Abstract.
Murine typhus is a flea-borne rickettsiosis caused by Rickettsia typhi. When severe, endothelial dysfunction can lead to acute kidney injury secondary to prerenal azotemia or acute tubular necrosis. Here, we describe an unusual cause of kidney injury during the course of murine typhus–focal segmental glomerulosclerosis.
INTRODUCTION
Murine typhus is an undifferentiated acute febrile illness caused by Rickettsia typhi, a Gram-negative obligately intracellular bacterium. Endemic to seaboard regions of the tropics and subtropics, the disease is maintained by Rattus spp. and transmitted by their fleas (Xenopsylla cheopis). In southern California and Texas, opossums and cat fleas (Ctenocephalides felis) are also presumed reservoirs and vectors, respectively. Rickettsia typhi is transmitted to humans by the inoculation of rickettsiae-laden flea feces onto flea bite sites or onto mucous membranes, disseminates via lymphohematogenous spread, and establishes infection within endothelial cells, where it exerts its pathogenic effects.1 As a consequence of endothelial injury, the cascade of increased vascular permeability and extravasation of intravascular fluid into the interstitium can lead to end-organ damage (e.g., encephalitis, pneumonitis, and acute kidney injury). In the case of the kidneys, hypovolemia and decreased perfusion lead to prerenal azotemia and, when severe or prolonged, can progress to cause acute tubular necrosis.2 To our knowledge, severe glomerular disease has not been documented during rickettsial infection. We herein describe a case of acute kidney injury, secondary to focal segmental glomerulosclerosis (FSGS), in a man with murine typhus.
CASE REPORT
A 40-year-old African-American man presented to the emergency department of the University of Texas Medical Branch on May 9, 2017 with a chief complaint of fever and myalgia for 7 days. Associated symptoms included anorexia, weight loss, dizziness, dyspnea, and diarrhea. He denied headache, sore throat, sinus congestion, rhinorrhea, cough, nausea, vomiting, abdominal pain, and arthralgia. He took acetaminophen and ibuprofen without relief.
The patient had no known medical problems and had normal renal function documented in 2006, with blood urea nitrogen and creatinine levels of 11 mg/dL (reference range, 7–23 mg/dL) and 1.26 m/dL (0.6–1.25 mg/dL), respectively (Table 1). He was on no long-standing medications. He lived in Galveston, Texas, worked delivering furniture, drank brandy daily for the past 10 years, used marijuana occasionally, and smoked cigars daily. Later, questioning revealed that opossums were living around his home, and his yard was infested with fleas. His family history was notable for a mother with FSGS requiring a kidney transplant. He denied recent travel or sick contacts.
Table 1.
Renal laboratory values at intervals throughout clinical course
| Laboratory value (reference range) | Admission: hospital day 1 (May 9, 2017) | Hospital day 5 (May 13, 2017) | Hospital day 9 (May 17, 2017) | Hospital day 13 (May 22, 2017) | Discharge: hospital day 21 (May 30, 2017) | Subsequent visit (December 2, 2018) |
|---|---|---|---|---|---|---|
| BUN (7–23 mg/dL) | 21 | 61 | 124 | 148 | 110 | 21 |
| Creatinine (0.6–1.25 mg/dL) | 1.38 | 6.24 | 12.52 | 16.11 | 8.46 | 2.07 |
| Creatine kinase (33–194 U/L) | 3,930 | 1,017 | 916 | – | – | – |
| 24-Hour urine protein (< 0.1 g/day) | – | 4.6 | – | – | – | – |
On examination, his temperature was 38.1°C, blood pressure 115/87 mm of Hg, heart rate 108 beats/minute, and respiratory rate 18 breaths/minute. Examination of his skin revealed a faint macular rash on his trunk and lower extremities that was difficult to discern because of darkly pigmented skin. Otherwise, physical examination was unremarkable. Initial laboratory testing revealed a leukocyte count of 8,930 cells/mm3 (4,200–10,700 cells/mm3), hemoglobin of 16.5 g/dL (12.2–16.4 g/dL), platelet count of 67,000/mm3 (150,000–328,000/mm3), serum sodium of 124 mmol/L (135–145 mmol/L), blood urea nitrogen of 21 mg/dL, serum creatinine of 1.38 mg/dL, aspartate aminotransferase of 331 U/L (13–40 U/L), alanine aminotransferase of 186 U/L (9–51 U/L), creatine kinase (CK) of 3,930 U/L (33–194 U/L), and lactic acid of 2.31 mmol/L (0.50–2.20 mmol/L). Urinalysis was not indicative of infection. Influenza A and B, and respiratory syncytial virus panel were negative. Blood cultures revealed no bacterial growth. Clostridium difficile stool toxin and fecal culture were negative. Serology for HIV, hepatitis B, hepatitis C, Epstein–Barr virus, and syphilis were negative. Glucose-6-phosphate dehydrogenase testing revealed no deficiency. Ultrasonography of his abdomen showed a normal appearing liver without gallstones and increased echogenicity of the kidneys.
While on the medical service, he continued to experience fever with an unrevealing infectious disease workup. On hospital day 5 (day 12 of illness), murine typhus was suspected, and doxycycline 100 mg twice daily was initiated; he defervesced approximately 24 hours later. The indirect immunofluorescence assay for typhus group IgG was reactive at 1:256 on hospital day 5 (day 12 of illness) and 1:1,024 on hospital day 9 (day 16 of illness). The CK, which was at its peak at presentation, steadily decreased to the last recorded value of 916 U/L on hospital day 10 (day 17 of illness) (Table 1). Despite resolution of symptoms, his renal function continued to decline with a peak blood urea nitrogen of 148 mg/dL, creatinine of 16.1 mg/dL, and nephrotic range proteinuria (4.6 g/day) without hematuria on hospital day 13 (day 20 of illness). Dialysis was initiated on hospital day 14 (day 21 of illness) where he only received only two sessions, and a renal biopsy was performed.
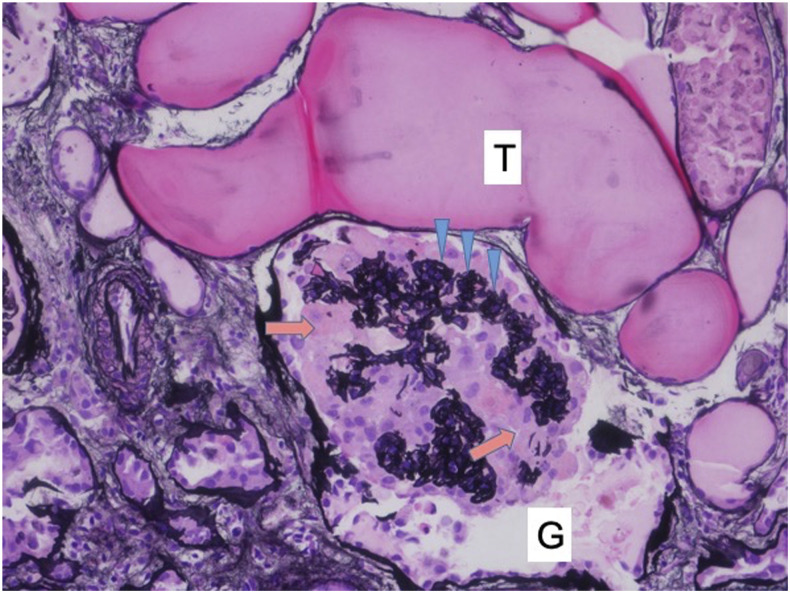
Pathologic examination revealed the collapsing variant of FSGS (Figure 1). Findings included the following: collapse of glomerular basement membranes, hyperplasia and proliferation of visceral epithelial cells, dilated Bowman’s space, microcystic dilation of tubules with proteinaceous luminal casts, edematous interstitium with mild inflammation composed of plasma cells, mild fibrous intimal thickening of arteries, mild arteriolar hyalinosis, and mild peritubular capillaritis. Genetic testing of the biopsy specimen for apolipoproteinL1 (APOL1) gene risk alleles was performed (Arkana Laboratories, Little Rock, AR). The patient exhibited two sequence variants in APOL1, known as G1 and G2, that confer increased risk for APOL1-associated nephropathy.
Figure 1.
Collapsing glomerulopathy: A glomerulus (G) showing collapsed glomerular basement membrane (arrowheads) with overlying proliferating epithelial cells (arrows). Microcystic dilatation of the tubule (T) containing proteinaceous material (Jones stain; ×200). This figure appears in color at www.ajtmh.org.
The patient was discharged after 22 days of hospitalization (29 days after onset of illness) on prednisone 60 mg daily. At discharge, his creatinine was 8.46 mg/dL. He was lost to follow up but was later seen in the emergency department the following year (February 12, 2018) for shoulder pain. Laboratory evaluation showed improvement in his renal function with a creatinine of 2.07 mg/dL and urinalysis with 100 mg/dL of protein.
DISCUSSION
Renal manifestations associated with rickettsial infection—especially when severe—are well established. During Rocky Mountain spotted fever (RMSF), the most severe rickettsiosis (caused by Rickettsia rickettsii), kidney injury occurs as a result of hypovolemia-induced prerenal azotemia, and when hypotension is prominent, acute tubular necrosis develops.2–5 The renal pathology associated with infection with the genus Rickettsia has been well described in fatal RMSF, where histologic analysis revealed perivascular interstitial nephritis associated with multifocal vasculitis of peritubular capillaries and venules (mostly in the outer medulla and corticomedullary junction). These pathologic findings correlate with the distribution of rickettsiae within the tissue.2
Although murine typhus is less severe than RMSF (untreated case fatality rate in the United States of approximately 1% versus 20–30%, respectively),6,7 renal manifestations have also been described. Abnormalities in the urinalysis include mild proteinuria and microhematuria, but the incidence of these findings, when urinalysis is performed, is variable. In a case series describing murine typhus in children, elevated blood urea nitrogen-to-creatinine ratio was noted, but neither elevations in creatinine nor abnormalities on urinalysis were described.8 Despite the mild proteinuria described in some cases of murine typhus,5 glomerular lesions have not been detected histologically,2,5 and to our knowledge, significant glomerular disease has not been previously linked to murine typhus or other rickettsioses. Severe renal failure—at times necessitating renal replacement therapy—as a result of murine typhus has been described,3,4,9 but with the exception of those who died with other severe manifestations (e.g., encephalitis and pneumonitis), recovery of renal function is the usual outcome.3,4,10
African Americans have a higher prevalence of chronic kidney disease than Americans of European descent. Genetic factors driving this excessive burden of renal disease, including collapsing FSGS, have been linked to genetic variations in APOL1, which are particularly common in African Americans (present in approximately 34%).11,12 The aforementioned G1 and G2 alleles of APOL1 represent a pair of missense variants, corresponding to two amino acid substitutions, and a six base pair deletion, corresponding to the deletion of two amino acids, respectively.13,14 Wild-type APOL1 is a serum factor that lyses Trypanosoma brucei brucei, but the agent of East African trypanosomiasis, Trypanosoma brucei rhodesiense, is not adversely affected. Interestingly, APOL1 with the G1 and G2 alleles exhibits lytic activity against T. b. rhodesiense.14 Thus, despite the predisposition for chronic kidney disease, there may have been a selective advantage for these alleles in areas where T. b. rhodesiense infection is endemic.
In the setting of the risk variant APOL1, it has been proposed that a physiologic stress may be necessary to trigger the cascade of events that cause renal disease. Induction of inflammatory cytokines (e.g., tumor necrosis factor-α and interferon-γ [IFN-γ ]) during the course of infection may promote this process.15 Indeed, the collapsing variant of FSGS has been described in association with a number of infections: HIV, hepatitis B, hepatitis C, cytomegalovirus, parvovirus B19, tuberculosis, Campylobacter enteritis, lymphatic filariasis, and visceral leishmaniasis.16 A recent report from Brazil revealed a small cohort with collapsing FSGS in those infected with Zika and dengue viruses (albeit high-risk APOL1 variants were not found among these patients).17 Finally, collapsing FSGS has been described in a cohort following the therapeutic use of IFN-α, -β, and -γ.18 Follow-up analysis revealed that all in this cohort had the high-risk APOL1 genotype, and in vitro experiments demonstrated that antiviral pathways are important in the induction of APOL1-associated nephropathy.19 The full mechanisms of podocyte injury in collapsing FSGS related to APOL1 variants are yet to be elucidated.15
As documented in both humans and animal models, infection with R. typhi is associated with elevated IFN-γ.20,21 The mean value of IFN-γ in those with murine typhus has been reported to be approximately 600 pg/mL (during the acute phase of illness).21 To compare with two other prevalent undifferentiated febrile illnesses, dengue and malaria, mean IFN-γ levels have been reported to be approximately 25 pg/mL and 231 pg/mL, respectively.22,23 Production of this cytokine is crucial to the clearance of rickettsial organisms and development of a protective immune response.20,24 We hypothesize that the patient’s genetic predisposition, in the setting of elevated cytokines (e.g., IFN-γ) as a result of murine typhus, led to the development of collapsing FSGS. In addition to infectious syndromes already linked to the development of collapsing FSGS, other infections, such as that demonstrated in this case of murine typhus, should be considered as possible triggers in those genetically susceptible.
Acknowledgments:
We would like to thank David Walker for his thoughtful review and Jon Wilson of Arkana Laboratories for assisting with APOL1 variant testing.
REFERENCES
- 1.Civen R, Ngo V, 2008. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis 46: 913–918. [DOI] [PubMed] [Google Scholar]
- 2.Walker DH, Mattern WD, 1979. Acute renal failure in Rocky Mountain spotted fever. Arch Intern Med 139: 443–448. [PubMed] [Google Scholar]
- 3.Hernandez Cabrera M, Angel-Moreno A, Santana E, Bolanos M, Frances A, Martin-Sanchez MS, Perez-Arellano JL, 2004. Murine typhus with renal involvement in Canary Islands, Spain. Emerg Infect Dis 10: 740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal T, Michaeli D, 1977. Murine typhus and spotted fever in Israel in the seventies. Infection 5: 82–84. [DOI] [PubMed] [Google Scholar]
- 5.Shaked Y, Shpilberg O, Samra Y, 1994. Involvement of the kidneys in Mediterranean spotted fever and murine typhus. Q J Med 87: 103–107. [PubMed] [Google Scholar]
- 6.Stuart BM, Pullen RL, 1945. Endemic (murine) typhus fever: clinical observations of 180 cases. Ann Intern Med 23: 520–536. [Google Scholar]
- 7.Hattwick MA, Retailliau H, O'Brien RJ, Slutzker M, Fontaine RE, Hanson B, 1978. Fatal Rocky mountain spotted fever. JAMA 240: 1499–1503. [PubMed] [Google Scholar]
- 8.Whiteford SF, Taylor JP, Dumler JS, 2001. Clinical, laboratory, and epidemiologic features of murine typhus in 97 Texas children. Arch Pediatr Adolesc Med 155: 396–400. [DOI] [PubMed] [Google Scholar]
- 9.Silpapojakul K, Chayakul P, Krisanapan S, Silpapojakul K, 1993. Murine typhus in Thailand: clinical features, diagnosis and treatment. Q J Med 86: 43–47. [PubMed] [Google Scholar]
- 10.Whelton A, Donadio JV, Jr., Elisberg BL, 1968. Acute renal failure complicating rickettsial infections in glucose-6-phosphate dehydrogenase-deficient individuals. Ann Intern Med 69: 323–328. [DOI] [PubMed] [Google Scholar]
- 11.Genovese G, Friedman DJ, Pollak MR, 2013. APOL1 variants and kidney disease in people of recent African ancestry. Nat Rev Nephrol 9: 240–244. [DOI] [PubMed] [Google Scholar]
- 12.Pollak MR, 2014. Familial FSGS. Adv Chronic Kidney Dis 21: 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzur S, et al. 2010. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genovese G, et al. 2010. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K, 2016. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transpl 31: 349–358. [DOI] [PubMed] [Google Scholar]
- 16.Dettmar AK, Oh J, 2016. Infection-related focal segmental glomerulosclerosis in children. Biomed Res Int 2016: 7351964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araujo SA, Cordeiro TME, Belisario AR, Araujo RFA, Marinho PES, Kroon EG, de Oliveira DB, Teixeira MM, Simoes ESAC, 2019. First report of collapsing variant of focal segmental glomerulosclerosis triggered by arbovirus: dengue and Zika virus infection. Clin Kidney J 12: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markowitz GS, Nasr SH, Stokes MB, D'Agati VD, 2010. Treatment with IFN-α, -β, or -γ is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols B, et al. 2015. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caro-Gomez E, Gazi M, Cespedes MA, Goez Y, Teixeira B, Valbuena G, 2014. Phenotype of the anti-Rickettsia CD8(+) T cell response suggests cellular correlates of protection for the assessment of novel antigens. Vaccine 32: 4960–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauch J, Eisermann P, Noack B, Mehlhoop U, Muntau B, Schafer J, Tappe D, 2018. Typhus group rickettsiosis, Germany, 2010–2017(1). Emerg Infect Dis 24: 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priyadarshini D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S, Mokashi N, Vaidya D, Shah PS, Cecilia D, 2010. Clinical findings and pro-inflammatory cytokines in dengue patients in western India: a facility-based study. PLoS One 5: e8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tangteerawatana P, Pichyangkul S, Hayano M, Kalambaheti T, Looareesuwan S, Troye-Blomberg M, Khusmith S, 2007. Relative levels of IL4 and IFN-gamma in complicated malaria: association with IL4 polymorphism and peripheral parasitemia. Acta Trop 101: 258–265. [DOI] [PubMed] [Google Scholar]
- 24.Walker DH, Popov VL, Feng HM, 2000. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab Invest 80: 1361–1372. [DOI] [PubMed] [Google Scholar]