Abstract
Nicotinamide (Nam, amide form of niacin acid or nicotinate), a precursor for nicotinamide adenine dinucleotide (NAD+), is important for normal physiological function of organisms. Nam also suppresses mobilization of Ca2+ from sarcoplasmic reticulum into cytoplasm through inhibiting ADP-ribose cyclase. Previously, we have demonstrated that a pharmacological dose of Nam normalizes maternal blood pressure in mouse models of preeclampsia, a pregnancy related hypertensive disorder. We hypothesized that Nam could decrease blood pressure in hypertensive conditions unrelated to pregnancy. Nam at a dose of 500 mg/kg/day was given to wild type (WT) mice treated with L-NAME, endothelial nitric oxide synthase (eNOS)-null and renin transgenic (Renin-Tg) mice via drinking water. Blood pressure was measured by tail-cuff at different stages of treatment. The function and structure of kidneys of WT mice with L-NAME were determined at the end of the study. The gene expression of markers of inflammation and fibrosis in the kidneys of WT mice with L-NAME was also measured. Nam effectively prevented increase in blood pressure in L-NAME treated mice and decreased elevated blood pressure in eNOS-null mice. However, it did not alter high blood pressure in Renin-Tg mice. Nam prevented increase in urinary albumin excretion and collagen deposit in kidneys of WT mice treated with L-NAME. In addition, Nam significantly decreased the mRNA levels of the markers of inflammation and fibrosis in the kidneys of WT mice treated with L-NAME. Nam may execute beneficial effects on hypertensive conditions associated with eNOS dysfunction via suppressing inflammation. Because Nam is generally regarded as safe in humans, it merits further evaluation for the tailored treatment for the subgroup of hypertensive cases associated with impaired eNOS system.
Keywords: Nicotinamide, Mice, L-NAME, Blood pressure, Endothelial nitric oxide synthase
Introduction
Nicotinamide (Nam, amide form of niacin acid or nicotinate) along with niacin acid and nicotinamide riboside are components of an essential vitamin (vitamin B3). These compounds and tryptophan are precursors for nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP) in most living organisms [1]. The classical function of NAD+/NADP is to act as a coenzyme for hydride-transfer enzymes, which is central to metabolism, including energy production and synthesis of fatty acids, cholesterol, and steroids. Insufficiency of NAD+/NADP resulting from deficiency of its precursors can lead to clinical manifestation including fatigue, pigmented skin rash, and oral ulcerations [2]. In severe deficiency, patients develop pellagra which is characterized by cutaneous rashes and dermatitis, diarrhea, and dementia [1]. Elvehjem demonstrated that Nam and niacin acid had anti-pellagragenic effects in 1937 [3]. The recommended daily allowance of vitamin B3 is13–16 mg per day.
Besides its function as coenzyme, NAD+ also is a substrate of NAD+-consuming enzymes that cleave the N-glycosidic bond between the Nam moiety and the ADP-ribose moiety. One or more ADP-ribose moieties are transferred to certain proteins which are reversibly modified in order to form signaling compounds from NAD+ and NADP [1,4]. For example, ADP-ribosyl cyclase catalyzes the conversion of NAD+ to cyclic ADP-ribose (cADPR) and Nam. While cADPR mobilizes intracellular Ca2+ from sarcoplasmic reticulum via actions on ryanodine receptors [5], Nam inhibits ADP-ribosyl cyclase [6] which can be activated by the endothlin-1 (ET-1)/endothelin receptor system and other factors [7]. Recently, we have reported that Nam decreases maternal blood pressure and improves maternal kidney function and structures in mouse models of preeclampsia via inhibiting ADP-ribosyl cyclase [8]. Preeclampsia is a hypertensive disorder that occurs in pregnant women [9]. The molecular mechanisms of this condition is largely unknown and there is no effective therapeutic strategy available.
In the current study, we hypothesized that Nam may also lower blood pressure in broader spectrum of hypertensive conditions. Therefore, we tested the blood pressure lowering effects of Nam on hypertension caused by inhibiting endothelial nitric oxide synthase (eNOS). eNOS/nitric oxide (NO) plays an important role in blood pressure regulation, as evidenced by genetic deletion of Nos3 (encoding eNOS) gene leading to hypertension in mice [10,11]. In addition, pharmacological inhibition of eNOS leads to hypertension as well. Although NG-nitro-L-arginine methyl ester (L-NAME) is a potent non-selective inhibitor of all three isoforms of NOS [eNOS, inducible nitric oxide synthase (iNOS) and neuronal nitric oxide synthase (nMOS)], it has been widely used to induce hypertension due to inhibiting eNOS [12–14]. Our results demonstrate that Nam prevents against increase in blood pressure and kidney injury in L-NAME treated wild type (WT) mice, and decreases elevated blood pressure in eNOS-null mice. In addition, Nam suppresses inflammation and fibrosis in kidneys of WT mice treated with L-NAME
Materials and Methods
Mice
Wild type male (WT) mice (C57BL/6J), Renin-Tg [129S/ SvEv-Tg(Alb1-Ren)2Unc/CofJ, JAX: 007853] male mice and eNOS-null female and male mice (on the C57BL/6J background) [15] at age of 12–16 weeks were housed in standard cages on a 12h light/dark cycle and were allowed free access to food and water. All experiments were carried out in accordance with the National Institutes of Health guideline for use and care of experimental animals, as approved by the IACUC of the University of North Carolina at Chapel Hill.
WT mice with L-NAME and/or Nam treatment
WT mice were randomly enrolled into four groups: 1) control: mice received only vehicle (water), 2) Nam: mice were administered with Nam (#72340, Sigma) at a dose of 500 mg/kg/day in drinking water (0.3% weight/volume) [8], 3) L-NAME : mice were treated with L-NAME (#483125, Sigma) at dose of 50 mg/kg/day in drinking water (0.03% weight/volume) [14], 4) L-NAME + Nam: mice were treated with both L-NAME and Nam via drinking water at dose described in 2) and 3) respectively. After 2 months treatment, mice were euthanized, and body fluids and tissues were collected for analysis.
Renin Tg and eNOS-null mice with Nam treatment
Renin Tg and eNOS-null mice were randomly enrolled into either control (vehicle: water) or Nam (same dose as described above) groups. Blood pressure was measured before and after 1-month of treatment.
Blood pressure measurement
The blood pressure of mice was determined via transmission photoplethysmography and an occlusion tail cuff (BP-2000 Blood Pressure Analysis System; Visitech Systems, NC) as described previously [16].
Urinary albumin
Urine was collected by massaging the bladder at one time, and urinary albumin concentration and creatinine were determined using commercially available kits (Exocell Inc., Philadephia, PA) as described previously [17].
Morphological examination
Kidney tissues were fixed with 4% paraformaldehyde, paraffin sectioned (5 μm), and stained with hematoxylin and eosin (H&E), or with Masson’s Trichrome [17].
Quantitative RT-PCR
Total RNA from tissues was extracted using Trizol (Life Technologies, St. Paul, MN) following the manufacturer’s instruction. mRNA was quantified with TaqMan real-time quantitative RT-PCR (7500 real time PCR system, Applied Biosystems, Foster City, CA) by using the one-step RT-PCR Kit (Bio Rad, Hercules, CA) with Hprt as the reference gene in each reaction [18].
Statistical analysis
Data are presented as mean ± SEM. Multifactorial ANOVA test was used to assess statistical significance with the program JMP 14.0 (SAS Institute Inc. Cary, NC). Post hoc analyses were done using the Tukey–Kramer Honest Significant Difference test.
Results
Nam prevents increase in blood pressure in wild type (WT) mice chronically treated with L-NAME
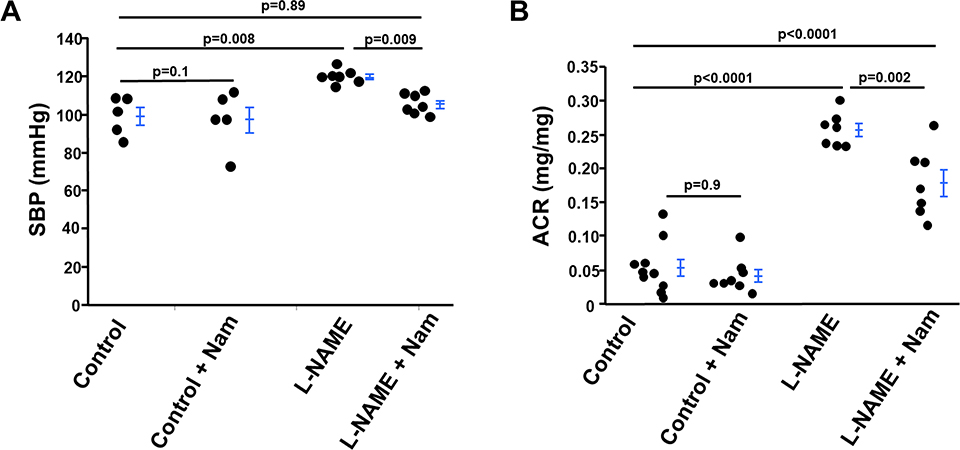
We tested the effect of Nam on hypertension induced by L-NAME treatment. After treatment with L-NAME for 2 months at a dose of 50 mg/kg/day, the blood pressure of WT mice increased approximately 20 mmHg, consistent with previous reports [14], and Nam restored the blood pressure to normal control levels (Figure 1). Nam alone had no effect on blood pressure of WT control mice without L-NAME.
Figure 1 : Nicotinamide (Nam) protects against increase in blood pressure and urinary albumin excretion in wild type (WT) mice treated with L-NAME.
(A) Systolic blood pressure (SBP) of four groups of mice was measured after 2-months of treatment. n≥5. (B) Urinary albumin/ creatinine ratio (ACR) was determined after 2-months of treatment n≥7.
Neither Nam nor L-NAME had effect on body weight, and heart and kidney weight in WT mice under the treatment regimen of this study (Table 1).
Table 1:
Characteristics of different groups of mice.
| Control | Control+Nam | L-NAME | L-NAME+Nam | |
|---|---|---|---|---|
| Body weight (g) | 32.4 ± 0.8 | 33.5 ± 0.8 | 32.4 ± 1.1 | 34.6 ± 0.8 |
| Kidney weight (g) | 0.198 ± 0.01 | 0.194 ± 0.004 | 0.178 ± 0.01 | 0.199 ± 0.007 |
| Heart weight (g) | 0.172 ± 0.002 | 0.168 ± 0.01 | 0.170 ± 0.008 | 0.176 ± 0.0061 |
Mice were randomly enrolled into four different groups as described in “Materials and Method” section. Two months after treatment, mice were euthanized and plasma and kidneys were collected for the analysis. n≥5.
Nam improves kidney function in wild type (WT) mice chronically treated with L-NAME
After treatment with L-NAME for 2 months at a dose of 50 mg/ kg/day, kidney function was damaged as indicated by the increased urinary protein excretion as measured by albumin/creatine ratio (ACR), while Nam significantly dampened this induction. Nam had no effect on ACR in WT control mice (Figure 1B).
Nam suppresses inflammation in kidneys of wild type (WT) mice induced by L-NAME
Our previous study [8] and pilot data of the current study have not shown any effect of Nam on WT control mice, therefore, we only investigated the difference between mice treated with L-NAME and mice treated with L-NAME plus Nam.
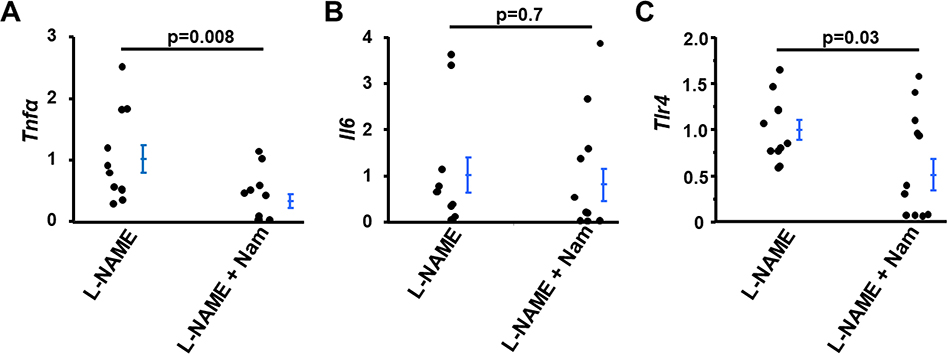
L-NAME induces tumor necrosis factor-α (TNFα) expression in kidneys and other tissues [13,14] and Nam has been shown to inhibit TNFα signaling induced by lipopolysaccharide (LPS) [19]. We, therefore, examined the effect of co-treatment with Nam on the expression of the Tnfα gene in the kidneys of mice treated with L-NAME. As shown in Figure 2A, mRNA levels of Tnfα in kidneys of mice co-treated with L-NAME and Nam was 30% that in kidneys of mice treated with L-NAME only (p<0.008). In contrast, Nam co-treatment did not affect the mRNA levels of Il6 (encoding Interleukin 6, IL6) in the kidneys of WT mice treated with L-NAME (Figure 2B), consistent with Fukuzawa et al. report that Nam had no significant effect on IL6 production induced by LPS [19].
Figure 2: Nicotinamide (Nam) co-treatment suppressed the inflammatory gene expression of Tnfα and Tlr4 but not Il6 in kidneys of WT mice treated with L-NAME.
mRNA levels of Tnfα (A), Il6 (B) and Tlr4 (C). n≥10.
Toll-like receptor4 (TLR4) is an important contributor of the innate immune system and is involved in the pathological changes induced by L-NAME [14,20]. Therefore, we examined the effect of Nam on the mRNA level of Tlr4. Like its effect on mRNA level of Tnfα, the mRNA levels of Tlr4 in kidneys of WT mice co-treated with Nam and L-NAME were markedly lower than that of the L-NAME only treatment group by 50% (p=0.03, Figure 2C).
Nam suppresses fibrosis in kidneys of WT mice induced by L-NAME
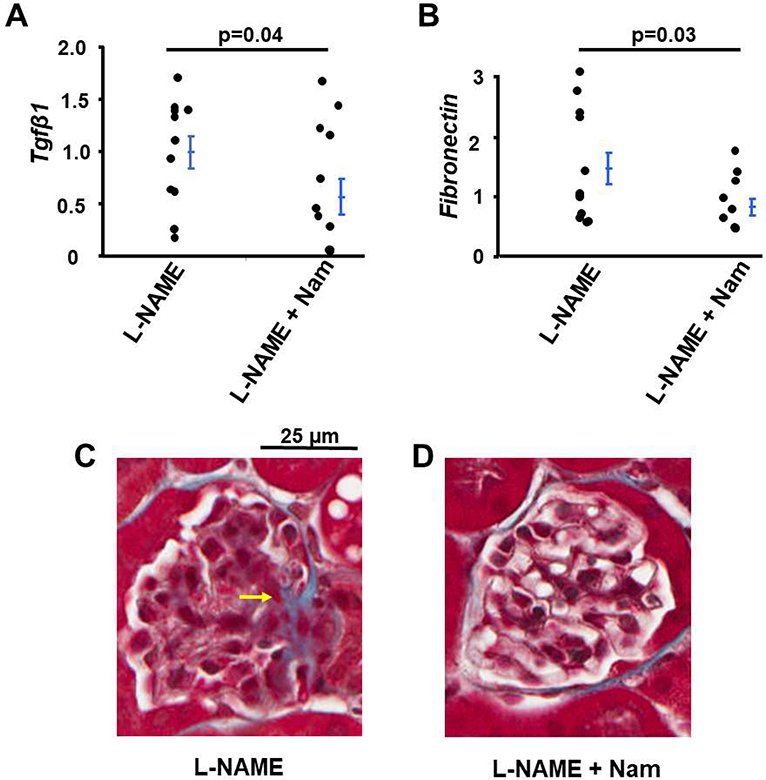
L-NAME treatment leads to kidney fibrosis [13] and TGFβ1 is thought to play a role in the pathogenesis of fibrosis caused by L-NAME [21]. We examined the effect of Nam on kidney fibrosis of mice co-treated with L-NAME. The mRNA levels of Tgfβ1 and Fibronectin in the kidneys of WT mice co-treated with Nam and L-NAME were about 50% of those treated with L-NAME only (Figures 3A and 3B). In addition, Nam decreased collagen deposition detected by Masson’s Trichrome staining in kidneys of WT mice with L-NAME treatment (Figures 3C and 3D).
Figure 3: Nicotinamide (Nam) co-treatment suppressed the kidney fibrosis in WT mice treated with L-NAME.
mRNA levels of markers of fibrosis genes (A) Tgfβ1, (B) Fibronectin.n≥10 (C) Representative Masson’s Trichrome stain of kidneys in the two different groups of mice. Yellow arrow: fibrosis in a glomerulus which was not present in kidneys of mice treated with L-NAME plus Nam.
Nam decreases blood pressure in eNOS-null mice
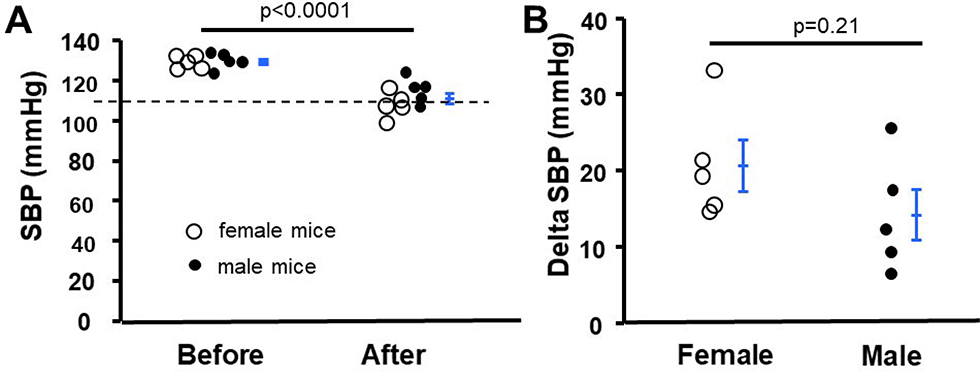
Genetically lacking eNOS results in hypertension in mice [10,11]. We tested the blood pressure lowering effect of Nam on this hypertensive mouse model. Before Nam treatment, the systolic blood pressure (SBP) of eNOS-null mice was 129.2 ± 1.1 mmHg, the SBP significantly decreased to 111.3 ± 2.3 mmHg after 1-month treatment (p<0.0001, Figure 4A). Nam effectively decreased blood pressure in both female and male eNOS-null mice, although female mice tended to decrease more (p=0.21, Figure 4B). Because eNOS-null mice do not develop obvious kidney problems [15], we did not examine the kidney function and structure in these mice.
Figure 4: Nicotinamide (Nam) decreased elevated blood pressure in eNOS-null mice.
(A) Systolic blood pressure (SBP) was measured by tail-cuff before and 1-month after treatment. n=5 female and 5 male mice. Broken line indicates the value of SBP of WT mice at the same age. (B) The difference of SBP before and 1-month after Nam treatment in male and female mice. n=5.
Taken together, we demonstrated that Nam had antihypertensive effects in mice with impaired eNOS function.
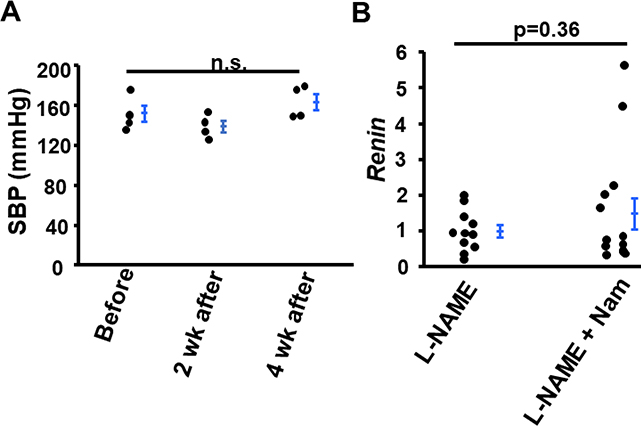
Nam did not alter blood pressure in Rein-Tg mice and did not alter the expression of Renin gene in kidneys of wild type (WT) mice treated with L-NAME
We next tested whether Nam decreased blood pressure in mice in which hypertension was not resulting from eNOS dysfunction. Therefore, we treated Renin-Tg mice with Nam for 4 weeks and blood pressure was measured before, 2 and 4 weeks after treatment. The Renin-Tg mice express renin ectopically at a constant high level in the liver which leads to elevated plasma levels of prorenin and active renin. The transgenic mice display high blood pressure, and kidney damage [22]. Before Nam treatment, the SBP of Renin-Tg mice was 152.4 ± 8.0 mmHg. At 2 weeks and 4 weeks after treatment, the SBP was 139.2 ± 5.9 and 163.7 ± 8.0 mmHg, respectively (Figure 5A). Nam did not decrease elevated blood pressure in mice caused by over-expressing renin [22].
Figure 5:
Nicotinamide (Nam) did not alter blood pressure in Renin Tg mice and mRNA level of Renin in kidneys of WT mice treated with L-NAME. (A) Systolic blood pressure (SBP) was measured before, 2-weeks and 4-weeks after Nam treatment. n.s.: not significant. n=4. (B) mRNA level of Renin. n≥10.
Because the renin-angiotensin system (RAS) is involved in L-NAME induced kidney lesions, we determined the mRNA level of Renin in the kidneys. Nam had no effect on the gene expression (Figure 5B).
Discussion
In the current study, we demonstrated that Nam effectively decreased blood pressure in mice whose blood pressure were above normal due to pharmacological inhibition of eNOS with L-NAME, or due to genetic lack of Nos3. Co-treatment with Nam prevented the decline of kidney function in WT mice treated with L-NAME, as judged by lower urinary albumin excretion. In addition, Nam suppressed kidney inflammation and fibrosis induced by L-NAME.
Nam is generally considered safe by the Food and Drug Administration (FDA), and daily doses of over 3 g are generally well tolerated [23]. The dose used in our study with mice translates to approximately 2.5 g/day in a person whose body weight is 60kg [24]. Nam appears to be a well-tolerated medication with broad applications. For example, Nam has been used to treat acne vulgaris and other skin problems for more than 50 years [25,26]. Although the precise mechanism is not very clear, inhibition of proinflammatory cytokine pathways has been proposed as the underlying mechanism of its beneficial effects in the skin conditions [25]. Because Nam is known to inhibit poly-ADP-ribose-polymerase-1 (PARP-1) and regulate DNA repair, it has potential as a prophylaxis against nonmelanoma skin cancer [27,28]. In other diseases, there are clinical trials to test the role of Nam in Alzheimer’s Disease (NCT00580931) and preeclampsia (NCT03419364). More recently, beneficial effects of Nam have been explored in animals with aims to treat human diseases in the future such as age-related macular degeneration [29] and glaucoma [30].
Hypertension is a worldwide health challenge as result of its high prevalence and concomitant risks for stroke and cardiovascular disease. Numerous genetic and environmental factors are involved in pathogenesis and progression of hypertension [31]. In human studies, NOS3 (encoding eNOS) polymorphisms are associated with hypertension [32–35]. However, there is no tailored therapeutic regiment for hypertension associated with impaired eNOS/NO. In this study, we show that Nam effectively decreased blood pressure in hypertensive mice with impaired eNOS function genetically or pharmacologically without detrimental effects. In contrast, our experiments showed that Nam had no BP-lowering effects in severe hypertension caused by an unregulated overproduction of renin in mice. Taken together, our data suggest that this economic well-tolerated small molecule has tremendous potential as a treatment for the hypertensive condition related to eNOS dysfunction.
Both Inflammation and innate immunity play a role in the development of hypertension [20,36,37]. In humans, eNOS polymorphisms associated with hypertension also modulates the inflammatory response [38]. TLR4 signaling modulates blood pressure in L-NAME-induced hypertension, and Sollinger et al reported that Tlr4-null mice are protected against blood pressure increases by L-NAME [14]. Nam has been shown to possess anti-inflammation properties [19,39]. Our current data show that co-treatment with Nam suppressed the mRNA levels of Tnα and Tlr4 in kidneys of mice induced by L-NAME. Thus, the anti-inflammation could be one of the mechanisms by which Nam decreases blood pressure.
In our previous work we have shown that Nam decreases maternal blood pressure through inhibiting ADP-ribose cyclase in preeclamptic mice. This could also be a mechanism by which Nam moderates the development of hypertension in mice lacking eNOS. Further study is needed to elucidate it [8].
Some reagents that decrease the elevated blood pressure induced by L-NAME are thought to work through preserving eNOS/ NO [40]. However, our current study shows that Nam effectively decreases blood pressure in eNOS-null mice, and the mRNA level of Nos3 is not increased in the kidneys of WT mice treated with L-NAME and Nam (1.00 ± 030 in kidneys with L-NAME vs. 0.28 ± 0.08 in kidneys with L-NAME + Nam, p=0.02), suggesting that Nam decreases blood pressure in an eNOS/NO-independent manner.
L-NAME causes proteinuria and kidney fibrosis [13] and the mechanisms of the chronic pathological changes are more complex than simple inhibition of endothelial NO synthesis. Upregulation of RAS is thought to play an important role in fibrosis of tissues including the heart [41] and kidney [13]. In the current study, we observed that Nam partially corrected the urinary albumin excretion but had no effect on the mRNA level of Renin in the kidneys of WT mice treated with L-NAME. These data suggest that Nam could resolve the kidney problems associated with eNOS dysfunction but have no effects on pathological changes induced by uncontrolled upregulation of RAS.
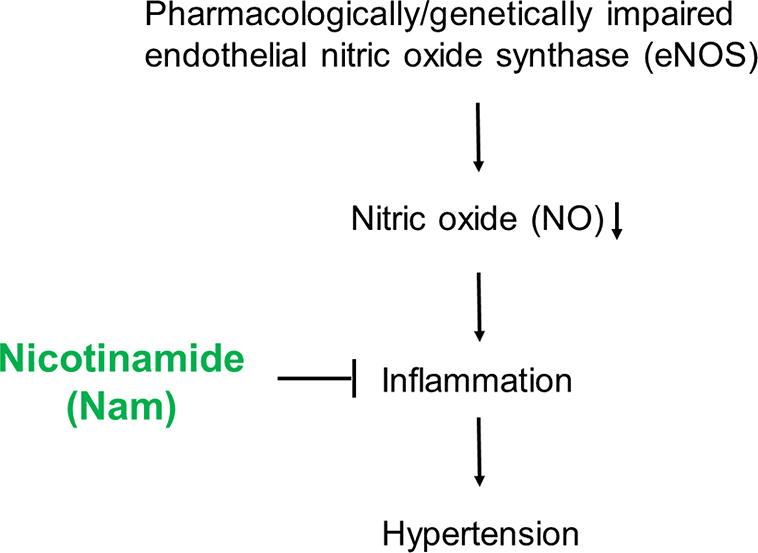
In summary, we have demonstrated that a pharmacological dose of Nam normalizes blood pressure in mice with impaired eNOS function either pharmacologically or genetically, and has partial benefits on kidney problems induced by L-NAME. Because Nam is generally regarded as safe in humans, it merits further evaluation as a treatment of human hypertension associated with eNOS dysfunction (Figure 6).
Figure 6:
Schematic illustration of the potential mechanism by which nicotinamide (Nam) executes its beneficial effect.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01HL049277) to N.M-S. The histology core facility at UNC is supported by NIH Grant DK 034987.
References
- 1.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 2008. Aug 21;28:115–30. [DOI] [PubMed] [Google Scholar]
- 2.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009. Sep;14(9):3446–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elvehjem CA, Madden RJ, Strong FM, Woolley D. Relation of nicotinic acid and nicotinic acid amide to canine black tongue. Journal of the American Chemical Society. 1937. Sep;59(9):1767–8. [Google Scholar]
- 4.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends in Biochemical Sciences. 2007. Jan 1;32(1):12–9. [DOI] [PubMed] [Google Scholar]
- 5.Arendshorst WJ, Thai TL. Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Current Opinion in Nephrology and Hypertension. 2009. Jan 1;18(1):40–9. [DOI] [PubMed] [Google Scholar]
- 6.SETHI JK, EMPSON RM, GALIONE A. Nicotinamide inhibits cyclic ADP-ribose-mediated calcium signalling in sea urchin eggs. Biochemical Journal. 1996. Oct 15;319(2):613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thai TL, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptors mediate endothelin ETA and ETB receptor-induced renal vasoconstriction in vivo. American Journal of Physiology-Renal Physiology. 2008. Aug;295(2):F360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Fushima T, Oyanagi G, Townley-Tilson HD, Sato E, Nakada H, et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proceedings of the National Academy of Sciences. 2016. Nov 22;113(47):13450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology. 2009. Jun;24(3):147–58. [DOI] [PubMed] [Google Scholar]
- 10.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences. 1996. Nov 12;93(23):13176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995. Sep;377(6546):239–42. [DOI] [PubMed] [Google Scholar]
- 12.Lahera VI, Salom MG, Miranda-Guardiola FA, Moncada SA, Romero JC. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. American Journal of Physiology-Renal Physiology. 1991. Dec 1;261(6):F1033–7. [DOI] [PubMed] [Google Scholar]
- 13.Giani JF, Janjulia T, Kamat N, Seth DM, Blackwell WL, Shah KH, et al. Renal angiotensin-converting enzyme is essential for the hypertension induced by nitric oxide synthesis inhibition. Journal of the American Society of Nephrology. 2014. Dec 1;25(12):2752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sollinger D, Eißler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, et al. Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovascular research. 2014. Mar 1;101(3):464–72. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Wang CH, Wang JG, Thai T, Boysen G, Xu L, et al. Elevated tissue factor expression contributes to exacerbated diabetic nephropathy in mice lacking eNOS fed a high fat diet. Journal of Thrombosis and Haemostasis. 2010. Oct;8(10):2122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagaman JR, John S, Xu L, Smithies O, Maeda N. An improved technique for tail-cuff blood pressure measurements with darktailed mice. Journal of the American Association for Laboratory Animal Science. 2005. Sep 15;44(5):43–6. [PubMed] [Google Scholar]
- 17.Li F, Kakoki M, Smid M, Boggess K, Wilder J, Hiller S, et al. Causative effects of genetically determined high maternal/fetal endothelin-1 on preeclampsia-like conditions in mice. Hypertension. 2018. May;71(5):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Bahnson EM, Wilder J, Siletzky R, Hagaman J, Nickekeit V, Hiller S, Ayesha A, Feng L, Levine JS, Takahashi N. Oral high dose vitamin B12 decreases renal superoxide and post-ischemia/reperfusion injury in mice. Redox biology. 2020. Mar 10:101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuzawa M, Satoh J, Muto G, Muto Y, Nishimura S, Miyaguchi S, et al. Inhibitory effect of nicotinamide on in vitro and in vivo production of tumor necrosis factor-α. Immunology letters. 1997. Oct 1;59(1):7–11. [DOI] [PubMed] [Google Scholar]
- 20.Eißler R, Schmaderer C, Rusai K, Kühne L, Sollinger D, Lahmer T, et al. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertension Research. 2011. May;34(5):551–8. [DOI] [PubMed] [Google Scholar]
- 21.Mihout F, Shweke N, Bigé N, Jouanneau C, Dussaule JC, Ronco P, et al. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF‐β1 synthesis. The Journal of pathology. 2011. Jan;223(1):37–45. [DOI] [PubMed] [Google Scholar]
- 22.Caron KM, James LR, Kim HS, Morham SG, Lopez ML, Gomez RA, et al. A genetically clamped renin transgene for the induction of hypertension. Proceedings of the National Academy of Sciences. 2002. Jun 11;99(12):8248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA, ENDIT Group. Safety of high-dose nicotinamide: a review. Diabetologia. 2000. Oct 1;43(11):1337–45. [DOI] [PubMed] [Google Scholar]
- 24.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. Journal of basic and clinical pharmacy. 2016. Mar;7(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niren NM. Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review. Cutis. 2006. Jan;77(1 Suppl):11–6. [PubMed] [Google Scholar]
- 26.Forbat E, Al‐Niaimi F, Ali FR. Use of nicotinamide in dermatology. Clinical and Experimental Dermatology. 2017. Mar;42(2):137–44. [DOI] [PubMed] [Google Scholar]
- 27.Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. New England Journal of Medicine. 2015. Oct 22;373(17):1618–26. [DOI] [PubMed] [Google Scholar]
- 28.Surjana D, Halliday GM, Martin AJ, Moloney FJ, Damian DL. Oral nicotinamide reduces actinic keratoses in phase II doubleblinded randomized controlled trials. The Journal of Investigative Dermatology. 2012. May 1;132(5):1497. [DOI] [PubMed] [Google Scholar]
- 29.Saini JS, Corneo B, Miller JD, Kiehl TR, Wang Q, Boles NC, et al. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. 2017. May 4;20(5):635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017. Feb 17;355(6326):756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smithies O Many little things: one geneticist’s view of complex diseases. Nature reviews genetics. 2005. May;6(5):419–25. [DOI] [PubMed] [Google Scholar]
- 32.Hong Z, Pan L, Ma Z, Zhu Y, Hong Z. Combined effects of cigarette smoking, alcohol drinking and eNOS Glu298Asp polymorphism on blood pressure in Chinese male hypertensive subjects. Tobacco induced diseases. 2019;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neto AB, Farias MC, Vasconcelos NB, Xavier AF Jr, Assunção ML, Ferreira HS. Prevalence of endothelial nitric oxide synthase (ENOS) gene G894T polymorphism and its association with hypertension: a population-based study with Brazilian women. Archives of medical sciences. Atherosclerotic Diseases. 2019;4:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassereddine S, Idrissi HH, Habbal R, Abouelfath R, Korch F, Haraka M, Karkar A, Nadifi S. The polymorphism G894 T of endothelial nitric oxide synthase (eNOS) gene is associated with susceptibility to essential hypertension (EH) in Morocco. BMC Medical Genetics. 2018. Dec;19(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamil S, Erdmann J, Abdalrahman IB, Mohamed AO. Association of NOS3 gene polymorphisms with essential hypertension in Sudanese patients: a case control study. BMC Medical Genetics. 2017. Dec;18(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007. Oct 1;204(10):2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II–infused macrophage colony-stimulating factor–deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005. Oct 1;25(10):2106–13. [DOI] [PubMed] [Google Scholar]
- 38.Maurer P, Barbisan F, Azzolin VF, Berro LF, Montagner R, Duarte MM, da Cruz IB, Manfredini V, Piccoli JD Polymorphism eNOS Glu298Asp modulates the inflammatory response of human peripheral blood mononuclear cells. Cytokine. 2020. Jan 1;125:154812. [DOI] [PubMed] [Google Scholar]
- 39.Villeda-González JD, Gómez-Olivares JL, Baiza-Gutman LA, Manuel-Apolinar L, Damasio-Santana L, Millán-Pacheco C, et al. Nicotinamide reduces inflammation and oxidative stress via the cholinergic system in fructose-induced metabolic syndrome in rats. Life Sciences. 2020. Mar 31:117585. [DOI] [PubMed] [Google Scholar]
- 40.Ding L, Cheng P, Wang L, Hu J, Zhang YX, Cai GW, et al. The protective effects of polysaccharide extract from Xin-Ji-Er-Kang formula on Ang II-induced HUVECs injury, L-NAME-induced hypertension and cardiovascular remodeling in mice. BMC Complementary and Alternative Medicine. 2019. Dec;19(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda O, Tsutsui M, Morishita T, Tanimoto A, Horiuchi M, Tasaki H, Huang PL, Sasaguri Y, Yanagihara N, Nakashima Y. Longterm treatment with Nω-nitro-L-arginine methyl ester causes arteriosclerotic coronary lesions in endothelial nitric oxide synthase-deficient mice. Circulation. 2002. Sep 24;106(13):1729–35. [DOI] [PubMed] [Google Scholar]