Abstract
Context
Chronic opioid use may lead to adrenal insufficiency because of central suppression of the hypothalamic-pituitary-adrenal axis. However, the prevalence of opioid-induced adrenal insufficiency (OIAI) is unclear.
Objective
To determine the prevalence of OIAI and to identify predictors for the development of OIAI in patients taking opioids for chronic pain.
Design
Cross-sectional study, 2016-2018.
Setting
Referral center.
Patients
Adult patients taking chronic opioids admitted to the Pain Rehabilitation Center.
Main outcome measure
Diagnosis of OIAI was considered if positive case detection (cortisol < 10 mcg/dL, ACTH < 15 pg/mL, and dehydroepiandrosterone sulfate < 25 mcg/dL), and confirmed after endocrine evaluation. Daily morphine milligram equivalent (MME) was calculated.
Results
In 102 patients (median age, 53 years [range, 22-83], 67% women), median daily MME was 60 mg (3-840), and median opioid therapy duration was 60 months (3-360). Abnormal case detection testing was found in 11 (10.8%) patients, and diagnosis of OIAI was made in 9 (9%). Patients with OIAI were on a higher daily MME (median, 140 [20-392] mg vs 57 [3-840] mg, P = 0.1), and demonstrated a 4 times higher cumulative opioid exposure (median of 13,440 vs 3120 mg*months, P = 0.03). No patient taking <MME of 20 mg/day developed OIAI (sensitivity of 100% for MME > 20 mg); however, specificity of MME cutoff >20 mg was only 19%. After opioid discontinuation, 6/7 patients recovered adrenal function.
Conclusion
The prevalence of OIAI was 9%, with MME cumulative exposure being the only predictor for OIAI development. Patients on MME of 20 mg/day and above should be monitored for OIAI.
Keywords: diagnosis, MME, morphine, pituitary, pain, cortisol
Opioids are important agents in the treatment algorithm of acute pain management and are still frequently used in chronic pain (1, 2). Despite ongoing efforts to minimize prescriptions, chronic opioid use remains widespread (3-5). Several adverse effects of opioids, such as constipation, sedation, and nausea are well recognized; however, despite recent reports, opioid-induced endocrine dysfunction continues to be underappreciated, in particular opioid-induced adrenal insufficiency (OIAI) (6).
Opioid-induced endocrinopathies occur primarily as a consequence of hypothalamic-pituitary suppression (7-9), the most common of which is opioid-induced hypogonadism reported in 75% to 89% of men and 23% to 67% of women (10-13) taking chronic opioids. Several case reports and small heterogeneous studies have reported suppression of the hypothalamic-pituitary-adrenal (HPA) axis in patients taking opioids leading to OIAI, and occasionally even adrenal crisis (13-17). Symptoms of adrenal insufficiency may overlap with those of chronic noncancer pain, making their utility in the assessment of OIAI challenging (17). In addition, the impact of possible superimposed OIAI in overall symptomatology of those with chronic noncancer pain is not known. However, OIAI appears to be reversible as recovery of HPA axis following cessation of opioids has been described (17).
The reported prevalence of OIAI varies widely between 8% and 50% (13, 18-21), estimated to be 15% in a recent meta-analysis of 5 studies composed of 205 patients on differing opioid regimens (22). In addition, the method of diagnosis of OIAI varied and included a combination of subnormal serum cortisol and inappropriate/low ACTH, abnormal peak cortisol after cosyntropin stimulation, CRH stimulation, overnight metyrapone test, or insulin tolerance test (ITT). The lack of established patient or opioid therapy specific risk factors for the development of OIAI is further hampering the recognition of OIAI. Although several risk factors for the development of opioid-induced hypogonadism have been reported, including long-acting opioids (23) or the use of high-dose opioids (10), only 1 study to date has reported a 22.5% prevalence of OIAI in patients taking ≥25 morphine milligram equivalent (MME)/day (24), suggesting a dose-dependent effect.
In this study, we have applied a simple and feasible case detection approach in a large consecutive cohort of patients treated with chronic opioids, taking advantage of a collaborative effort between the Pain Rehabilitation Center and Adrenal clinic. Our objectives were (1) to determine the prevalence of OIAI and characterize abnormalities in HPA axis assessments in a prospectively enrolled cohort of patients taking chronic opioids, (2) to identify risk factors for the development of OIAI, and to (3) to determine any relationship between HPA axis abnormalities and success of opioid cessation.
Methods
Subjects
Patients admitted to the Pain Rehabilitation Center at Mayo Clinic, Rochester, between 2016 and 2018, were eligible for participation. Only those patients who had provided authorization for research use of their medical record were included following Mayo Clinic institutional review board approval of the study. Inclusion criteria were: (1) age ≥18 years, (2) intermittent or continuous opioid use of at least 90 days, and (3) morning cortisol, dehydroepiandrosterone sulphate (DHEAS), and ACTH measurement on admission. Patients with known pituitary or adrenal dysfunction or exogenous oral or injectable glucocorticoid use within 3 months or topical use within 48 hours were excluded. As a part of the Pain Rehabilitation Center evaluation protocol, all patients with cortisol < 10 mcg/dL, ACTH < 15 pg/mL, and DHEAS < 25 mcg/dL were offered endocrine evaluation. The cutoffs were chosen after discussion with members of the adrenal endocrine group, after reviewing the literature (13, 19-21), as well as informed by the data from patients diagnosed with OIAI at our institution (2006-2016) (17). Diagnosis of OIAI was made based on endocrine evaluation including clinical and biochemical assessment, as well as exclusion of alternative etiologies of adrenal insufficiency. Each patient’s opioid daily dose was obtained and converted into an MME. Medical records were reviewed for additional information. As a part of Pain Rehabilitation Center program, all patients taper and discontinue opioid therapy, undergo assessments of quality of life (36-Item Short Form Health Survey [SF-36]), physical function (5-minute walk), and pain intensity assessment at the time of program admission as well as at discharge (3 weeks later).
Self-reported measurements at pain rehabilitation clinic
Adjustment to chronic pain
The Pain Severity and Pain Interference subscales of the West Haven-Yale Multidimensional Pain Inventory1 were used in all patients and have been previously assessed for reliability and validity (25, 26). Possible scores range from 0 to 6 for each subscale, with higher scores representing greater symptom severity and functional impairment, respectively.
Quality of life
The Medical Outcomes Study, SF-36 (27) is a measure of 8 domains of health-related quality of life, including: general health perceptions, physical health functions, mental health functioning, role limitations because of emotional problems, role limitations from physical health, bodily pain, vitality, and social functioning. The subscales can be combined into 2 summary scores: mental health-related quality of life and physical health-related quality of life. For this study, the general health perceptions scale and the 2 quality of life composite scores were used. Items are rated on a Likert-type scale, which are then transformed into percentages (0-100). Lower scores reflect worse quality of life. Research supports strong psychometric properties for the measure, including high convergence with clinical data (28).
Physical performance measure
A 5-minute walk test was used as a direct measure of physical performance. The 5-minute walk test measured walking speed and yielded a measure of distance traveled with good test-retest reliability (29).
Laboratory analysis
All patients using chronic opioids had morning cortisol, ACTH, and DHEAS measurement performed on program admission collected before 10 am and before opioid taper, and without holding the usual opioid therapy. Cortisol was measured using a competitive binding immunoenzymatic assay on the UniCel DxI 800 (Beckman Coulter, Brea, CA). ACTH was measured using Roche Elecsys ACTH assays (Los Angeles, CA). DHEAS was measured by automated chemiluminescent competitive immunoassay on the IMMULITE 2000 platform (Siemens Diagnostics, Tarrytown, NY).
Cosyntropin stimulation testing
For those patients with abnormal results, cosyntropin stimulation testing (CST) was subsequently performed in the morning (8-10 am) at the Endocrine Testing Center, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, at Mayo Clinic, Rochester, MN. An IV catheter was inserted into an antecubital vein for blood sample collections. Cosyntropin (Mylan Institutional, Amsterdam, Netherlands) was reconstituted with 2 mL 0.9% sodium chloride and administered over 30 seconds. Samples at baseline and 30 and 60 minutes following injection were obtained and analyzed for total cortisol measurements. The peak cortisol concentration was defined as the highest cortisol value after cosyntropin administration. The diagnosis of AI was considered on the basis of a peak total cortisol level of <18 mg/dL (30, 31).
Data analysis
Descriptive statistics were used to determine median and ranges, whereas categorical data were shown as a number of patients and percentage of sample. To assess the associations between variables nonparametric Wilcoxon/Kruskal-Wallis test was used for continuous variables and the χ 2 test was used for categorical variables. Subgroup analysis was performed on patients with and without OIAI. P values < 0.05 were considered significant. Data was analyzed using JMP software, version 10.
Results
Patients
In total, 102 patients (median age, 54 years [range, 24-83], 66 [65%] women) met the inclusion criteria (Table 1). The most common reason for opioid use was back pain (38, 37%), followed by the lower and upper extremity pain (22, 22%), headache (10, 10%), and fibromyalgia pain (9, 9%). The most commonly used opioid agents were oxycodone (46, 45%), hydrocodone (33, 32%), fentanyl patch (19, 19%), and tramadol (16, 16%), with 40 patients (39%) using multiple types of opioids at the time of admission (Table 2). The median MME dose was 60 mg (range, 3-840) and median duration of opioid use was 60 months (range, 3-360; Table 1). On admission, patients’ pain severity and interference were high, whereas general health and mental and physical health scores on the SF-36 survey were low (Table 3). By the end of the 3 weeks of pain rehabilitation intervention, 101 (99%) patients were able to discontinue opioid therapy. At discharge, patients improved their pain and quality of life, and demonstrated a better physical performance as measured by the 5-minute walk (Table 3).
Table 1.
Comparison of Patients with OIAI and Without OIAI
| All Patients | Patients with Diagnosis of OIAI | Patients without Diagnosis of OIAI | P Value | |
|---|---|---|---|---|
| N | 102 | 9 | 93 | |
| Women, n (%) | 66 (65) | 7 (77.8) | 59 (63.4) | 0.39 |
| Caucasian, n (%) | 93 (91) | 8 (89) | 85 (91.4) | 0.79 |
| Age, years median (range) | 54 (24-83) | 54 (24-83) | 53 (47-75) | 0.58 |
| BMI, kg/m2 median (range) | 28.6 (17.4-46) | 29.9 (33.5-45.6) | 28.6 (17.4-46) | 0.66 |
| MME, mg median (range) | 60 (3-840) | 140 (20-392) | 57 (3-840) | 0.06 |
| Duration of opioid use, months median (range) | 60 (3-360) | 96 (6-204) | 60 (3-360) | 0.19 |
| Cumulative opioid exposure, mg*months median (range) | 3600 (30-156720) | 13440 (120-36000) | 3120 (30-156720) | 0.03 |
| Cortisol, mcg/dL median (range) | 13 (1-37) | 5.4 (1-9.9) | 14 (2.4-37) | <0.0001 |
| ACTH, pg/mL median (range) | 19 (5-97) | 11 (5-15) | 21 (7-97) | 0.0002 |
| DHEAS, mcg/dL median (range) | 60 (<15-379) | 17.3 (<15-24) | 64 (<15-379) | <0.0001 |
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; MME, morphine equivalent; OIAI, opioid-induced adrenal insufficiency.
Table 2.
Type of Opioid and the Reason for Opioid Use
| Type of opioid used, n (%) | |
|---|---|
| Oxycodone | 46 (45) |
| Hydrocodone | 33 (32) |
| Tramadol | 16 (16) |
| Fentanyl patch | 19 (19) |
| Hydromorphone | 10 (10) |
| Morphine | 9 (9) |
| Codeine | 2 (2) |
| Tapentadol | 3 (3) |
| Methadone | 2 (2) |
| Oxymorphone | 1 (1) |
| Buprenorphine | 2 (2) |
| Meperidine | 1 (1) |
| Multiple opioids | 40 (39) |
| Primary reason for opioid use, n (%) | |
| Back pain | 38 (37) |
| Lower extremity pain | 11 (11) |
| Upper extremity pain | 11 (11) |
| Headache | 10 (10) |
| Fibromyalgia | 9 (9) |
| Generalized pain (does not meet fibromyalgia criteria) | 7 (7) |
| Abdominal pain | 7 (7) |
| Neck pain | 6 (6) |
| Facial pain | 5 (5) |
| Pelvic pain | 2 (2) |
| Chest wall pain | 2 (2) |
Table 3.
Pain, Quality of Life, and Physical Performance Measurements at Admission and Discharge from the Pain Rehabilitation Center
| Assessments | Admission (Day 1) | Discharge (Day 21) | ||||
|---|---|---|---|---|---|---|
| All | OIAI | Non-OIAI | All | OIAI | Non-OIAI | |
| N | 102 | 9 | 93 | 102 | 9 | 93 |
| Pain severity,a median (range) | 4.7 (1.67-6) | 4.8 (3.3-5.7) | 4.7 (1.7-6) | 3 (0-6)a | 3 (1-4)b | 3 (0-6)a |
| Pain interference,a median (range) | 4.7 (1.22-6) | 4 (2.6-5.4) | 4.78 (1.2-6) | 3 (0-6)a | 2.1 (1.4-4.3)b | 3 (0-6)a |
| General health,b median (range) | 34.5 (0-82) | 34 (4-82) | 34 (0-80) | 66 (12-100)a | 66 (44-96)b | 66 (0-100)a |
| Mental health,b median (range) | 26.1 (3-78.2) | 30.7 (8.4-79.2) | 25.5 (3-75.3) | 71.1 (15.9-96.5)a | 81.9 (55.4-92.8)b | 69.1 (15.9-96.5)a |
| Physical health,b median (range) | 31.9 (3.9-86.8) | 39.8 (13.3-69.4) | 31.8 (3.9-88.8) | 64.3 (11.6-97.5)a | 80.6 (45.9-90)b | 63 (11.6-97.5)a |
| 5-minute walk, feet median (range) | 1203 (175-2008) | 1270 (640-1899) | 1168 (175-2008) | 1436 (200-2159)a | 1410 (1200-1790)c | 1436 (200-2159)a |
a Subscales of the West-Haven Yale Multidimensional Pain Inventory, report on a 0-6 subscale with higher scores representing increased symptom severity.
b Subscales of the Short Form-36 initially report using a Likert scale and the transformed to percentages with lower scores representing worse quality of life.
Assessments at the time of discharge versus admission: aP < 0.0001, bP < 0.01; cP > 0.05.
Abbreviation: OIAI, opioid-induced adrenal insufficiency.
Adrenal function
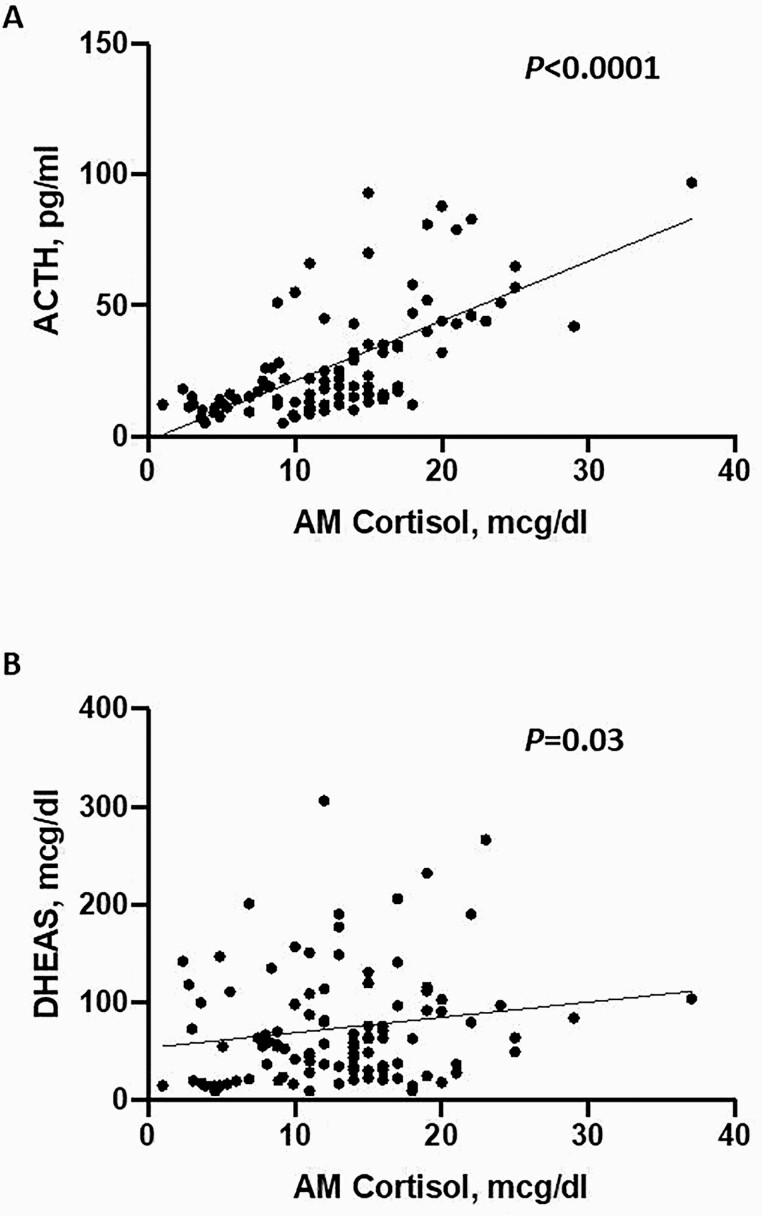
Median morning cortisol concentration was 13 mcg/dL (range, 1-37), median ACTH was 19 pg/mL (range, 5-97), and median DHEAS was 60 mcg/dL (range, <15-379). Morning cortisol concentrations were directly correlated with both DHEAS (P = 0.03) and ACTH (P < 0.0001) (Fig. 1A, B).
Figure 1.
Association of cortisol to (A) ACTH and to (B) DHEAS. am cortisol, morning cortisol; DHEAS, dehydroepiandrosterone sulfate.
Of 102 patients, 11 (10.8%) demonstrated abnormal case detection testing with ACTH ≤ 15 pg/mL, DHEAS ≤ 25 mcg/dL, and cortisol ≤ 10 mcg/dL, and were referred for endocrine evaluation. Diagnosis of OIAI was made in 9 (9%) patients: in 6 based on initial abnormal case detection combined with high clinical suspicion because of symptoms of adrenal insufficiency, and in 3, also based on abnormal peak cortisol after stimulation with cosyntropin (Table 4). All 9 patients were initiated on hydrocortisone. In 2 patients with abnormal case detection testing, clinical suspicion for OIAI was low, and during a cosyntropin stimulation test ordered by the treating endocrinologist in both cases, peak cortisol was normal. Potential risk factors for the development of OIAI were assessed (Table 1). Cumulative opioid exposure was significantly higher in those with OIAI compared with those without (median cumulative opioid exposure, 13,440 mg*months [range 120-36,000] vs 3120 mg*months [30-21,240] in patients without OIAI; P = 0.03). However, median daily MME (140 mg [20-392] vs 57 mg [3-840], P = 0.06) or median duration of opioid use (96 months [6-204] vs 60 [3-360] months, P = 0.19) did not differ between those who developed OIAI compared with those who did not. Additionally, no difference in development of OIAI was noted according to sex or age. Cortisol, ACTH, and DHEAS concentrations were significantly lower in patients diagnosed with OIAI when compared with the rest of patients (Table 1). No patient taking < 20 mg/day of MME developed OIAI (sensitivity of 100% for MME > 20 mg); however, the specificity of >20 mg/day MME as a cutoff to predict OIAI development was only 19%.
Table 4.
Characteristics of Patients Diagnosed with Opioid-Induced Adrenal Insufficiency
| Pt | Sex/age | Opioid type, MME dose and duration | Reasons for opioid therapy | Adrenal function assessment | Outcome of the pain rehabilitation intervention | Adrenal insufficiency management and recovery |
|---|---|---|---|---|---|---|
| 1 | F, 76 | Hydrocodone | Back and lower extremity pain | AM Cortisol 4.5 µg/dL | Failure to taper and discontinue opioid therapy | Hydrocortisone 20 mg daily initiated |
| 80 MME mg | ||||||
| At 3-month follow up: persistent adrenal insufficiency | ||||||
| 204 months | ||||||
| ACTH 8.9 pg/mL | ||||||
| DHEAS < 15 µg/dL | ||||||
| Peak cortisol during CST 16 µg/dL | ||||||
| 2 | F, 54 | Oxycodone | Back and lower extremity pain | am cortisol 6.9 µg/dL | Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| 30 MME mg | ||||||
| 144 months | At 4-month follow up, hydrocortisone was discontinued as adrenal function recovered on reassessment | |||||
| ACTH 15 pg/mL | ||||||
| DHEAS 22 µg/dL | ||||||
| 3 | M, 69 | Oxycodone, hydrocodone | Back and lower extremity pain | am cortisol 6 µg/dL | Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| 300 MME mg | At 13-month follow up, hydrocortisone was discontinued as adrenal function recovered on reassessment | |||||
| ACTH 14 pg/mL | ||||||
| 120 months | ||||||
| DHEAS 20.1 µg/dL | ||||||
| 4 | F, 51 | Fentanyl | Back, lower extremity, and generalized pain | am cortisol 5.4 µg/dL | Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| 140 MME mg | ||||||
| 96 months | At 5-month follow up, persistent adrenal insufficiency | |||||
| ACTH 11 pg/mL | ||||||
| DHEAS 17.3 µg/dL | ||||||
| 5 | F, 48 | Hydrocodone | Lower extremity pain |
am cortisol 9.9 µg/dL ACTH 8.2 pg/mL DHEAS 17 µg/dL |
Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| Tramadol | ||||||
| 20 MME mg | At 14-month follow up, hydrocortisone was discontinued as adrenal function recovered on reassessment | |||||
| 6 months | ||||||
| 6 | F, 60 | Oxycodone | Lower extremity pain |
am cortisol 3.9 µg/dL ACTH 5.1 pg/mL DHEAS < 15 µg/dL |
Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| No follow up at our institution | ||||||
| 392 MME mg | ||||||
| 72 months | ||||||
| 7 | F, 50 | Oxycodone | Generalized pain |
am cortisol 1.8 µg/dL ACTH 12 pg/mL Peak cortisol during CST 17 µg/dL |
Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| Oxymorphone | ||||||
| 240 MME mg | At 6-month follow up, hydrocortisone was discontinued as adrenal function recovered on reassessment | |||||
| 48 months | ||||||
| 8 | M, 52 |
Hydromorphone 48 MME mg 60 months |
Chronic headache | am cortisol < 1.0 µg/dL | Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| At 12-month follow up, hydrocortisone was discontinued as adrenal function recovered on reassessment | ||||||
| ACTH 12 pg/mL | ||||||
| DHEAS < 15 µg/dL | ||||||
| Peak cortisol during CST 16 µg/dL | ||||||
| 9 | F, 58 |
Oxycodone Hydromorphone 218 MME mg 108 months |
Back, lower, and upper extremity pain, and headache | am cortisol 9.2 µg/dL | Successful discontinuation of opioids | Hydrocortisone 20 mg daily initiated |
| ACTH 5 pg/mL | ||||||
| DHEAS 24 µg/dL | ||||||
| At 1-month follow up, hydrocortisone was discontinued as adrenal function recovered on reassessment |
Abbreviations: am, morning; CST, cosyntropin stimulation test; DHEAS, dehydroepiandrosterone sulphate; MME, morphine equivalents.
Baseline morning cortisol, ACTH and DHEAS concentrations, or diagnosis of OIAI were not associated with baseline quality of life scores, pain scores, or physical function measure by 5-minute test (data not shown).
Follow up
At the time of completion of the 3-week Pain Rehabilitation Program, all patients demonstrated improvements in pain, quality of life, and physical function (Table 3). Patients diagnosed with OIAI who were treated with hydrocortisone during the 3-week program showed similar improvement on follow-up assessment when compared with patients without OIAI.
Following the Pain Rehabilitation Program, 9 patients with OIAI continued on hydrocortisone. One patient who failed to discontinue opioids continued to demonstrate persistent adrenal insufficiency at the time of last follow up (Table 4). Of the remaining 8 patients who successfully discontinued opioid therapy, 6 recovered adrenal function when reassessed at 1 to 14 months, and hydrocortisone was discontinued. One patient has successfully discontinued opioids, and although improved clinically, has not recovered from adrenal insufficiency at the time of last follow up. Another patient was lost to follow up.
Discussion
In this cross-sectional case detection study with prospective enrollment, the prevalence of OIAI among those with chronic noncancer-related pain was 9%. Cumulative opioid exposure was the only identified predictor of OIAI and the daily 20 mg MME cutoff demonstrated a sensitivity of 100% to diagnose OIAI, but specificity of only 19%. We showed that all patients experienced a significant improvement in pain, quality of life, and physical function with the pain rehabilitation program intervention without significant differences between those diagnosed with OIAI and initiated on glucocorticoid replacement therapy and those without OIAI. The majority of patients diagnosed with OIAI recovered adrenal function after opioid discontinuation.
Several primarily retrospective studies have assessed the prevalence of adrenal insufficiency in chronic opioid users with chronic pain, demonstrating a prevalence of possible OIAI of 8% to 50% (Table 5). In a recent meta-analysis, the weighted mean prevalence of OIAI was 15%, and when limited to the 2 studies that used ITT to assess the HPA axis status, prevalence of OIAI was 24% (22). The wide range of OIAI prevalence is explained by a very high heterogeneity in the studies, including patient selection (with therapies including intrathecal opioids (13), duration of opioid use >3 or 6 months (21, 24), dose of opioid (18, 24) and methods of adrenal insufficiency assessment (dynamic testing, baseline testing, use of different cutoffs). Abnormal morning cortisol concentrations have been reported in 9% to 35% patients taking chronic opioids, low DHEAS in 35% to 43%, low ACTH in 6% to 26%, and abnormal peak cortisol during CST in 10% to 15% of patients taking chronic opioids (13, 18-21, 24, 32). In our study, using a predefined protocol that includes a combined cortisol, DHEAS, and ACTH testing, we found that 11% of our cohort had abnormal testing, and 9% were ultimately diagnosed with OIAI after a thorough endocrine evaluation.
Table 5.
Summary of the Literature Regarding Opioid-Induced Adrenal Insufficiency
| Author, Year | No. of Patients on Opioid Therapy | Daily MME (mg) | Duration of Opioid Therapy | Prevalence of OIAI |
|---|---|---|---|---|
| Abs et al, 200013 | 73 patients with chronic noncancer pain | Mean 24 ± 15 (range, 3-75) | Mean 26.6 ± 16.3 (range, 3-61) months | • 9.2%: am cortisol < 5 µg/dL |
| • 26.4%: ACTH < 10 ng/l | ||||
| • 19.7%: 24-h urinary free cortisol < 20 µg/24 h | ||||
| • 14.8%: Peak cortisol < 18 µg/dL during ITT | ||||
| Rhodin et al, 201019 | 39 patients with chronic noncancer pain | Male: mean 1596 (range, 320-3840) | >12 months | • 43%: DHEAS < normal range for age |
| • 33%: Peak cortisol < 550 nmol/L during CRH stimulation | ||||
| Female: mean 1322 (range, 240-2760) | ||||
| Nenke et al, 201520 | 17 patients with chronic noncancer pain | Mean daily 124 ± 95 | >1 month | • 29.4%: am cortisol < 5 µg/dL |
| 10%: peak cortisol < 550 nmol/L during CST (performed only in 10 patients) | ||||
| Valverde-Filho et al 201521 | 18 patients on intrathecal opioid therapy and 18 patients on oral opioid therapy with chronic noncancer pain | Median intrathecal: 3.5 (IQR 1.5-5) | Median intrathecal: 24 months | • 33% (intrathecal) and 22% (oral): am cortisol < 5 µg/dL |
| • 33% (intrathecal) and 50% (oral): peak cortisol < 18 µg/dL during ITT | ||||
| Median oral: 12 months | ||||
| Median oral: 30 (IQR 30-40) | ||||
| Gibb et al, 201618 | 48 patients with chronic noncancer pain | Median daily: 86 (40-153) | >6 months | • 8.3%: am cortisol < 100 nmol/L |
| • 6.3%: Peak cortisol < 430 nmol/L during CST | ||||
| Merdin et al, 201633 | 20 patients with chronic cancer-associated pain | Median daily: 180 (10-420) | >1 month | • 15%: am cortisol < 4.3 µg/dL |
| • 5.6%: ACTH: undetectable | ||||
| Lamprecht et al, 201824 | 40 patients with chronic noncancer pain | Median daily: 74 (25-265) | Median: 4 years (1-25 years) | • 35%: am cortisol < 5 µg/dL |
| • 35%: DHEAS <1 µmol/L in women and <2 µmol/L in men | ||||
| • 22.5% based on CST or metyrapone test (15%: peak cortisol < 500 nmol/L during CST; 7.5%: 11 deoxycortisol < 200 nmol/L on oral metyrapone test) | ||||
| Current study | 102 patients with chronic noncancer pain | Median daily: 60 (3-840) | Median: 60 (3-360) months | • 11.8%: am cortisol < 5 µg/dL |
| • 38.2%: ACTH < 15 ng/L | ||||
| • 21.6%: DHEAS < 25 µg/dL | ||||
| • 9% based on am cortisol < 10 µg/dL, DHEAS < 25 µg/dL, ACTH < 15 ng/L, and endocrine evaluation |
Abbreviations: CST, cosyntropin stimulation test; DHEAS, dehydroepiandrosterone sulfate; ITT, insulin tolerance test; MME, morphine equivalent; OIAI, opioid-induced adrenal insufficiency.
Although the ITT is considered to be the gold standard, it is not always available, is expensive, and is contraindicated in some patients (eg, cardiovascular disease, seizures). Additionally, metyrapone has limited availability (and investigated only in 1 study (24), whereas CST (1 mcg or 250 mcg) utilization is dependent on chronicity of hypopituitarism and has a suboptimal sensitivity, especially in partial forms of secondary adrenal insufficiency (30). Therefore, no single detection strategy is optimal or convenient. Several studies with varying morning serum cortisol cutoffs suggest that morning cortisol can be used to predict response to CST or ITT (33-35). In addition, DHEAS offers supportive evidence of decreased ACTH availability when measured and found to be concurrently low (36). We defined OIAI strictly as a low morning cortisol (<10 mcg/dL), with a low ACTH (15 pg/mL) and DHEAS (25 mcg/dL). However, this definition may have led to an underestimation of those with milder forms of OIAI.
The risk factors associated with the development of OIAI are not clear. Studies examining factors for the development of opioid-induced hypogonadism have suggested that the type of opioid, higher doses, use of long-acting opioids, and longer duration of opioid use may be risk factors for hypogonadism (23, 37). In studies assessing OIAI, Lambrecht et al observed a higher daily MME among those with OIAI compared with those without (median of 100 mg vs 60 mg) (24). Assessment of short-term use of opioids in volunteers without pain, demonstrated that increasing opioid dose or long-acting opioids were associated with lower levels of cortisol or ACTH (38, 39). Contrary to this, however, several other studies have not shown an association between daily MME and the development of OIAI (13, 18). In our study, cumulative opioid exposure, defined as the duration of opioid use × daily MME, was higher in those with OIAI compared with those without. In addition, no patient taking <20 MME per day was diagnosed with OIAI. This may explain the differing observations in studies that assess opioid dose or duration of opioid use independently as opposed to opioid exposure which provides a cumulative exposure estimate.
Several case reports and 1 retrospective study have demonstrated that OIAI is reversible following opioid cessation (14, 17, 40). In our study, we found that 6 of 7 patients who were able to discontinue opioids and had available follow-up recovered from adrenal insufficiency and were able to stop hydrocortisone. Therefore, when possible, opioid discontinuation should be encouraged, and glucocorticoid replacement should be continued until recovery has been confirmed.
There are several limitations to our study. Use of an arbitrary strict case detection strategy and lack of dynamic testing, such as ITT, may have influenced our prevalence estimate. Normal cortisol response during CST in several patients may not completely exclude adrenal insufficiency (30). All endocrine testing was performed in the morning before opioid taper, and patients were asked not to hold any opioid doses for testing. Because opioid therapy was not withheld on the day of testing, the prevalence of OIAI could be overestimated. Studies looking at the difference between acute and chronic effects of opioids and risk for OIAI are necessary. Baseline evaluation of patients did not include a systematic assessment of the HPA axis, which is known to adversely affect the majority of patients on chronic opioids. Therefore, this may have affected our ability to ascertain appreciable differences in measures of quality of like, adjustment to pain, or functional capacity in those with OIAI compared with those without OIAI. Last, the exact duration of opioid therapy and dose was estimated based on patient recollection and medical records and therefore may have been subject to recall and information bias. The strength of our study is uniform predefined case detection strategy that is feasible to assimilate into clinical care, combined endocrine evaluation in the adrenal clinic that allowed integration of clinical expertise in addition to laboratory workup to achieve the diagnosis of OIAI, and availability of follow-up information for most patients with OIAI. Further prospective studies are needed to confirm the association of opioid dose and duration with development of OIAI.
In conclusion, using a case detection strategy of baseline testing with ACTH, DHEAS, and cortisol, followed by the endocrine evaluation, we demonstrated a 9% prevalence of OIAI. We showed that cumulative opioid exposure was a significant predictor of OIAI. Patients taking <20 MME per day do not need further evaluation for OIAI. In contrast, clinical evaluation and case detection testing should be considered in any patient treated with chronic opioids with >20 MME per day.
Acknowledgments
Financial Support: This research was supported by the Catalyst Award for Advancing in Academics from Mayo Clinic (I.B.) and partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award K23DK121888 (to I.B.). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health.
Glossary
Abbreviations
- CST
cosyntropin stimulation testing
- DHEAS
dehydroepiandrosterone sulfate
- HPA
hypothalamic-pituitary-adrenal
- ITT
insulin tolerance test
- MME
morphine milligram equivalent
- OIAI
opioid-induced adrenal insufficiency
- SF-36
Short Form 36.
Additional Information
Disclosure Summary: I.B. reports advisory board participation with Corcept and HRA Pharma and consultancy with ClinCor outside the submitted work. D.D. reports advisory board participation and research support from Corcept.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Roehler, DR. Annual surveillance report of drug-related risks and outcomes. https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf. 2019. Accessed March 1, 2020 .
- 2. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315(15):1624-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller GF, Guy GP, Zhang K, Mikosz CA, Xu L. Prevalence of nonopioid and opioid prescriptions among commercially insured patients with chronic pain. Pain Med. 2019;20(10):1948-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. John WS, Wu LT. Chronic non-cancer pain among adults with substance use disorders: prevalence, characteristics, and association with opioid overdose and healthcare utilization. Drug Alcohol Depend. 2020;209:107902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saeed ZI, Bancos I, Donegan D. Current knowledge and practices of heath care professionals on opioid-induced adrenal insufficiency. Endocr Pract. 2019;25(10):1012-1021. [DOI] [PubMed] [Google Scholar]
- 7. Aloisi AM, Aurilio C, Bachiocco V, et al. Endocrine consequences of opioid therapy. Psychoneuroendocrinology. 2009;34(Suppl 1):S162-S168. [DOI] [PubMed] [Google Scholar]
- 8. Aloisi AM, Ceccarelli I, Fiorenzani P, et al. Aromatase and 5-alpha reductase gene expression: modulation by pain and morphine treatment in male rats. Mol Pain. 2010;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuong QC, Friedman A, Plante C. Modulation of viewpoint effects in object recognition by shape and motion cues. Perception. 2009;38(11):1628-1648. [DOI] [PubMed] [Google Scholar]
- 10. Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3(5):377-384. [DOI] [PubMed] [Google Scholar]
- 11. Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain. 2008;9(1):28-36. [DOI] [PubMed] [Google Scholar]
- 12. Fraser LA, Morrison D, Morley-Forster P, et al. Oral opioids for chronic non-cancer pain: higher prevalence of hypogonadism in men than in women. Exp Clin Endocrinol Diabetes. 2009;117(1):38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222. [DOI] [PubMed] [Google Scholar]
- 14. Oltmanns KM, Fehm HL, Peters A. Chronic fentanyl application induces adrenocortical insufficiency. J Intern Med. 2005;257(5):478-480. [DOI] [PubMed] [Google Scholar]
- 15. Schimke KE, Greminger P, Brändle M. Secondary adrenal insufficiency due to opiate therapy–another differential diagnosis worth consideration. Exp Clin Endocrinol Diabetes. 2009;117(10):649-651. [DOI] [PubMed] [Google Scholar]
- 16. Debono M, Chan S, Rolfe C, Jones TH. Tramadol-induced adrenal insufficiency. Eur J Clin Pharmacol. 2011;67(8):865-867. [DOI] [PubMed] [Google Scholar]
- 17. Li T, Donegan D, Michael Hooten W, Bancos I. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020. doi: 10.4158/EP-2020-0297 [DOI] [PubMed] [Google Scholar]
- 18. Gibb FW, Stewart A, Walker BR, Strachan MW. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016;85(6):831-835. [DOI] [PubMed] [Google Scholar]
- 19. Rhodin A, Stridsberg M, Gordh T. Opioid endocrinopathy: a clinical problem in patients with chronic pain and long-term oral opioid treatment. Clin J Pain. 2010;26(5):374-380. [DOI] [PubMed] [Google Scholar]
- 20. Nenke MA, Haylock CL, Rankin W, et al. Low-dose hydrocortisone replacement improves wellbeing and pain tolerance in chronic pain patients with opioid-induced hypocortisolemic responses. A pilot randomized, placebo-controlled trial. Psychoneuroendocrinology. 2015;56:157-167. [DOI] [PubMed] [Google Scholar]
- 21. Valverde-Filho J, da Cunha Neto MB, Fonoff ET, Meirelles Ede S, Teixeira MJ. Chronic spinal and oral morphine-induced neuroendocrine and metabolic changes in noncancer pain patients. Pain Med. 2015;16(4):715-725. [DOI] [PubMed] [Google Scholar]
- 22. de Vries F, Bruin M, Lobatto DJ, et al. Opioids and their endocrine effects: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105(3):1020-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubinstein A, Carpenter DM. Elucidating risk factors for androgen deficiency associated with daily opioid use. Am J Med. 2014;127(12):1195-1201. [DOI] [PubMed] [Google Scholar]
- 24. Lamprecht A, Sorbello J, Jang C, Torpy DJ, Inder WJ. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018;179(6):353-362. [DOI] [PubMed] [Google Scholar]
- 25. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23(4):345-356. [DOI] [PubMed] [Google Scholar]
- 26. Verra ML, Angst F, Staal JB, et al. Reliability of the Multidimensional Pain Inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ware JE, Sherbourne CD, Davies AR. Developing and testing the MOS 20-Item Short-Form Health Survey. In: Stewart AL, Ware Jr, JE, eds. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992: Chap.16, p. 277-290.
- 28. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247-263. [DOI] [PubMed] [Google Scholar]
- 29. Simmonds MJ, Olson SL, Jones S, et al. Psychometric characteristics and clinical usefulness of physical performance tests in patients with low back pain. Spine (Phila Pa 1976). 1998;23(22):2412-2421. [DOI] [PubMed] [Google Scholar]
- 30. Ospina NS, Al Nofal A, Bancos I, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-434. [DOI] [PubMed] [Google Scholar]
- 31. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216-226. [DOI] [PubMed] [Google Scholar]
- 32. Merdin A, Merdin FA, Gündüz Ş, Bozcuk H, Coşkun HŞ. Opioid endocrinopathy: a clinical problem in patients with cancer pain. Exp Ther Med. 2016;11(5):1819-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidt IL, Lahner H, Mann K, Petersenn S. Diagnosis of adrenal insufficiency: evaluation of the corticotropin-releasing hormone test and Basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. J Clin Endocrinol Metab. 2003;88(9):4193-4198. [DOI] [PubMed] [Google Scholar]
- 34. Yip CE, Stewart SA, Imran F, et al. The role of morning basal serum cortisol in assessment of hypothalamic pituitary-adrenal axis. Clin Invest Med. 2013;36(4):E216-E222. [DOI] [PubMed] [Google Scholar]
- 35. Karaca Z, Tanriverdi F, Atmaca H, et al. Can basal cortisol measurement be an alternative to the insulin tolerance test in the assessment of the hypothalamic-pituitary-adrenal axis before and after pituitary surgery? Eur J Endocrinol. 2010;163(3):377-382. [DOI] [PubMed] [Google Scholar]
- 36. Charoensri S, Chailurkit L, Muntham D, Bunnag P. Serum dehydroepiandrosterone sulfate in assessing the integrity of the hypothalamic-pituitary-adrenal axis. J Clin Transl Endocrinol. 2017;7:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rubinstein AL, Carpenter DM. Association between commonly prescribed opioids and androgen deficiency in men: a retrospective cohort analysis. Pain Med. 2017;18(4):637-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allolio B, Schulte HM, Deuss U, Kallabis D, Hamel E, Winkelman W. Effect of oral morphine and naloxone on pituitary-adrenal response in man induced by human corticotropin-releasing hormone. Acta Endocrinol (Copenh). 1987;114(4):509-514. [DOI] [PubMed] [Google Scholar]
- 39. Palm S, Moenig H, Maier C. Effects of oral treatment with sustained release morphine tablets on hypothalamic-pituitary-adrenal axis. Methods Find Exp Clin Pharmacol. 1997;19(4): 269-273. [PubMed] [Google Scholar]
- 40. Tabet EJ, Clarke AJ, Twigg SM. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019;12(12):e230551. doi: 10.1136/bcr-2019-230551 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.