline arm and 2 in the placebo arm (P = 0.53) were judged to be potentially related to the study medication. Table 2 summarizes the aggregate RT–related toxicity observed, by treatment group. Differences between groups were not statistically significant for any item. Two patients in the minocycline arm required feeding tube placement during RT, versus 5 in the placebo arm (P = 0.21).
Table 2.
Adverse events, by treatment group: proportion of patients with peak toxicity grade ≥ 2
| Adverse event | Minocycline (n = 20) | Placebo (n = 20) |
|---|---|---|
| Atrial fibrillation | 5% | 0% |
| Diarrhea | 5% | 0% |
| Dizziness | 5% | 5% |
| Fever | 5% | 0% |
| Lethargy | 5% | 0% |
| Nausea | 0% | 10% |
| Vomiting | 5% | 5% |
| Mucositisa | 100% | 100% |
| Dermatitis | 50% | 65% |
| Fatigue | 20% | 25% |
| Esophagitis | 5% | 0% |
| Pulmonary | 0% | 0% |
| Cardiac | 0% | 0% |
| Motor neuropathy | 0% | 0% |
| Sensory neuropathy | 0% | 0% |
| Pain | 35% | 50% |
% peak grade 3 by treatment group: minocycline = 75%; placebo = 65%
Primary endpoint
The average daily AUCs for the 5 preselected symptoms (based on the composite of dry mouth, lack of appetite, fatigue, pain, and drowsiness) during RT were 3.1 (SD 1.0) for the minocycline group and 3.7 (SD 1.7) for the placebo group. The absolute group difference of 0.6 was not statistically significant (95% CI – 0.26 to 1.53; P = 0.16) and was equivalent to a standardized effect size of 0.37. This indicates that the minocycline group had a reduced average daily AUC of 0.37 SD compared with the placebo group.
Exploratory analyses
Group differences in individual symptoms
The effect size comparisons in AUCs for individual symptoms favored minocycline but were not statistically significant.
Table 3 shows a trend of favorable effect in the minocycline group for individual symptoms and symptom interference during the 6–7 weeks of RT. The group effect size difference was largest for numbness, followed by choking, vomiting, problem with voice, and disturbed sleep, which differed from the preselected top 5 symptoms (dry mouth, lack of appetite, fatigue, pain, and drowsiness).
Table 3.
Group effect sizes for MDASI-HN symptom and interference items, area under the curve sorted by decreasing magnitude
| Baseline | Minocycline | Placebo | SD at baseline | Group comparison effect sizea |
|---|---|---|---|---|
| Symptoms | ||||
| Numbness | 48.76 | 63.95 | 26.34 | 0.35 |
| Choking/coughing | 57.83 | 78.50 | 34.16 | 0.30 |
| Vomiting | 52.80 | 72.18 | 31.45 | 0.30 |
| Difficulty with voice/speech | 59.20 | 79.10 | 36.18 | 0.29 |
| Disturbed sleep | 58.75 | 89.00 | 34.46 | 0.27 |
| Pain | 64.90 | 104.55 | 40.30 | 0.24 |
| Nausea | 59.87 | 87.83 | 41.19 | 0.21 |
| Lack of appetite | 64.40 | 106.50 | 42.65 | 0.20 |
| Distress | 55.46 | 85.13 | 38.12 | 0.20 |
| Fatigue | 63.90 | 106.70 | 42.64 | 0.20 |
| Drowsiness | 63.54 | 100.40 | 44.23 | 0.19 |
| Shortness of breath | 47.61 | 71.13 | 34.08 | 0.19 |
| Difficulty swallowing/chewing | 63.22 | 106.80 | 43.36 | 0.19 |
| Problem with teeth/gums | 56.66 | 85.15 | 41.81 | 0.17 |
| Skin pain/burning/rash | 53.93 | 88.33 | 40.44 | 0.15 |
| Problem with mucus | 62.91 | 114.45 | 45.92 | 0.15 |
| Sadness | 51.18 | 80.75 | 39.31 | 0.15 |
| Difficulty remembering | 45.92 | 72.73 | 35.83 | 0.14 |
| Mouth/throat sores | 59.99 | 109.28 | 48.57 | 0.10 |
| Dry mouth | 62.62 | 125.10 | 49.56 | 0.10 |
| Problem with tasting food | 63.34 | 136.00 | 50.97 | 0.09 |
| Constipation | 50.36 | 85.28 | 51.99 | 0.02 |
| Symptom interference | ||||
| Enjoyment of life | 64.23 | 115.18 | 96.15 | 0.30 |
| Walking | 54.55 | 88.50 | 73.95 | 0.27 |
| Activity | 60.57 | 112.85 | 97.08 | 0.26 |
| Work | 64.85 | 109.23 | 95.00 | 0.22 |
| Mood | 58.72 | 102.10 | 89.75 | 0.21 |
| Relations with others | 58.86 | 93.40 | 85.83 | 0.13 |
SD standard deviation
Positive effect sizes indicate that the minocycline group had less-severe symptom/interference than the placebo group. The effect sizes ranged from small to moderate by item, and all were favorable toward minocycline
Group differences in symptom interference
Similar to the individual symptoms, symptom interference AUCs were smaller for the minocycline group. Table 3 demonstrates that the group effect size difference was largest for enjoyment of life, followed by walking, activity, work, mood, and relations with others.
Group differences during and after therapy
After the initiation of RT, a core set of systemic symptoms (component score of disturbed sleep, lack of appetite, fatigue, pain, and drowsiness) and local head and neck symptoms (component score of dry mouth, taste change, mucus, mouth sores, and chewing problems) rapidly increased and peaked around the time of therapy completion. In piecewise linear mixed–effects models, the minocycline and placebo groups differed significantly in the severity of the core systemic symptoms (P < 0.005) but not in the severity of local head and neck symptoms (P < 0.13). Figure 2 presents longitudinal curves for the component scores of the systemic core symptoms by group, adjusting for the effects of relevant covariates via linear mixed models that included PRO data beyond the RT period, for a total of 12–13 weeks.
Fig. 2.
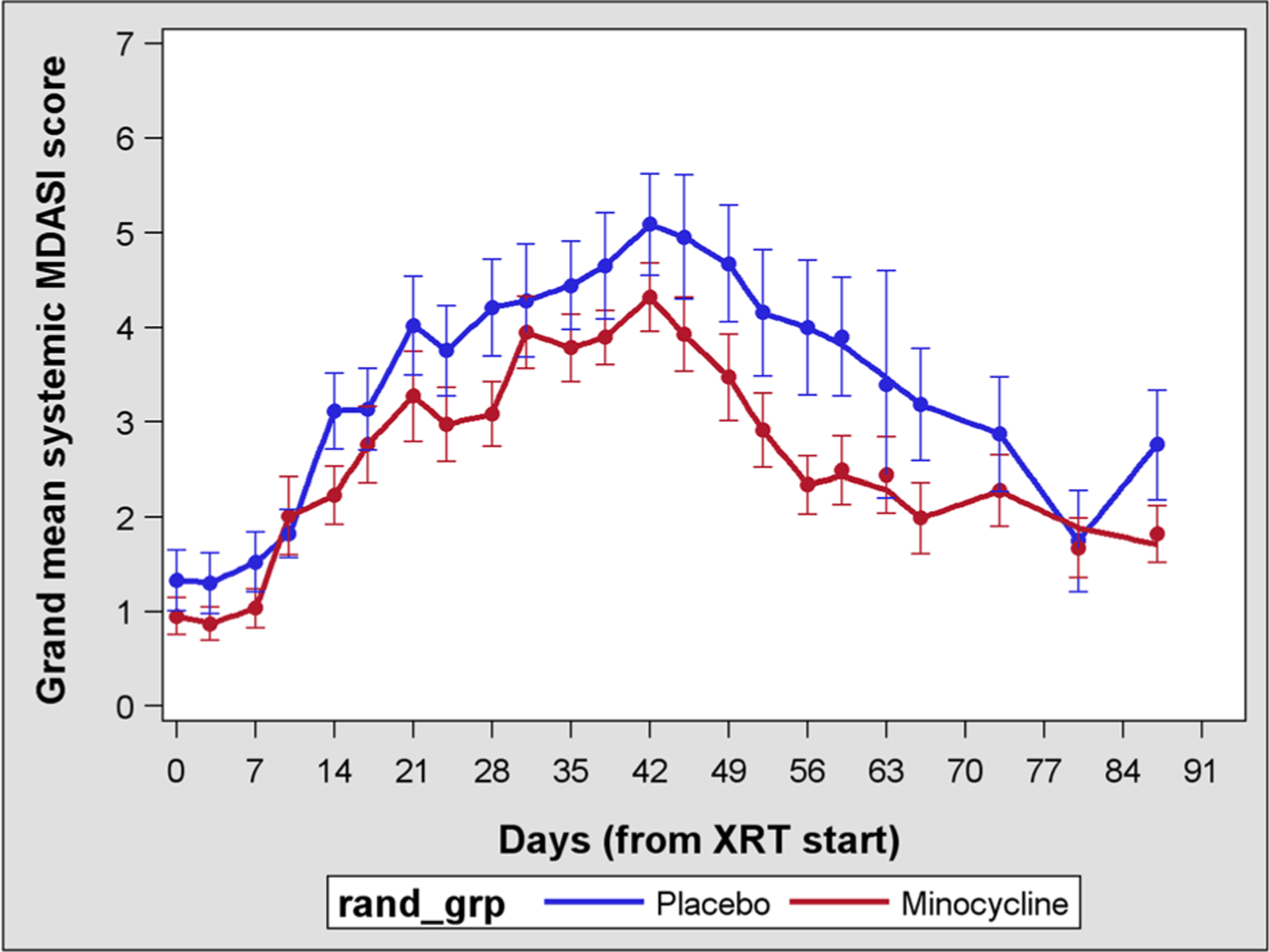
Longitudinal curves for systemic symptoms over 12 weeks
Discussion
In this study of patients with HNC receiving RT, we investigated a safe, widely prescribed anti-inflammatory agent (minocycline) that has shown an ability to reduce symptoms in other diseases such as inflammatory acne and rheumatoid arthritis [7, 9]. In oncology, several studies [18–23] have indicated that increased inflammation is associated with increases in symptoms during various cancer therapies. We performed this study to see if the acute treatment–related symptoms of patients with head and neck cancer receiving RT might be reduced by the administration of minocycline during active treatment.
We found that minocycline during RT for HNC was feasible and well tolerated. No potentially study medication-related grade 3 adverse events were reported. Although 7 patients (2 in the minocycline group and 5 in the placebo group) discontinued therapy, this was unrelated to the use of minocycline. Our primary endpoint (AUC of preselected symptoms during the treatment period, comparing minocycline with placebo) was not statistically significant, and with a corresponding effect size of 0.37, favored minocycline, yet our prespecified effect size of 0.70 was not obtained. Although exploratory studies typically set a relatively large target effect size, an effect size of ~ 1/3 could have clinical importance in this disease site with overall high symptom burden, particularly given the lack of effective symptom reduction therapies.
Nonetheless, our exploratory analyses revealed larger effect sizes for some symptoms after the end of RT. In fact, under the linear mixed model, there was a statistically significant difference in the severity of the core systemic symptoms between the minocycline and placebo groups (P < 0.005), but not in the severity of local head and neck symptoms (P < 0.13), when we included symptoms beyond the 6–7-week treatment period. This is a somewhat expected finding, given that mucositis-related and other acute symptoms directly attributable to RT could overwhelm any modest (but still potentially meaningful) drug effect. Even though our primary endpoint focused solely on symptoms during RT, rapidity of recovery and/or return to baseline are important clinical measures of patient outcomes/toxicity. Our finding of reduction in systemic symptoms in the minocycline group during the early recovery period (posttreatment) highlights the potential of focusing on symptom-recovery kinetics in future studies or extending the drug period through the recovery phase to see if these differences could be further magnified.
Although we did not see statistically significant differences in symptom severity between minocycline and placebo during the treatment period in this small trial, we did observe symptom reduction in the immediate posttreatment period that might be associated with minocycline. We also observed a positive effect of minocycline on systemic symptoms (e.g., pain, fatigue) that might be expected, given that preclinical data demonstrate an effect on symptoms known to be associated with inflammation/sickness behavior [5]. It also worth noting that every symptom and interference item numerically favored minocycline over placebo. Altogether, these findings may support pursuit of a larger trial with a longer study medication period. Several study limitations should be noted, including lack of correlative inflammatory biomarkers, relatively larger number of unilateral compared to bilateral neck RT cases, lack of opioid utilization data, and lack of utilization and compliance with available supportive services, any of which could have influenced these results.
In designing future trials, important considerations are the length of study drug/intervention, optimal minocycline dose, and timing of symptom assessment and the choice of symptoms to include as the primary outcome. As illustrated here, we observed significant differences when we included an additional 6 weeks of posttherapy symptoms (for a total assessment period of 12–13 weeks) instead of only including symptoms reported during the 6–7 weeks of treatment. The choice of symptom set also has an effect. We selected a set of 5 symptoms a priori on the basis of previous research, only to find that a completely different set of symptoms were more severe for this particular cohort.
Conclusion
Minocycline was not effective in reducing either local or systemic symptoms during the acute treatment period but did show reduction in systemic symptoms (disturbed sleep, reduced appetite, fatigue, pain, and drowsiness) during the 6– 7-weeks posttreatment. Given that this may be of potential benefit to patients, additional exploration of minocycline for reduction of treatment-related symptoms may be warranted. Future trials of minocycline should include the posttreatment recovery period.
Acknowledgments
The authors extend special thanks to Jeanie F. Woodruff, BS, ELS, for editorial comments.
Grant or financial support
Supported in part by an Institutional Research Grant from The University of Texas MD Anderson Cancer Center and by grants from the National Cancer Institute of the National Institutes of Health: grants R01 CA026582 and P01 CA124787 (PI: Charles Cleeland); the Paul Calabresi Clinical Trial Program Subaward of NCI grant K12 CA088084 (PI: Robert Bast); and the MD Anderson Cancer Center Support (Core) Grant P30 CA016672 (PI: Peter Pisters).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of interest
The MD Anderson Symptom Inventory is copyrighted and licensed by The University of Texas MD Anderson Cancer Center and Charles S. Cleeland. Xin Shelley Wang is the spouse of Charles S. Cleeland.
Previous presentation Presented in part at the American Society for Clinical Oncology (ASCO) Annual Meeting; Chicago, Illinois, 06/2014.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Garden AS, Kies MS, Morrison WH, Weber RS, Frank SJ, Glisson BS, Gunn GB, Beadle BM, Ang KK, Rosenthal DI, Sturgis EM (2013) Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol 8(21). 10.1186/1748-717X-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal DI, Mendoza TR, Fuller CD, Hutcheson KA, Wang XS, Hanna EY, Lu C, Garden AS, Morrison WH, Cleeland CS, Gunn GB (2014) Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 120(13):1975–1984. 10.1002/cncr.28672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goepfert RP, Fuller CD, Gunn GB, Hanna EY, Lewin JS, Zaveri JS, Hubbard RM, Barrow MP, Hutcheson KA (2017) Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck 39(11):2151–2158. 10.1002/hed.24879 [DOI] [PubMed] [Google Scholar]
- 4.Mirghani H, Blanchard P (2018) Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol 8:4–11. 10.1016/j.ctro.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dantzer R, Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21(2): 153–160. 10.1016/j.bbi.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantzer R, Meagher MW, Cleeland CS (2012) Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol 9(7):414–426. 10.1038/nrclinonc.2012.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrido-Mesa N, Zarzuelo A, Galvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169(2):337–352. 10.1111/bph.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CJ, Sayles H, Mikuls TR, Michaud K (2011) Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res Ther 13(5):R168 10.1186/ar3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Dell JR, Blakely KW, Mallek JA, Eckhoff PJ, Leff RD, Wees SJ, Sems KM, Fernandez AM, Palmer WR, Klassen LW, Paulsen GA, Haire CE, Moore GF (2001) Treatment of early seropositive rheumatoid arthritis: a two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis Rheum 44(10): 2235–2241 [DOI] [PubMed] [Google Scholar]
- 10.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21(8):2580–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plane JM, Shen Y, Pleasure DE, Deng W (2010) Prospects for minocycline neuroprotection. Arch Neurol 67(12):1442–1448. 10.1001/archneurol.2010.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Yuan K, Schluesener H (2013) Impact of minocycline on neurodegenerative diseases in rodents: a meta-analysis. Rev Neurosci 24(5):553–562. 10.1515/revneuro-2013-0040 [DOI] [PubMed] [Google Scholar]
- 13.Bonelli RM, Hodl AK, Hofmann P, Kapfhammer HP (2004) Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol 19(6):337–342 [DOI] [PubMed] [Google Scholar]
- 14.Leporini C, Saullo F, Filippelli G, Sorrentino A, Lucia M, Perri G, Gattuta GL, Infusino S, Toscano R, Dima G, Olivito V, Paletta L, Bottoni U, De Sarro G (2013) Management of dermatologic toxicities associated with monoclonal antibody epidermal growth factor receptor inhibitors: a case review. J Pharmacol Pharmacother 4(Suppl 1):S78–S85. 10.4103/0976-500X.120966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melosky B, Anderson H, Burkes RL, Chu Q, Hao D, Ho V, Ho C, Lam W, Lee CW, Leighl NB, Murray N, Sun S, Winston R, Laskin JJ (2016) Pan Canadian Rash Trial: a randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol 34(8):810–815. 10.1200/JCO.2015.62.3918 [DOI] [PubMed] [Google Scholar]
- 16.Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ (2012) Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135(Pt 4):1224–1236. 10.1093/brain/aws072 [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal DI, Mendoza TR, Chambers MS, Asper JA, Gning I, Kies MS, Weber RS, Lewin JS, Garden AS, Ang KK, X SW, Cleeland CS (2007) Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck 29(10): 923–931. 10.1002/hed.20602 [DOI] [PubMed] [Google Scholar]
- 18.Shi Q, Wang XS, Li G, Shah ND, Orlowski RZ, Williams LA, Mendoza TR, Cleeland CS (2015) Racial/ethnic disparities in inflammatory gene single-nucleotide polymorphisms as predictors of a high risk for symptom burden in patients with multiple myeloma 1 year after diagnosis. Cancer 121(7):1138–1146. 10.1002/cncr.29154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XS, Shi Q, Shah ND, Heijnen CJ, Cohen EN, Reuben JM, Orlowski RZ, Qazilbash MH, Johnson VE, Williams LA, Mendoza TR, Cleeland CS (2014) Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res 20(5):1366–1374. 10.1158/1078-0432.CCR-13-2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z (2010) Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun 24(6):968–974. 10.1016/j.bbi.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, Shi Q, Mobley GM, Woodruff JF, Cleeland CS (2012) Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun 26(5):699–705. 10.1016/j.bbi.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood LJ, Weymann K (2013) Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care 7(1):54–59. 10.1097/SPC.0b013e32835dabe3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwekkeboom KL, Tostrud L, Costanzo E, Coe CL, Serlin RC, Ward SE, Zhang Y (2018) The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manag 55(5):1286–1295. 10.1016/j.jpainsymman.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


