Abstract
Thrombotic thrombocytopaenic purpura (TTP) is a life-threatening thrombotic microangiopathy characterised by microangiopathic haemolytic anaemia, thrombocytopaenia and organ ischaemia. TTP is caused by a severe functional deficiency of ADAMTS13 activity. We describe a 10-year-old girl presenting anaemia and thrombocytopaenia with schistocytes. Urine protein to creatinine ratio was within nephrotic range. ADAMTS13 activity was 0%, and no anti-ADAMTS13 antibodies were found. A renal biopsy showed deposits of IgG, C3 and C1q in the capillary membrane, compatible with class V lupus nephritis. Therapeutic plasma exchange (TPE) was performed in conjunction with therapy consisting of steroids and mycophenolate mofetil. After 11 months of follow-up, the patient remains in remission with normal ADAMTS13 activity. Although acquired TTP is a rare finding in children, differential diagnosis of thrombotic microangiopathy should include ADAMTS13 and the assay should be performed early. TTP treatment is based on TPE, although the underlying disease must be ruled out to optimise treatment and prevent relapse.
Keywords: haematology (drugs and medicines), paediatric prescribing
Background
Thrombotic thrombocytopaenic purpura (TTP) is a rare type of thrombotic microangiopathy (TMA). TMA syndromes are defined by microangiopathic haemolytic anaemia with the presence of characteristic schistocytes on blood smear, platelet consumption thrombocytopaenia and organ ischaemia related to disseminated microvascular platelet-rich thrombi.
TMA is classified based on aetiology.1–3 Primary TMA syndromes are those in which the aetiology is well known. The presence of a defined abnormality, such as ADAMTS13 deficiency, may not be clinically significant unless another condition, such as an inflammatory disorder, precipitates an acute TMA episode. Primary TMA includes the following:
TTP caused by severe functional deficiency (<5%–10%) of ADAMTS13 protease, which is required for the cleavage of the high molecular weight multimers of von Willebrand factor, leading to the formation of platelet-rich microthrombi in the microcirculation. Most paediatric cases are genetic, via inherited biallelic mutations of the ADAMTS13 gene, although the deficiency is acquired by means of autoantibodies to ADAMTS13 in most adult cases (>95%). Acquired TTP is a rare finding in children.4
TMA due to Shiga toxin, also called ST-haemolytic uraemic syndrome (HUS), is the most frequent type found in children.
Complement-mediated TMA results from complement dysregulation, either hereditary (ie, caused by mutations in genes that encode proteins involved in this pathway) or acquired due to autoantibodies to the complement. Anticomplement therapy with eculizumab is currently the most effective treatment option.5
On the other hand, secondary TMA syndromes stem from an imbalance between immunity, coagulation and complement in certain patients with an underlying condition.
In children, TTP is a very uncommon disease (~5% of all TTP cases), and congenital TTP is more prevalent than the acquired form.4 TTP is unique in that it rarely causes severe acute kidney injury. Hereditary TTP presents clinically as TMA and thrombocytopaenia, usually with neurological abnormalities; however, the clinical features of acquired TTP vary.3 Other TMA syndromes are more frequently reported in children, such as Shiga toxin-producing Escherichia coli HUS typically with acute kidney injury. Despite the challenge they pose, these entities must be included in the differential diagnosis. Although paediatric HUS has a clinical presentation resembling that of TTP, its pathophysiology and management are completely distinct. ADAMTS13 activity is typically normal in children with HUS, while severe ADAMTS13 deficiency is a defining characteristic of children with TTP. Thus, measurement of ADAMTS13 activity and a search for inhibitor antibodies are crucial to rule out the presence of HUS.6 In the absence of appropriate treatment, TTP is a life-threatening disease. Therapeutic plasma exchange (TPE) should be started as soon as possible in patients with clinical features of the disease. The use of steroids is recommended if TTP is mediated by autoantibodies.1 2 Secondary TTP triggered by an underlying disease or condition such as cancer, autoimmune disease or transplant should be ruled out in order to optimise treatment. Complete remission is defined as the reversal of clinical manifestations and thrombocytopaenia (>150×109/L for at least 2 consecutive days). Relapse entails the reappearance of clinical manifestations and/or thrombocytopaenia after 30 consecutive days of complete remission, and flare-up is understood as a worsening of clinical manifestations and/or thrombocytopaenia during treatment or over the 30 days following complete remission.7 Long-term follow-up including monitoring of ADAMTS13 activity is mandatory.4
Case presentation
We present the case of a 10-year-old girl of Asian descent. On admission to the intensive care unit of our hospital due to suspected TMA, her general condition was fair, although she complained of vomiting and presented arterial hypertension. A complete blood count revealed anaemia and severe thrombocytopaenia with schistocytes on peripheral blood smear. Results of blood coagulation testing were normal, and a direct antiglobulin test was negative. Renal function and neurological examination were normal. Screening for hepatitis B, hepatitis C and HIV viruses was negative. ADAMTS13 enzyme activity was 0%, indicating a severe ADAMTS13 deficiency, which was later confirmed with a repeat TECHNOZYM ADAMTS13 Activity ELISA Kit. A search for anti-ADAMTS13 antibodies was also initiated. ADAMTS13 inhibitors were determined by measuring residual ADAMTS13 activity in 50:50 mixtures with normal plasma,6 producing a negative result. Due to the possible diagnosis of congenital TTP, sequencing of the ADAMTS13 gene was performed, although no variants associated with congenital TTP were detected. Response to transfusion of plasma was suboptimal, so we performed one session of TPE combined with steroid therapy (3 days of intravenous methylprednisolone followed by prednisone 1 mg/kg/day). Following this, the patient reached complete remission. After she was discharged, oral steroid therapy was gradually reduced and eventually discontinued.
Twenty days after discontinuing steroid treatment, the patient presented to our institution with epistaxis and purpuric lesions on the legs. Blood testing showed anaemia (haemoglobin 10.2 g/L), schistocytes, lactate dehydrogenase 776 U/L, aspartate aminotransferase 65 U/L, total bilirubin 2.56 mg/dL, uric acid 5.8 mg/dL and severe thrombocytopaenia (platelet count 12×109/L).
Complementary studies revealed a urine protein to creatinine ratio of 3.7 (nephrotic range) and decreased complement C3 and C4 levels; antinuclear antibodies (ANA) were positive, including positive results for the anti-Ro52 and anti-La antibodies.
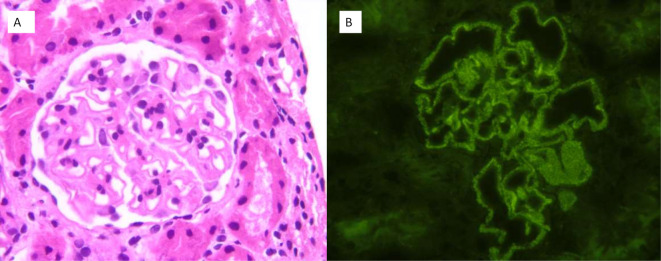
A renal biopsy was performed when the patient achieved haematological response, evidencing granular deposits of IgG, C3 and C1q in the capillary membrane, compatible with class V lupus nephritis (figure 1).
Figure 1.
(A) Renal cortex without histologically relevant findings in the interstitial, tubular, vascular or glomerular compartment (HE ×400). (B) The direct immunofluorescence evidences granular membrane deposition for IgG, C3 and C1q, showing no deposition of IgA, IgM or C4 (IgG ×400).
Investigations
Complementary studies performed on diagnosis revealed a urine protein to creatinine ratio of 3.7 (nephrotic range), low complement C3 and C4 levels, and positive results for ANA, including anti-Ro52 and anti-La antibodies.
A renal biopsy showed granular deposits of IgG, C and C1q in the capillary membrane, compatible with class V lupus nephritis (figure 1). In light of the histopathological results, we decided to add mycophenolate mofetil (MMF) to the treatment and then initiated gradual reduction of steroid therapy.
Treatment
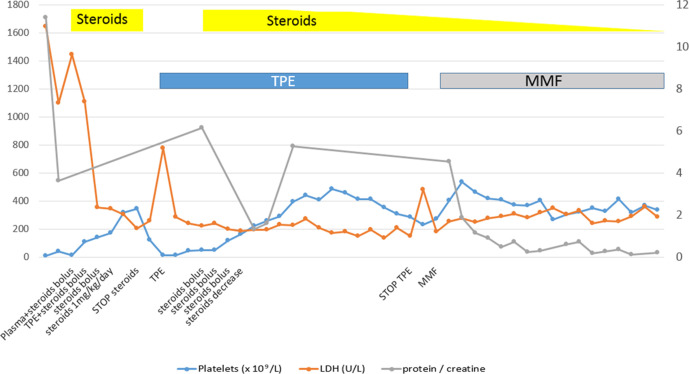
Due to possible reactivation of TTP, four units of plasma were transfused without response and daily TPE was restarted. As this measure failed to produce the desired effect, we decided to combine this approach with steroid treatment (3 days of intravenous methylprednisolone followed by oral prednisone at 2 mg/kg/day for 8 days and subsequently 1 mg/kg/day). The patient achieved haematological response (>150×109/L platelets for 2 consecutive days) after five sessions of TPE and steroids, after which TPE was administered at gradually increasing intervals. A total of 14 TPE sessions were administered. A renal biopsy was performed when the patient achieved haematological response. As the histological report contained findings compatible with class V lupus nephritis, we decided to add MMF 500 mg/12 hours to the treatment followed by a gradual withdrawal of steroid therapy. The protein to creatinine ratio only improved after sustained steroid and MMF treatment (figure 2). In addition to plasma exchange, sustained glucocorticoids and immunosuppressive agents are also important in the treatment of TTP secondary to systemic lupus erythematosus (SLE) (TTP-SLE).6
Figure 2.
Trend of platelet count, LDH level and proteinuria in relation to therapeutic management. LDH, lactate dehydrogenase; MMF, mycophenolate mofetil; TPE, therapeutic plasma exchange.
Outcome and follow-up
Currently, after 11 months of follow-up with continued treatment consisting of MMF and low-dose steroids, the patient is in clinical remission with normal ADAMTS13 activity (>95%).
Discussion
TMA encompasses a group of heterogeneous entities characterised by microangiopathic haemolytic anaemia, thrombocytopaenia, fever and organ involvement. All forms of TMA present with vascular damage, which triggers formation of thrombi in the microcirculation leading to mechanical haemolysis and organ ischaemia.
TTP is a type of TMA caused by severe functional deficiency (<5%–10%) of ADAMTS13 protease, which is required for the cleavage of the high molecular weight multimers of von Willebrand factor, leading to the formation of platelet-rich microthrombi in the microcirculation. Most paediatric cases are genetic, via inherited biallelic mutations of the ADAMTS13 gene, although the deficiency is acquired by means of autoantibodies to ADAMTS13 in most adult cases (>95%). Acquired TTP is a rare finding in children, and in some patients may be triggered by an underlying condition and classified as a secondary TMA.
Secondary TMA syndromes derive from an imbalance between immunity, coagulation and complement due to an underlying condition. As evidenced by this case, TMA can present in association with autoimmune diseases such as SLE. TMA occurs in 6%–9% of patients with SLE, and may be triggered by an infection, pregnancy or lupus flare.8 The pathogenesis of SLE-associated TTP is related to the autoimmune response or vascular damage induced by platelet aggregation or endothelial damage. Studies have reported the presence of antiendothelial, antiplatelet and anti-ADAMTS13 antibodies in this context, thus pointing to a role played by an autoimmune mechanism in its pathogenesis.9 Usually, the activity of ADAMTS13 is not significantly reduced.10 However, in 21% of cases there may be a severe deficit of ADAMTS13 activity attributable to autoantibodies11; in such patients, it may be considered that TTP is triggered by the underlying disease. SLE also may have associated complement-mediated TMA and could be a separate entity.12
Regarding TTP secondary to SLE, a previous study reported that most patients had a diagnosis of SLE prior to TTP onset, whereas only 15% of patients had SLE and TTP diagnosed simultaneously.13 In another study, 60% of patients were diagnosed with TTP-SLE simultaneously.9 In our case, the age and atypical presentation together with the severe deficit of ADAMTS13, with no presence of mutations or anti-ADAMTS13 autoantibodies, posed a challenge. Findings of proteinuria and autoimmunity studies guided us to the diagnosis of SLE, which was confirmed by renal biopsy. More than 64% of children with TTP-SLE present lupus-related markers, such as positive double-stranded DNA (dsDNA) or low levels of serum C3 or C4,9 as in our patient. In a recent review in adults, the most common type of histopathological renal damage in TTP-SLE was class IV (57.7%), followed by class V (11.5%), class I (5.8%) and TMA (5.8%). The renal biopsy performed in this patient indicated class V lupus nephritis. According to the criteria of Systemic Lupus International Collaborating Clinics, patients presenting biopsy-proven nephritis compatible with SLE in the presence of ANAs or anti-dsDNA antibodies are classified as having SLE.14
Our final diagnosis was acquired TTP caused by SLE, which responded to combination therapy (TPE, steroids and MMF). Although we were unable to confirm the existence of anti-ADAMTS13 antibodies, the recovery of normal ADAMTS13 activity during remission and the absence of anomalies in the ADAMTS13 gene led us to rule out congenital TTP. Negative results for inhibitory anti-ADAMTS13 autoantibodies at diagnosis have been described in a previous case of TTP.6 15 However, this undetectable inhibitor in some patients with acquired ADAMTS13 deficiency remains unexplained and suggests false negatives or unknown mechanisms for acquired TTP.15
The aetiopathogenesis of TTP in SLE is not completely understood, although it appears that complement and functional deficiency of ADAMTS13 secondary to cytokines and autoantibodies are involved. Several cases of TTP triggered by SLE have been described in the literature, which poses a diagnostic challenge, especially in children with no history of SLE.16
If TTP is suspected, ADAMTS13 activity should be measured before plasma exchange, and treatment based on TPE should be started as soon as possible. Based on the results of additional tests, it is mandatory to re-evaluate the case to determine the causal factor in order to improve prognosis and avoid relapse. Combined therapies have been found to be more appropriate in TTP-SLE.9 Thus, plasma exchange and immunosuppressant therapy that combines steroids and maintenance with cytotoxic agents such as MMF should be administered; in patients who have a contraindication to or cannot tolerate MMF, we suggest treatment with intravenous cyclophosphamide or a calcineurin inhibitor rather than other immunosuppressive drugs.
Learning points.
Thrombotic thrombocytopaenic purpura (TTP) is a life-threatening thrombotic microangiopathy characterised by microangiopathic haemolytic anaemia, thrombocytopaenia and organ ischaemia, and is caused by a severe functional deficiency in ADAMTS13 protease.
If TTP is suspected, activity of ADAMTS13 should be measured before initiating plasma exchange; treatment based on therapeutic plasma exchange should be started as soon as possible.
It is important to determine the secondary cause of TTP by performing an immunological study including antinuclear antibodies (ANAs), C3 and C4.
ANAs are highly sensitive but less specific antibodies to systemic lupus erythematosus, so the presence of hypocomplementaemia supports the diagnosis of lupus.
With the results of additional tests, it is mandatory to re-evaluate the case in order to determine the possible underlying trigger, thus improving prognosis and avoiding relapse.
Acknowledgments
Special thanks to Dr Jorge Martínez Nieto for technical and scientific support and Dr Lorenzo Antúnez for help with writing. We also thank the nurses and technicians who contributed to the care and treatment of the patient, as well as all the professionals involved, especially Dr Julián Sevilla, Dr Andrés Urquía Renque, Dr Eva Gálvez, Dr Josune Zubicaray and Dr Cristina Aparicio. Finally, our thanks to the patient and her family.
Footnotes
Contributors: IAOJ: summary of clinical record, writing and editing. CDLC: design. AMA: review. ES: writing, review and translation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Arrizabalaga B, Ataúlfo González F. Eritropatología. Ambos Marketing Services, 2017. [Google Scholar]
- 2.Fernández JAP, González-Porras JR, Arranz JM. Hemostasia Y Trombosis. manual Práctico. Arán. Madrid, España, 2017. [Google Scholar]
- 3.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med Overseas Ed 2014;371:654–66. 10.1056/NEJMra1312353 [DOI] [PubMed] [Google Scholar]
- 4.Joly BS, Coppo P, Veyradier A. Pediatric thrombotic thrombocytopenic purpura. Eur J Haematol 2018;101:425–34. 10.1111/ejh.13107 [DOI] [PubMed] [Google Scholar]
- 5.Réti M, Farkas P, Csuka D, et al. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost 2012;10:791–8. 10.1111/j.1538-7836.2012.04674.x [DOI] [PubMed] [Google Scholar]
- 6.Ferrari S, Scheiflinger F, Rieger M, et al. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 1 de abril de 2007;109:2815–22. [DOI] [PubMed] [Google Scholar]
- 7.Vesely SK, George JN, Lämmle B, et al. Adamts13 activity in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 2003;102:60–8. 10.1182/blood-2003-01-0193 [DOI] [PubMed] [Google Scholar]
- 8.Kello N, Khoury LE, Marder G, et al. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: case series and review of literature. Semin Arthritis Rheum 2019;49:74–83. 10.1016/j.semarthrit.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Jiang J-J, Wang C-Y, et al. Clinical features and prognosis of patients with thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a review of 25 cases. Ital J Pediatr 2019;45:55. 10.1186/s13052-019-0641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuyama T, Kuwana M, Matsumoto M, et al. Heterogeneous pathogenic processes of thrombotic microangiopathies in patients with connective tissue diseases. Thromb Haemost 2009;102:371–8. 10.1160/TH08-12-0825 [DOI] [PubMed] [Google Scholar]
- 11.Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara medical university during 1998-2008. Intern Med 2010;49:7–15. 10.2169/internalmedicine.49.2706 [DOI] [PubMed] [Google Scholar]
- 12.Boneparth A, Moorthy LN, Weiss L, et al. Complement inhibition in the treatment of SLE-Associated thrombotic thrombocytopenic purpura. Glob Pediatr Health 2015;:;2:2333794X1557015. 2. 10.1177/2333794X15570150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musio F, Bohen EM, Yuan CM, et al. Review of thrombotic thrombocytopenic purpura in the setting of systemic lupus erythematosus. Semin Arthritis Rheum 1998;28:1–19. 10.1016/S0049-0172(98)80023-1 [DOI] [PubMed] [Google Scholar]
- 14.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veyradier A, Obert B, Houllier A, et al. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood 2001;98:1765–72. 10.1182/blood.V98.6.1765 [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, An X, Li Y, et al. Clinical features and prognostic factors of thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a literature review of 105 cases from 1999 to 2011. Clin Rheumatol 2014;33:419–27. 10.1007/s10067-013-2312-5 [DOI] [PMC free article] [PubMed] [Google Scholar]