To the Editor:
Inhaled corticosteroids in combination with long-acting β-agonists (ICS/LABA) are commonly used to treat fixed and variable obstructive lung diseases (1–6). Current treatment guidelines are based on clinical trials with restricted data before ICS/LABA initiation. Research on the trajectories of respiratory symptoms before and after ICS/LABA initiation is limited. Fire Department of the City of New York (FDNY) rescue/recovery workers experienced a massive irritant exposure after the collapse of the World Trade Center (WTC) on September 11, 2001 (9/11), resulting in increased rates of respiratory symptoms, as well as an acute drop in lung function associated with reactive airway disease and fixed airflow obstruction (7–13). Using longitudinal data on respiratory symptoms, the aims of the present study were to analyze changes in dyspnea before and after ICS/LABA initiation and to determine whether time between WTC exposure and treatment initiation was associated with treatment response.
Methods
The source population consisted of 9,638 male firefighters who were employed by FDNY on 9/11, first arrived at the WTC site between 9/11 and September 24, 2001, and underwent at least three routine medical monitoring examinations between 9/11 and September 10, 2018. The study population (N = 1,073; 11% of the source population) consisted of those who had ICS/LABA treatment for longer than 2 years after 9/11 and had at least one modified Medical Research Council (mMRC) dyspnea scale score (14) before and at least two mMRC scores after ICS/LABA initiation. The study population completed 7,835 medical monitoring questionnaires, including mMRC scores, between August 1, 2005 and September 10, 2018. Written informed consent was provided by all participants.
Demographics, height, weight, smoking status, initial arrival time at the WTC site (WTC exposure level), and spirometric measurements were retrieved from the FDNY employee database and/or assessed during routine medical monitoring examinations. Medication data were obtained from the FDNY electronic medical record and/or pharmacy claims data. Treatment duration was defined as the interval between first and latest fill dates of ICS/LABA. Multivariable-adjusted logistic regression determined variables associated with being in the study population of ICS/LABA-treated individuals versus not receiving ICS/LABA treatment (n = 6,721). Individuals classified as “responders” to ICS/LABA were those who had an mMRC slope less than 0 after treatment initiation; “nonresponders” had an mMRC slope greater than or equal to 0. Longitudinal mMRC scores in responders and nonresponders were estimated using linear mixed effects models with random intercepts, with categorized year from ICS/LABA initiation, age, body mass index (BMI), and race as fixed effects. Multivariable logistic regression assessed pretreatment mMRC and time from 9/11 to treatment initiation as predictors for treatment response, adjusting for age and BMI pretreatment, race, ever-smoking status, and WTC exposure level.
Data analyses were performed using SAS version 9.4 software (SAS Institute). Figures were created with Prism 8 software (GraphPad Software).
Results
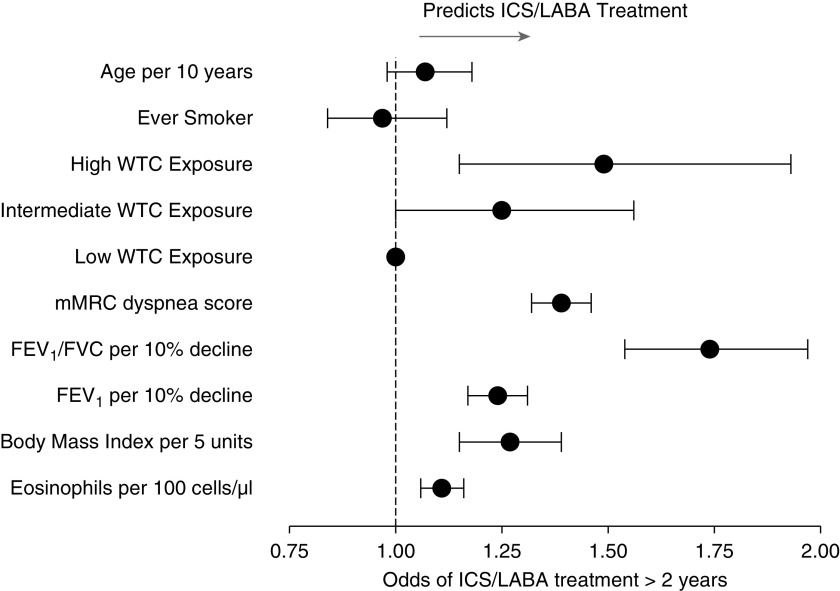
The study population included 1,073 individuals who received ICS/LABA therapy for more than 2 years and had at least one mMRC score before and two mMRC scores after ICS/LABA initiation. The mean (± standard deviation) numbers of mMRC scores were 4 (±2) before ICS/LABA initiation and 6 (±3) after ICS/LABA initiation. Individuals from the study population had higher WTC exposure, first post-9/11 mMRC score, blood eosinophils, and BMI and lower first post-9/11 lung function than those who did not receive ICS/LABA treatment (Figure 1).
Figure 1.
Forest plot showing variables associated with being included in the study population and receiving inhaled corticosteroid (ICS)/long-acting β-agonist (LABA) treatment for longer than 2 years versus not receiving ICS/LABA treatment (n = 7,777). Results shown are from a multivariable logistic regression analysis performed to determine the associations between first post-9/11 medical monitoring data and ICS/LABA treatment for longer than 2 years (odds ratios and 95% confidence intervals [bars]); data are also adjusted for race. FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; mMRC = modified Medical Research Council dyspnea scale; WTC = World Trade Center.
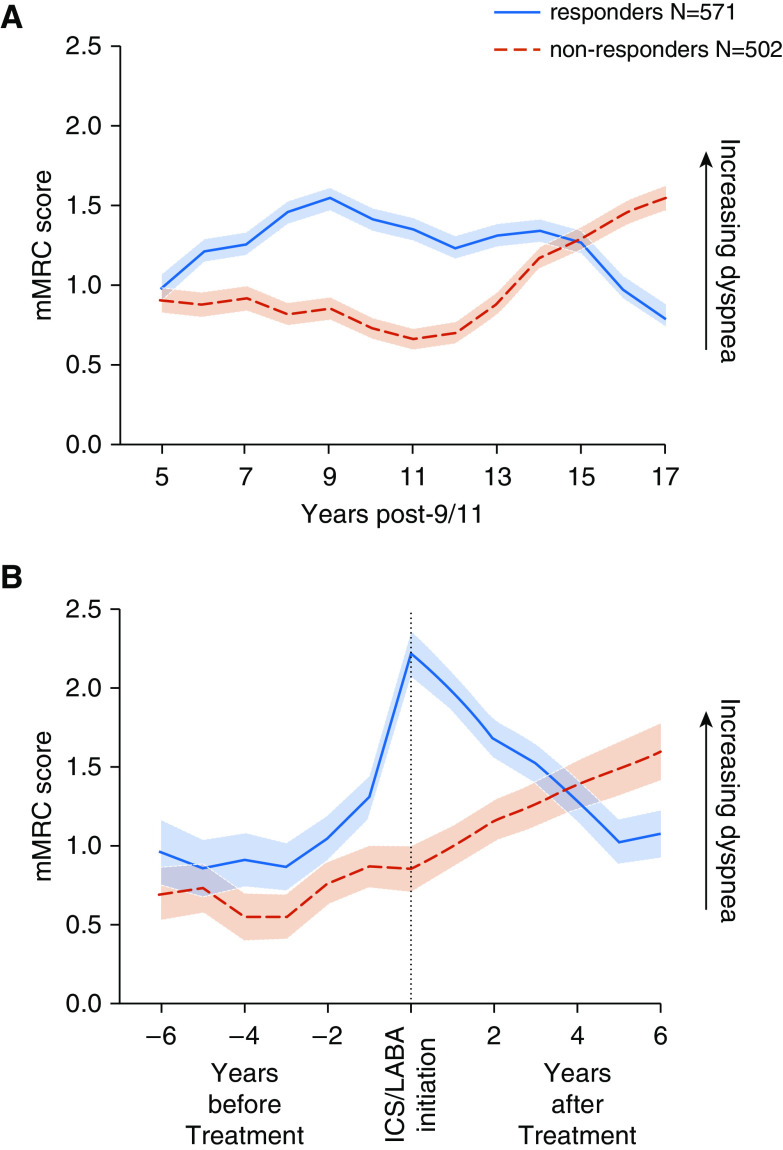
Responders (571 of 1,073; 53%) were more likely to be ever-smokers (36% vs. 33%) but had pretreatment lung function similar to that of nonresponders (forced expiratory volume in 1 second [FEV1] percent predicted, 89.3 ± 4% vs. 89.2 ± 13.7%; FEV1/FVC forced vital capacity, 77.3 ± 6.0% vs. 76.8 ± 6.4%). Nonresponders (502 of 1,073; 47%) had a gradual rise in mMRC scores starting 11 years after WTC exposure (Figure 2A), culminating in worse dyspnea score at the end of longitudinal follow-up (1.55; 95% confidence interval [CI], 1.40–1.70; vs. 0.79; 95% CI, 0.64–0.94, respectively). When we assessed dyspnea trajectory relative to treatment initiation (Figure 2B), we observed that responders had a sharp increase in mMRC scores before treatment and a subsequent decrease. Nonresponders, however, had a gradual rise in mMRC scores before treatment initiation, which continued to increase during treatment.
Figure 2.
Longitudinal modified Medical Research Council (mMRC) dyspnea scale scores and 95% confidence intervals in linear mixed effects models, stratified by responder type. mMRC scores were estimated using linear mixed effects models with random intercepts, with categorized year, age, body mass index, and race as fixed effects. The trajectory of the responder group is shown as a solid blue line, and the trajectory in the nonresponders is shown as a broken red line. (A) Trajectories of mMRC scores relative to September 11, 2001 (9/11). Nonresponders had a gradual rise in mMRC scores starting 11 years after WTC exposure, culminating in worse dyspnea score at the end of longitudinal follow-up. (B) Trajectories of mMRC scores relative to treatment initiation. Responders had a sharp increase in mMRC scores before treatment, followed by a subsequent decrease. Nonresponders, however, had a gradual rise in mMRC scores before treatment initiation, which continued to increase after inhaled corticosteroid/long-acting β-agonist (ICS/LABA) initiation.
In an adjusted multivariable model, increased time between 9/11 and treatment initiation was a strong predictor of nonresponse to therapy (Table 1). A higher pretreatment mMRC, however, was significantly associated with a favorable response to treatment.
Table 1.
Multivariable logistic regression predicting response to ICS/LABA treatment (N = 1,073)
| Variable | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Time from 9/11 to treatment initiation, per 5 yr | 0.43 | 0.34 | 0.55 | <0.001 |
| mMRC score*, per 1 point | 1.21 | 1.10 | 1.33 | <0.001 |
| Age*, per 10 yr | 1.06 | 0.87 | 1.28 | 0.60 |
| Smoking, ever vs. never | 1.17 | 0.89 | 1.53 | 0.26 |
| BMI*, per 5 units | 1.05 | 1.01 | 1.08 | 0.005 |
| WTC exposure (reference = low exposure) | ||||
| High exposure | 0.74 | 0.45 | 1.22 | 0.24 |
| Intermediate exposure | 0.74 | 0.48 | 1.13 | 0.16 |
Definition of abbreviations: 9/11 = September 11, 2001; BMI = body mass index; CI = confidence interval; ICS/LABA = inhaled corticosteroid and long-acting β agonist therapy; mMRC = modified Medical Research Council dyspnea scale; WTC = World Trade Center.
Pretreatment.
Discussion
This study produced longitudinal, patient-reported data on dyspnea from 1,073 previously healthy WTC-exposed firefighters who received more than 2 years of ICS/LABA treatment. The risk factors for treatment were similar to risk factors for obstructive airway disease in this cohort (7–10). We observed heterogeneity in dyspnea response, with only 53% of treated individuals responding to treatment. We found that responders had rapidly increasing dyspnea, as defined by mMRC score, in the 3 years before treatment initiation. Notably, in responders, dyspnea improved for 5 years after treatment initiation, returning to a level similar to baseline. Nonresponders had gradually increasing dyspnea in the 3 years before treatment, which continued to increase during the first 5 years after treatment initiation. This finding suggests that clinical trials with patient-reported outcomes may benefit from longer follow-up than that used in most randomized clinical trials.
Our study revealed pronounced differences in the trajectory of dyspnea in responders and nonresponders to ICS/LABA treatment. Responders presented earlier after WTC exposure, and higher pretreatment mMRC score predicted favorable treatment response, whereas nonresponders had a longer time between WTC exposure and symptom onset or treatment initiation. This difference in onset of symptoms suggests that nonresponders might have a different endotype of obstructive airway disease that is less responsive to ICS/LABA therapy. The lack of response to ICS/LABA therapy in those with later-onset dyspnea might be indicative of a less inflammatory type of disease.
One limitation of this study may include generalizability to other affected individuals, because this single-center study of a massively dust-exposed cohort included only previously healthy males. We also acknowledge that there may be unmeasured confounding, which is possible in all observational studies. In addition, although regression to the mean might also contribute to the difference in symptom score trajectories between responders and nonresponders, the greater symptom burden of nonresponders at the end of follow-up suggests that regression to the mean is unlikely to be the sole or even main explanation for the observed effect.
Conclusions
This longitudinal study showed that almost half of irritant-exposed patients had worsening dyspnea after ICS/LABA initiation. Treatment benefited more symptomatic individuals who initiated ICS/LABA treatment sooner after WTC exposure.
Supplementary Material
Footnotes
Supported by National Institute for Occupational Safety and Health (NIOSH) contracts 200-2011-39383, 200-2011-39378, 200-2017-93426, and 200-2017-93326 and NIOSH grants U01 OH011302 and U01OH011682.
Author Contributions: M.D.W. had full access to all of the data in the study and agrees to be accountable for all aspects of the work so that questions related to the accuracy and integrity of the research are appropriately investigated and resolved. B.P. and M.D.W. conceived of the study and designed it in conjunction with L.L., R.Z.-O., C.B.H., and D.J.P. B.P., A.S., R.Z.-O., T.S., and M.D.W. analyzed and interpreted the data. B.P. and M.D.W. drafted the first manuscript with critical revisions from L.L., A.S., R.Z.-O., C.B.H., M.J.F., T.S., M.P.W., H.W.C., and D.J.P. All authors approved the final manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46:622–639. doi: 10.1183/13993003.00853-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, et al. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 4.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui SH, Pavord ID, Barnes NC, Guasconi A, Lettis S, Pascoe S, et al. Blood eosinophils: a biomarker of COPD exacerbation reduction with inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2018;13:3669–3676. doi: 10.2147/COPD.S179425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 7.Zeig-Owens R, Singh A, Aldrich TK, Hall CB, Schwartz T, Webber MP, et al. Blood leukocyte concentrations, FEV1 decline, and airflow limitation: a 15-year longitudinal study of World Trade Center-exposed firefighters. Ann Am Thorac Soc. 2018;15:173–183. doi: 10.1513/AnnalsATS.201703-276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362:1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Liu C, Putman B, Zeig-Owens R, Hall CB, Schwartz T, et al. Predictors of asthma/COPD overlap in FDNY firefighters with World Trade Center dust exposure: a longitudinal study. Chest. 2018;154:1301–1310. doi: 10.1016/j.chest.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiden MD, Ferrier N, Nolan A, Rom WN, Comfort A, Gustave J, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137:566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lioy PJ, Weisel CP, Millette JR, Eisenreich S, Vallero D, Offenberg J, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110:703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weakley J, Webber MP, Gustave J, Kelly K, Cohen HW, Hall CB, et al. Trends in respiratory diagnoses and symptoms of firefighters exposed to the World Trade Center disaster: 2005-2010. Prev Med. 2011;53:364–369. doi: 10.1016/j.ypmed.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Webber MP, Gustave J, Lee R, Niles JK, Kelly K, Cohen HW, et al. Trends in respiratory symptoms of firefighters exposed to the World Trade Center disaster: 2001-2005. Environ Health Perspect. 2009;117:975–980. doi: 10.1289/ehp.0800291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.