Abstract
Recent reports of similar patterns of brain electrical activity (Electroencephalogram: EEG) during action execution and observation recorded from scalp locations over motor-related regions in infants and adults have raised the possibility that two foundational abilities—controlling one’s own intentional actions and perceiving others’ actions—may be integrally related during ontogeny. However, to our knowledge, there are no published reports of the relations between developments in motor skill (i.e., recording actual motor skill performance) and EEG during both action execution and action observation. In the present study we collected EEG from 21 9-month-olds who were given opportunities to reach for toys and who also observed an experimenter reach for toys. Event-related desynchronization (ERD) was computed from the EEG during the reaching events. We assessed infants’ reaching-grasping competence, including reach latency, errors, preshaping of the hand, and bimanual reaches, and found that desynchronization recorded in scalp electrodes over motor-related regions during action observation was associated with action competence during execution. Infants who were more competent reachers, compared to less competent reachers, exhibited greater ERD while observing reaching-grasping. These results provide initial evidence for an early emerging neural system integrating one’s own actions with the perception of others’ actions.
The notion that two foundational abilities—the control of one’s own intentional actions and the meaningful perception of others’ actions—are integrally related has gained momentum and opened debate in behavioral and neuroscience fields in recent years. The mu rhythm, an electroencephalogram (EEG) rhythm found over sensorimotor areas of the scalp, has been associated with both motor behavior and the perception of biological movement (Cochin, Barthelemy, Lejeune, Roux, & Martineau, 1998; Lepage & Theoret, 2006; Pfurtscheller, Neuper, Andrew, & Edlinger, 1997). From a developmental perspective, both action production and perception systems undergo fundamental developments in infancy; thus, because EEG is a relatively infant-friendly tool, studying the development of this rhythm and its potential mirroring properties can shed light on neurocognitive mechanisms that drive early development.
During the first postnatal year, infants undergo dramatic developments in the control of goal-directed action. In the production of manual actions, infants progress from relatively crude batting movements, to more efficient, visually-guided object-directed reaching by 4 to 6 months of age (e.g., Bushnell, 1985; von Hofsten, 1979). By 9 to 12 months, infants’ manual actions become highly skilled, with increased efficiency in movements, prospective control, and fine motor adjustments to target objects (Claxton, Keen, & McCarty, 2003; Clearfield & Thelen, 2001; von Hofsten, 2004; von Hofsten & Ronnqvist, 1988).
In the first year, infants also begin to analyze others’ actions in meaningful ways, for example, visually anticipating action outcomes and selectively encoding the goal structure of others’ actions (Cannon & Woodward, 2012; Gredebäck, Stasiewicz, Falck-Ytter, Rosander, & von Hofsten, 2009; Kanakogi & Itakura, 2010; Woodward, Sommerville, Gerson, Henderson, & Buresh, 2009; Southgate, Begus, Lloyd-Fox, di Gangi, & Hamilton, 2014). Critically, developments in infants’ action control are correlated with, and render changes in their analysis of others’ actions (Cannon, Woodward, Gredebäck, von Hofsten, & Turek, 2012; Gerson & Woodward, 2014; Hunnius & Bekkering, 2014; Kanakogi & Itakura, 2010; Sommerville, Woodward, & Needham, 2005). For example, 3-month-olds given experience with “sticky mittens” (i.e., allowing infants to obtain objects themselves) were more likely to perceive another’s reach to a toy in terms of the goal structure, compared to infants who did not receive prior training (Sommerville et al., 2005). Similarly, 10-month-olds who successfully executed a means-end act themselves—cloth-pulling to retrieve a toy—were more likely to interpret another’s similar action as goal-directed than infants who were less successful in the action themselves (Sommerville & Woodward, 2005). Motor competence also influences the observation of reaching and grasping: 10-month-olds understood the functional consequences of another’s precision grasp only if they were capable of performing precision grasps themselves (Loucks & Sommerville, 2012). Less is known about the neural correlates that may coincide with the development of these action-perception links in the first year.
The discovery of mirror neurons—neurons that fire during execution and observation of goal-directed acts—in the monkey motor cortex has largely contributed to the broader notion of a neurocognitive mirroring mechanism for understanding psychological phenomena across diverse domains and populations (Rizzolatti & Craighero, 2004). EEG is a useful tool for studying the development of neural mirroring. Specifically, the mu rhythm, an endogenous rhythm in the alpha frequency band over central electrode sites, can be functionally defined by its signature desynchronization in EEG power during the execution of an intentional motor act. This rhythm has been associated with sensorimotor activity in human adults (Arnstein et al., 2011; Ritter, Moosmann, & Villringer, 2009) and, because similar topographic desynchronization patterns have been found during action observation, has been suggested to reflect the activity of a mirroring system in human adults (Muthukumaraswamy, Johnson, & McNair, 2004; Pineda, Allison, & Vankov, 2000).
The emergence of a central EEG rhythm, thought to be mu, was reported in human infants approximately 9 to 10 months of age (Marshall, Bar-Haim, & Fox, 2002). More recently, a number of studies report human and monkey infants exhibit desynchronization in the 5–9 Hz frequency band, during action observation (Ferrari et al., 2012; Marshall et al., 2011; Nystrom, Ljunghammar, Rosander, & von Hofsten, 2011; Southgate et al., 2009; Southgate, Johnson, El Karoui, & Csibra, 2010; van Elk, van Schie, Hunnius, Vesper, & Bekkering, 2008), and action production (Ferrari et al., 2012; Marshall et al., 2011; Marshall, Saby, & Meltzoff, 2013; Southgate et al., 2009, 2010). For example, human infants exhibit desynchronization during the execution and observation of reaching acts at 9 months (Southgate et al., 2009) and the execution and observation of button-pressing acts by 14 months (Marshall et al., 2011). Thus, the neural correlates of action perception may be measured during infancy using EEG.
It remains unclear, however, whether and the extent to which the desynchronization patterns in infants can be used as a marker to track the developing mirror mechanism. Adult mu rhythm desynchronization during execution and observation is reported to be specific to central sites, consistent with the view that it reflects motor system activity (Babiloni et al., 1999; Muthukumaraswamy & Johnson, 2004, Muthukumaraswamy et al., 2004; Pfurtscheller et al., 1997). In contrast, infant findings often show desynchronization is more distributed across the scalp during action observation, and sometimes during action production (Marshall et al., 2011; Saby et al., 2012; van Elk et al., 2008; see Marshall & Meltzoff, 2011 for a discussion). It is therefore important to understand how much this desynchronization over central electrodes could relate to the development of the motor system.
From this perspective, in fact, little is known about whether mu rhythm relates to the development of motor abilities during infancy. In adults, motor learning affects the neural response to observed actions, as measured by functional magnetic resonance imaging (fMRI; e.g., Calvo-Merino et al., 2005), magnetoencephalography (MEG; e.g., Jarvelainen, Schurmann, & Hari, 2004), and EEG (e.g., Orgs, Dombrowski, Heil, & Jansen-Osmann, 2008). Action expertise for specific actions is associated with heightened responses of motor-related areas during the observation of those actions (Calvo-Merino et al., 2005; Cannon et al., 2014; Hadjidimitriou et al., 2011; Jarvelainen et al., 2004; Orgs et al., 2008). For example, greater mu desynchronization was reported in dancers, compared to non-dancers, during the observation of dance movements, but not during the observation of everyday movements, familiar to both groups (Orgs et al., 2008). One adult study measured qualitative aspects of action expertise, in which there was a positive association between a motor skill—drawing—and the amount of desynchronization at central sites while observing drawing (Marshall, Bouquet, Shipley, & Young, 2009). However, to date there is limited evidence, particularly with respect to development, to clarify whether and how the quality of one’s own motor competence is determinant in modifying the EEG response during action perception.
Based on these considerations, it is possible that if mu rhythm desynchronization in infants reflects neural recruitment specific to one’s own motor representations, then the strength of mu desynchronization during action perception should vary as a function of the quality of infants’ motor abilities for that particular act. That is, developments in infants’ motor skill should predict the strength of their desynchronization during action observation, and desynchronization during action production may reflect variations in infants’ motor skill.
Several recent experiments offer initial, but inconclusive evidence concerning the relations between motor learning and mu rhythm desynchronization in infants. For example, infants given one week of training shaking a particular rattle subsequently showed attenuated power in the mu band (6–8 Hz) when hearing that rattle sound as compared to sounds not associated with a familiar action (Paulus et al., 2012). In addition, the amount of training infants received with the rattles correlated with their degree of attenuation at 6–7 Hz. However, there was no measure of neural activity during action execution, nor was there a measure of infants’ action production skill. In addition, although there were comparisons of power across conditions, there were not, however, comparisons of desynchronization with respect to a resting baseline, leaving open questions concerning the nature and specificity of the neural response (see Cuevas, Cannon, Yoo, & Fox, 2014).
A second example comes from a study in which 14-month-olds were given experience lifting heavy and light (weight) objects. Differential patterns of mu desynchronization (6–9 Hz) between the conditions were found at central sites, as well as differential desynchronization when infants observed someone else lifting the same objects (Marshall et al., 2013). These effects, however, did not reach conventional levels of statistical significance, and therefore seem to suggest that very brief, short-term action experience does not always lead to a detectable effect on mu rhythm desynchronization during action observation in infants.
Lastly, the effects of long-term, spontaneous developmental changes in mu rhythm suppression were investigated in 14- to 16-month-old infants who viewed films of other infants crawling or walking (van Elk et al., 2008). Greater suppression was reported when infants viewed crawling compared to walking, at two sites (FCz and Cz) in the mu frequency band (7–9 Hz). This differential suppression may be due to the fact that, at this age, crawling, compared to walking, is better established in infants’ motor repertoire. Further, EEG power in the mu band correlated with the length of time (as estimated by parents) that infants had been crawling, but no relations were found between suppression and the amount of time infants had been walking. These findings suggest relations between motor development, as measured by amount of experience, and mu rhythm activity developing well into the second year.
Taken together, prior findings suggest that changes in motor skill during infancy may relate to mu rhythm suppression, but no study, to our knowledge, has investigated the relations between developments in motor skill—beyond “amount” of experience measures (i.e., recording actual motor skill performance)—and EEG activity during both action execution and action observation. In the current study, we addressed this gap in the literature by exploring the relations between mu rhythm desynchronization and spontaneous development in infants’ reaching-grasping competence at 9 months of age.
Infants’ reaching-grasping competence has been quantified in previous studies using various metrics, including the frequency or proportion of grasp attempts with errors, including misjudgments of target distance or position (Fetters & Todd, 1987; McDonnell, 1975; Sclafani, Simpson, Suomi, & Ferrari, under review). Greater competence has been associated with slower latencies to initiate reaches, and faster latencies to complete reach-grasps (Berthier & Carrico, 2010; Barrett, Traupmann, & Needham, 2008; Newman, Atkinson, & Braddick, 2001; Sacrey & Wishaw, 2012). Appropriate hand preshaping, including matching the opening and orientation of the fingers to the size of the object, reflects increased reach-grasp competence (Berthier & Carrico, 2010; Fagard, 2000; Halverson, 1931; Newell, McDonald, & Baillargeon, 1993; Sacrey & Wishaw, 2012; Siddiqui, 1995; von Hofsten & Rönnqvist, 1988; Lockman et al., 1984; von Hofsten & Fazel-Zandy, 1984). Infants also produce more one-handed reach-grasps with age, as opposed to bimanual reaches, reflecting increased motor coordination (Bruner, 1970; Fagard, 1998; Fagard & Jacquet, 1989; Fagard & Pezé, 1997; Michel et al., 1985; Rochat, 1992). All of these developments are the product of months of motor practice during which infants’ actions become progressively more organized with respect to their goals. These changes in infants’ motor control, however, have yet to be studied empirically using neurophysiological measures.
In the present study we measured EEG desynchronization during both the execution and observation of a reaching-grasping action. We asked whether individual differences in reach-grasp skills that are associated with EEG desynchronization. We coded infants’ motor behavior during the execution of reaches and chose measures previously used to assess reaching-grasping competence, as reviewed above, including reach-grasp latencies, errors, bimanual reaches, and hand preshaping. Given the mu band’s proposed role in action processing, we predicted that reach-grasping competence would predict EEG desynchronization, particularly over central scalp locations.
Method
Participants
The final sample included 21 full-term (minimum 37 week gestation), 9-month-old infants (13 females, 8 males; M = 9 months; 3 days, SD = 14 days, range = 8;14–10;3). An additional 14 infants were tested but excluded from the analyses (n = 11 males, 3 females, M = 9;12, SD = 11 days) due to fussiness (n = 2), fewer than five artifact-free segments in one or more condition (n = 8), fewer than five segments after the calculation of ERD and removal of trial outliers, as described in the section Event-Related Desynchronization (ERD) analysis (n = 2), and technical difficulties (n = 2).
Experimental set-up
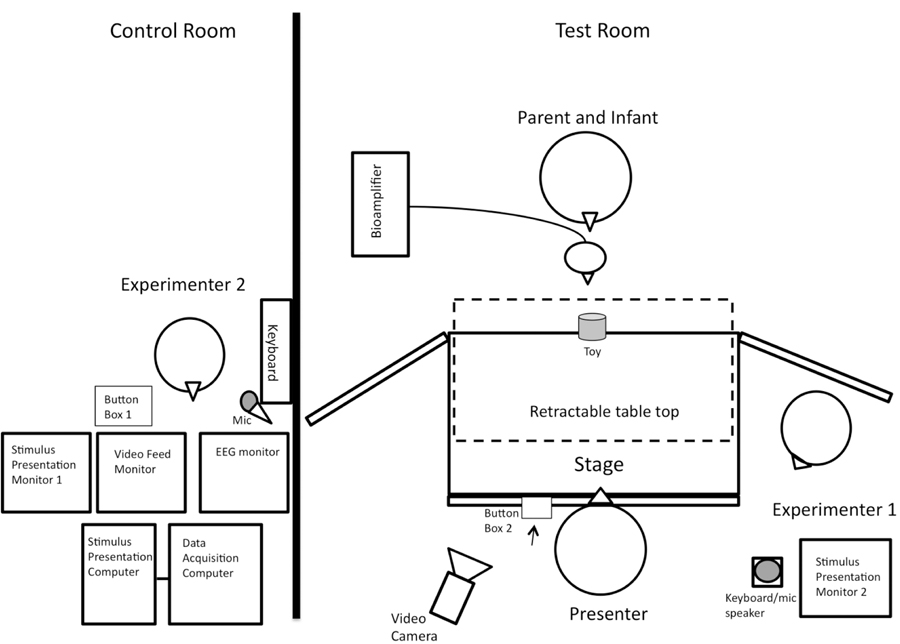
Figure 1 displays the general layout of equipment and experimenter placement in the test room and the adjacent experimenter control room. In the test room was a black puppet stage (99 cm wide x 61 cm deep x 84 cm tall) on a table top with a taupe curtain hanging in front that was raised at the start of each trial. Areas immediately surrounding the stage were covered with black panel curtains to hide experimenters and equipment during the session. A video camera behind the presenter was focused on the infant when the curtain was raised and was also angled to capture key events during the trials.
Figure 1.
Set-up of experimenter control room (left) and participant test room (right).
In a separate but adjacent room, Experimenter 2 monitored the live video feed of the infant’s behavior. A PC running E-prime 2.0 software, networked to the EEG acquisition computer, sent event marks via button press that were placed into the EEG data stream. Experimenter 2 pressed button box 1 to signal the start of each trial immediately after the curtain was raised and the infant attended to the stage center. If the infant was inattentive at the start, Experimenter 2 pressed a couple of keys on a piano keyboard attached to a speaker behind the stage in the testing room to play sounds and redirect the infant’s attention towards the stage. Trial status was displayed on the Stimulus Presentation Monitor 1 (in the control room) and Monitor 2 (in the test room). Experimenter 2 was also able to communicate via a microphone to Experimenter 1 between trials when necessary.
Experimenter 1 monitored the Stimulus Presentation Monitor 2 for trial status cues and, based on this information, raised and lowered the curtain to begin and end trials. Experimenter 1 also checked in with the caregiver and infant between trials and retrieved toys from the infant as needed. A third experimenter, the presenter, was a female who, during observation trials, reached to grasp a toy. The presenter pressed button box 2 (under the table and out of view from the infant) to mark the start and end of observation trials (described below). During intertrial intervals when the curtain was down, the presenter set up the picture or toy for the baseline and execution trials, respectively, and remained out of the infant’s view under the table during data collection for these trials.
Stimuli and procedure
All infants sat on their caregiver’s lap approximately 40 cm from the edge of the stage. A trial set consisted of both an execution and an observation condition, each preceded by a three-second baseline period (See Figure 2). For the baseline periods, the curtain was raised to reveal a white foam core board (28 × 23 cm) with a black shape or pattern at the center of the stage. Baseline recording began as soon as the infant gazed toward the display, marked by Experimenter 2’s button press, and ended after three seconds elapsed. The curtain was then lowered.
Figure 2.
Depiction of trial events. From left panel to right: Observe Baseline (OB), Observation grasp, Execute Baseline (EB), Execution grasp.
During the observation condition, the curtain was raised to reveal the presenter sitting across from the infant. The presenter first made eye contact with the infant to ensure attention at the beginning of the reach and then shifted their gaze to a toy centered on the stage (approximately 29 cm from the stage edge). The presenter then pressed button box 2 with her left hand (out of the infant’s view) to mark the beginning of the event and reached with her right hand to pick up a toy and gave it a brief shake upon retrieval. Finally, the presenter pressed button box 2 to mark the end of the event and the curtain was lowered.
During the execution condition, the toy was placed approximately 12 cm from the infant’s side of the stage. As the curtain was raised, the hidden presenter pushed the table top toward the infant (via drawer casters) to within reaching distance (see Figure 1). Experimenter 2 pressed button box 1 to mark the start of the event upon seeing the infant on camera. Infants were given approximately 60 s to reach for the toy. Trial end was signaled via Experimenter 2’s button press approximately two seconds after infants picked up the toy or grasped and moved the toy for approximately two seconds.
Observation/execution order presentation was pseudo-randomized, with trial set 1 always involving the observation condition before the execution condition. Ten unique baseline pictures and toys were presented, with the same pictures presented twice within the set, and the same toy used in the observation and execution trials within a set. Infants completed an average of 13 trial sets (SD = 4). Toys and baseline picture orders were randomized across infants.
Behavioral coding for EEG segmentation
Video was recorded at a resolution of 640 × 480 pixels and at a frame rate of 30 Hz, allowing accuracy of coding to within approximately 33 ms for each behavior of interest. Two independent coders viewed each video offline, frame-by-frame, and identified the times in which the presenter (observation condition) and the infant (execution condition) made the first contact to the toy that resulted in obtainment of the toy (i.e., infant’s hand in direct physical contact with toy until toy was grasped and lifted up from the table or, in the cases where the toy was not lifted, multiple fingers were wrapped around toy). Inter-rater agreement, within three frames, was achieved on 90% of the execution trials and 89% of the observation trials. The EEG data were then segmented around these contact events. For each subject the first 10 trials were analyzed to ensure the integrity of the timing synchronization between EEG and video was maintained. Baseline and observation trials in which the infant or caregiver appeared to make a reach, gesture, or grasping motion were eliminated from further analysis. Eighteen trial segments from a total of six participants in the final sample were excluded for this reason.
Reach and grasp measures
Because 9-month-old infants vary in their prior experience grasping toys, and are variable in their reach and grasp behaviors, we examined additional qualitative aspects of infant motor behavior during the execution trials. For these behavioral measures, the two independent coders overlapped in their coding of at least of 5 participants (23%).
Latency to Reach.
We calculated the speed with which infants reached in two ways: as the average amount of time from the start of the reach until the hand first contacted the toy (M = 1,860 ms, SD = 1,430, range: 613 to 6,327), and as the average amount of time from the start of the reach until the hand contacted the toy and resulted in a grasp (M = 3,266 ms, SD = 2438, range: 1,118 to 9,631). Inter-rater agreement (I-RA) on the timing of reach start was within 250 ms on 89% of trials. These two measures of reach latency were highly correlated (r = .67, p = .001), so they were transformed to z scores and added together into a latency composite score to capture infants’ average reaching speed.
Reach-Grasp Errors.
We assessed reaching errors in two ways. First, we quantified reaching errors by the average number of errors per trial in the first two trials, including distance errors (M = .38, SD = .67, range: 0 to 2.5, I-RA = 95%) and position errors (M = .21, SD = .34, range: 0 to 1, I-RA = 96%). Distance errors consisted of misjudgments of toy distance (e.g., did not reach far enough or reached beyond the toy). Position errors consisted of misjudgments of toy position (e.g., reached to the right or left of the toy). These two errors were correlated (r = .50, p = .019), so they were transformed into z scores and added together for an initial error composite score to capture infants’ initial reach-grasp accuracy.
Preshaping.
Infants’ preshaping was assessed with four measures: the number of trials in which the palm was the first part of the hand to touch the toy (M = 1.81, SD = 1.60, range: 0 to 6), the number of trials in which extended fingers were the first part of the hand to touch the toy (M = 4.67, SD = 2.08, range: 2 to 9), the number of trials in which the fingers were curled, or “preshaped” (M = 2.81, SD = 2.23, range: 0 to 7), and the total number of trials in which the preshaped fingers matched the affordances of the toy being grasped (M = 1.9, SD = 1.9; range: 0 to 6) I-RA for type of preshaping was achieved on 91% of trials. The four measures of preshaping were correlated, rs ranged from −.50 to .86, ps < .05, so all measures were transformed into z scores and combined (preshape matches and fingers curled were added, and then palm touch and extended fingers were subtracted), to capture infants’ overall tendencies to preshape during their reaches.
Bimanual Reaches.
The extent to which infants used one or two hands during reaching was assessed in three ways: First, by the number of trials in which two hands were used at the time of first touching the toy (i.e., both hands touched the toy at the same time) (M = .19, SD = .60; range: 0 to 2, I-RA = 100%); second, by the number of trials in which two hands were used at the time of first touching the toy that resulted in a grasp (M = .86, SD = 1.24; range: 0 to 4, I-RA = 99%); and third, the number of trials in which both hands were reaching at the time of first toy touch (i.e., at the time of first touch with one hand, the second hand was approaching the toy) (M = 1.29, SD = 1.59; range: 0 to 5, I-RA = 100%). All three of these measures were correlated, rs > .44, ps < .045, so they were transformed into z scores and added together for a bimanual reach composite score, to capture infants’ overall use of both hands in reaching-grasping.
Correlations Among Composites.
Composites scores were not correlated with each other, ps > .24, therefore we retained each of these four composite measures as distinct aspects of reach-grasp skill.
EEG acquisition and pre-processing
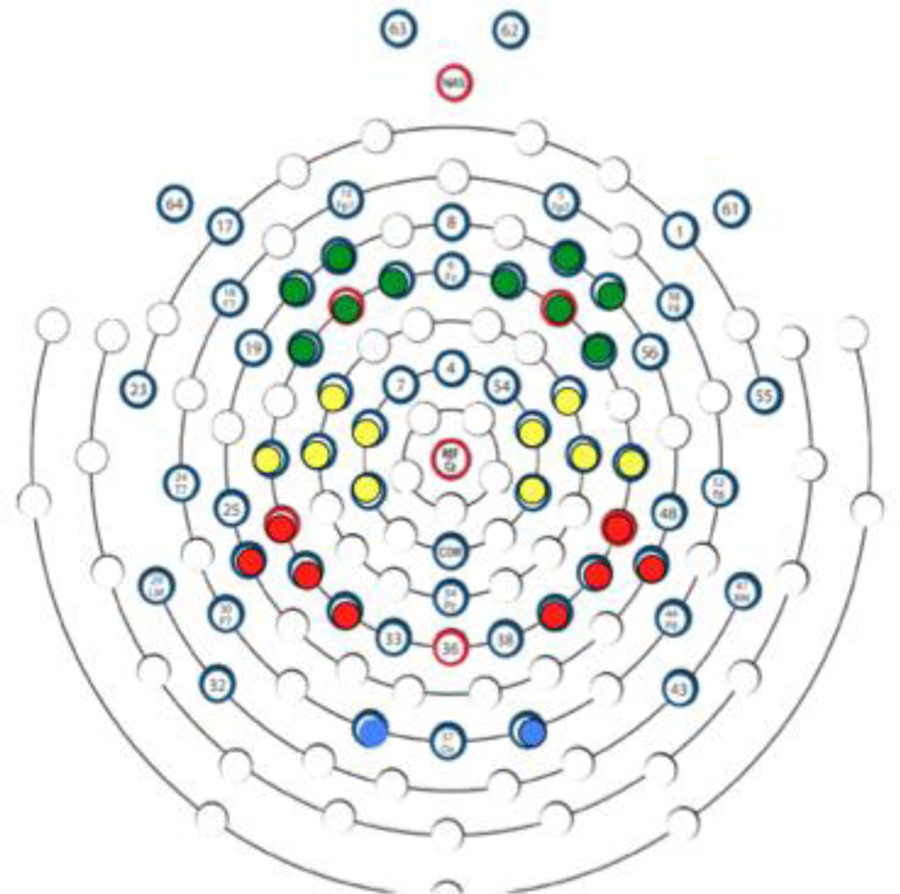
EEG was recorded using a 64-channel Geodesic Sensor Net and sampled at 500 Hz via EGI software (Net Station v4.3; Electrical Geodesics, Inc., Eugene, OR). Channels 61–64, above and below the eyes, were removed from the net and thus data were collected from 60 channels referenced to the vertex, then re-referenced offline to an average reference. Channels 23 and 55 did not always sit properly in front of the ear (at the tragus) and were excluded from the average reference, leaving 58 active channels. Impedance values for all EEG channels were below 100 kΩ at the start of data acquisition. The primary channels of interest with respect to EEG desynchronization were the clusters that corresponded to central sites in the 10/20 system (C3: 15, 16, 20, 21, 22; and C4: 41, 49, 50, 51, 53). Because we also wanted to know whether suppression of EEG power during the observation or execution of a grasp was specific to the central region or distributed across the scalp, channels near midline Frontal (F3: 9, 11, 12, 13,14; and F4: 2, 3, 57, 59, 60), Parietal (P3: 26, 27, 28, 34; and P4: 40, 42, 45, 46), and Occipital (O1: 35; and O2: 39) regions were also analyzed (Figure 3).
Figure 3.
Sensor layout of electrodes analyzed, viewed from above (anterior at top, posterior at bottom): Green = Frontal, Yellow = Central, Red = Parietal, Blue = Occipital.
For the purposes of identifying bad channels and removing artifact, the data were filtered offline using a finite impulse response (FIR) bandpass filter using 0.3 – 100 Hz filter settings. Observation and execution trials were segmented 1,000 ms before and after the toy touch that resulted in a grasp. Baseline trials were initially segmented 3,000 ms after the trial began, then the cleanest 1,000 ms from visual inspection were selected for further analysis. If the entire 3,000 ms baseline period was artifact free, then the first 1,000 ms were selected. All segments were then run through an artifact detection tool in Netstation to flag channels exceeding ±250 μV, and were then hand inspected to identify channels affected by motor and/or ocular artifact. Segments in which 15% or more of the channels were affected by artifact were eliminated from further analysis. The resulting number of artifact free segments per infant were as follows: execution-baseline (the baseline trials that preceded execution trials): M = 8.86, SD = 1.42; observation-baseline (those that preceded the observation trials): M = 8.65, SD = 1.23, execution trials: M = 8.28, SD = 1.27; and observation: M = 8.96, SD = 1.15.
ERD computation was based on an approach described by Pfurtscheller and Lopes da Silva (1999) and implemented with infants by Marshall et al. (2011).
The original (unfiltered, unsegmented) EEG data were band pass filtered at 5–10 Hz to isolate and maintain the integrity of the 6–9 Hz band. Data were then segmented as described above, the bad channels identified were interpolated and bad segments removed, and resulting segments were average referenced. Using MATLAB v7.13 data were squared, and average power over 125 ms bins was computed. In the baseline segments, the 125 ms bins were averaged resulting in one aggregated execution-baseline (EB) and one aggregated observation-baseline (OB) score for each participant. To calculate the ERD, these aggregated baseline scores were compared to each observation (O) and execution (E) trial at each 125 ms interval using the formulas: ((E-EB)/EB)*100 and ((O-OB)/OB)*100 for the percent change from baseline during execution and observation, respectively. Negative values indicate percent desynchronization, whereas positive values indicate percent of event-related synchronization (ERS). These epoched 125 ms ERD/ERS values were then averaged over the length of each individual observation or execution segment (2,000 ms). At this stage, we removed any segments that were statistical outliers for that participant. Statistical outliers were calculated using a similar method to Marshall and colleagues- values greater than 1.5 times the interquartile range (IQR) from the median (Marshall et al., 2011; Saby et al., 2012). This resulted in an exclusion of two participants, as reported above, whom, with the exclusion of additional segments, fell below the five segment minimum standard, leaving 21 infants in the final sample.
Results
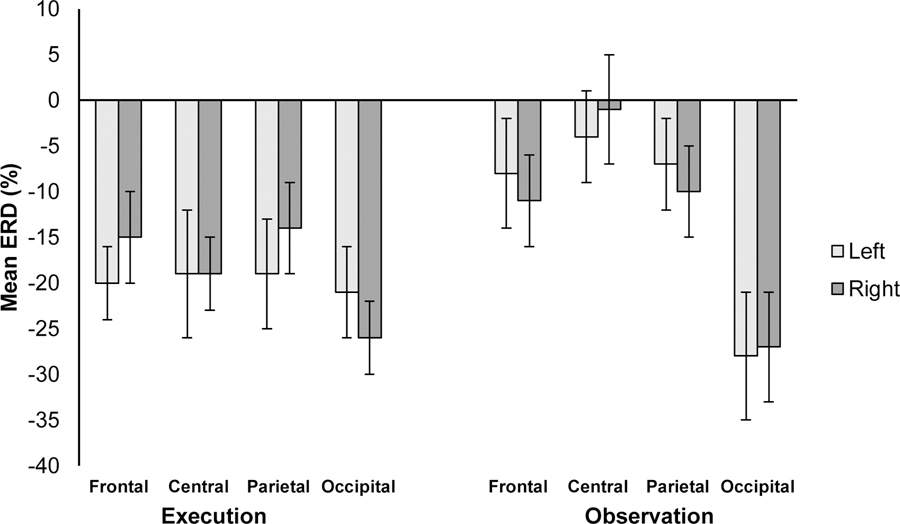
We first examined whether there was ERD to execution of a reach and observation of a reach. We computed a Region (frontal, central, parietal, occipital) × Hemisphere (right, left) × Condition (observation, execution) omnibus repeated-measures analysis of covariance (ANCOVA) with Age as a covariate. Figure 4 depicts the means for each region, hemisphere, and condition. There was a significant effect of Condition, F(1, 19) = 9.18, p = .007, ηp2 = .34, qualified by an Age × Condition interaction, F(1, 19) = 8.72, p = .008, ηp2 = .33. Across the scalp, there was more ERD in the execution condition (M = −19.12, SE = 3.22) than the observation condition (M = −12.02, SE = 3.22). Regressions of Age on each Condition indicated older infants showed more scalp-wide desynchronization during observation than younger infants, F(1, 19) = 9.63, p = .006, r = −.58. There was no relation between Age and ERD in the execution condition (p > .51). There were no other main effects or interactions. One sample t-tests compared to chance, zero, indicated significant ERD during execution trials at all sites (ps < .05) and during Observation in right frontal and right/left occipital sites (ps < .05).
Figure 4.
Mean percentage of change from baseline (ERD) for each hemisphere (Left/Right), Region (Frontal, Central, Parietal, Occipital) and Condition (Execution, Observation) in the 6–9 Hz band. Error bars indicate ±1 Standard Error.
Reach-grasp competence and ERD during execution and observation
We used hierarchical multiple regression to examine whether four composite measures of reach-grasp competence predicted ERD specifically in the central region during the execution and/or observation conditions. To account for multiple comparisons across the scalp, we applied a Bonferroni correction significance criterion of α < .00625 (or α =.05/8; 2 conditions x 4 scalp locations) to each of 8 regressions. As reported above, age was also related to ERD in the observation condition, and therefore was entered at the first level of regression. Measures of reach-grasp competence—reach latency, reach-grasp errors, preshaping, and bimanual reaching—were entered in the second block of the regression to test the prediction that reach-grasp competence significantly predicted ERD above and beyond the age variable. No predictions were made regarding which measure of motor skill competence should best predict ERD at central sites.
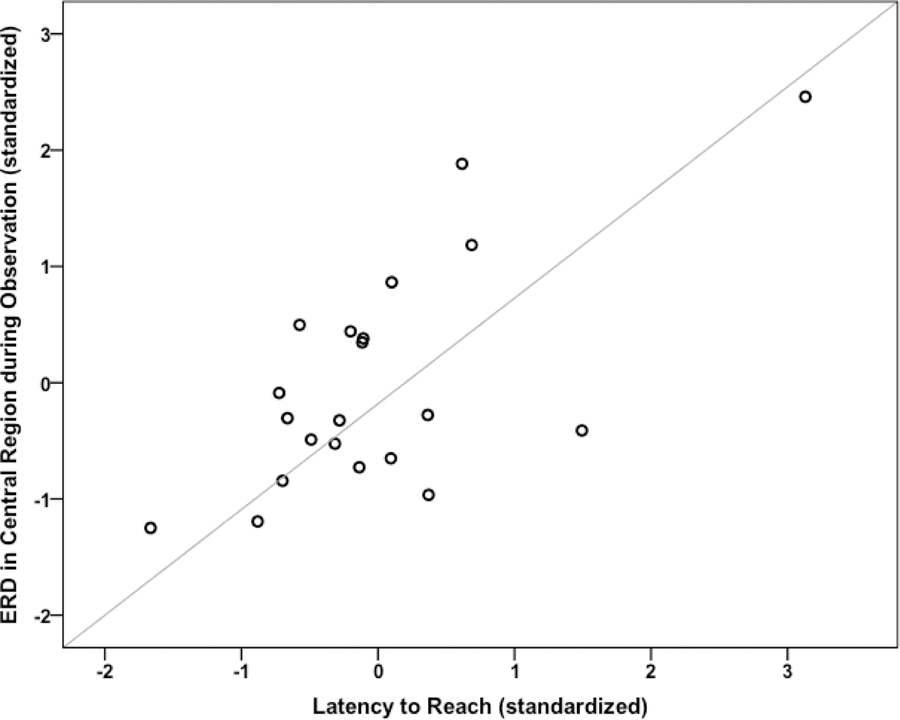
Of the eight comparisons, only central ERD during observation withstood the Bonferroni correction. Age significantly predicted central ERD in the first block, F(1,19) = 13.18, p = .002, R2 = .41. The addition of the Reach-grasp competence composites (reach latency, errors, preshaping, and bimanual reaching) made a unique and significant contribution above and beyond age alone, ΔR2 = .32, ΔF(4, 15) = 4.54, p = .013. Thus, the final model, including Age and Reach-grasp competence predicted 73.3% of the variance in ERD for the central region, F(5, 14) = 8.23, p = .001. Examination of the beta weights indicated Age (b = −55.10, SE = 10.32, t(20) = 5.34, p < .001) and the reach latency composite (b = 6.67, SE = 2.02, t(20) = 3.30, p < .001) significantly contributed to the model. Figure 5 depicts the relation between central ERD during observation and reach latency with age-controlled.
Figure 5.
Scatter plot showing the partial correlation between 6–9 Hz ERD in central electrode sites during action observation and the reach latency composite score controlling for infants age, rp (18) = .67, p < .001.
The same models used to predict ERD at all other regions (frontal, parietal, and occipital) were not significant during observation (ps > .008), nor any region, including central, during execution (ps > .31). See Supplementary Materials for tables of each regression and corresponding beta values.
Discussion
There is growing evidence of a link between action experience and cortical activity while observing actions in human infants (Gerson, Bekkering, & Hunnius, in press; Lloyd-Fox et al., in press; Marshall et al., 2013, Paulus et al., 2012; Saby et al., 2012; van Elk et al., 2008). The current study clarifies the relations between motor competence and meaningful action perception, and suggests that the quality of one’s motor actions is associated with the strength of EEG mu suppression while observing those actions in others. No study, to our knowledge, has investigated the relations between developments in motor skill beyond “amount” of experience measures (i.e., recording actual motor skill performance), and the mu rhythm during both action execution and action observation. To test this, we collected EEG from 9-month-olds who were given opportunities to reach for toys and who also observed an experimenter reach for toys. We assessed infants’ reaching-grasping competence, including reach latency, errors, hand preshaping, and bimanual reaching, measures of infants’ reach-grasp competence. We found that developments in motor competence largely contributed to greater desynchronization in the mu frequency band over central electrodes during action observation, above and beyond other aspects of maturity such as age. Specifically, infants who were more competent reachers—and particularly those who quickly executed reaching actions—exhibited greater EEG desynchronization while observing a reaching action compared to infants who were less skilled. What is clear is that, overall, there is a unique pattern of EEG activity during reach-grasp observation that is associated with infants’ own reach-grasp competence.
We found EEG desynchronization was particularly robust in the 6–9 Hz band during execution, thus suggesting a neural signature for the established links between action and perception systems (e.g., Cannon et al., 2012; Gerson & Woodward, 2014; Sommerville & Woodward, 2005; Sommerville et al., 2005). Beyond chronological age, these findings suggest that the maturation of reaching-grasping motor skills may contribute to action perception reflected via the magnitude of EEG reactivity. This link may underlie infants’ implementation of an action plan and may facilitate the prediction and understanding of others’ actions.
We predicted that a functionally linked action-perception system would result in relations between motor competence and ERD at central sites, during both action execution and observation. We found that during grasp execution, when ERD was greatest in magnitude, mu desynchronization was not related to motor competence. It is possible that mu ERD during execution is too coarse a measure to detect subtle differences associated with grasping maturity.
Although we made no predictions regarding the magnitude of ERD between execution and observation, not surprisingly, we found greater ERD during execution. Tests of the magnitude of desynchronization from baseline for each condition at each region indicated significant desynchronization at all scalp locations, except for action observation at central and parietal sites. In contrast to adults, who typically exhibit the most pronounced mu rhythm desynchronization over central electrodes (e.g., Babiloni et al., 1999; Muthukumaraswamy et al., 2004), our data suggest that infants have a wider distribution of EEG alpha activity across the scalp. However, our findings clearly indicate that even though the EEG desynchronization is not specific to the central sites, only the pattern of activity at central sites is linked to motor skill, thus suggesting that even at a young age, the EEG activity in the central electrodes can be used as an indirect measurement of the activity of the motor system.
The current data of course are limited with respect to being correlational in nature. More studies should systematically manipulate the amount of experience an infant has with a motor skill (e.g., Gerson et al., in press; Paulus et al., 2012) but additionally examine variation in the target motor skill controlling the amount of experience. Such studies will shed light on the critical aspects of experience that modulate mu activity early in development.
The current study also addresses a methodological issue concerning the topography of infants’ EEG suppression during action and observation (Cuevas et al. 2014). We focused on the functionally defined 6–9 Hz band, which desynchronized during execution of the grasp. We did not see mu ERD recorded over central sites during both execution and observation of a reach. Rather, there was desynchronization at multiple sites across the scalp to both of these conditions. These data suggest that at 9-months the links between action and perception as indexed exclusively by mu ERD are not yet tightly coupled. While previous studies have focused on the central mu rhythm, those that report data at other scalp locations suggest that this motor-related neural signature may be more broadly distributed in young infants and children (Marshall et al., 2011; Saby et al., 2012; Stapel et al., 2010; van Elk et al., 2008).
There are other considerations that should be taken into account to explain the topographic distribution of EEG suppression in 9-month-olds. First, EEG studies in adults show that alpha desynchronization occurs across the scalp during tasks that require a certain level of attention or effort (Klimesch, 1999). Particularly in infancy, and within the context of an engaging experimental situation, infant attention to the toys and their own actions, as well as to the experimenter’s motor action, may be driving, in part, the suppression of EEG alpha activity across the scalp. Second, there is growing evidence that a mirroring system may not be confined solely to the motor cortex (i.e., premotor and primary motor cortex), but rather comprises an active fronto-parietal network (e.g., Arnstein et al., 2011; Buccino et al., 2004, Dinstein, Hasson, Rubin, & Heeger, 2007; Molenberghs, Cunnington, & Mattingly, 2012; see also Bonini et al., 2010, 2011 and Vigneswaran et al., 2013, for comparison with single cell recording findings in the monkey). Future studies should continue to report findings across the scalp to inform our understanding of whether there is a developmental trajectory for topographical specificity. This information will also help to clarify the definition of the mu rhythm with respect to developing action and perception systems, and whether mu rhythm is a viable measure for tracking the development of action-perception links in infancy.
In sum, the current study supports the idea that motor competence is associated with EEG suppression during goal-directed action observation in 9-month-old infants. These data support the proposal that desynchronization during action observation may reflect a developmental precursor of the mu rhythm reported in human adults, and particularly supports the proposed contribution of action expertise (e.g., Cannon et al., 2014; Orgs et al, 2008). Results of the present study provide evidence for an underlying neural system relating one’s own action experiences with action perception that emerges within the first year of life. Thus, the data here lend support to developmental theories that emphasize the critical role of action experience as a developmental driver of action perception and social learning.
Supplementary Material
Research Highlights.
The control of intentional actions and the meaningful perception of others’ actions are integrally related during ontogeny.
A neural mirror system predicts that developments in infants’ motor competence should be associated with the strength of EEG mu desynchronization during action observation.
To explore this, 9-month-old infants reached for toys and observed an experimenter reaching for toys while we recorded EEG activity in the mu frequency band.
We found greater desynchronization in scalp electrodes located over motor-related regions during action observation was associated with greater reaching-grasping competence, suggesting an early emerging neural system integrating one’s own actions with the perception of others’ actions.
Acknowledgments
This research was supported by an NIH grant (NICHD P01 HD064653) to ALW. We thank L. Brand, T. Tavassolie, and K. Finch for assistance in collecting and coding the data, as well as the many undergraduate research assistants at the UMD Child Development Lab. We are especially grateful to the families who participated in this work.
References
- Arnstein D, Cui F, Keysers C, Maurits NM, & Gazzola V (2011). μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. Journal of Neuroscience, 31, 14243–14249. 10.1523/JNEUROSCI.0963-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, et al. (2012). The dynamics of sensorimotor cortical oscillations during observation of hand movements: An EEG study. PLoS ONE, 7, e37534 10.1371/journal.pone.0037534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, et al. (1999). Human movement-related potentials vs desynchronization of EEG alpha rhythm: A high-resolution EEG study. NeuroImage, 10, 658–665. 10.1006/nimg.1999.0504 [DOI] [PubMed] [Google Scholar]
- Berthier NE, & Carrico RL (2010). Visual information and object size in infant reaching. Infant Behavior and Development, 33(4), 555–566. 10.1016/j.infbeh.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi FU, Luciano S, Ferrari PF, & Fogassi L (2010). Ventral premotor and inferior parietal cortices differently contribute to action organization and intention understanding. Cerebral Cortex, 20(6), 1372–1385. 10.1093/cercor/bhp200 [DOI] [PubMed] [Google Scholar]
- Bonini L, Serventi FU, Simone L, Rozzi S, Ferrari PF, & Fogassi L (2011). Grasping neurons of monkey parietal and premotor cortices encode action goals at distinct levels of abstraction during complex action sequences. The Journal of Neuroscience, 31(15), 5876–5886. 10.1523/JNEUROSCI.5186-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner JS (1970). The growth and structure of skill, In Connolly K (Ed.), Mechanisms of motor skill development. New York: Academic Press; pp. 63–94. [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. (2001). Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience, 13, 400–404. 10.1111/j.1460-9568.2001.01385.x [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, & Haggard P (2005). Action observation and acquired motor skills: an FMRI study with expert dancers. Cerebral cortex, 15, 1243–1249. 10.1093/cercor/bhi007 [DOI] [PubMed] [Google Scholar]
- Cannon EN, & Woodward AL (2012). Infants generate goal-based action predictions. Developmental Science, 15, 292–298. 10.1111/j.1467-7687.2011.01127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Woodward AL, Gredebäck G, von Hofsten C, & Turek C (2012). Action production influences 12-month-old infants’ attention to others’ actions. Developmental Science, 15, 35–42. 10.1111/j.1467-7687.2011.01095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Yoo KH, Vanderwert RE, Ferrari PF, Woodward AL & Fox NA (2014). Action experience, more than observation, influences mu rhythm desynchronization. PLoS ONE 9, e92002 10.1371/journal.pone.0092002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton LJ, Keen R, & McCarty ME (2003). Evidence of motor planning in infant reaching behavior. Psychological Science, 14, 354–356. 10.1111/1467-9280.24421 [DOI] [PubMed] [Google Scholar]
- Clearfield MW & Thelen E (2001). Stability and flexibility in the acquisition of skilled movement In Nelson C & Luciano M (Eds.) Handbook of Developmental Cognitive Neuroscience, pp. 253–266. Cambridge, MA: MIT Press. [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, & Martineau J (1998). Perception of motion and qEEG activity in human adults. Electroencephalography and Clinical Neurophysiology, 107, 287–295. 10.1016/S0013-4694(98)00071-6 [DOI] [PubMed] [Google Scholar]
- Csibra G (2007). Action mirroring and action interpretation: An alternative account In: Haggard P, Rosetti Y, & Kawato M (Eds.), Sensorimotor Foundations of Higher Cognition. Attention and Performance XXII (pp. 435–459). Oxford: Oxford University Press. [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, & Phillips JP (2014). Functional connectivity in the developing brain: A longitudinal study from 4 to 9months of age. NeuroImage, 84, 169–180. 10.1016/j.neuroimage.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, & Rizzolatti G (1992). Understanding motor events: A neurophysiological study. Experimental Brain Research, 91, 176–180. 10.1007/BF00230027 [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, & Heeger DJ (2007). Brain areas selective for both observed and executed movements. Journal of Neurophysiology, 98, 1415–1427. 10.1152/jn.00238.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard J (1998). Changes in grasping skills and the emergence of bimanual coordination during the first year of life In Connolly KJ (Ed.), The psychobiology of the hand, Clinics in developmental medicine, no. 147. pp. 123–143. London: Cambridge University Press. [Google Scholar]
- Fagard J (2000). Linked proximal and distal changes in the reaching behavior of 5-to 12-month-old human infants grasping objects of different sizes. Infant Behavior and Development, 23(3), 317–329. 10.1016/S0163-6383(01)00047-9 [DOI] [Google Scholar]
- Fagard J, & Jacquet AY (1989). Onset of bimanual coordination and symmetry versus asymmetry of movement. Infant Behavior and Development, 12(2), 229–235. 10.1016/0163-6383(89)90009-X [DOI] [Google Scholar]
- Fagard J, & Pezé A (1997). Age changes in interlimb coupling and the development of bimanual coordination. Journal of Motor Behavior, 29(3), 199–208. 10.1080/00222899709600835 [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Vanderwert RE, Paukner A, Bower S, Suomi SJ, & Fox NA (2012). Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys Journal of Cognitive Neuroscience, 24, 1165–1172. 10.1162/jocn_a_00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetters L, & Todd J (1987). Quantitative assessment of infant reaching movements. Journal of Motor Behavior, 19(2), 147–166. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, & Rizzolatti G (2005). Parietal lobe: from action organization to intention understanding. Science, 308 (5722), 662–667. 10.1126/science.1106138 [DOI] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Avesani M, Cerini R, Milanese F, Gasparini A, Acler M, Mucelli RP, Fiaschi A, Manganotti P, 2008. EEG and fMRI coregistration to investigate the cortical oscillatory activities during finger movement. Brain topography 21, 100–111. 10.1007/s10548-008-0058-1 [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, & Rizzolatti G (1996). Action recognition in the premotor cortex. Brain, 119, 593–609. 10.1093/brain/119.2.593 [DOI] [PubMed] [Google Scholar]
- Gerson SA, Bekkering H, & Hunnius S (in press). Short-term motor training, but not observational training, alters neurocognitive mechanisms of action processing in infancy. Journal of Cognitive Neuroscience. [DOI] [PubMed]
- Gerson SA, & Woodward AL (2014). Learning from their own actions: The unique effect of producing actions on infants’ action understanding. Child Development, 85(1), 264–277. 10.1111/cdev.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck G, Stasiewicz D, Falck-Ytter T, Rosander K, & von Hofsten C (2009). Action type and goal type modulate goal-directed gaze shifts in 14-month-old infants. Developmental Psychology, 45, 1190–1194. [DOI] [PubMed] [Google Scholar]
- Hadjidimitriou SK, Zacharakis AI, Doulgeris PC, Panoulas KJ, Hadjileontiadis LJ, & Panas SM (2011). Revealing action representation processes in audio perception using fractal EEG analysis. IEEE Transactions on Biomedical Engineering, 58, 1120–1129. [DOI] [PubMed] [Google Scholar]
- Halverson HM (1931). An experimental study of prehension in infants by means of systematic cinema records. Genetic Psychology Monographs, 10, 107–286. [Google Scholar]
- Hunnius S, & Bekkering H (2014). What are you doing? How active and observational experience shape infants’ action understanding. Philosophical Transactions of the Royal Society B, 369, 1–9. 10.1098/rstb.2013.0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvelainen J, Schurmann M, & Hari R (2004). Activation of the human primary motor cortex during observation of tool use. NeuroImage, 23, 187–192. [DOI] [PubMed] [Google Scholar]
- Kanakogi Y, & Itakura S (2011). Developmental correspondence between action prediction and motor ability in early infancy. Nature Communications, 2, 341 10.1038/ncomms1342 [DOI] [PubMed] [Google Scholar]
- Klimesch W (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews, 29, 169–195. 10.1016/S0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- Lepage J-F & Theoret H (2006). EEG evidence for the presence of an action observation-execution matching system in children. European Journal of Neuroscience, 23, 2505–2510. 10.1111/j.1460-9568.2006.04769.x [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Wu R, Richards JE, Elwell CE, & Johnson MH (in press). Cortical activation to action perception is associated with action production abilities in young infants. Cerebral Cortex. 10.1093/cercor/bht207 [DOI] [PMC free article] [PubMed]
- Lockman JJ, Ashmead DH, & Bushnell EW (1984). The development of anticipatory hand orientation during infancy. Journal of Experimental Child Psychology, 37(1), 176–186. 10.1016/0022-0965(84)90065-1 [DOI] [PubMed] [Google Scholar]
- Loucks J, & Sommerville JA (2012). The role of motor experience in understanding action function: The case of the precision grasp. Child Development, 83(3), 801–809. 10.1111/j.1467-8624.2012.01735.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareschal D, Johnson MH, Sirois S, Spratling M, Thomas MS, & Westermann G (2007). Neuroconstructivism: How the brain constructs cognition (Vol.1). Oxford: Oxford University Press; 10.1093/acprof:oso/9780198529910.001.0001 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113, 1199–1208. 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bouquet CA, Shipley TF, & Young T (2009). Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia, 47(10), 2100–2106. 10.1016/j.neuropsychologia.2009.03.022 [DOI] [PubMed] [Google Scholar]
- Marshall PJ & Meltzoff AN (2011). Neural mirroring systems: Exploring the EEG mu rhythm in infancy. Developmental Cognitive Neuroscience, 1, 110–123. 10.1016/j.dcn.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Young T, & Meltzoff AN (2011). Neural correlates of action observation and execution in 14-month-old infants: An event-related EEG desynchronization study. Developmental Science, 14, 474–480. 10.1111/j.1467-7687.2010.00991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall Saby, & Meltzoff (2013). Infant brain responses to object weight: Exploring goal-directed actions and self-experience. Infancy, 18(6), 942–960. 10.1111/infa.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell PM (1975). The development of visually guided reaching. Perception & Psychophysics, 18(3), 181–185. 10.3758/BF03205963 [DOI] [Google Scholar]
- Michel GF, Ovrut MR, & Harkins DA (1985). Hand-use preference for reaching and object manipulation in 6-through 13-month-old infants. Genetic, Social, and General Psychology Monographs, 111(4), 407–427. [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, & Mattingley JB (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience & Biobehavioral Reviews, 36, 341–349. 10.1016/j.neubiorev.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD & Johnson BW (2004). Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology, 41, 152–156. 10.1046/j.1469-8986.2003.00129.x [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, & McNair NA (2004). Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research, 19, 195–201. 10.1016/j.cogbrainres.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Newell KM, McDonald PV, & Baillargeon R (1993). Body scale and infant grip configurations. Developmental Psychobiology, 26(4), 195–205. 10.1002/dev.420260403 [DOI] [PubMed] [Google Scholar]
- Newman C, Atkinson J, & Braddick O (2001). The development of reaching and looking preferences in infants to objects of different sizes. Developmental Psychology, 37(4), 561–572. 10.1037/0012-1649.37.4.561 [DOI] [PubMed] [Google Scholar]
- Nystrom P (2008). The infant mirror neuron system studied with high density EEG. Social Neuroscience, 3, 334–347. 10.1080/17470910701563665 [DOI] [PubMed] [Google Scholar]
- Nyström P, Ljunghammar T, Rosander K, & von Hofsten C (2011). Using mu rhythm desynchronization to measure mirror neuron activity in infants. Developmental Science, 14(2), 327–335. 10.1111/j.1467-7687.2010.00979.x [DOI] [PubMed] [Google Scholar]
- Orgs G, Dombrowski JH, Heil M, & Jansen-Osmann P (2008). Expertise in dance modulates alpha/beta event-related desynchronization during action observation. European Journal of Neuroscience, 27(12), 3380–3384. 10.1111/j.1460-9568.2008.06271.x [DOI] [PubMed] [Google Scholar]
- Paulus M, Hunnius S, van Elk M, & Bekkering H (2012). How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: Electrophysiological evidence for action-effect binding in infancy. Developmental Cognitive Neuroscience, 2, 90–96. 10.1016/j.dcn.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, & Lopes da Silva FH (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology, 110, 1842–1857. 10.1016/S1388-2457(99)00141-8 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, & Neuper C (1997). Motor imagery activates primary sensorimotor area in humans. Neuroscience Letters, 239, 65–68. 10.1016/S0304-3940(97)00889-6 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper Ch., Andrew C, & Edlinger G (1997). Foot and hand area mu rhythms. International Journal of Psychophysiology, 26, 121–135. 10.1016/S0167-8760(97)00760-5 [DOI] [PubMed] [Google Scholar]
- Pineda JA, Allison BZ, & Vankov A (2000). The effects of self-movement, observation, and imagination on μ rhythms and readiness potentials (RP’s): toward a brain-computer interface (BCI). Rehabilitation Engineering, IEEE Transactions on, 8, 219–222. 10.1109/86.847822 [DOI] [PubMed] [Google Scholar]
- Pineda J (2005). The functional significance of mu rhythms: Translating “hearing” and “seeing” into “doing.” Brain Research Reviews, 50, 57–68. 10.1016/j.brainresrev.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, & Villringer A (2009). Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping, 30, 1168–1187. 10.1002/hbm.20585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G & Craighero L (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. 10.1146/annurev.neuro.27.070203.144230 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, & Fogassi L (1996). Premotor cortex and the recognition of motor actions. Cognitive Brain Research, 3, 131–141. [DOI] [PubMed] [Google Scholar]
- Rochat P (1992). Self-sitting and reaching in 5-to 8-month-old infants: The impact of posture and its development on early eye-hand coordination. Journal of Motor Behavior, 24(2), 210–220. 10.1080/00222895.1992.9941616 [DOI] [PubMed] [Google Scholar]
- Rochat MJ, Caruana F, Jezzini A, Escola L, Intskirveli I, Grammont F, … Umiltà MA (2010). Responses of mirror neurons in area F5 to hand and tool grasping observation. Experimental Brain Research, 204, 605–616. 10.1007/s00221-010-2329-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, Marshall PJ, & Meltzoff AN (2012). Neural correlates of being imitated: An EEG study in preverbal infants. Social Neuroscience, 7, 650–661. 10.1080/17470919.2012.691429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LAR, & Whishaw IQ (2012). Subsystems of sensory attention for skilled reaching: vision for transport and pre-shaping and somatosensation for grasping, withdrawal and release. Behavioural Brain Research, 231(2), 356–365. 10.1016/j.bbr.2011.07.031 [DOI] [PubMed] [Google Scholar]
- Sclafani V, Simpson EA, Suomi SJ, & Ferrari PF (under review). Development of space perception in relation to the maturation of the motor system in infant rhesus macaques (Macaca mulatta). Neuropsychologia. [DOI] [PMC free article] [PubMed]
- Siddiqui A (1995). Object size as a determinant of grasping in infancy. The Journal of Genetic Psychology, 156(3), 345–358. 10.1080/00221325.1995.9914828 [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, & Needham A (2005). Action experience alters 3-month-old infants’ perception of others actions. Cognition, 96, B1–B11. 10.1016/j.cognition.2004.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Begus K, Lloyd-Fox S, di Gangi V, & Hamilton A (2014). Goal representation in the infant brain. NeuroImage, 85, 294–301. 10.1016/j.neuroimage.2013.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, & Csibra G (2010). Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science, 21, 355–359. 10.1177/0956797610362058 [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, & Csibra G (2009). Predictive motor activation during action observation in human infants. Biology Letters, 5, 769–772. 10.1098/rsbl.2009.0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, & Needham A (2005). Action experience alters 3-month-old infants’ perception of others’ actions. Cognition, 96, B1–B11. 10.1016/j.cognition.2004.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapel JC, Hunnius S, van Elk M, & Bekkering H (2010). Motor activation during observation of unusual versus ordinary actions in infancy. Social Neuroscience, 5, 451–460. 10.1080/17470919.2010.490667 [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, & Hatsopoulos NG (2007). Congruent activity during action and action observation in motor cortex. The Journal of Neuroscience, 27(48), 13241–13250. 10.1523/JNEUROSCI.2895-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiltà MA, Escola L, Intskirveli I, Grammont F, Rochat MJ, Caruana F, … Rizzolatti G (2008). When pliers become fingers in the monkey motor system. Proceedings of the National Academy of Sciences, 105, 2209–2213. 10.1073/pnas.0705985105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Vesper C, & Bekkering H (2008). You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage, 43, 808–814. 10.1016/j.neuroimage.2008.07.057 [DOI] [PubMed] [Google Scholar]
- Vanderwert RE, Ferrari PF, Paukner A, Bower SB, Fox NA, & Suomi SJ (2012). Spectral characteristics of the newborn rhesus macaque EEG reflect functional cortical activity. Physiology & Behavior, 107, 787–791. 10.1016/j.physbeh.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Fox NA, & Ferrari PF (2012). The mirror mechanism and mu rhythm in social development. Neuroscience Letters, 540, 15–20. 10.1016/j.neulet.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G, Philipp R, Lemon RN, & Kraskov A (2013). M1 corticospinal mirror neurons and their role in movement suppression. Current Biology, 23, 236–43. 10.1016/j.cub.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C (2004). An action perspective on motor development. Trends in Cognitive Science, 8, 266–272. 10.1016/j.tics.2004.04.002 [DOI] [PubMed] [Google Scholar]
- von Hofsten C, & Fazel-Zandy S (1984). Development of visually guided hand orientation in reaching. Journal of Experimental Child Psychology, 38(2), 208–219. 10.1016/0022-0965(84)90122-X [DOI] [PubMed] [Google Scholar]
- von Hofsten C & Ronnqvist L (1988). Preparation for grasping an object: A development study. Journal of Experimental Psychology: Human Perception and Performance, 14, 610–621. 10.1037/0096-1523.14.4.610 [DOI] [PubMed] [Google Scholar]
- Virji-Babul N, Rose A, Moiseeva N, & Makan N (2012). Neural correlates of action understanding in infants: Influence of motor experience. Brain and Behavior, 2, 237–242. 10.1002/brb3.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warreyn P, Ruysschaert L, Wiesema JR, Handl A, Pattyn G, & Roeyers H (2013). Infants’ mu suppression during the observation of real and mimicked goal-directed actions. Developmental Science, 16(2), 173–185. 10.1111/desc.12014 [DOI] [PubMed] [Google Scholar]
- Woodward AL, Sommerville JA, Gerson S, Henderson AME, & Buresh J (2009). The emergence of intention attribution in infancy In Ross B (Ed.) The Psychology of Learning and Motivation, 51, 187–222. Academic Press; 10.1016/S0079-7421(09)51006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.