Abstract
Objectives
We sought to determine whether smokers with oral cavity squamous cell carcinoma (OCSCC) have tumors with more adverse pathological features than in non-smokers, and whether or not these are predictive of outcomes.
Materials/Methods
We retrospectively identified 163 patients with AJCC stages I-IVa OCSCC diagnosed between 2005–2015, and treated with curative intent. A pathological risk score (PRS) was calculated using the NCCN adverse risk factors: positive margin, extracapsular extension of lymph node metastases, pT3 or pT4 primary, N2 or N3 nodal disease, perineural invasion and lymphovascular space invasion. Multivariable models were constructed to determine independent predictors of overall survival (OS), recurrence-free survival (RFS), and PRS.
Results
108 (66.26%) were smokers, and 55 non-smokers. Three-year actuarial OS and RFS were 62% and 68% in smokers, and 81% and 69% in non-smokers, respectively (p=0.06 and p=0.63). Smokers were more likely to have advanced disease stage and tumors with aggressive pathological features than non-smokers. Smokers had significantly worse PRS (mean ± SD; 2.38 ± 2.19, median; 2.00) than non-smokers (0.89 ± 1.21, 0.00) (p <0.001). Older age, higher PRS and smoking status were independent predictors of OS. Smoking or PRS did not predict for worse RFS. On multivariate analysis, independent predictors of PRS were smoking status, and grade (p <0.001).
Conclusion
In patients with OCSCC, smokers have more aggressive disease as evidenced by more adverse pathological features than non-smokers. Moreover, smoking is an independent predictor of OS, but not RFS. The PRS is a significant predictor of OS and needs validation in future studies.
Keywords: Oral cavity, oral cancer, head and neck cancer, squamous cell carcinoma, smoking, tobacco, prognosis, survival, pathology
Introduction
The annual incidence of oral cavity squamous cell carcinoma (OCSCC) is approximately 32,000 new cases with around 6,500 expected deaths. [1]
Incidence of oral cancers has decreased overall in the United States in the past fifty years, [2,3] although this trend varies by oral cavity subsite: incidence was shown to be stable from 1973 to 2004 for oral tongue cancers, but significantly decreased during that period for cancers of gingiva, floor of mouth, palate and other subsites of the oral cavity. [3] This decreasing trend is thought to be due to decreased tobacco use in the United States. [4] Cigarette smoking is indeed the most commonly cited risk factor for OCSCC, and tobacco users have consistently been shown to be at a 5- to 25-fold higher risk than non-smokers, with a clear dose-response relationship. [5,6] Alcohol consumption works synergistically with tobacco smoking and increases the risk of OCSCC by several folds; a greater than multiplicative effect between these two risk factors has been demonstrated. [7] Because around 75% of OCSCC are attributable to these exposures, [4] the World Health Organization (WHO) has established the Global Oral Health Programme in an attempt to combat modifiable risk factors, the most important of which is smoking. [8]
Based on the National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) Program 2005–2011, the five-year overall survival (OS) for OCSCC is 63%, ranging from 83% for early stages to 38% for advanced stages. [9]
The management of OCSCC relies heavily on a multidisciplinary approach, and a close collaboration between surgeons, medical oncologists and radiation oncologists. For early-stage lesions, surgery is the mainstay of treatment. [10] The presence of certain pathological features, such as lymphovascular space invasion (LVSI), perineural invasion (PNI), extracapsular extension (ECE) of lymph node metastasis, inadequate resection margins, multiple involved neck lymph nodes, advanced pathologic T stage (pT3 or pT4), has been consistently shown to adversely affect outcomes in patients with head and neck squamous cell carcinoma, including OCSCC. [11–17]
In patients with these adverse features, the National Comprehensive Cancer Network (NCCN) recommends adjuvant radiation therapy or chemo-radiation (NCCN). [10] Concurrent chemotherapy is recommended in the presence of positive margins or extracapsular extension of lymph node metastasis. This recommendation is based on the Radiation Therapy Oncology Group (RTOG) 9501/European Organization for Research and Treatment of Cancer (EORTC) 22931 combined analysis. [18–20]
To the best of our knowledge, the relationship between smoking and all of these adverse clinicopathologic features in OCSCC has never been thoroughly investigated. The aim of our study is to determine whether smoking predicts for more aggressive pathological features and worse survival outcomes in patients with OCSCC.
Materials and methods
Patients
We identified 163 patients with AJCC stages I-IVa OCSCC diagnosed between 2005 and 2015 and treated with curative intent at our institution. Patients with squamous cell carcinoma involving the following subsites were included: buccal mucosa, upper and lower alveolar ridge, retromolar trigone, floor of mouth, hard palate, and anterior two-thirds of the tongue. All patients underwent surgical resection of the primary tumor with or without unilateral/bilateral neck dissection, with or without adjuvant therapy (radiation therapy (RT) or radiation therapy with concurrent chemotherapy (RT/CT)), depending on the presence of risk factors and after multidisciplinary tumor board discussion. A retrospective medical chart review was conducted identifying various clinico-pathologic variables, including information on smoking status assessed pre-operatively.
A pathological risk score was calculated using the NCCN-defined adverse risk factors: [10] 1 point was assigned to each of the following: pT3 or pT4 primary, N2 or N3 nodal disease, PNI and LVSI, and 2 points were assigned to positive margin as well as to ECE of lymph node metastases, because the presence of these last two parameters would be an indication for trimodality therapy. The sum of these points resulted in a total possible score of 0–8 with a higher score indicating worse pathological features.
Statistical analysis
Differences in participant characteristics were compared by smoking status using Chi-Square or Fisher’s exact tests for categorical variables and Wilcoxon Rank Sum for continuous variables. Univariate survival analysis was performed using Cox regression for continuous variables, and Kaplan-Meier and log-rank test for categorical variables. OS was calculated from date of diagnosis to date of death or last contact, and recurrence-free survival (RFS) was calculated from date of diagnosis to date of disease recurrence, death or last contact.
A multivariate analysis (MVA) using Cox regression models was performed to determine independent predictors of OS and RFS endpoints. A multivariable linear regression model was built to study the association between different predictive variables and pathological risk score. Multivariable models were selected by first including any predictor with a univariate p-value <0.2 and then employing stepwise selection. We constructed two different multivariable models for each of the OS and RFS outcomes: one model excluding the pathological risk score variable, while including all of its individual components, and a second model including the pathological risk score variable, and with all of its six score components excluded to avoid collinearity. All variables in the final models were significant at p < 0.05.
In order to determine whether smokers were more likely to receive adjuvant treatment (RT or RT/CT) than non-smokers given a specific pathological score, we studied the interaction between smoking status and pathological risk score, with postoperative treatment as the outcome of interest in this final logistic regression analysis.
All analyses were performed using SAS 9.4 and statistical significance was set at p<0.05.
Results
Patients’ characteristics
Patients’ characteristics are shown in Table 1. Patients’ population median age was 60 (range, 25–93). 108 (66.26%) patients in our cohort were smokers, and 55 were non-smokers. On univariate analysis, smokers were more likely to use alcohol (60.2% in smokers versus 34.6%, p<0.01), and more likely to have advanced disease stage and tumors with aggressive pathologic features than non-smokers: 38.9% pT3-pT4 in smokers versus 18.2% in non-smokers; 38.0% N2 in smokers versus 7.3% in non-smokers; 27.8% ECE of LN metastases in smokers versus 7.3% in non-smokers; 29.6% LVSI in smokers versus 10.9% in non-smokers; and 47.2% PNI in smokers versus 16.4% in non-smokers, all with p<0.01. Consequently, adjuvant concurrent chemo-radiation was more commonly used in smokers (38.9%) than non-smokers (20%) (borderline significance, p=0.057).
Table 1:
Characteristics of the study population of 163 patients with oral cavity squamous cell carcinoma
| Variables | Patient groups |
P | ||
|---|---|---|---|---|
| Entire sample (n=163), n (%), mean ± SD, median | Smokers (n=108), n (%), mean ± SD, median | Nonsmokers (n=55), n (%), mean ± SD, median | ||
| Sex | ||||
| Male | 104 (63.8) | 74 (68.5) | 30 (54.6) | 0.079 |
| Female | 59 (36.2) | 34 (31.5) | 25 (45.4) | |
| Race | ||||
| Caucasian | 91 (55.8) | 59 (54.6) | 32 (58.2) | 0.617 |
| African-American | 29 (17.8) | 18 (16.7) | 11 (20.0) | |
| Other/unknown | 43 (26.4) | 31 (28.7) | 12 (21.8) | |
| Age | ||||
| ≤50 | 36 (22.1) | 25 (23.2) | 11 (20.0) | 0.647 |
| >50 | 127 (77.9) | 83 (76.8) | 44 (80.0) | |
| Alcohol use | ||||
| Yes | 84 (51.5) | 65 (60.2) | 19 (34.6) | 0.002 |
| No | 79 (48.5) | 43 (39.8) | 36 (65.4) | |
| Oral cavity subsite | ||||
| Oral tongue | 92 (56.4) | 54 (50.0) | 38 (69.1) | 0.052 |
| Floor of mouth | 43 (26.4) | 36 (33.3) | 7 (12.7) | |
| Alveolar ridge | 9 (5.5) | 6 (5.6) | 3 (5.5) | |
| Retromolar trigone | 5 (3.1) | 3 (2.8) | 2 (3.6) | |
| Other | 14 (8.6) | 9 (8.3) | 4 (7.3) | |
| Pathological tumor T status | ||||
| T1-T2 | 111 (68.1) | 66 (61.1) | 45 (81.8) | 0.007 |
| T3-T4 | 52 (31.9) | 42 (38.9) | 10 (18.2) | |
| Pathological nodal status | ||||
| N0 | 98 (60.1) | 53 (49.0) | 45 (81.8) | <0.001 |
| N1 | 20 (12.3) | 14 (13.0) | 6 (10.9) | |
| N2 | 45 (27.6) | 41 (38.0) | 4 (7.3) | |
| Tumor AJCC TNM stage | ||||
| I | 57 (35.0) | 28 (25.9) | 29 (52.7) | 0.001 |
| II | 27 (16.6) | 16 (14.8) | 11 (20.0) | |
| III | 17 (10.4) | 13 (12.0) | 4 (7.3) | |
| IVa | 62 (38.0) | 51 (47.2) | 11 (20.0) | |
| Pathological findings | ||||
| Positive SM | 22 (13.5) | 16 (14.8) | 6 (10.9) | 0.746 |
| ECE of LN metastasis | 34 (20.9) | 30 (27.8) | 4 (7.3) | 0.002 |
| LVSI | 38 (23.3) | 32 (29.6) | 6 (10.9) | 0.008 |
| PNI | 60 (36.8) | 51 (47.2) | 9 (16.4) | <0001 |
| Total pathological risk score | 1.88±2.04, 1 | 2.39±2.19, 2 | 0.89±1.21 | <0.001 |
| Treatment | ||||
| Surgery alone | 70 (43.0) | 39 (36.1) | 31 (56.4) | 0.057 |
| Surgery + RT | 40 (24.5) | 27 (25) | 13 (23.6) | |
| Surgery + RT/CT* | 53 (32.5) | 42 (38.9) | 11 (20) | |
Refers to platinum-based chemotherapy or immunotherapy with cetuximab concurrent with radiation therapy. SD=Standard deviation, AJCC=American Joint Committee on cancer, SM=surgical margins, ECE=Extracapsular extension, LN=Lymph node, LVSI=Lymphovascular space invasion, PNI=Perineural invasion, RT=Radiation therapy, CT=Chemotherapy, TNM=Tumor/node/metastasis
Survival analysis
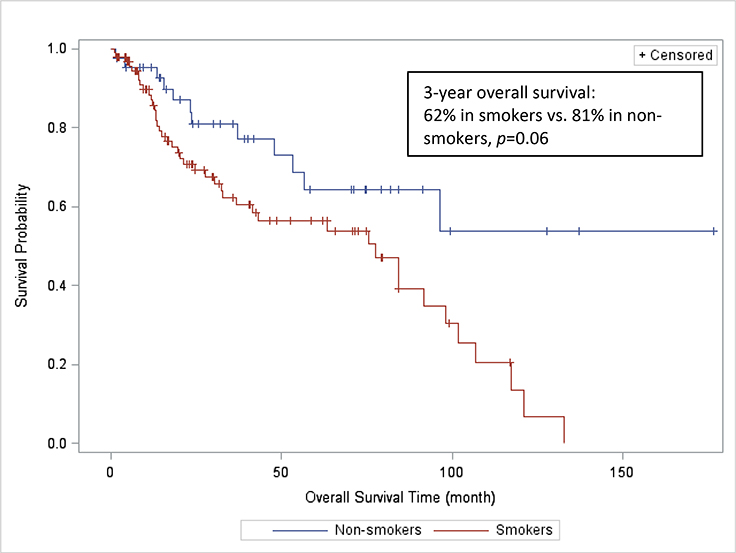
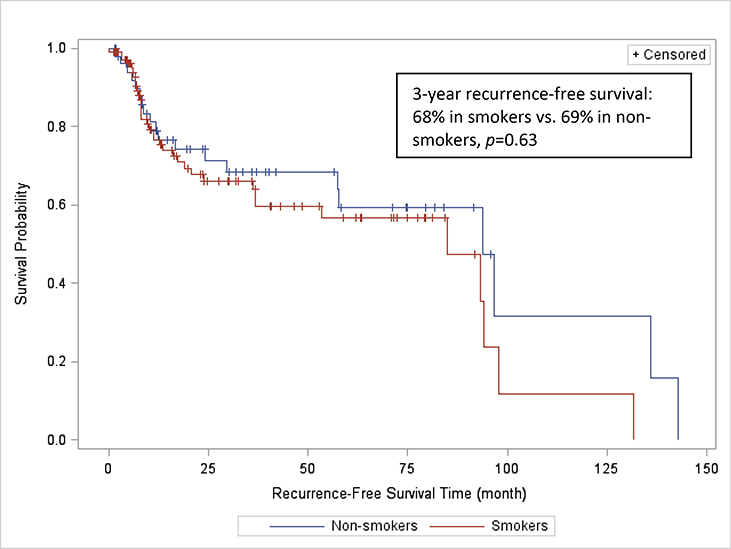
143 patients (99 smokers and 44 non-smokers) were included in the survival analysis. The remaining 20 patients were not included in the survival analysis due to insufficient follow-up time. After a median follow-up of 44.5 months, 55 deaths were recorded (43 (43.4%) in smokers, and 12 (27.3 %) in non-smokers). Three-year actuarial OS was 62% and 81% in smokers and non-smokers, respectively (p=0.06) (Figure 1). Three-year actuarial RFS was 68% and 69% in smokers and non-smokers, respectively (p=0.63) (Figure 2).
FIGURE 1:
Overall survival rates of smokers and non-smokers with squamous cell carcinoma of the oral cavity
FIGURE 2:
Recurrence-free survival rates of smokers and non-smokers with squamous cell carcinoma of the oral cavity
Table 2 depicts the first multivariable model, which includes the six NCCN adverse risk factors and excludes the pathological risk score. In the entire sample, older age, presence of LVSI (HR=2.27, 95% CI 1.28–4.02), presence of PNI (2.19, 95% CI 1.13–4.24), and nodal status (N2 versus N0: HR=3.47, 95% CI 1.70–7.10) were independent predictors of OS. The presence of LVSI, female gender, advanced T stage and nodal status significantly predicted for worse recurrence-free survival (RFS). No independent predictors of RFS were found in this MVA model in the entire sample. In particular, smoking did not predict for worse RFS. In smokers, the presence of ECE and nodal status significantly predicted for worse OS in this MVA model. Positive margins and nodal status were independent predictors of RFS in smokers.
Table 2:
Multivariate analysis of survival outcomes in the entire sample and in the subset of smokers
| Variables | HR (95% CI) |
|
|---|---|---|
| Overall survival | Recurrence-free survival | |
| Entire sample (n=163) | ||
| Age (continuous) | 1.05 (1.03–1.08) | - |
| Sex (female vs. male) | - | 1.81 (1.01–3.25) |
| LVSI | 2.27 (1.28–4.02) | 2.05 (1.07–3.94) |
| PNI | 2.19 (1.13–4.24) | - |
| Nodal status | ||
| N2 versus N0 | 3.47 (1.70–7.10) | 2.67 (1.25–5.69) |
| N1 versus N0 | 1.49 (0.60–3.73) | 1.96 (0.75–5.13) |
| T status | - | |
| T4 versus T1 | 1.06 (0.46–2.44) | |
| T3 versus T1 | 3.97 (1.53–10.32) | |
| T2 versus T1 | 0.73 (0.34–1.53) | |
| Smokers (n=108) | ||
| Age (continuous) | 1.04 (1.01–1.07) | - |
| ECE | 2.18 (1.01–4.71) | - |
| Nodal status | ||
| N2 versus N0 | 3.73 (1.35–10.29) | 2.55 (1.19–5.48) |
| N1 versus N0 | 1.78 (0.57–5.52) | 1.28 (0.35–4.67) |
| Margin status | - | |
| Positive versus close (<0.5 cm) | 4.88 (1.32–18.07) | |
| Positive versus negative (≥0.5 cm) | 2.22 (1.01–4.86) | |
Description: This second model includes the pathological risk score as a continuous variable and excludes all of its individual factors. We only included in this table the predictive variables that were found to be significantly associated with survival outcomes. HR=Hazard ratio, CI=Confidence interval, LVSI=Lymphovascular space invasion, PNI=Perineural invasion, ECE=Extracapsular extension of lymph node metastasis
Table 3 consists of the second multivariable model, which includes pathological risk score as a continuous variable and excludes its individual components. In the entire sample, older age, smoking status, and worse pathological risk score were independent predictors of OS. The HR for death for smokers versus non-smokers was 2.28 (95% CI 1.12–4.64). Also, for every point increase in the pathological risk score, the risk of death increased by 1.50 fold (95% CI 1.30–1.72). Similarly to the first MVA model, smoking status did not predict for worse RFS.
Table 3:
Multivariate analysis of survival outcomes in the entire sample and in the subset of smokers
| Variables | HR (95% CI) |
|
|---|---|---|
| Overall survival | RFS | |
| Entire sample (n=163) | ||
| Smoking | 2.28 (1.12–4.64) | - |
| Age (continuous) | 1.06 (1.03–1.09) | - |
| Pathological risk score (continuous) | 1.50 (1.30–1.72) | - |
| Smokers (n=108) | ||
| Age (continuous) | 1.05 (1.01–1.08) | - |
| Pathological risk score (continuous) | 1.56 (1.33–1.83) | - |
Description: This first model includes the six NCCN adverse risk factors and excludes the pathological risk score. We only included in this table the predictive variables that were found to be significantly associated with survival outcomes. HR=Hazard ratio, CI=Confidence interval, RFS=Recurrence-free survival, NCCN=National Comprehensive Cancer Network
Older age and higher pathological risk score predicted for worse OS in smokers. There were no independent predictors of RFS in smokers in this model.
Pathological risk score
Smokers had a significantly worse pathological risk score (mean ± SD; 2.38 ± 2.19, median; 2.00) than non-smokers (0.89 ± 1.21, 0.00) (p <0.001) on univariate analysis. On multivariate analysis, independent predictors of pathological risk score were smoking status, and grade (p <0.01). Smokers were not more likely to receive adjuvant treatment based on adverse pathological score than non-smokers (p for interaction=0.224).
Discussion
To our knowledge, this is the first study on the impact of smoking on a specific set of pathological features that are used to guide adjuvant therapy.
Our study showed that smoking is an independent predictor of worse pathological score, as defined in this study. Smokers seem to have significantly more aggressive disease as evidenced by more adverse pathological features than non-smokers. Some experts have argued that head and neck squamous cell carcinoma (HNSCC) in non-smokers is a ‘distinct clinical and molecular entity’. Koch et al. have shown that most non-smokers with oral cavity tumors had an oral tongue primary, while most of the oral cancers in smokers involved the floor of mouth. [21] In our study, there was a borderline difference in the distribution of oral cavity subsites on univariate analysis between smokers and non-smokers (69.1% oral tongue in non-smokers versus 50% in smokers; 12.7% floor of mouth in non-smokers versus 33.3% in smokers, p=0.052). Also, in this same study by Koch, tumors of non-smokers harbored fewer genetic alterations than tumors in non-smokers. [21] It has been established that “carcinogens leave fingerprints”, in the form of mutations of tumor suppressor genes and oncogenes, among others. [22] Smoking is no exception to this rule; tobacco has been associated with high frequency of p53 mutations in patients with HNSCC, and these mutations confer resistance to apoptosis. [23] A recently published paper studying the relationship between expression of matrix metalloproteinases (MMP) 2 and 9 and the clinicopathological features of OSCC revealed that patients with a smoking history had higher expression of MMP 2 and 9, two proteins that play a role in extracellular matrix (ECM) degradation and cell migration. [24] Similarly, a novel protein, migration and invasion enhancer 1 (MIEN1), which facilitates cell migration and invasion, was shown to be correlated with smoking and associated with significantly lower survival. [25] These are all examples of molecular “fingerprints” caused by tobacco smoking. Whether smoking-related alterations in the above-mentioned and other molecular targets translate into more aggressive histopathological features has only been investigated superficially so far. An earlier study showed significant correlation between tobacco smoking and an empirically calculated histological score, incorporating eight different cellular morphological features, such as cytoplasmic and nuclear differentiation, growth pattern, mitosis and depth of invasion. [26] Another study on oral cancer in women supported the association between cigarette smoking and mitotic activity. [27] Nevertheless, none of these histopathological features has been shown to have a direct impact on survival, and none is directly implicated in decision-making. No studies were found on the association between smoking and the presence of the NCCN adverse clinicopathological risk factors taken as a whole. These risk factors were established following the two major EORTC 22931 and RTOG 9501 trials. [18–20] Specific mutations leading to cell migration and mitosis could explain the more aggressive pathology found in smokers. Whether smoking status should be incorporated into patient management decisions, and whether smokers require intensification of treatment need further evaluation. Future studies should focus on comparing molecular alterations in smokers and non-smokers in order to better understand the mechanisms of carcinogenesis in these two populations, and help in more effectively managing patients with OCSCC. The field of prognostic molecular signatures is still relatively new, and treatment paradigms for OCSCC still rely heavily on tumor stage. Future directions include whole-genome sequencing to study specific targetable mutations in smokers and non-smokers for molecular therapies [28–30] and possibly prognostication.
In our study, smoking was shown to be an independent predictor of OS in patients with OCSCC, corroborating findings in other studies on HNSCC, and on OCSCC specifically. In a study by Fortin et al. of 1,871 HNSCC patients, including 343 patients with primaries in the oral cavity, smoking was shown to have a detrimental effect on local control and overall survival. [31] Similarly, in a cohort of 504 patients with HNSCC, pretreatment smoking status was the strongest predictor of survival, out of an array of selected health behaviors (including alcohol, physical activity, diet and sleep patterns), with both current smokers (HR= 2.4; 95% CI 1.3 to 4.4) and former smokers (HR = 2.0; 95% CI 1.2 to 3.5) showing significantly poorer survival than non-smokers. [32] A Japanese retrospective study of 222 OCSCC patients showed that pretreatment smoking was associated with significantly worse survival among patients treated with definitive chemoradiation or radiation therapy. [33]
The pathological risk score that we used in this study, although not a validated tool, was helpful in assessing the predictive capacity of the NCCN clinicopathological features taken as a whole, and not as individual, isolated parameters. We assigned arbitrary weights to each of the six NCCN risk factors, with more weights assigned to ECE of lymph node metastasis and positive margins, two risk factors that would entail the addition of concurrent chemotherapy to adjuvant radiation. Interestingly, this tool was shown to be an independent predictor of OS, but not of RFS, in the entire sample and in the subgroup of smokers, after adjusting for all other predictive variables. We would not recommend the use of our scoring system before further validation in future studies. A number of histological scoring systems have been devised in the past, like the invasive cell grading (ICG), which is a significant, and reproducible predictor of survival, and was validated in OCSCC. [34,35] Another scoring system used in OCSCC is the Risk Model devised by Brandwein-Gensler et al., incorporating three histological features: perineural invasion, lymphocytic host response, and worst pattern of invasion. [36–38] Interestingly, patients with early-stage tumors, who might otherwise have been managed with surgery alone, may be offered multi-modality therapy based on a high Brandwein-Gensler et al.’s risk score indicating a high propensity for recurrence. [36–39] A modified tumor classification including histopathological features has been proposed as an alternative to the AJCC tumor classification, [40,41] as the paramount importance of histological prognosticators is being increasingly appreciated.
We recognize that our study has some important limitations. One major limitation lies in the inherent biases of a retrospective study design. A multicenter prospective study would be needed to validate our results in a larger sample of patients. Also, we only recorded pretreatment smoking status, and did not gather information on whether patients continued smoking during treatment. This could have some impact on our results, as other studies have shown that smoking during radiation therapy was associated with worse prognosis. [42,43] This effect has been postulated to be related to hypoxia which confers resistance to radiotherapy. [44]
One of our study’s strengths is that complete follow-up and information on vital status for all of the patients included in this study were present, as well as details on the different treatments received. Another important study strength relates to our institution’s compliance with quality assurance measures in the treatment of head and neck cancers. [45] In particular, we use standardized histopathology reporting by a head and neck pathologist (CK), following the College of American Pathologists protocol for examination of specimens from patients with OCSCC. [46] Also, all head and neck cancer cases are presented in our multidisciplinary tumor boards, and treatment recommendations follow the NCCN and other well-established national guidelines. [45]
In summary, smoking is an independent predictor of OS, but not RFS in patients with OCSCC. Moreover, smokers have more aggressive disease as evidenced by more adverse pathological features than non-smokers. In future clinical trials, smoking status should be used to stratify patients with OCSCC. The pathological risk score, as defined in our study, seems to be a valuable and easy tool in the evaluation of important clinicopathological features in OCSCC, and was shown in our study to be significantly associated with OS. However, it would require further validation in randomized controlled trials.
Acknowledgements
The training of the first author was in part supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health under Award Number D43 TW009118. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66(1): 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Shiboski CH, Shiboski SC, Silverman S Jr. Trends in oral cancer rates in the United States, 1973–1996. Community Dent Oral Epidemiol. 2000; 28(4): 249–56. [DOI] [PubMed] [Google Scholar]
- [3].Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008; 26(4): 612–9. [DOI] [PubMed] [Google Scholar]
- [4].Blot WJ, Devesa SS, McLaughlin JK, Fraumeni JF Jr. Oral and pharyngeal cancers. Cancer Surv. 1994; 19-20: 23–42. [PubMed] [Google Scholar]
- [5].Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011; 23(8): 633–41. [DOI] [PubMed] [Google Scholar]
- [6].Macfarlane GJ, Zheng T, Marshall JR, Boffetta P, Niu S, Brasure J, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol. 1995; 31b(3): 181–7. [DOI] [PubMed] [Google Scholar]
- [7].Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between Tobacco and Alcohol Use and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009; 18(2): 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol. 2009; 45(4–5): 454–60. [DOI] [PubMed] [Google Scholar]
- [9].Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. [Internet]. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute; Bethesda, MD: [cited 2016 Apr 13]. Available from: http://seer.cancer.gov/csr/1975_2012/. [Google Scholar]
- [10].National Comprehensive Cancer Network: Head and Neck Cancers (version I.2015) [cited 2016 Apr 13]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. [DOI] [PMC free article] [PubMed]
- [11].Jardim JF, Francisco AL, Gondak R, Damascena A, Kowalski LP. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015; 44(1): 23–8. [DOI] [PubMed] [Google Scholar]
- [12].Fagan JJ, Collins B, Barnes L, D’Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998; 124(6): 637–40. [DOI] [PubMed] [Google Scholar]
- [13].Soo KC, Carter RL, O’Brien CJ, Barr L, Bliss JM, Shaw HJ. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986; 96(10): 1145–8. [DOI] [PubMed] [Google Scholar]
- [14].Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA. Prognostic factors of neck node metastasis. Clin Otolaryngol Allied Sci. 1982; 7(3): 185–92. [DOI] [PubMed] [Google Scholar]
- [15].Greenberg JS, Fowler R, Gomez J, Mo V, Roberts D, El Naggar AK, et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003; 97(6): 1464–70. [DOI] [PubMed] [Google Scholar]
- [16].Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001; 92(12): 3030–6. [DOI] [PubMed] [Google Scholar]
- [17].Peters LJ, Goepfert H, Ang KK, Byers RM, Maor MH, Guillamondegui O, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993; 26(1): 3–11. [DOI] [PubMed] [Google Scholar]
- [18].Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004; 350(19): 1945–1952. [DOI] [PubMed] [Google Scholar]
- [19].Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012; 84(5): 1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005; 27(10): 843–50. [DOI] [PubMed] [Google Scholar]
- [21].Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999; 109(10): 1544–51. [DOI] [PubMed] [Google Scholar]
- [22].Vogelstein B, Kinzler KW. Carcinogens leave fingerprints. Nature. 1992; 355(6357): 209–10. [DOI] [PubMed] [Google Scholar]
- [23].Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995; 332(11): 712–7. [DOI] [PubMed] [Google Scholar]
- [24].Jafarian AH, Vazife Mostaan L, Mohammadian Roshan N, Khazaeni K, Parsazad S, Gilan H. Relationship between the Expression of Matrix Metalloproteinase and Clinicopathologic Features in Oral Squamous Cell Carcinoma. Iran J Otorhinolaryngol. 2015; 27(80): 219–23. [PMC free article] [PubMed] [Google Scholar]
- [25].Rajendiran S, Kpetemey M, Maji S, Gibbs LD, Dasgupta S, Mantsch R, et al. MIEN1 promotes oral cancer progression and implicates poor overall survival. Cancer Biol Ther. 2015; 16(6): 876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bundgaard T, Bentzen SM, Sogaard H. Histological differentiation of oral squamous cell cancer in relation to tobacco smoking. Eur J Cancer B Oral Oncol. 1995; 31B(2): 118–21. [DOI] [PubMed] [Google Scholar]
- [27].Wey PD, Lotz MJ, Triedman LJ. Oral cancer in women nonusers of tobacco and alcohol. Cancer. 1987; 60(7): 1644–50. [DOI] [PubMed] [Google Scholar]
- [28].Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J Clin Oncol. 2015; 33(29): 3269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011; 333(6046): 1154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011; 333(6046): 1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2009; 74(4): 1062–9. [DOI] [PubMed] [Google Scholar]
- [32].Duffy SA, Ronis DL, McLean S, Fowler KE, Gruber SB, Wolf GT, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009; 27(12): 1969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kawakita D, Hosono S, Ito H, Oze I, Watanabe M, Hanai N, et al. Impact of smoking status on clinical outcome in oral cavity cancer patients. Oral Oncol. 2012; 48(2): 186–91. [DOI] [PubMed] [Google Scholar]
- [34].Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989; 18(8): 432–7. [DOI] [PubMed] [Google Scholar]
- [35].Bryne M, Nielsen K, Koppang HS, Dabelsteen E. Reproducibility of two malignancy grading systems with reportedly prognostic value for oral cancer patients. J Oral Pathol Med. 1991; 20(8): 369–72. [DOI] [PubMed] [Google Scholar]
- [36].Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005; 29(2): 167–78. [DOI] [PubMed] [Google Scholar]
- [37].Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010; 34(5): 676–88. [DOI] [PubMed] [Google Scholar]
- [38].Li Y, Bai S, Carroll W, Dayan D, Dort JC, Heller K, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013; 7(3): 211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vered M, Dayan D, Dobriyan A, Yahalom R, Shalmon B, Barshack I, et al. Oral tongue squamous cell carcinoma: recurrent disease is associated with histopathologic risk score and young age. J Cancer Res Clin Oncol. 2010; 136(7): 1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee CC, Ho HC, Su YC, Yu CH, Yang CC. Modified tumor classification with Inclusion of tumor characteristics improves discrimination and prediction accuracy in oral and hypopharyngeal cancer patients who underwent surgery. Medicine (Baltimore). 2015; 94(27): e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brandwein-Gensler M, Wei S. Envisioning the next WHO head and neck classification. Head Neck Pathol. 2014; 8(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993; 328(3): 159–63. [DOI] [PubMed] [Google Scholar]
- [43].Chen AM, Chen LM, Vaughan A, Sreeraman R, Farwell DG, Luu Q, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011; 79(2): 414–9. [DOI] [PubMed] [Google Scholar]
- [44].Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991; 126(9): 1131–4. [DOI] [PubMed] [Google Scholar]
- [45].Gayar OH, Yu Y, Chang S, Ghanem T, Hall F, Siddiqui F. Compliance with Quality Assurance Measures for Improving Oropharyngeal Cancer Patient Care (abstr.). Int J Radiat Oncol Biol Phys. 2014; 90: S511. [Google Scholar]
- [46].Seethala RR, Weinreb I, Carlson DL, McHugh JB, Harrison LB, Richardson MS, et al. [Internet]. Protocol for the Examination of Specimens from Patients with Carcinomas of the Lip and Oral Cavity, v. 3.2.0.0 [cited 2016 Apr 13]. Available from: http://www.cap.org/ShowProperty?nodePath=/UCMCon/ContributionFolders/WebContent/pdf/liporalcaversion-13protocol.pdf.