Abstract
This paper provides quantitative data on the distributions of enteroendocrine cells (EEC), defined by the hormones they contain, patterns of colocalisation between hormones and EEC relations to nerve fibres in the rat gastric mucosa. The rat stomach has three mucosal types: non-glandular stratified squamous epithelium of the fundus and esophageal groove, a region of oxyntic glands in the corpus, and pyloric glands of the antrum and pylorus. Ghrelin and histamine were both contained in closed cells, not contacting the lumen, and were most numerous in the corpus. Gastrin cells were confined to the antrum, and 5-hydroxytryptamine (5-HT) and somatostatin cells were more frequent in the antrum than the corpus. Most somatostatin cells had basal processes that in the antrum commonly contacted gastrin cells. Peptide YY (PYY) cells were rare and mainly in the antrum. The only numerous colocalisations were 5-HT and histamine, PYY and gastrin and gastrin and histamine in the antrum, but each of these populations was small. Peptide-containing nerve fibres were found in the mucosa. One of the most common types was vasoactive intestinal peptide (VIP) fibres. High-resolution analysis showed that ghrelin cells were closely and selectively approached by VIP fibres. In contrast, gastrin cells were not selectively innervated by VIP or CGRP fibres. The study indicates that there are distinct populations of gastric EEC and selective innervation of ghrelin cells. It also shows that, in contrast to EEC of the small intestine, the majority of EEC within the stomach contained only a single hormone.
Keywords: Enteroendocrine cells, Gastrointestinal hormones, Ghrelin, 5-hydroxytryptamine, Somatostatin, Histamine, Enteric neurons
Introduction
Gastrointestinal hormones play powerful roles in regulating digestive and metabolic functions, appetite and food intake (Furness et al. 2013; Gribble and Reimann 2016; Clemmensen et al. 2017; Husted et al. 2017; Worthington et al. 2018). The major gastric hormones that have been identified are gastrin, ghrelin, 5-HT, somatostatin and histamine, that have been described as being located in separate cells (Solcia et al. 2000). However, recent studies, primarily in the last 6 years, have revealed that the majority of EEC of the small intestine and colon contain multiple hormones and their gene transcripts, some cells expressing three or more hormones (Egerod et al. 2012; Habib et al. 2012; Sykaras et al. 2014; Cho et al. 2015; Fothergill et al. 2017). Colocalization of hormones in the major classes of gastric endocrine cells has been investigated in a small number of studies. A significant overlap between gastrin and histidine decarboxylase (HDC), a marker of ECL cells, was found in EEC of the mouse gastric antrum (Walker et al. 2013). Of HDC cells, 30% had gastrin immunoreactivity and 17% of gastrin cells were HDC positive. Reynaud et al. (2016) investigated colocalization with 5-HT in mouse stomach and found that very few cells were immunoreactive for both 5-HT and either ghrelin (fewer than 1% of ghrelin cells), gastrin (about 2% of gastrin cells) or somatostatin about 15% of somatostatin cells in the antrum were 5-HT positive). In addition, ghrelin cells also express nesfatin-1 (Stengel et al. 2013). In human stomach, colocalization has been investigated in the fundus and corpus; the only substantial overlap observed was that about 25% of 5-HT cells were a subpopulation of ECL cells (Fakhry et al. 2019). Gastrin and HDC were found to be colocalized in a subpopulation of rat EEC (Hunyady et al. 1998).
To date, there has been no comprehensive study of EEC in the rat stomach, although the rat has been used extensively for studies of gastric physiology and signalling between the stomach and the CNS (Schubert et al. 1982; Phillips and Powley 1998; Furness et al. 2001; Mumphrey et al. 2013; Lu et al. 2017). The rat has also provided a model of choice for the investigation of gastric innervation, including the identification of different classes of fibres innervating the gastric mucosa (Berthoud and Powley 1992; Ekblad et al. 2000; Zheng and Berthoud 2000; Powley et al. 2011, 2014).
In the current study, we have determined the regional distributions of EEC defined by their hormonal content. In the case of ECL cells, histidine decarboxylase, in the synthesising pathway for histamine, was used for localisation. We have quantified patterns of colocalization and proximities of ghrelin and gastrin containing EEC to chemically identified nerve fibre types.
Methods
Experiments were conducted on Sprague-Dawley rats of 250–350 g. Procedures were approved by the University of Melbourne Animal Ethics Committee. Rats were supplied with food and water ad libitum prior to the experiments.
Triple label immunohistochemistry
Rats were anaesthetised with a mixture of ketamine (55 mg/kg) and xylazine (9 mg/kg) prior to being perfused transcardially with heparinised phosphate buffered saline (PBS: 0.15 M NaCl, 0.01 M sodium phosphate buffer, pH 7.2) followed by fixative (2% formaldehyde, 0.2% picric acid in 0.1 M sodium phosphate buffer, pH 7.0). The stomach was removed, dissected, and post-fixed overnight at 4 °C in the same fixative, before being cleared with 3 × 10 min washes in dimethyl sulfoxide, 3 × 10 min washes in PBS and then stored in PBS-sucrose-azide (30% sucrose, 0.1% sodium azide in PBS) at 4 °C. Tissue was then equilibrated overnight at 4 °C in a 1:1 solution of PBS-sucrose-azide and OCT compound (Tissue Tek, Elkhart, IN, USA) before being embedded and frozen in OCT. Tissue from the antrum and corpus was analysed from four animals.
Cryosections (12 μm) were cut onto Superfrost Plus microscope slides (Menzel-Glaser; Thermo Fisher, Scoresby, VIC, Australia), air dried for 1 h and then blocked in a solution of 10% normal horse serum with 1% Triton-X100 in PBS for 30 min at room temperature. Mixtures of up to three primary antibodies from different species were diluted according to Table 1 and applied to slides overnight at 4 °C. Slides were washed with three changes of PBS and then incubated with an appropriate mixture of Alexa Fluor–labelled secondary antibodies for 90 min at room temperature. After a further three changes of PBS, coverslips were applied with Dako fluorescence mounting medium (Agilent, Tullamarine, VIC, Australia). Samples for which the primary antibodies were omitted were analysed to investigate background staining and autofluorescence and were used to set appropriate thresholds for quantification. Slides were examined and imaged using an Axio Imager microscope (Zeiss, Sydney, Australia), an LSM800 or an LSM880 confocal microscope (Zeiss).
Table 1.
List of primary antibodies used and their respective dilutions
| Target | Host species | Dilution | Antibody code, source and/or reference. Research Resource Identifier (RRID) when available |
|---|---|---|---|
| Calbindin | Rabbit | 1:2000 | R8701 (Furness et al. 1989) |
| CGRP (calcitonin gene-related peptide) | Sheep | 1:1000 | #1780 (Kestell et al. 2015) |
| DBH (dopamine beta-hydroxylase) | Rabbit | 1:400 | 2D4 (Costa and Furness 1984) |
| Gastrin | Rabbit | 1:3000 | #8007 (gift from Dr. Jens Rehfeld). RRID Ab_2762851 |
| Gastrin-CCK (cholecystokinin) | Mouse | 1:2700 | #28.2 (Kovacs et al. 1997). RRID Ab_2650429 |
| Ghrelin | Chicken | 1:800 | #ab15861 (Pustovit et al. 2017) RRID Ab_2041392 |
| Ghrelin | Rabbit | 1:10000 | #RY1601 (Mizutani et al. 2009). RRID Ab_2767291 |
| GRP (gastrin releasing peptide = bombesin) | Rabbit | 1:500 | Moody and Pert 1979 |
| HDC (histidine decarboxylase) | Rabbit | 1:2000 | #16045 Progen Biotechnik GmbH, Heidelberg, Germany. RRID Ab_1541512 |
| H+/K+ ATPase (hydrogen/ potassium ATPase: the proton pump) | Mouse | 1:200 | #12.18 (Smolka et al. 2000) |
| 5-HT (5-hydroxytryptamine = serotonin) | Rabbit | 1: 1000 | #20080 Immunostar, Hudson, WI, USA. RRID Ab_572263 |
| 5-HT | Goat | 1: 5000–1:10000 | #20079, Immunostar (Cho et al. 2014). RRID Ab_572262 |
| PYY (peptide tyrosine tyrosine) | Rabbit | 1:200 | #HPA010973 Sigma-Aldrich, Castle Hill, NSW, Australia. RRID Ab_1855194 |
| Somatostatin | Mouse | 1:1000 | #S895 (Buchan et al. 1985) |
| Somatostatin | Sheep | 1:3000 | #AS01 (gift from Dr. Arthur Shulkes) |
| Tuj1 (Neuron-specific Class III β-tubulin) | Mouse | 1:1000 | TUBB3 Biolegend, San Diego, CA, USA |
| VAChT (vesicular acetylcholine transporter) | Goat | 1:4000 | #1624 (Weihe et al. 2005) |
| VIP (vasoactive intestinal peptide) | Rabbit | 1:400 | #7913 (Furness et al. 1981) |
| VIP | Mouse | 1:1000 | V31ASC (Accili et al. 1995) |
Free-floating section immunohistochemistry
Cryosections (50 μm) were cut and placed into PBS as free-floating sections in a 48-well plate. The PBS was then aspirated and replaced with blocking solution (10% normal horse serum with 1% Triton-X100 in PBS) and incubated for three nights at 4 °C. Sections were then incubated in a mixture of primary antibodies (Table 1) for three nights at 4 °C, washed and then incubated in Alexa Fluor–labelled secondary antibodies for a further three nights at 4 °C. Slides were mounted with ProLong Diamond Antifade Mountant (Life Technologies, Mulgrave, VIC, Australia) using #1.5 thickness coverslips and sealed with nail polish.
Image quantification
Sections for cell counts were imaged as tile scans with a nominal optical thickness of 7.7 μm using a × 10 objective on the LSM800 confocal microscope (Zeiss). A 1.5-mm wide region from each imaged section, which contained the full thickness of the mucosa, was selected and each separate channel, as well as a merged image, was converted to tif format for analysis in Fiji (http://imagej.nih.gov/ij/). Images were converted to greyscale and the pixel scale was entered from microscope metadata. Cells from each channel were manually circled and were counted as positive if their mean grey value for pixel intensity was above a threshold determined from negative control images. The total mucosal area was also measured in order to determine the cell density (positive cells per mm2 of mucosa). The thickness of the mucosa was measured from the micrographs (five measurements from n = 4 rats).
Free-floating sections for analysis of distances between EEC and nerve fibres were imaged on the LSM880. A low-resolution preview scan was obtained in the channel with cell body labelling and then 10 cells from the top half of the mucosa and 10 cells from the bottom half of the mucosa were chosen per section (two sections per animal, four animals). Each chosen cell was then imaged in all three channels as a superresolution z-stack using the fast Airyscan mode and a 63× oil objective with a 70×70-μm field of view through the whole section. Images were deconvoluted using the Zen (Zeiss) three-dimensional Airyscan processing function and then imported into Imaris (Bitplane AG, Zurich, Switzerland) for three-dimensional analysis. A 3D surface was rendered for each labelled object and the Distance Transformation XTension was used to generate a heat map, assigning a pixel intensity to represent the distance between that pixel and the closest fibre surface in micrometre enabling us to determine the distance from the edge of the cell to the edge of the closest nerve fibre in the field of view.
Results
Enteroendocrine cell types, locations and colocalisation of markers
EEC in the glandular mucosa of the rat stomach with immunoreactivities for gastrin, ghrelin, PYY, somatostatin, 5-HT and histidine decarboxylase were observed (Figs. 1, 2, and 3). The fundus and the esophageal groove in the rat have a stratified squamous lining without any glands or enteroendocrine cells. Only the corpus and antrum had glandular linings. The corpus had a thickness of 599 ± 50 μm and most of the length of each gland was dominated by parietal cells that were identified by their immunoreactivity for the proton pump, H+/K+ ATPase (Fig. 1a). Internal to this parietal cell layer was a band containing chief cells that were recognised by their larger size and lack of autofluorescence when compared with parietal cells (Fig. 1). Some scattered parietal cells occurred in the chief cell layer (Fig. 1a). The antral mucosa was thinner, 214 ± 13 μm, and antral glands were shorter (Fig. 2c–f) and did not contain parietal cells, but, unlike the corpus, contained gastrin cells (Fig. 2f). A transition zone was found between the antrum and corpus. It contained parietal cells in lower numbers than the corpus and gastrin cells in lower numbers than the antrum.
Fig. 1.
Regions of the mucosa of the gastric corpus, defined by (a), the parietal cell marker, the proton pump (H/K ATPase) (a′), the positions of ghrelin immunoreactive EEC (closed triangles) and (a″), the positions of ECL cells (arrows), localised by immunoreactivity for histidine decarboxylase (HDC). The dotted lines at the top and bottom indicate the luminal (external) ends of the glands, where mucous cells are located, and the basal ends, where there are numerous chief cells. The horizontal dotted line is the approximate boundary between the parietal cell-dominated oxyntic gland parts and the chief cell parts of the glands. There were a small number of parietal cells in the chief cell part, and some chief cells at the base of the parietal cell area. An example area in which ghrelin and ECL cells are close to parietal cells is circled. The luminal and submucosal ends of the glands are indicated in (a). Merge image (a′′′)
Fig. 2.
EEC in the gastric corpus (a, b) and antrum (c–f). In the corpus, the most common cell types are the ghrelin cells (open triangles, a) and the HDC-immunoreactive ECL cells (closed triangles (b)). Somatostatin cells, which commonly have processes, are less common (arrows (a, b)), as are 5-HT cells (star (a)). In the antrum, 5-HT cells (star (c)), somatostatin cells (arrows (c, d)) and gastrin cells, that form a band near the base of the mucosa (open triangles (f)), are common cell types. HDC-immunoreactive ECL cells (closed triangles (d, f)), Ghrelin cells (open triangles (c)) and PYY cells (open triangles (e)) are rarer
Fig. 3.
Mucosal regions and distributions of EEC in the lining of the rat stomach. (a) Rat stomach that has been opened along its greater curvature and pinned flat with its internal surface uppermost. The regions are readily distinguished by colour and texture. (b–g) Semi-quantitative representations of the distributions of EEC in the mucosa of the rat stomach. (h) Quantitation of EEC densities in the antrum and corpus
The corpus contained a large number of ghrelin cells (69 ± 7 per mm2) and numerous somatostatin cells (49 ± 7 per mm2), but fewer 5-HT cells (14 ± 3 per mm2), very few PYY cells (0.2 ± 0.2 per mm2) and no gastrin cells (Figs. 1 and 2). There were also numerous ECL cells, identified by HDC immunoreactivity, in the corpus (298 ± 26 per mm2). In contrast, the antrum contained a very large number of gastrin cells (274 ± 34 per mm2) and numerous somatostatin (105 ± 18 per mm2), 5-HT (68 ± 12 per mm2) and ECL cells (51 ± 17 per mm2), similar numbers of ghrelin cells (45.9 ± 6.6 per mm2) and fewer PYY cells (26 ± 3 per mm2; Figs. 2 and 3).
The relationships of the different gastric regions and the relative numbers of EEC in the different regions are depicted in Fig. 3. The mucosal regions of the rat stomach, the fundus and esophageal groove, the corpus and the antrum are shown in Fig. 3a. In the rat, there was no obvious equivalent of the cardiac glands that are located adjacent to the esophagogastric junction in human.
Cell morphologies and locations
Ghrelin cells were almost all round or ovoid, and located at the base of the epithelium, without processes that extended to the gland lumen, i.e. they were closed cells (Fig. 4e). They were very commonly located between parietal cells and the base of the mucosal epithelium, and sometimes were situated within invaginations of the parietal cells (Fig. 4e). Somatostatin cells commonly had one or more basal processes, both in the antrum and corpus (Figs. 4a, c). The processes were aligned with the basal surfaces of the epithelial cells. In the antrum, these processes were commonly close to gastrin cells (Fig. 4a, a′, a″). ECL (HDC immunoreactive) cells were elongated, flattened cells at the bases of the epithelial cell layer (Fig. 4f). They were commonly closely apposed to parietal cells in the corpus (Fig. 1, circled region) and also occurred in the region of the gland dominated by chief cells, at the bases of the corpus glands; they were also frequent in the antrum (Fig. 2), where parietal cells do not occur. ECL cells did not communicate with the gastric lumen. Some, but not all ECL cells, were immunoreactive for calbindin, as is also seen in the human stomach (Furness et al. 1989). Many 5-HT cells were round or ovoid closed cells, but a significant number, about 20% in the half of the mucosa towards the lumen, were flask-shaped with an apparent luminal contact (Fig. 4d). The majority of gastrin cells were typical open, flask-shaped cells, but a few cells with prominent basal processes, as described in the mouse stomach (Frick et al. 2016), were also encountered (Figs. 2f and 4b). The relatively rare PYY cells also had flask-like, open-type EEC shapes (Fig. 2e).
Fig. 4.
EEC morphologies and relationships. (a, a′, a″) Gastrin and somatostatin cells in the antrum. Somatostatin cells have basal processes (arrows) that in nearly every case come close to gastrin cells, but not all gastrin cells had closely related somatostatin cells. (b) A gastrin cell with a basal process. A minority of gastrin cells had long processes. (c, c′, c″) A ghrelin cell and somatostatin cells in the corpus. The ghrelin cell is a round closed cell, whereas the somatostatin cells have processes that partly wrap the gland. Unlike the consistent relationships of somatostatin and gastrin cells (a″), somatostatin and ghrelin cells do not form close relationships. (d) An example of a 5-HT cell in the corpus with processes. The majority of 5-HT cells were closed, round or oval cells. (e, e′, e″, e″) Relationship between a ghrelin immunoreactive EEC and a parietal cell. These were often close to each other and sometimes, as in this case, a parietal cell partly enveloped a ghrelin cell (shown as a 3 dimensional reconstruction in (e′, e″, e‴)). (f, f′, f″) Calbindin immunoreactivity of ECL cells. A subpopulation of ECL cells, revealed by anti-HDC, was immunoreactive for calbindin. Calbindin negative ECL cells are indicated by the closed triangles. The dotted lines indicate the basal ends of the glands
Patterns of colocalization
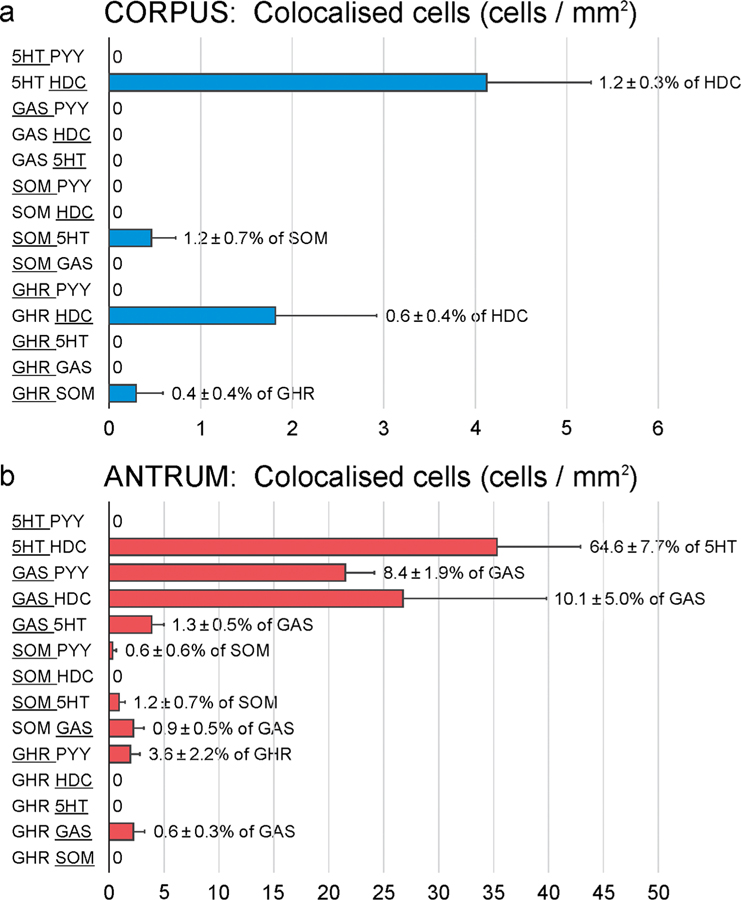
Because previous studies in other parts of the GI tract have indicated that several hormones can be present in the same EEC cell, we examined colocalisation of EEC cell markers using multiple label immunofluorescence (Fig. 5). There was generally very little overlap in the EEC cell markers, with the number of cells containing more than one marker in the corpus being fewer than 1 per mm2 and commonly zero for almost all of the marker combinations (Fig. 6). The only overlaps that were numerous were 5-HT and histidine decarboxylase, 4 cells/mm2 in the corpus and 35 cells/mm2 in the antrum (51% of antral ECL cells were 5-HT immunoreactive). A high proportion of PYY cells were gastrin positive (69.4 ± 10.3%), but these represented fewer than 10% of gastrin cells (Fig. 6). Overlap was found between gastrin and HDC, as previously reported in mouse stomach (Walker et al. 2013). Combinations of antibodies were used to search for triple-labelled EEC, but these were very rare. An example of triple labelling is shown in Fig. 5c.
Fig. 5.
Colocalisation between hormones in gastric EEC. (a and b) examples of colocalisation of histidine decarboxylase (HDC) with 5-HT in the corpus and antrum. The ECL process ran at the base of the mucosal epithelium. This is one of the most common overlaps. (c) Example of a cell triple labelled for gastrin, ghrelin and PYY and another cell with ghrelin alone. Triple-labelled cells were very rare. (d) Example of a cell with colocalised gastrin and PYY. An adjacent cell only contains gastrin
Fig. 6.
Quantitation of overlaps between hormones in the rat gastric corpus (a) and antrum (b). At left the double labelling combinations that were investigated are indicated. A range of triple label combinations were also examined (e.g. Fig. 5) but very few triple-labelled cells were found. ‘0’ against a combination indicates that no colocalisation of this combination was found. Note the differences in the scales on the X-axes, between (a) and (b). The proportion of the larger group of cells that contained the other hormone is indicated to the right of the bar. The larger group is indicated by the underlining. Data is mean ± SEM, n = 3 gastric samples
To determine whether different cell types were more or less abundant at different depths in the mucosa, we divided the height of the mucosa in each section evenly into ‘luminal’ (external) and ‘basal’ (internal) and counted the cells present in each. We found that the majority of EECs were present in the internal half of the mucosa, particularly in the antrum, where fewer than 15% of EECs were found in the external half. This was not the case, however, for 5-HT cells in the corpus, which although distributed throughout the mucosa were more abundant in the external half in this stomach region.
Immunohistochemistry of nerve fibres in the mucosa of the rat stomach
Immunohistochemistry for markers of mucosal nerve fibres was performed to investigate relative fibre densities and the relationship between EEC and the fibres. Antibodies to VIP, VAChT, GRP (mammalian bombesin), CGRP, Tuj-1, DBH and TH (Table 1) all labelled nerve fibres in the mucosa of the stomach. Tuj-1 (neuron-specific Class III β-tubulin) is a pan-neuronal marker that is assumed to label all gastric nerve fibres. VIP and GRP immunoreactive fibres were the most numerous (Fig. 7), with NPY and VAChT being less common and CGRP fibres only seen occasionally. DBH and TH positive fibres were rare. Individual axons were varicose and were commonly intertwined in nerve fibre bundles. For this reason, it is difficult to estimate the actual numbers of nerve fibres in the mucosa, particularly for the more numerous axon types.
Fig. 7.
Nerve fibres in the mucosa at moderate power, showing nerve fibre bundles containing VIP, GRP and NPY axons. Rows (a and b) VIP and GRP axons were in the same bundles in the mucosa (examples at arrows). Some of these bundles also contained NPY fibres. VIP and NPY fibres are seen in the muscularis mucosae (mm) and NPY fibres are seen around arterioles (art) in the submucosa (a, a″, b″). The arteriole in (a and a″) has innervation from both VIP and NPY immunoreactive fibres
At low power, there appeared to be a large degree of overlap between all fibre types (Fig. 7). However, at higher power, it became clear that different nerve fibres run together, intertwined in small nerve bundles in the mucosa, and that fibres did not always show colocalization. VIP, GRP and NPY were generally in the same nerve bundles (Fig. 7), and in some cases were colocalized in the same varicosities of axons innervating the mucosa (Fig. 8). VAChT and CGRP axons ran in the lamina propria with the VIP, GRP and NPY fibre bundles. There were similar numbers of VAChT and VIP fibres, but these labels were not colocalised. Likewise, CGRP fibres were separate from the other chemically defined fibres. NPY immunoreactive fibres formed a plexus around submucosal arterioles (Fig. 7b″, b‴).
Fig. 8.
Simultaneous labelling of nerve fibre bundles in the gastric mucosa. Axons ran closely together in the same nerve fibre bundles. (a, b) High power views of closely related GRP, VIP and NPY axon varicosities. (a) Showing one neurotransmitter at a time. (b) Showing neurotransmitters in pairs. Some varicosities contain only one neurotransmitter (arrowheads), others 2 or three neurotransmitters (arrows). (c) VIP and cholinergic (VAChT) axons run close together, but are separate axon varicosities (arrowheads). (d) VIP and CGPR axons also run close together, but are separate (arrowheads)
The noradrenergic fibres in the mucosa were localised using antibodies against enzymes in the synthesizing pathways for noradrenaline, anti-tyrosine hydroxylase (TH) and anti-dopamine β-hydroxylase (DBH) antibodies. These revealed a very sparse innervation of the mucosa, fibres around arterioles (Fig. 9a, b) and small arteries in the submucosa (Fig. 9c) and fibres in the muscularis mucosae and circular muscle (Fig. 9b).
Fig. 9.
Noradrenergic innervation of the gastric mucosa. Immunoreactivities for dopamine β-hydroxylase (DBH (a, c)) and tyrosine hydroxylase (TH (b)) were used to locate noradrenergic axons. The most prominent innervation was of arterioles (a) and small arteries (art). The internal elastic lamina of the artery is autofluorescent (asterisk (c)). There were very few axons in the mucosa. Immunoglobulins in the anti-TH antiserum bound to immune cells (asterisk, b). Axons were in the muscularis mucosae (mm) and circular muscle (cm). Images from corpus
Relationship of nerve fibres to mucosal cell types in rat stomach
The most common types of nerve fibres in the mucosa were VIP axons, which generally ran in nerve bundles with closely intertwined GRP and NPY axons, including axons with colocalisations between VIP, GRP and NPY. We chose VIP fibres to investigate the relationship between nerve fibre types and EEC cells, because when we scrutinised the sections, the only nerve fibre/EEC relationship that appeared selective was of VIP/GRP/NPY fibre bundles and ghrelin cells. We decided to investigate whether this relationship was quantifiable, and how it compared with a relationship that appeared not to be selective, VIP fibres and gastrin cells. We measured the distance from each ghrelin cell in the corpus and gastrin cell in the antrum to the closest VIP axon. For comparison, we measured distances to the closest CGRP fibre for ghrelin cells in the corpus and gastrin cells in the antrum (Fig. 10). VIP fibres were, on average, much closer to ghrelin cells than were CGRP fibres (Fig. 10) and about 50% of ghrelin cells had a VIP axon within 2 μm, compared with fewer than 5% with such close approaches by CGRP axons (Fig. 10a). For gastrin cells, there appeared to be no favoured innervation by VIP or CGRP axons (Fig. 10c).
Fig. 10.
Relationships of nerve fibres to ghrelin and gastrin cells. (a) Relationship of a VIP immunoreactive axon to a ghrelin cell: original confocal image at left (Z-stack), Imaris 3-D surface rendered image at right. (b) Quantitative data: 50% of ghrelin cells had a VIP axon within 2 mm. There was no selective innervation by CGRP fibres. (c) Relationships of VIP and CGRP axons to gastrin cells. In contrast to the VIP innervation of ghrelin cells, there is no selective innervation of the gastrin cells
Discussion
This study showed that defined sub-classes of gastric EEC were concentrated in particular gastric regions (Fig. 3). Histamine-synthesising ECL cells and ghrelin cells were more numerous in the corpus than antrum and 5-HT and somatostatin cells were more common in the antrum. Gastrin cells were confined to the antrum. PYY cells were rare and mainly in the antrum. Similar to rat, 5-HT cells were about sixfold more numerous in the mouse antrum (11.9 ± 3.4 cells/mm2) than in the mouse corpus (1.7 ± 0.4 cells/mm2) (Reynaud et al. 2016). Likewise, in the human stomach, 5-HT cells are more numerous in the antrum (Ito et al. 1986). Similar to rat, in human, ghrelin cells are dominant in the corpus and are rarer in the antrum (Rindi et al. 2002). In human, they are also present in the fundus. The distribution of somatostatin cells in rat, in which we found a higher density in the antrum, contrasts to human, for which the corpus shows a greater density (Kasacka et al. 2012; Choi et al. 2014). In rats, the gastrin cells are concentrated in a band towards the base of the mucosa, whereas they are more spread across the mucosa in human (Miller et al. 1989; Choi et al. 2014).
In contrast to the colocalisation of hormones that occurs in al most all EEC of t he sm all int estine (see “Introduction”), the majority of gastric EEC contained only a single hormone. The only notable colocalisations were 5-HT and HDC in a subpopulation of ECL cells, PYY and gastrin, and gastrin and histamine in the antrum, but each of these populations was small. 5-HT has also been reported in a subpopulation of ECL cells in the human stomach (Fakhry et al. 2019). A common colocalization, that we did not investigate, is between ghrelin and nesfatin-1 in gastric EEC (Stengel et al. 2013).
Ghrelin cells
Ghrelin, released from the gastric EEC, has an important role in signalling hunger, and thus stimulating appetite, and it also increases gastric emptying in humans and laboratory animals (Levin et al. 2006; Kojima and Kangawa 2010; Avau et al. 2013). Ghrelin-containing endocrine cells were of the closed type, that is, they did not contact the lumen, a feature that has been reported previously in rat and human (Date et al. 2000; Dornonville De La Cour et al. 2001; Fakhry et al. 2019). Thus, ghrelin cells probably do not react directly to the luminal content, but may react to nutrients that are absorbed by the gastrointestinal tract, possibly indirectly through neurons that innervate these EEC (Williams et al. 2003; Steensels et al. 2016). The most numerous chemically defined nerve fibre type that we found in the mucosa, VIP axons, may have a functional interaction with the ghrelin cells, as VIP1 receptor gene expression is enriched in gastric ghrelin cells (Engelstoft et al. 2013). Consistent with this hypothesis, we found by high-resolution confocal analysis that ghrelin cells were often closely approached by VIP fibres, with about 50% of ghrelin cells having a VIP fibre within 2 μm. Because of the tight bundling of fibres, this implies that GRP, NPY and VAChT fibres are also close to the ghrelin cells. Previous studies in the rat have shown that the dense VIP innervation of the mucosa was not reduced by vagotomy or removing the celiac and superior mesenteric ganglia, thus interrupting the sympathetic supply to the rat stomach (Ekblad et al. 1985). The majority of the VIP fibre innervation of the mucosa was thus deduced to arise from neurons of the gastric enteric ganglia. Many of the VIP neurons are innervated by the vagus but the vagus nerve does not appear to be involved in the regulation of ghrelin release (Veedfald et al. 2018). Thus, if VIP neurons influence ghrelin release, they may do this through peripheral reflex pathways. This is consistent with levels of circulating ghrelin being controlled by feedback from the duodenum, through neural circuits or duodenal hormones (Williams et al. 2003; Steensels et al. 2016). Circulating nutrients, that reach the stomach, such as free fatty acids could also influence the ghrelin cells because they express appropriate fatty acid receptors (FFAR2 and FFAR4) whose activation inhibits ghrelin secretion (Engelstoft et al. 2013; Gong et al. 2013).
GRP is contained in many of the same nerve fibres as VIP in the rat gastric mucosa (Berthoud 1996; Ekblad et al. 2000), but GRP receptor gene expression is not enriched in ghrelin cells (Engelstoft et al. 2013). There is enrichment of expression for CGRP (RAMP1 + calcitonin receptor) and NPY2 receptor genes in gastric ghrelin cells of the mouse. NPY nerve fibres were generally in the same nerve bundles as GRP/VIP fibres (Fig. 7), and thus, NPY can be expected to reach the ghrelin cells. However, we did not find a close association between CGRP containing nerve fibres and ghrelin cells, indicating that receptor gene expression and proximity of fibres containing the natural ligand for the receptor does not always occur. Interestingly, the ghrelin cells are not responsive to acetylcholine (Engelstoft et al. 2013), suggesting that they are not targets for the cholinergic (VAChT immunoreactive) axons in the mucosa.
Many of the ghrelin cells were sandwiched behind parietal cells and were even partly enveloped by parietal cells (Fig. 4e). This close proximity between the two was also seen in the human gastric mucosa (Tanaka-Shintani and Watanabe 2005; Fakhry et al. 2019). However, ghrelin does not directly affect acid secretion (Dornonville de la Cour et al. 2004). The increase in acid secretion by high doses of ghrelin is indirect, via vagally mediated release of histamine (Yakabi et al. 2006). Thus, the close relationships of ghrelin and parietal cells may not be indicative of a function of ghrelin in regulation of parietal cells. The positioning of ghrelin cells close to the blood vessels of the lamina propria may be so that they are exposed to circulating factors, for example from the duodenum (see above).
It is notable that ghrelin-containing EEC is concentrated in the corpus. Ghrelin reduces feelings of nausea (Sanger and Furness 2016) and electrical stimuli that are applied to the corpus of patients with gastroparesis reduce nausea and vomiting (Wo et al. 2016). It is feasible that the anti-nauseant effect of gastric electrical stimulation is exerted by ghrelin released from the stimulated region or that the stimulus acts on vagal afferents that innervate the ghrelin cells and connect with nausea control circuits in the lower brain stem. Agonists acting at ghrelin receptors increase gastric emptying and this effect may contribute to the relief of nausea, but trials of ghrelin receptor agonists in patients with gastroparesis have not demonstrated significant symptom relief, even when increased gastric emptying occurred (Avau et al. 2013).
The appetite-stimulating effects of ghrelin are mediated by signalling from ghrelin-containing gastric EEC to vagal afferent nerve fibres. This is demonstrated by experiments in rodents, in which transection of the vagus prevents the orexigenic effect of ghrelin (Asakawa et al. 2001; Date et al. 2002), and in humans with truncal vagotomy in whom ghrelin did not increase food intake, in contrast to people with the vagi intact (le Roux et al. 2005). Chemical markers of the endings of the vagal afferent neurons that are responsive to ghrelin have not yet been identified, but the endings are presumed to come close to the ghrelin-containing gastric EEC.
Somatostatin cells
Somatostatin cells were characterised by their multiple basal processes. In the antrum, these processes came close to gastrin cells, appearing to make specific contact (Fig. 4a), as previously reported (Larsson et al. 1979). These are functional connections, somatostatin being a physiological regulator that provides chronic restraint of gastrin secretion, a restraint that is increased when antral acidity increases (Schubert and Peura 2008). Basal processes were also prominent for somatostatin cells in the corpus; these were close to parietal cells and ECL cells. Consistent with this, somatostatin released in proximity to the parietal cells inhibits acid production (Schubert et al. 1988) and somatostatin inhibits histamine release, pointing to an inhibitory control of ECL cells that would also reduce acid production (Chuang et al. 1993; Vuyyuru et al. 1997). Like other gastric EEC, the majority of somatostatin cells are of the closed type (Hauso et al. 2007; Fakhry et al. 2019).
5-HT cells
We found that 5-HT cells were a mixture of round or ovoid closed cells and open cells, and a previous paper had reported that 5-HT cells in the rat stomach were open cells (Yu et al. 2001). In the corpus, 5-HT cells were closely apposed to parietal cells. This can be related to the inhibition of acid secretion by 5-HT, a direct action that is not nerve mediated (Canfield and Spencer 1983; LePard et al. 1996).
Gastric 5-HT may also have roles in causing satiety and promoting nausea and vomiting. Both nausea and vomiting are precipitated within minutes of oral cytotoxic drugs being taken, suggesting a gastric site of action (Andrews et al. 1998). 5-HT3 receptor blockers antagonise these effects in human and in experimental animals. Bilateral vagotomy substantially reduced nausea and vomiting, suggesting that toxins release 5-HT from gastric EEC that acts on 5-HT3 receptors on the endings of vagal afferent neurons (Andrews et al. 1990).
The 5-HT cells in the stomach may also provide satiety signals. Agonists of 5-HT receptors and drugs that inhibit 5-HT metabolism (thus increasing its availability) decrease food intake; conversely, antagonists of 5-HT receptors increase feeding (Blundell 1986; Cooper and Dourish 1990). It is feasible that the satiety effect is aversive, caused by a nauseant effect of gastric 5-HT.
ECL cells
ECL cells were very numerous in the corpus, where their role to stimulate the parietal cells to secrete acid is well established (Schubert and Peura 2008). In addition, we found numerous ECL cells in the antrum, remote from parietal cells. Many of these antral cells also contained 5-HT. At this time, we do not know their roles.
In conclusion, this study documents and quantifies the differences in EEC populations between the corpus and antrum in the rat. Unlike EEC in the small intestine, a low proportion of gastric EEC expresses two or more hormones. We found a selective innervation of ghrelin cells by VIP axons.
Acknowledgements
We thank Josiane Fakhry for helpful comments on the manuscript.
Funding This work was supported by NIH (SPARC) grant ID # OT2OD023847 (PI Terry Powley) to JBF.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Informed consent None required.
Ethical approval Procedures were approved by the University of Melbourne Animal Ethics Committee (ethics approval number 1614002). All applicable National and Institutional guidelines for the care and use of animals were followed.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Accili EA, Dhatt N, Buchan AMJ (1995) Neural somatostatin, vasoactive intestinal polypeptide and substance P in canine and human jejunum. Neurosci Lett 185:37–40 [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L (1990) The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol 68:325–345 [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Naylor RJ, Joss RA (1998) Neuropharmacology of emesis and its relevance to anti-emetic therapy. Support Care Cancer 6:197–203 [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M (2001) Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120:337–345 [DOI] [PubMed] [Google Scholar]
- Avau B, Carbone F, Tack J, Depoortere I (2013) Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil 25:720–732 [DOI] [PubMed] [Google Scholar]
- Berthoud H-R (1996) Morphological analysis of vagal input to gastrin releasing peptide and vasoactive intestinal peptide containing neurons in the rat glandular stomach. J Comp Neurol 370:61–70 [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Powley TL (1992) Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319:261–276 [DOI] [PubMed] [Google Scholar]
- Blundell JE (1986) Serotonin manipulations and the structure of feeding behaviour. Appetite 7:39–56 [DOI] [PubMed] [Google Scholar]
- Buchan AMJ, Sikora LKJ, Levy JG, McIntosh CHS, Dyck I, Brown JC (1985) An immunocytochemical investigation with monoclonal antibodies to somatostatin. Histochemistry 83:175–180 [DOI] [PubMed] [Google Scholar]
- Canfield SP, Spencer JE (1983) The inhibitory effects of 5-hydroxytryptamine on gastric acid secretion by the rat isolated stomach. Br J Pharmacol 78:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-J, Callaghan B, Bron R, Bravo DM, Furness JB (2014) Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res 356:77–82 [DOI] [PubMed] [Google Scholar]
- Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB (2015) Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res 359:693–698 [DOI] [PubMed] [Google Scholar]
- Choi E, Roland JT, Barlow BJ, O’Neal R, Rich AE, Nam KT, Shi C, Goldenring JR (2014) Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 63:1711–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C-N, Tanner M, Lloyd KCK, Wong H, Soll AH (1993) Endogenous somatostatin inhibits histamine release from canine gastric mucosal cells in primary culture. Am J Physiol Gastrointest Liver Physiol 28:G521–G525 [DOI] [PubMed] [Google Scholar]
- Clemmensen C, MT D, Woods SC, Berthoud H-R, Seeley RJ, Tschöp MH (2017) Gut-brain cross-talk in metabolic control. Cell 168:758–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Dourish CT (1990) Multiple cholecystokinin (CCK) receptors and CCK-monoamine interactions are instrumental in the control of feeding. Physiol Behav 48:849–857 [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB (1984) Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience 13: 911–919 [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261 [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123:1120–1128 [DOI] [PubMed] [Google Scholar]
- Dornonville De La Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao C-M, Chen D, Håkanson R (2001) A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept 99:141–150 [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Lindström E, Norlén P, Håkanson R (2004) Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept 120:23–32 [DOI] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV et al. (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Ekelund M, Graffner H, Håkanson R, Sundler F (1985) Peptide-containing nerve fibres in the stomach wall of rat and mouse. Gastroenterology 89:73–85 [DOI] [PubMed] [Google Scholar]
- Ekblad E, M Q, Sundler F (2000) Innervation of the gastric mucosa. Microsc Res Tech 48:241–257 [DOI] [PubMed] [Google Scholar]
- Engelstoft MS, Park W-M, Sakata I et al. (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2:376–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhry J, Stebbing MJ, Hunne B, Bayguinov Y, Ward SM, Sasse KC, Callaghan B, McQuade RM, Furness JB (2019) Relationships of endocrine cells to each other and to other cell types in the human gastric fundus and corpus. Cell Tissue Res 376:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB (2017) Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158:2113–2123 [DOI] [PubMed] [Google Scholar]
- Frick C, Rettenberger AT, Lunz ML, Breer H (2016) Complex morphology of gastrin-releasing G-cells in the antral region of the mouse stomach. Cell Tissue Res 366:301–310 [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M, Walsh JH (1981) Evidence for and significance of the projection of VIP neurons from the myenteric plexus to the taenia coli in the guinea-pig. Gastroenterology 80:1557–1561 [PubMed] [Google Scholar]
- Furness JB, Padbury RTA, Baimbridge KG, Skinner JM, Lawson DEM (1989) Calbindin immunoreactivity is a characteristic of enterochromaffin- like cells ECL cells of the human stomach. Histochemistry 92:449–451 [DOI] [PubMed] [Google Scholar]
- Furness JB, Koopmans HS, Robbins HL, Clerc N, Tobin JM, Morris MJ (2001) Effects of vagal and splanchnic section on food intake, weight, serum leptin and hypothalamic neuropeptide Y in rat. Auton Neurosci 92:28–36 [DOI] [PubMed] [Google Scholar]
- Furness JB, Rivera LR, Cho H-J, Bravo DM, Callaghan B (2013) The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10:729–740 [DOI] [PubMed] [Google Scholar]
- Gong Z, Yoshimura M, Aizawa S, Kurotani R, Zigman JM, Sakai T, Sakata I (2013) G-protein coupled receptor 120 (GPR120) signaling regulates ghrelin secretion in vivo and in vitro. Am J Physiol Endocrinol Metab 306:E28–E35 [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F (2016) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78:277–299 [DOI] [PubMed] [Google Scholar]
- Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153: 3054–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauso Ø, Gustafsson BI, Waldum HL (2007) Long slender cytoplasmic extensions: a common feature of neuroendocrine cells? J Neuroendocrinol 19:739–742 [DOI] [PubMed] [Google Scholar]
- Hunyady B, Zólyomi A, Hoffman BJ, Mezey E (1998) Gastrin-producing endocrine cells: a novel source of histamine in the rat stomach. Endocrinology 139:4404–4415 [DOI] [PubMed] [Google Scholar]
- Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW (2017) GPCR-mediated signaling of metabolites. Cell Metab 25:777–796 [DOI] [PubMed] [Google Scholar]
- Ito H, Yokozaki H, Tokumo K, Nakajo S, Tahara E (1986) Serotonin-containing EC cells in normal human gastric mucosa and in gastritis. Virchows Archiv A 409:313–323 [DOI] [PubMed] [Google Scholar]
- Kasacka I, Łebkowski W, Janiuk I, Łapińska J, Lewandowska A (2012) Immunohistochemical identification and localisation of gastrin and somatostatin in endocrine cells of human pyloric gastric mucosa. Folia Morphol (Warsz) 71:39–44 [PubMed] [Google Scholar]
- Kestell GR, Anderson RL, Clarke JN, Haberberger RV, Gibbins IL (2015) Primary afferent neurons containing calcitonin gene-related peptide but not substance P in forepaw skin, dorsal root ganglia, and spinal cord of mice. J Comp Neurol 523:2555–2569 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K (2010) Ghrelin: more than endogenous growth hormone secretagogue. Ann N Y Acad Sci 1200:140–148 [DOI] [PubMed] [Google Scholar]
- Kovacs TO, Lloyd KC, Lawson DC (1997) Inhibition of sham feeding-stimulated acid secretion in dogs by immunoneutralization of gastrin. Am J Physiol Gastrointest Liver Physiol 273:G399–G403 [DOI] [PubMed] [Google Scholar]
- Larsson LI, Goltermann N, De Magistris L, Rehfeld JF, Schwarz TW (1979) Somatostatin cell processess as pathways for paracrine secretion. Science 205:1393–1395 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA, Bloom SR (2005) Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 90:4521–4524 [DOI] [PubMed] [Google Scholar]
- LePard KJ, Chi J, Mohammed JR, Gidener S, Stephens RL Jr (1996) Gastric antisecretory effect of serotonin: quantitation of release and site of action. Am J Physiol Endocrinol Metab 271:E669–E677 [DOI] [PubMed] [Google Scholar]
- Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM, Näslund E (2006) Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab 91:3296–3302 [DOI] [PubMed] [Google Scholar]
- Lu K-H, Cao J, Thomas Oleson S, Powley TL, Liu Z (2017) Contrast-enhanced magnetic resonance imaging of gastric emptying and motility in rats. IEEE Trans Biomed Eng 64:2546–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AS, Furness JB, Costa M (1989) The relationship between gastrin cells and bombesin-like immunoreactive nerve fibres in the gastric antral mucosa of guinea-pig, rat, dog and man. Cell Tissue Res 257: 171–178 [DOI] [PubMed] [Google Scholar]
- Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M (2009) Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297: G974–G980 [DOI] [PubMed] [Google Scholar]
- Moody TW, Pert CB (1979) Bombesin-like peptides in rat brains: quantitation and biochemical characterization. Biochem Biophys Res Commun 90:7–14 [DOI] [PubMed] [Google Scholar]
- Mumphrey MB, Patterson LM, Zheng H, Berthoud H-R (2013) Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil 25:e70–e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL (1998) Gastric volume detection after selective vagotomies in rats. Am J Physiol Regul Integr Comp Physiol 271: R766–R769 [DOI] [PubMed] [Google Scholar]
- Powley TL, Spaulding RA, Haglof SA (2011) Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519:644–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Hudson CN, McAdams JL, Baronowsky EA, Martin FN, Mason JK, Phillips RJ (2014) Organization of vagal afferents in pylorus: mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton Neurosci 183:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustovit RV, Callaghan B, Ringuet MT, Kerr NF, Hunne B, Smyth IM, Pietra C, Furness JB (2017) Evidence that central pathways that mediate defecation utilize ghrelin receptors but do not require endogenous ghrelin. Physiol Rep 5:e13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud Y, Fakhry J, Fothergill L, Callaghan B, Ringuet MT, Hunne B, Bravo DM, Furness JB (2016) The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res 364:489–497 [DOI] [PubMed] [Google Scholar]
- Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E (2002) Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol 117:511–519 [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Furness JB (2016) Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 19:38–48 [DOI] [PubMed] [Google Scholar]
- Schubert ML, Peura DA (2008) Control of gastric acid secretion in health and disease. Gastroenterology 134:1842–1860 [DOI] [PubMed] [Google Scholar]
- Schubert ML, Bitar KN, Makhlouf GM (1982) Regulation of gastrin and somatostatin secretion by cholinergic and noncholinergic intramural neurons. Am J Physiol Gastrointest Liver Physiol 243:G442–G447 [DOI] [PubMed] [Google Scholar]
- Schubert ML, Edwards NF, Makhlouf GM (1988) Regulation of gastric somatostatin secretion in the mouse by luminal acidity: a local feedback mechanism. Gastroenterology 94:317–322 [DOI] [PubMed] [Google Scholar]
- Smolka AJ, Larsen KA, Hammond CE (2000) Location of a cytoplasmic epitope for monoclonal antibody HK 12.18 on H,K-ATPase α sub-unit. Biochem Biophys Res Commun 273:942–947 [DOI] [PubMed] [Google Scholar]
- Solcia E, Rindi G, Buffa R, Fiocca R, Capella C (2000) Gastric endocrine cells: types, function and growth. Regul Pept 93:31–35 [DOI] [PubMed] [Google Scholar]
- Steensels S, Vancleef L, Depoortere I (2016) The sweetener-sensing mechanisms of the ghrelin cell. Nutrients 8:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Hofmann T, Goebel-Stengel M, Lembke V, Ahnis A, Elbelt U, Lambrecht NWG, Ordemann J, Klapp BF, Kobelt P (2013) Ghrelin and NUCB2/nesfatin-1 are expressed in the same gastric cell and differentially correlated with body mass index in obese subjects. Histochem Cell Biol 139:909–918 [DOI] [PubMed] [Google Scholar]
- Sykaras AG, Demenis C, Cheng L, Pisitkun T, Mclaughlin JT, Fenton RA, Smith CP (2014) Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155:3339–3351 [DOI] [PubMed] [Google Scholar]
- Tanaka-Shintani M, Watanabe M (2005) Distribution of ghrelin-immunoreactive cells in human gastric mucosa: comparison with that of parietal cells. J Gastroenterol 40:345–349 [DOI] [PubMed] [Google Scholar]
- Veedfald S, Plamboeck A, Hartmann B, Vilsbøll T, Knop FK, Deacon CF, Svendsen LB, Holst JJ (2018) Ghrelin secretion in humans - a role for the vagus nerve? Neurogastroenterol Motil 30:e13295. [DOI] [PubMed] [Google Scholar]
- Vuyyuru L, Harrington L, Arimura A, Schubert ML (1997) Reciprocal inhibitory paracrine pathways link histamine and somatostatin secretion in the fundus of the stomach. Am J Physiol Gastrointest Liver Physiol 273:G106–G111 [DOI] [PubMed] [Google Scholar]
- Walker AK, Park W-M, Chuang J-C, Perello M, Sakata I, Osborne-Lawrence S, Zigman JM (2013) Characterization of gastric and neuronal histaminergic populations using a transgenic mouse model. PLoS One 8:e60276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Schütz B, Hartschuh W, Anlauf M, Schäfer MK, Eiden LE (2005) Coexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous system. J Comp Neurol 492:370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Cummings DE, Grill HJ, Kaplan JM (2003) Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 144:2765–2767 [DOI] [PubMed] [Google Scholar]
- Wo JM, Nowak TV, Waseem S, Ward MP (2016) Gastric electrical stimulation for gastroparesis and chronic unexplained nausea and vomiting. Curr Treat Options Gastroenterol 14:386–400 [DOI] [PubMed] [Google Scholar]
- Worthington JJ, Reimann F, Gribble FM (2018) Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol 11:3–20 [DOI] [PubMed] [Google Scholar]
- Yakabi K, Ro S, Onouhi T, Tanaka T, Ohno S, Miura S, Johno Y, Takayama K (2006) Histamine mediates the stimulatory action of ghrelin on acid secretion in rat stomach. Dig Dis Sci 51:1313–1321 [DOI] [PubMed] [Google Scholar]
- Yu P-L, Fujimura M, Hayashi N, Nakamura T, Fujimiya M (2001) Mechanisms in regulating the release of serotonin from the perfused rat stomach. Am J Physiol Gastrointest Liver Physiol 280:G1099–G1105 [DOI] [PubMed] [Google Scholar]
- Zheng H, Berthoud H-R (2000) Functional vagal input to gastric myenteric plexus as assessed by vagal stimulation-induced Fos expression. Am J Physiol Gastrointest Liver Physiol 279:G73–G81 [DOI] [PubMed] [Google Scholar]