Abstract
Objective
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors known to produce and secrete high levels of circulating catecholamines and their metabolites. The biochemical characteristics of these tumors can be used to divide them into three major phenotypes. The adrenergic, noradrenergic and dopaminergic phenotypes are defined by predominant elevations in epinephrine and metanephrine, norepinephrine and normetanephrine, and dopamine and 3-methoxytyramine, respectively. There are over 15 well-identified tumor-susceptibility genes responsible for approximately 40% of the cases. The objective of this review article is to outline specific genotype/biochemical phenotype relationships.
Methods
Literature review.
Results
None.
Conclusion
Biochemical phenotype of PPGL is determined by the underlying genetic mutation and the associated molecular pathway. Identification of genotype/biochemical relationships is valuable in prioritizing testing for specific genes, making treatment decisions and monitoring disease progression.
INTRODUCTION
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors that arise from either chromaffin cells of the adrenal medulla or their neural crest progenitors located outside of the adrenal gland, respectively. Sympathetic PPGLs are chromaffin-positive tumors, known to frequently produce considerable amounts of catecholamines, and in approximately 80% of patients, they are found in the adrenal medulla (1,2). Nearly 20% of these tumors are located outside of the adrenal glands, in the prevertebral and paravertebral sympathetic ganglia of the chest, abdomen, and pelvis (1). In contrast, most parasympathetic PPGLs are chromaffin-negative tumors, only 4% of which secrete catecholamines and are confined to the regions of the head and neck (2).
Approximately half of adrenal PPGLs have been shown to produce norepinephrine almost exclusively, whereas the other half produces both epinephrine and norepinephrine in varying quantities (3–5). Based on biochemical secretory patterns, PPGLs can be characterized into three different phenotypes. PPGLs with the noradrenergic phenotype predominantly produce norepinephrine, whereas those with the adrenergic phenotype predominantly produce epinephrine (3,6,7). A third, rare biochemical phenotype is composed of PPGLs mainly producing dopamine (8–12).
Due to their highest diagnostic sensitivity among other biochemical methods, it is now widely established that measurements of catecholamine metabolites, plasma free metanephrines, or urinary fractionated metanephrines are the recommended initial tests (13–15). These results, in addition to plasma 3-methoxytyramine (3-MT), can be used to accurately establish the biochemical phenotype of the tumor (10–12). Neglecting the secretory status of these tumors predisposes patients to serious and potentially life-threatening cardiovascular complications due to catecholamine excess, including severe hypertension, acute myocardial infarction, cardiac arrhythmias, pulmonary edema, heart failure due to toxic cardiomyopathy, and shock (16). Nearly 40% of PPGLs have been attributed to germline mutations in over 15 different tumor-susceptibility genes, making these tumors a highly heritable form of neoplasia (17–21). Given this high frequency of genetic mutations, all patients with PPGLs should undergo genetic screening (22). Patients with a positive family history, young age at diagnosis, bilateral adrenal PPGLs, and metastatic or multifocal PPGLs have a high likelihood of a hereditary etiology (22).
The clinical practice guidelines summarized decisional algorithms for genetic screening and suggested targeted genetic testing based on specific clinical features (22). However, with genetic research advancing rapidly, next-generation sequencing of targeted gene panels has now become the recommended method of genetic screening in PPGL patients (23). While this technique is now more affordable and is being increasingly adopted at advanced health care institutions, limitations regarding access to advanced technology and insurance-specific health care coverage still exist worldwide. In such scenarios, careful analysis of biochemical results combined with other clinical features become critical in prioritizing specific genes in PPGL patients. This review summarizes established research and provides an update on specific genotype/biochemical phenotype relationships in PPGLs.
BIOCHEMICAL PHENOTYPES
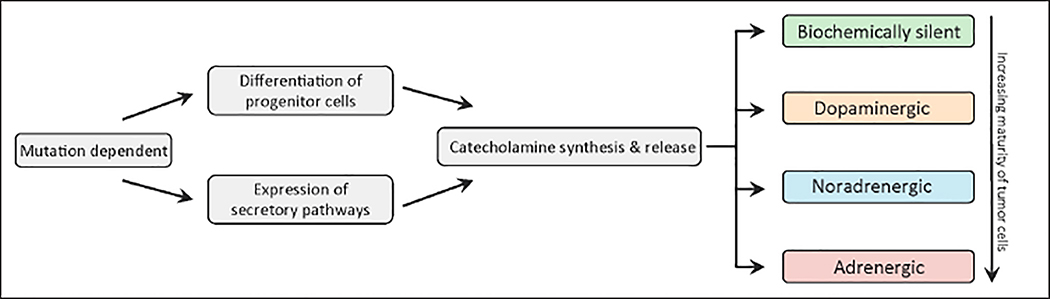
Patients with PPGLs present with a wide variety of nonspecific symptoms, which makes it challenging for physicians to make a timely diagnosis. The clinical presentation is determined by the biochemical profile of the tumor. A majority of patients exhibit typical manifestations of catecholamine excess, including either sustained or paroxysmal episodes of hypertension, sweating, headache, anxiety, and palpitations (2,24). In addition, patients may also present with pallor, flushing, nausea, vomiting, tremors, and fatigue (2,24). Symptoms may be precipitated due to several external factors, including a tyramine-rich diet; use of specific drugs such as histamine, monoamine oxidase inhibitors, and tricyclic antidepressants; anesthesia and diagnostic procedures; and less frequently, anxiety (25). In 10 to 20% of cases, however, patients may be entirely asymptomatic, with PPGLs discovered as adrenal incidentalomas on imaging studies (24). Variable expression of biosynthetic enzymes, highly influenced by the underlying mutation, leads to profound differences in the types and amounts of catecholamines being produced (5). This is accomplished by mutation-dependent differentiation of progenitor cells, which regulates the expression of enzymes involved in the synthesis of catecholamines. Moreover, regulatory and constitutive secretory pathways, which are also genotype dependent, contribute to variations in the catecholamine content displayed by the tumors (26). These characteristic behaviors result in three distinct biochemical phenotypes: noradrenergic, adrenergic, and dopaminergic, which are defined by predominant elevations in epinephrine, norepinephrine, and dopamine, respectively. In addition, there is also an extremely rare subset of PPGLs that do not produce and secrete catecholamines at all and are referred to as biochemically silent. Pathways for the synthesis of catecholamines and their metabolism are shown in Figure 1 A and B, respectively (25). In patients with PPGLs, metabolism of catecholamines within tumor cells accounts for more than 93% of elevated levels of free metanephrine and normetanephrine in the plasma (27). The intratumoral production and release of metanephrines occurs continuously and independently of catecholamine secretion (27), making them highly reliable disease markers. As suggested in preliminary studies, tumor progenitor cells which give rise to PPGLs with the adrenergic phenotype may be more differentiated than the noradrenergic phenotype, which in turn, may be more differentiated than the dopaminergic phenotype (Fig. 2) (6,28).
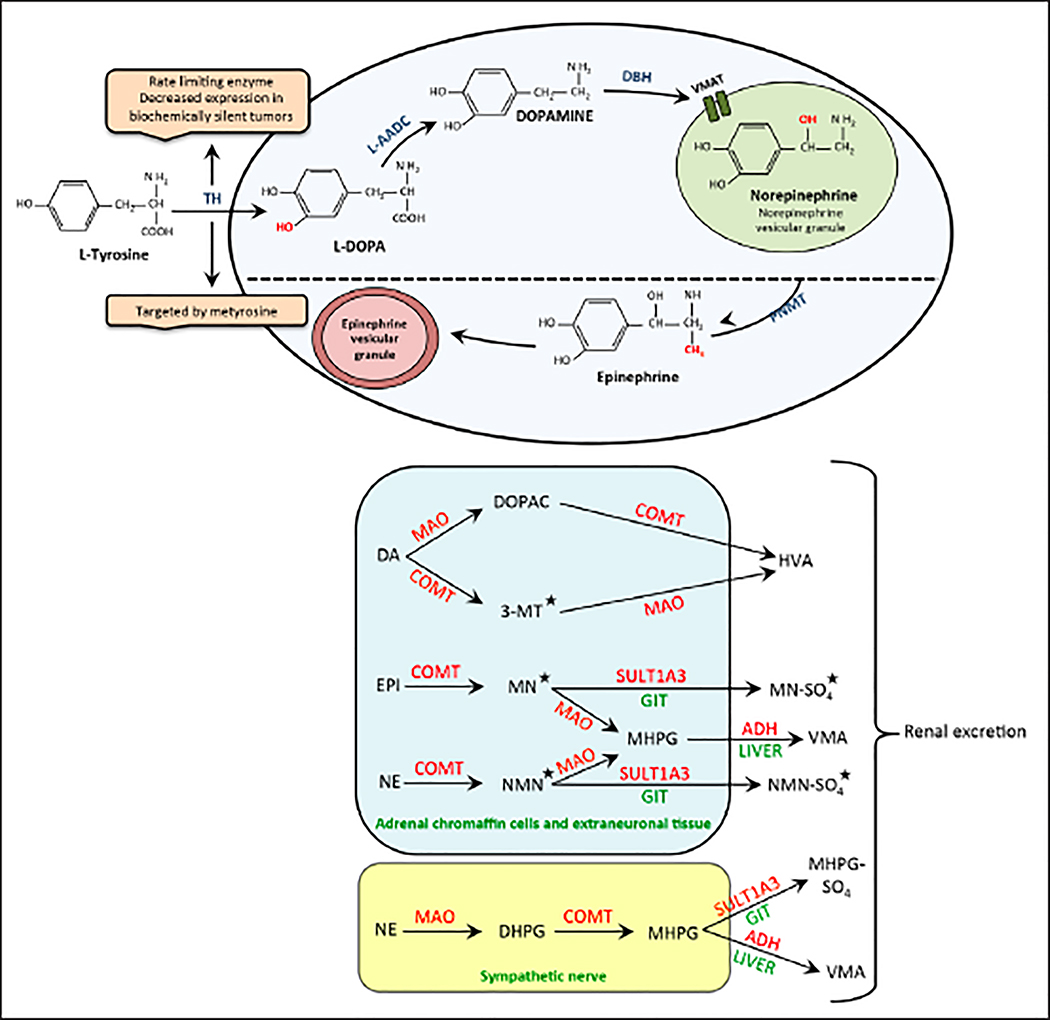
Fig. 1.
(A) Biosynthetic pathway of catecholamines. Norepinephrine vesicular storage granules are found in noradrenergic neurons and chromaffin cells of the adrenal medulla. Norepinephrine from these granules can passively leak back into the cytoplasm and be converted into epinephrine by the enzyme phenylethanolamine N-methyltransferase (PNMT). (B) Main pathways of catecholamine metabolism. Most of the norepinephrine (NE) released into the synaptic cleft translocates back into the neurons to either be sequestered in vesicles or metabolized. The remaining circulating NE is acted upon by the enzyme catechol-O-methyltransferase (COMT), restricted to extraneuronal tissues. Epinephrine (EPI) is mainly metabolized by COMT in the chromaffin cells of the adrenal medulla. All catecholamine metabolites, apart from vanillylmandelic acid (VMA) and homovanillic acid (HVA), are sulfate-conjugated by sulfotransferase type 1A3 (SULT1A3) in the gastrointestinal tract wall (GIT) prior to excretion. *Key metabolites that serve as disease markers. ADH = alcohol dehydrogenase; DA = dopamine; DBH = dopamine β-hydroxylase; DHPG = 3,4-dihydroxyphenylglycol; DOPAC = 3,4-dihydroxyphenylacetic acid; L-AADC = L-amino acid decarboxylase; MAO = monoamine oxidase; MHPG = 3-methoxy-4-hydroxyphenylglycol; MHPG-SO4 = 3-methoxy-4-hydroxyphenylglycol sulfate; MN = metanephrine; MN-SO4 = metanephrine sulfate; 3-MT = 3-methoxytyramine; NMN = normetanephrine; NMN-SO4 = normetanephrine sulfate; PNMT = phenylethanolamine N-methyltransferase; TH = tyrosine hydroxylase; VMAT = vesicular monoamine transporter.
Fig. 2.
Illustration of how a genetic mutation influences the biochemical phenotype of a tumor.
There is a positive correlation between tumor size and plasma and urinary concentrations of metanephrines, which is stronger than the positive relationship between tumor size and plasma or urinary catecholamine levels (4). Similarly, patients with metastatic or multifocal disease show a positive trend in the levels of catecholamines and metanephrines relative to the tumor burden (unpublished findings). Noradrenergic tumors present with larger size-dependent elevations in plasma and urinary catecholamines compared to adrenergic tumors (4). To our knowledge, no studies comparing genotype-specific variations in correlations between tumor size or burden and biochemical profiles have been published. A linear correlation is also seen between plasma chromogranin A levels and tumor mass (29).
Chromogranin A, a marker of neuroendocrine tumors, is stored in granules in the adrenal medulla and is released together with catecholamines from the tumor (30). Measurements of this secretory protein increase the sensitivity of plasma metanephrines in diagnosing patients with PPGLs, who do not have renal insufficiency (29,31). However, causes of iatrogenic elevations, such as the use of proton pump inhibitors or H2 blockers, should be ruled out before this is used as a reliable disease marker. In addition to being elevated in functional PPGLs (29,31), chromogranin A may also be secreted from nonfunctional tumors that do not produce or secrete catecholamines and their metabolites (32). Therefore, this protein is beneficial in not only the initial diagnosis of PPGLs but also as a marker of disease progression in biochemically silent tumors (32).
The action of different catecholamines on specific neurochemical receptors results in distinct clinical manifestations, which become targets of therapeutic blockade for symptomatic management and pre-operative preparation of patients undergoing surgery. Most benign and isolated malignant PPGLs can be resected through laparoscopic surgery (33). Some of these cases need conversion to an open procedure due to large tumor size or adherence to adjacent structures. Pre-operative optimization of patients’ hemodynamic status with α- and β-adrenoceptor blockade is crucial in order to reduce the risk of intra-operative and postoperative cardiovascular complications (2,22,34). β-Adrenoceptor blockade, when indicated to control tachyarrhythmia, should always be administered after adequate α-adrenoceptor blockade in order to prevent the unopposed action of epinephrine and norepinephrine on vascular α1-adrenoceptors, which may result in catastrophic hypertensive crisis (2,22,34). Moreover, calcium channel blockers and metyrosine, a competitive inhibitor of tyrosine hydroxylase (TH), can be used for further hemodynamic stabilization (22,34–36). Using metyrosine pre-operatively reportedly decreases intra-operative hemodynamic lability and postoperative cardiovascular complications (35,36). This drug may be particularly effective for patients with extremely elevated catecholamine levels and extensive metastatic disease with intractable signs and symptoms (34,37).
The Noradrenergic Phenotype
The noradrenergic phenotype comprises PPGLs that predominantly produce norepinephrine and are therefore characterized by elevated norepinephrine and normetanephrine levels (38). These tumors have an insignificant increase in epinephrine and metanephrine levels, in most cases, <5% over the total level of normetanephrine and metanephrine (4). Thus, this phenotype is most accurately determined based on the measurement of plasma free normetanephrine levels. While these tumors are primarily located in in extraadrenal areas, they may also be found within the adrenal glands (4). In contrast to the paroxysmal symptoms caused by the highly potent hormone epinephrine (39), these tumors that produce and secrete mainly norepinephrine are commonly associated with a decreased frequency of signs and symptoms related to catecholamine excess and present more commonly with nonepisodic hypertension (6).
Norepinephrine exerts its cardiovascular effects by working mainly on α1-adrenoceptors in the vasculature, with low activity on cardiac β1-adrenoceptors (Table 1). The action of norepinephrine on α1-adrenoceptors causes continuous vasoconstriction, resulting in sustained hypertension, the most common clinical manifestation of noradrenergic PPGLs (40,41). In a case series of 8 patients undergoing laparoscopic adrenalectomy, Col et al (33) reported greater intra-operative hemodynamic instability in patients with noradrenergic than adrenergic PPGLs. Peaks in blood pressure were attributed to an accelerated release of norepinephrine from the tumor (33), which may be precipitated by surgical manipulation, acute intratumoral hemorrhage or necrosis, and when there is an increase in intra-abdominal pressure (40). Reportedly, these patients may also rarely present with orthostatic hypotension (42) resulting from downregulation of α1-adrenoceptors due to chronically elevated norepinephrine levels, cosecretion of epinephrine from the tumor, excessive α-adrenoceptor blockade, or intravascular volume depletion.
Table 1.
Biochemical Phenotypes, Their Associated Clinical Presentations, and Recommended Therapies
| Biochemical phenotype | Primary receptor stimulated | Signs and symptoms | Clinical complications | Therapeutic approaches |
|---|---|---|---|---|
| Noradrenergic | α1 and β1 | Sustained HTN; Constipation; Sweating; Headache | Hypertensive crisis; Hypertensive encephalopathy; TIA and CVA; Intestinal ischemia; Optic neuropathy and retinopathy; Renal failure; Rhabdomyolysis | α-Adrenoceptor blockade; β-Adrenoceptor blockadea (for tachyarrhythmia); Calcium channel blockers;b Metyrosinec |
| Adrenergic | β2, α1 and α2 | Paroxysmal HTN; Palpitations; Anxiety; Diaphoresis; Flushing; Hyperglycemia; Hyperlipidemia | Severe hypotension; Hypertensive crisis; Cardiogenic shock | α- and β-Adrenoceptor;a Calcium channel blockers;b Metyrosinec |
| Dopaminergic | D1 and D2 | Usually asymptomatic; Hypotension; Diarrhea; Nausea and vomiting | Dehydration | Calcium channel blockers |
| Biochemically silent | -- | Asymptomatic | Possible hypertensive crisis during surgery | α- and/or β-Adrenoceptor blockade pre-operatively;d Calcium channel blockersd |
Abbreviations: CVA = cerebrovascular accident; HTN = hypertension; TIA = transient ischemic attack.
α-Adrenoceptor blockade should always be initiated before β-adrenoceptor blockade to prevent the unopposed action of catecholamines on α1-adrenoceptors.
Used for additional blood pressure control, in patients with intermittent hypertension, or those with adverse effects from α-adrenoceptor blockade.
Used for additional blood pressure control in patients with excessive catecholamines and metastatic disease. Metyrosine is also known as α-methyl-l-tyrosine or Demser.
There are conflicting views on the use of this therapy pre-operatively.
Goswami et al (43) reported a case of hypertensive encephalopathy that was attributed to sustained peripheral hypertension, resulting in an impaired autoregulatory response in the cerebral circulation. Additionally, anecdotal evidence suggests that norepinephrine can cause vasospasms of the cerebral circulation, resulting in either a transient ischemic episode or stroke (44). The vasoconstrictory effect of norepinephrine can also reportedly occur in ocular, gastrointestinal, and renal circulation, causing retinopathy and optic neuropathy, renal artery stenosis that may result in renal failure and intestinal necrosis and ischemia, respectively (41,45,46). Moreover, decreased blood supply to the skeletal muscles may cause rhabdomyolysis, resulting in further renal damage by causing myoglobinuria, which may lead to acute tubular necrosis (47). Apart from cardiovascular damage, norepinephrine significantly hampers intestinal motility, causing a decrease in peristalsis that results in constipation, sometimes intractable, and pseudo-obstruction, especially in patients with metastatic disease (48,49). Anecdotal evidence suggests that the increased intraluminal pressure may lead to bowel fissures, ulceration, bleeding, and ultimately, perforation, which can cause life-threatening abdominal sepsis (48). Furthermore, bowel necrosis caused by vasospasms in the intestinal circulation may also contribute to altered intestinal motility, increasing the likelihood of developing perforation (49).
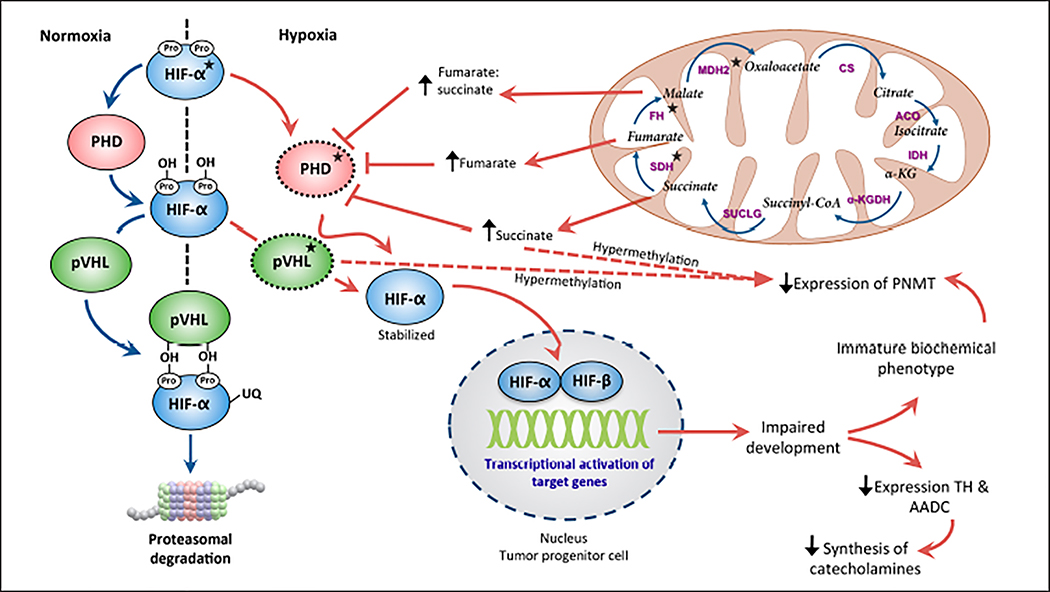
A typical noradrenergic phenotype is suggestive of mutations in the tumor suppressor von Hippel-Lindau (VHL) in VHL syndrome, succinate dehydrogenase (SDH) type A, B, C, or D, fumarate hydratase (FH), malate dehydrogenase, and endothelial pas domain protein 1/hypoxiainducible factor type 2A (EPAS1/HIF2A) genes (Table 2) (6,38,50–62). The pathogenesis of PPGLs harboring these mutations is characterized by a (pseudo)hypoxic signature and is referred to as cluster 1. Succinate dehydrogenase complex assembly factor 2 (SDHAF2)-mutated PPGLs have also been identified as part of cluster 1 (63). However, only limited evidence has been published on their biochemical characteristics in order to accurately classify them (63–65). This cluster involves stabilization of HIF, which is a transcription factor that mediates the hypoxia signaling cascade (Fig. 3) (66). The HIF-α subunit is regulated by cellular oxygen concentrations, and under normoxic conditions, it is hydroxylated by prolyl hydroxylase domain (PHD) proteins to be recognized by the VHL tumor suppressor protein for degradation (66). However, under hypoxic conditions, the expression of the HIF-α subunit is upregulated (66). HIF-α forms a heterodimer with HIF-β and binds to hypoxiaresponsive elements (66). This binding induces inappropriate transcription of target genes involved in tumorigenesis, leading to impaired development of tumor progenitor cells (66). Additionally, this pathway is linked to SDHx-mutated PPGLs via succinate, which when accumulated, competitively inhibits the action of PHDs and in turn, activates the HIF signaling pathway (67).
Table 2.
Genotype-Biochemical Phenotype Relationship
| Gene | Transmission | Biochemical profile | Most common PPGL locations | Bilateral PHEO | Malignancy | Most common clinical features/tumors |
|---|---|---|---|---|---|---|
| EPAS/HIF2A | Unknown | NE | Multifocal sPGLs | Possible | 29% | Somatostatinoma; polycythemia; ocular lesions |
| FH | AD | NE | Adrenal PPGL sPGL | Possible | 3/8 cases reported | Cutaneous and uterine leiomyoma; type 2 papillary RCC |
| MAX | AD, paternal | Intermediatea | Adrenal PPGL | 67% | 7–25% | Renal oncocytoma |
| MDH2 | AD | NE | sPGL | Unknown | Unknown | Unknown |
| NF1 | AD | EPI | Adrenal PPGL | 16% | ~12% | Cafè-au-lait spots; neurofibromas; axillary and inguinal skin freckling; optic gliomas; iris hamartomas; pseudoarthrosis |
| RET | AD | EPI | Adrenal PPGL | 50–80% | <5% | MEN2A: MTC, primary HPT MEN2B: MTC, Marfanoid habitus, mucosal ganglioneuromas |
| SDHA | AD | NE; NE + DA NS | sPGL HNPGL | Unknown | 0–14% | RCC; GIST; pituitary adenoma |
| SDHAF2 | AD, paternal | Unknowna | Multifocal HNPGLs | Not reported | Not reported | Not reported |
| SDHB | AD | NE; NE + DA NS | sPGL | Rare | 31–71% | RCC; GIST; pituitary adenoma; pulmonary chondroma |
| SDHC | AD | NS; DA NE; NE + DA | HNPGL sPGL | Unknown | 0–28% | RCC; GIST; pituitary adenoma |
| SDHD | AD, paternal | NE; NE + DA NS | sPGL HNPGL | Rare | <5% | RCC; GIST; pituitary adenoma; pulmonary chondroma |
| TMEM127 | AD | EPI | Adrenal PPGL | 33% | <5% | RCC |
| VHL | AD | NE | Adrenal PPGL | 50% | <5% | Hemangioblastoma; RCC; PNET; ELST |
Abbreviations: AD = autosomal dominant; DA = dopamine; ELST = endolymphatic sac tumor; EPI = epinephrine; GIST = gastrointestinal stromal tumor; HNPGL = head and neck paraganglioma; HPT = hyperparathyroidism; MTC = medullary thyroid carcinoma; NE = norepinephrine; NS = nonsecreting; PNET = pancreatic neuroendocrine tumor; PPGL = pheochromocytoma and/ or paraganglioma; RCC = renal cell carcinoma; sPGL = sympathetic paraganglioma.
Please refer to the text for additional detail.
Fig. 3.
Involvement of the hypoxia-inducible factor (HIF) signaling pathway and tricarboxylic acid (TCA) cycle substrates in determining the noradrenergic phenotype of the tumor. Mutations in genes encoding HIF-α, tumor suppressor von Hippel-Lindau (pVHL), succinate dehydrogenase (SDH), fumarate hydratase (FH), and malate dehydrogenase (MDH)2 lead to stabilization of HIF-α. TCA cycle substrate accumulation hypermethylates the promoter region of phenylethanolamine N-methyltransferase (PNMT) and decreases its expression. *Genes encoding these proteins are implicated in pheochromocytoma and paraganglioma pathogenesis. ACO = aconitase; CS = citrate synthase; IDH = isocitrate dehydrogenase; KGDH = ketoglutarate dehydrogenase; succinyl-CoA = succinyl coenzyme A; SUCLG = succinyl-CoA synthetase.
There is a crucial link between HIF-2α, an isoform of HIF-α expressed in neural crest derivatives, and the expression of biosynthetic enzymes such as TH and L-amino acid decarboxylase during the developmental stages of sympathoadrenal progenitor cells (68,69). Furthermore, immature progenitor cells with deregulated HIF-2α are more prone to tumorigenic influences such as impairment of apoptosis, leading to proliferation of poorly differentiated cells that do not express phenylethanolamine N-methyltransferase (PNMT) (70). Deficiency of key biosynthetic enzymes and impaired development of progenitor cells results in decreased synthesis of catecholamines and retention of an immature biochemical phenotype (6). Studies have demonstrated an epigenetic silencing pattern of the PNMT gene in patients with VHL and SDHx mutations. Hypermethylation of this gene’s promoter region results in markedly reduced levels of the PNMT enzyme (67,71). More specifically in SDHx mutations, accumulation of the oncometabolite succinate inhibits histone and DNA demethylases. This results in genomic hypermethylation, which provokes downregulation of genes such as PNMT implicated in chromaffin cell differentiation (71). Furthermore, independent epigenetic silencing of the PNMT gene has also been reported in sporadic noradrenergic PPGLs (72).
Further involvement of the tricarboxylic acid (TCA) cycle metabolites in the pathogenesis of PPGLs is evident by the discovery of loss-of-function mutations in the tumor suppressor FH and MDH2 (62,73). Accumulation of fumarate in patients with FH mutations inhibits the action of PHDs and leads to activation of the HIF signaling pathway (73). Additionally, PPGLs deficient in FH and MDH2 display transcriptional profiles and epigenetic hypermethylation patterns similar to SDHx-mutated PPGLs (62,71,73). Therefore, similarities in the tumorigenic mechanisms and the epigenetic remodeling profile associated with genetic mutations in this cluster result in downregulation of genes involved in progenitor cell differentiation (62,71,73).
VHL
PPGLs associated with VHL mainly produce norepinephrine and have solitary increases in normetanephrine levels, regardless of their proximity to the adrenal cortex (6,38,50). Evidence suggests that there are similarities in the genetic expression profiles seen in patients with noradrenergic sporadic PPGLs and in patients with VHL (7). In addition, there is a constant secretion of catecholamines that is unresponsive to secretagogues in PPGLs with a VHL mutation, pointing towards a poorly differentiated constitutive secretory pathway (26). This secretory behavior also explains the lack of cyclical symptoms in noradrenergic PPGLs.
SDH complex subunits A, B, C, and D (SDHA/B/C/D)
The SDH gene encodes a mitochondrial enzyme complex called succinate dehydrogenase, a key component of the TCA cycle, which catalyzes the conversion of succinate to fumarate (74). Based on case reports, patients with SDHA mutations present with noradrenergic adrenal and extra-adrenal PPGLs as well as biochemically silent head and neck paragangliomas (HNPGLs) (53,54). In our cohort of patients with SDHA mutations, we have noted aggressive behavior in both nonsecreting HNPGLs as well as noradrenergic extra-adrenal tumors, some of which also cosecrete dopamine (unpublished findings). A similar pattern has also been described in patients with SDHC mutations. To our best knowledge, only 25 patients with SDHC mutations have been reported worldwide (55–60). The majority of these patients present with HNPGLs, which may be nonfunctional, dopamine-secreting, or both dopamine- and norepinephrine-secreting (55–60). Additionally, noradrenergic extra-adrenal PPGLs, mainly located in the mediastinum, have also been reported (55–60). To our best knowledge, large studies regarding the utility of 3-MT as a tumor marker in patients with SDHA and SDHC have not been published.
The majority of patients with SDHB and SDHD mutations have elevations in normetanephrine levels, with over 67% also displaying high levels of plasma 3-MT (38,61). Adrenal PPGLs in patients with a SDHD mutation cause solitary increases in norepinephrine more frequently compared to HNPGLs (75). SDHD-associated HNPGLs may present with different biochemical patterns: biochemically silent, solely dopamine-secreting with elevated 3-MT/dopamine levels, solely norepinephrine-secreting, or norepinephrine-secreting with concurrent elevations in 3-MT (75). Most patients with SDHB and SDHD mutations reportedly present with hypertension as the primary manifestation of catecholamine overproduction (61,75). Space-occupying complications such as tinnitus and hearing loss in SDHD patients with HNPGLs and tumor-related pain and obstructive symptoms in SDHB patients are not uncommon (61,75).
EPAS1/HIF2A
A mutation in the EPAS1/HIF2A gene causes a defect in the proline residue at the hydroxylation site of HIF-2α, leading to its stabilization (51,52). Although initially considered to be a nonfamilial mutation, recent studies have shown the involvement of postzygotic mutational events that result in a widespread somatic mosaic pattern leading to variations in phenotypic expression and anecdotally may also be associated with germline mutations (76,77). Patients with a mutation in this gene present with significantly elevated plasma norepinephrine and normetanephrine levels owing to involvement of HIF-2α in catecholamine synthesis (51,52). The biochemical results and the presence of hypertension in these patients conform to the typical clinical picture characteristic of noradrenergic PPGLs.
FH and MDH2
FH is an enzyme in the TCA cycle that catalyzes the hydroxylation of fumarate to malate. The association between mutations in the FH gene and PPGLs was first identified in 2013, after which more gene analysis studies were performed and reported germline FH mutations in a total of eight cases worldwide (62,71,78). Castro-Vega et al (62) reported predominant normetanephrine elevations in 4 of 5 patients. We have noted similar findings in our cohort of patients with FH mutations, some of which also cosecrete dopamine (unpublished findings). Germline mutations in MDH2, which encodes the enzyme catalyzing the oxidation of malate to oxaloacetate, were also found to be a cause of PPGLs (73). In our cohort of patients with MDH2 mutations, we have noted a noradrenergic phenotype, similar to the biochemical phenotype resulting from mutations in other genes of this cluster (unpublished findings). Although much is yet to be discovered about the clinical aspects of PPGLs deficient in these enzymes, the similarities in the underlying tumorigenic mechanisms and epigenetic remodeling observed between the mutations in this cluster play an important role in the resultant biochemical phenotype.
SDHAF2
SDHAF2, encoded by the SDHAF2 gene, is required for flavination of the SDHA subunit, which is essential in forming a functional and stable SDH enzyme complex (63). Patients harboring a mutation in this gene most commonly present with multifocal HNPGLs (63–65). A study in 2014 identified for the first time 2 patients with SDHAF2 mutations and adrenal tumors (79). While one patient had higher normetanephrine than metanephrine levels, the other patient exhibited the reversed biochemical profile (79). To our knowledge, no other data have been published regarding the biochemical features of PPGLs in patients harboring a mutation in this gene (63–65). Therefore, the biochemical characteristics of patients with SDHAF2 mutations remain elusive. Therapeutic management for these patients should be based on their individual clinical presentation, cardiovascular status, and neurochemical characteristics.
Summary
Patients with normal metanephrine levels or elevated normetanephrine levels should undergo genetic screening for mutations in the aforementioned genes, especially if other syndromic features are absent. The location of PPGLs with the noradrenergic phenotype is typically extra-adrenal; however, they may also be limited only to the adrenal glands, especially in patients with VHL disease. With the adrenergic effects of norepinephrine resulting in hypertension being the key clinical manifestation, α-adrenoceptor blockade in these patients becomes the first line of therapy for both symptomatic and pre-operative management (13,34).
The Adrenergic Phenotype
PPGLs of the adrenergic phenotype are characterized by marked increases in metanephrine levels, representing an underlying predominant production and secretion of epinephrine (38). More specifically, the classification is predicated on an increase in the plasma level of free metanephrine, accounting for a minimum of 10% of the total plasma level increases in metanephrine and normetanephrine (4). Thus, this phenotype can be most accurately identified by measuring plasma metanephrine levels. Epinephrine, a highly potent circulating hormone, exerts its hemodynamic and metabolic effects by mainly acting on β2-adrenoceptors. Additionally, it exhibits a higher affinity for α1 and α2-adrenoceptors compared to norepinephrine (25).
Patients with PPGLs secreting mainly epinephrine more frequently have paroxysmal symptoms of hypertension, palpitations, anxiety, flushing, and sweating (39). This pattern has been hypothesized to be a result of rapid metabolism of circulating epinephrine and sophisticated secretory pathways associated with adrenergic PPGLs (39). These tumors are usually well differentiated, with mature exocytic machinery causing regulated secretion, as was displayed in predominantly epinephrine producing PPGLs associated with multiple endocrine neoplasia type 2 (MEN2) (26). The occurrence of hyperglycemia and hyperlipidemia associated with this phenotype is due to the diabetogenic effects of epinephrine on various enzymes involved in lipolysis, glycogenolysis, and gluconeogenesis (40).
Baxter et al (80) described one of the rare cases in which a patient with high levels of circulatory epinephrine developed profound hypotension and noncardiogenic shock. This was attributed to the vasodilatory effects of epinephrine, mediated through prominent β2-adrenergic stimulation (80). This was supported by the report of normal pulmonary capillary wedge pressure and cardiac output in the patient, representing normal cardiac function (80). Another mechanism of cardiovascular compromise in the adrenergic phenotype, based on anecdotal evidence, is through development of cardiogenic shock (81). This occurs most likely due to a compensatory downregulation of cardiac β-adrenoceptors in response to persistently elevated epinephrine levels and hypocalcemia, both of which lead to decreased cardiac contractility (81).
The adrenergic phenotype has been linked to the presence of an adrenal PPGL (82). Originally, this association was believed to be due to the increased expression and activation of the PNMT gene, stimulated in the presence of high glucocorticoid levels in the adjacent adrenal cortex. However, this mechanism did not provide an explanation for rare cases of extra-adrenal PPGLs, both primary and recurrent, to be producing epinephrine (4). An in vitro study by Carballeira et al (83) demonstrated the presence of enzymes involved in the biosynthesis of glucocorticoids in two extra-adrenal PPGLs, representing intratumoral production, which contributes to the adrenergic phenotype. Following this, Isobe et al (84) showed through further in vitro experiments that the presence of glucocorticoids may not be sufficient to yield the adrenergic phenotype if there is an absence of glucocorticoid receptors such as early growth response protein 1 (Egr-1) in the tumor tissue. However, this finding was not translated to a study conducted in patients with MEN2, who predominantly had adrenergic PPGLs and in patients with VHL, who predominantly had noradrenergic PPGLs (28). No significant differences were noted between the expressions of multiple glucocorticoid receptors, including Egr-1, in these two groups, suggesting a different underlying mechanism (28). Instead, it was shown that there is a higher expression of genes involved in maturation of neural crest progenitors in MEN2 compared to VHL tumors, favoring the hypothesis that the expression of the PNMT gene is derived from variations in differentiation of progenitor cells (28).
In addition to the rearranged during transfection (RET) proto-oncogene in MEN2, a typical adrenergic phenotype is also indicative of mutations in the neurofibromatosis type 1 (NF1) tumor-suppressor gene. Mutations in these genes are grouped together and referred to as cluster 2 because they cause activation of kinase signaling pathways, rat sarcoma oncogene/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase (i.e., the RAS/RAF/MAPK pathway) and phosphatidylinositol-3-kinase/RAC-alpha serine/threonine-protein kinase/mammalian target of rapamycin (i.e., the PI3K/AKT/mTOR pathway). Cluster 2 is also comprised of transmembrane protein 127 (TMEM127) and MYC-associated factor X (MAX), which encode other protein components of these cascades and were identified to be associated with PPGLs. Abnormal activation of these pathways leads to inhibition of apoptosis and promotes proliferation and tumor growth (85).
RET
MEN2 patients with PPGLs produce high amounts of epinephrine due to an increased expression of PNMT, which is also reflected in biochemical testing by high metanephrine levels (6,38). This is further supported by significant similarities observed in genetic expression profiles of sporadic adrenergic PPGLs and PPGLs in patients with MEN2 (7). Despite a high expression of TH and large amounts of tumor tissue catecholamines, 30 to 50% of patients with MEN2 do not exhibit symptoms of catecholamine excess (6). This may be attributed to specific secretagogue-dependent catecholamine release and tightly regulated mature secretory pathways (6,26). Furthermore, this nature of release also explains the characteristic paroxysmal clinical presentation of patients with adrenergic PPGLs.
NF1
Unlike other clinical aspects of this disease, PPGLs are usually diagnosed later, in the fifth decade of life, in 0.1 to 5.7% of patients with NF1 and in up to 50% of NF1 patients with hypertension (86). When feasible, the diagnosis of NF1 should be based on clinical diagnostic criteria, as the NF1 gene is challenging to sequence, owing to its large size. NF1-mutated PPGLs are characterized by pronounced elevations in metanephrine levels, representing an underlying production of epinephrine (38,70,87).
TMEM127
PPGLs with TMEM127 mutations have a transcriptional signature similar to PPGLs with mutations in the RET and NF1 genes (88,89). This finding correlates with the presence of predominantly high plasma metanephrine levels in patients with TMEM127 mutations, representing an underlying production of epinephrine (90,91).
MAX
The MAX gene impacts MYC/MAX dimerization, influencing numerous MYC downstream transcriptionally regulated pathways (92). In a study consisting of 23 patients with MAX mutations, the expression of PNMT and the levels of urinary metanephrine and tumor epinephrine (8.4%) were intermediate between the classic adrenergic and noradrenergic phenotypes (93). Patients reportedly present with at least 3-fold greater elevations in normetanephrine than metanephrine levels (93,94). Qin et al (95) classified MAX-mutated tumors as a subcluster within cluster 2 based on transcriptome profiling of genes involved in chromaffin cell differentiation. MAX-mutated PPGLs are rare and result in a distinct biochemical phenotype exhibiting larger increases in normetanephrine than metanephrine but intermediate increases in metanephrine levels, not typical of either the adrenergic or the noradrenergic phenotype (93,94).
Summary
Patients presenting with predominantly elevated levels of metanephrine should undergo genetic screening for RET and NF1 mutations. However, patients with these mutations are usually first diagnosed based on other syndromic features of the disease and may only require genetic testing to confirm the suspicion. Genetic screening for TMEM127 may be considered for adrenergic PPGLs once mutations in RET and NF1 have been ruled out. Since MAX-mutated tumors do not conform to the typical adrenergic phenotype, targeted genetic screening for this gene may be considered in cases of adrenal PPGLs when other susceptibility genes have been ruled out. Adrenergic PPGLs are typically located within the adrenal glands and are very rarely found in extra-adrenal locations. In addition to using α-adrenoceptor blockade, patients with adrenergic PPGLs should also be appropriately administered β-adrenoceptor blockade in order to block β1-adrenoceptor–mediated cardiac activity.
The Dopaminergic Phenotype
The dopaminergic phenotype is comprised of an extremely rare group of PPGLs that predominantly produce dopamine with negligible or only mild increases in the norepinephrine and epinephrine levels (9). Due to this secretory pattern, diagnostic measurements restricted to only plasma metanephrines fail to suggest the presence of a dopamine-producing PPGL and accurately indicate the neurochemical phenotype of the tumor. Therefore, the dopaminergic phenotype can be most accurately determined based on plasma dopamine and 3-MT levels (8,11,12). Nearly all of the dopamine in mammalian urine is formed in the renal cells from 3,4-dihydroxyphenylalanine derived from the circulation, making urinary dopamine levels unreliable markers of this phenotype (8). PPGLs of this subtype are mainly found in extra-adrenal locations and may be malignant, though adrenal tumors have also been reported (9,12). More specifically, HNPGLs, especially carotidbody tumors, more commonly produce dopamine, which is constantly metabolized into 3-MT (10,12,75). Therefore, in some cases, PPGLs only display high plasma 3-MT levels in the absence of elevated plasma dopamine (75).
In contrast to the classic symptoms of catecholamine excess seen with epinephrine- and norepinephrine-producing tumors, these tumors that produce and secrete mainly dopamine are most commonly asymptomatic or are associated with atypical manifestations (9). Patients have been reported to present with chronic diarrhea, nausea, vomiting, hypotension, weight loss, symptoms of large space-occupying lesions, such as pain with abdominal PPGLs and tinnitus and hearing loss with HNPGLs (9,12). This nonspecific clinical presentation may lead to a significant delay in diagnosis. Dopamine exerts its vasodilatory effects by working on D1 receptors located on vascular smooth muscle cells. Additionally, stimulation of D1-like receptors located in the gastrointestinal tract may be responsible for diarrhea seen in these patients (96). The presence of nausea and emesis may be related to the action of dopamine on the D2 receptors located in the central nervous system. This is supported by the cessation of these symptoms and the development of hypertension after tumor resection (9). The lack of hypertension and other associated symptoms can also be attributed to the relative absence of the production of norepinephrine and epinephrine in these patients. Moreover, this effect is accentuated by the stimulation of D2 receptors located on the presynaptic membranes of sympathetic ganglia, which inhibit the release of norepinephrine (40). This inhibition is unopposed after tumor resection and very rarely may result in rebound postoperative hypertension (97).
PPGLs of the dopaminergic phenotype have reduced levels of the enzyme dopamine β-hydroxylase, which results in dopamine accumulation and a decreased production of norepinephrine. This finding may be due to proliferation of poorly differentiated progenitor cells, as is seen in patients with metastatic disease and SDHB and SDHD mutations (98). Patients with these mutations show increases in dopamine and 3-MT levels in addition to elevations in normetanephrine levels (12,61,75). In a case series of 29 patients with SDHB mutations, only 1 patient with a para-adrenal PPGL was found to be exclusively dopamine secreting (61). The frequency of dopaminergic PPGLs in patients with a SDHD mutation remains unknown. In addition to being elevated in over two-thirds of patients with SDHB and SDHD mutations, 3-MT also serves as a marker of multifocal disease, extra-adrenal tumor location, and an increased risk of malignancy (11,38). To our best knowledge, no studies on correlations between 3-MT levels and the extent of metastatic PPGLs have been published. Recently, a case series of patients with SDHC mutations described 2 of 18 patients with exclusively dopamine secreting HNPGLs (56). Very rare cases of PPGLs in patients with NF1, VHL, and MEN2A displaying elevations in dopamine and/or 3-MT have also been reported (99–102).
Unlike adrenergic and noradrenergic PPGLs, patients with dopaminergic PPGLs who receive pre-operative α-adrenoceptor blockade reportedly develop severe hypotension and cardiovascular collapse both intra- and postoperatively (97). However, in the same series, 1 of 9 patients who did not receive pre-operative α-adrenoceptor blockade developed severe hypertension requiring intra-operative nicardipine (97). Three patients developed mild and transient hypertension with tumor manipulation (97). However, the basis of this observation is unclear. Although the Endocrine Society guidelines recommend monotherapy with calcium channels blockers in only certain situations (22), we have successfully used them as first-line therapy for pre-operative management of patients with dopamine-secreting tumors to ensure the highest patient safety at our institution (unpublished findings).
Biochemically Silent PPGLs
To our best knowledge, no large studies on the prevalence of biochemically silent PPGLs have been published. Based on the patient cohort at our institution and the available anecdotal evidence, biochemically silent PPGLs are an extremely rare subgroup and do not exhibit elevations in either catecholamines or metanephrines (103). Due to the absence of circulating catecholamines, patients do not present with symptoms of catecholamine excess, such as headache, palpitations, and sweating (103). Instead, the symptoms occur due to tumor mass effects, much later in the disease course (103).
These features are typical of isolated cases of HNPGLs, a large majority of which are seen in patients with SDHB and SDHD mutations (104). Of the 25 reported patients with SDHC-mutated tumors, nearly 50% presented with biochemically silent PPGLs, including both HNPGLs and mediastinal tumors (55–60). In addition, VHL, RET, NF1, SDHA, FH, and TMEM127 mutations have also been anecdotally associated with biochemically silent HNPGLs (54,62,64,65,91,105–108).
In 2008, Timmers et al (103) reported biochemically silent abdominal PPGLs in 4 patients with SDHB mutations. The tumor tissue from these patients demonstrated negligible levels of catecholamines due to the absence of the key biosynthetic enzyme, TH (103). The absence of TH and the inability to produce catecholamines was attributed to proliferation of immature neural crest progenitor cells that gave rise to the tumor (103). Due to negative biochemical testing and a lack of symptoms of catecholamine excess, there is a diagnostic delay, and these patients may present with metastatic disease (103). In the same series by Timmers et al (103), the plasma chromogranin A level remained consistently elevated and served as a disease marker. Further studies are needed regarding the utility of plasma chromogranin A as a tumor marker in biochemically silent PPGLs in patients with different genetic mutations. Despite a negligible amount of tissue and plasma catecholamines, patients with these PPGLs may develop hypertensive crisis both during induction of anesthesia and during the operation (109). Therefore, pre-operative α-adrenoceptor blockade (alone or in combination with β-adrenoceptor blockade) or calcium channel blockers may be considered with close monitoring of the cardiovascular status.
CONCLUSION
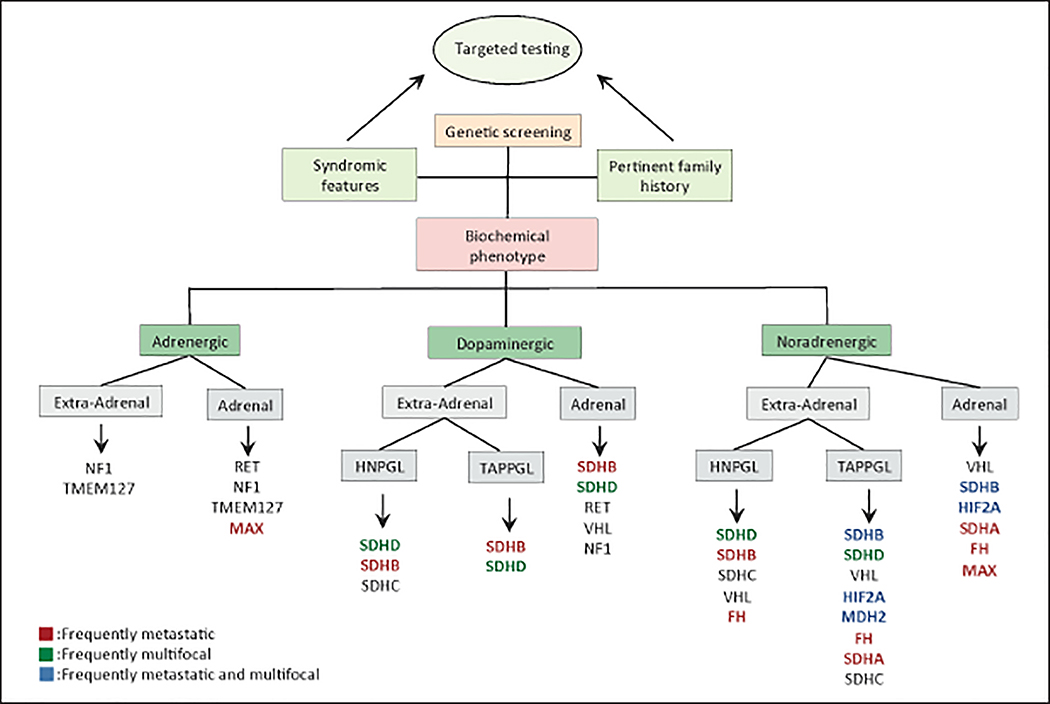
Biochemically active PPGLs are most accurately diagnosed and classified into different phenotypes based on the measurements of plasma metanephrines and urinary fractionated metanephrines in combination with plasma 3-MT levels. Biochemical properties of PPGLs are attributed to the underlying genetic mutation, which influences (i) differentiation of tumor progenitor cells, (ii) catecholamine biosynthetic enzymes, (iii) secretory pathways, and (iv) epigenetic remodeling profiles. Due to this association, specific genotype-biochemical phenotype relationships have been identified (Fig. 4). While next-generation sequencing of targeted gene panels has now become the recommended form of genetic screening, clinical feature-driven Sanger sequencing can be critical in making time-sensitive treatment decisions that are influenced by the presence or absence of specific genotype, such as SDHB. Additionally, identification of genotype-specific biochemical markers can be highly valuable in monitoring disease progression and determining treatment strategies.
Fig. 4.
Genotype-biochemical phenotype/location associations. Note: this chart may be used as a guide for time-sensitive genotype-dependent treatment options and targeted genetic screening when next-generation sequencing is not available. HNPGL = head and neck paraganglioma; TAPPGL = thoracic abdominal and pelvic paraganglioma.
ACKNOWLEDGMENT
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health. The authors would like to acknowledge the involvement of and feedback from the remaining members of the Adrenal Scientific Committee: Committee Chair, Dr. Karel Pacak; Dr. Aaron Vinik, Dr. Richard Auchus, Dr. Maria Fleseriu, Dr. Hans Ghayee, Dr. Amir Hamrahian, Dr. Christian Koch, Dr. Carl Malchoff, Dr. Barbra S. Miller, Dr. Phyllis Speiser, Dr. Anand Vaisya.
Abbreviations
- 3-MT
3-methoxytyramine
- EPAS1
endothelial pas domain protein 1
- FH
fumarate hydratase
- HIF2A
hypoxia inducible factor type 2A
- MEN2
multiple endocrine neoplasia type 2
- NF1
neurofibromatosis type 1
- PNMT
phenylethanolamine N-methyltransferase
- PPGL
pheochromocytoma and paraganglioma
- RET
rearranged during transfection
- SDH
succinate dehydrogenase
- SDHAF2
succinate dehydrogenase complex assembly factor 2
- TCA
tricarboxylic acid
- TH
tyrosine hydroxylase
- TMEM127
transmembrane protein 127
- VHL
von Hippel-Lindau
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001;134:315–329. [DOI] [PubMed] [Google Scholar]
- 2.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem. 2014;60:1486–1499. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhofer G, Lenders JW, Goldstein DS, et al. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin Chem. 2005;51:735–744. [DOI] [PubMed] [Google Scholar]
- 5.Kimura N, Miura Y, Nagatsu I, Nagura H. Catecholamine synthesizing enzymes in 70 cases of functioning and nonfunctioning phaeochromocytoma and extra-adrenal paraganglioma. Virchows Arch A Pathol Anat Histopathol. 1992;421:25–32. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhofer G, Walther MM, Huynh TT, et al. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86:1999–2008. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhofer G, Huynh TT, Pacak K, et al. Distinct gene expression profiles in norepinephrine- and epinephrineproducing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11:897–911. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhofer G, Goldstein DS, Sullivan P, et al. Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90:2068–2075. [DOI] [PubMed] [Google Scholar]
- 9.Dubois LA, Gray DK. Dopamine-secreting pheochromocytomas: in search of a syndrome. World J Surg. 2005;29:909–913. [DOI] [PubMed] [Google Scholar]
- 10.van Duinen N, Corssmit EP, de Jong WH, Brookman D, Kema IP, Romijn JA. Plasma levels of free metanephrines and 3-methoxytyramine indicate a higher number of biochemically active HNPGL than 24-h urinary excretion rates of catecholamines and metabolites. Eur J Endocrinol. 2013;169:377–382. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Horst-Schrivers AN, Osinga TE, Kema IP, Van Der Laan BF, Dullaart RP. Dopamine excess in patients with head and neck paragangliomas. Anticancer Res. 2010;30:5153–5158. [PubMed] [Google Scholar]
- 13.Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium October 2005 Nat Clin Pract Endocrinol Metab 2007;3:92–102. [DOI] [PubMed] [Google Scholar]
- 14.Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–1434. [DOI] [PubMed] [Google Scholar]
- 15.Lenders JW, Pacak K, Eisenhofer G. New advances in the biochemical diagnosis of pheochromocytoma: moving beyond catecholamines. Ann N Y Acad Sci. 2002;970:29–40. [DOI] [PubMed] [Google Scholar]
- 16.Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab. 2013;98:1100–1106. [DOI] [PubMed] [Google Scholar]
- 17.Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23:8812–8818. [DOI] [PubMed] [Google Scholar]
- 18.Bausch B, Borozdin W, Neumann HP, European-American Pheochromocytoma Study G. Clinical and genetic characteristics of patients with neurofibromatosis type 1 and pheochromocytoma. N Engl J Med. 2006;354:2729–2731. [DOI] [PubMed] [Google Scholar]
- 19.Welander J, Larsson C, Bäckdahl M, et al. Integrative genomics reveals frequent somatic NF1 mutations in sporadic pheochromocytomas. Hum Mol Genet. 2012;21:5406–5416. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. 2013;20:1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann HP, Bausch B, McWhinney SR, et al. Germline mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–1466. [DOI] [PubMed] [Google Scholar]
- 22.Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. [DOI] [PubMed] [Google Scholar]
- 23.NGS in PPGL Study Group, Toledo RA, Burnichon N, et al. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol 2017;13: 233–247. [DOI] [PubMed] [Google Scholar]
- 24.Kopetschke R, Slisko M, Kilisli A, et al. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol. 2009;161:355–361. [DOI] [PubMed] [Google Scholar]
- 25.Pacak K, Lenders JWM, Eisenhofer G. Pheochromocytoma: Diagnosis, Localization, and Treatment. Malden, MA: Oxford, Blackwell Pub.; 2007. [Google Scholar]
- 26.Eisenhofer G, Huynh TT, Elkahloun A, et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab. 2008;295:E1223–E1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhofer G, Keiser H, Friberg P, et al. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998;83:2175–2185. [DOI] [PubMed] [Google Scholar]
- 28.Huynh TT, Pacak K, Wong DL, et al. Transcriptional regulation of phenylethanolamine N-methyltransferase in pheochromocytomas from patients with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Ann N Y Acad Sci. 2006;1073:241–252. [DOI] [PubMed] [Google Scholar]
- 29.Giovanella L, Squin N, Ghelfo A, Ceriani L. Chromogranin A immunoradiometric assay in diagnosis of pheochromocytoma: comparison with plasma metanephrines and 123I-MIBG scan. Q J Nucl Med Mol Imaging. 2006;50:344–347. [PubMed] [Google Scholar]
- 30.Oconnor DT, Pandian MR, Cervenka J, Mezger MS, Parmer RJ. What is the source and disposition of chromogranin a (Cga) in normal human-plasma. Clin Res. 1987;35:A605–A605. [Google Scholar]
- 31.d’Herbomez M, Forzy G, Bauters C, et al. An analysis of the biochemical diagnosis of 66 pheochromocytomas. Eur J Endocrinol. 2007;156:569–575. [DOI] [PubMed] [Google Scholar]
- 32.Kimura N, Miura W, Noshiro T, et al. Plasma chromogranin A in pheochromocytoma, primary hyperparathyroidism and pituitary adenoma in comparison with catecholamine, parathyroid hormone and pituitary hormones. Endocr J. 1997;44:319–327. [DOI] [PubMed] [Google Scholar]
- 33.Col V, de Cannière L, Collard E, Michel L, Donckier J. Laparoscopic adrenalectomy for phaeochromocytoma: endocrinological and surgical aspects of a new therapeutic approach. Clin Endocrinol (Oxf). 1999;50:121–125. [DOI] [PubMed] [Google Scholar]
- 34.Pacak K Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069–4079. [DOI] [PubMed] [Google Scholar]
- 35.Wachtel H, Kennedy EH, Zaheer S, et al. Preoperative metyrosine improves cardiovascular outcomes for patients undergoing surgery for pheochromocytoma and paraganglioma. Ann Surg Oncol. 2015;22(suppl 3):S646–S654. [DOI] [PubMed] [Google Scholar]
- 36.Perry RR, Keiser HR, Norton JA, et al. Surgical management of pheochromocytoma with the use of metyrosine. Ann Surg. 1990;212:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchel O, Buu NT, Edwards DJ. Alternative catecholamine pathways after tyrosine hydroxylase inhibition in malignant pheochromocytoma. J Lab Clin Med. 1990;115:449–453. [PubMed] [Google Scholar]
- 38.Eisenhofer G, Lenders JW, Timmers H, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito Y, Fujimoto Y, Obara T. The role of epinephrine, norepinephrine, and dopamine in blood pressure disturbances in patients with pheochromocytoma. World J Surg. 1992;16:759–763; discussion 763–764. [DOI] [PubMed] [Google Scholar]
- 40.Pacak K Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am. 2011;40:295–311, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streeten DH, Anderson GH Jr. Mechanisms of orthostatic hypotension and tachycardia in patients with pheochromocytoma. Am J Hypertens. 1996;9:760–769. [DOI] [PubMed] [Google Scholar]
- 43.Goswami R, Tandon N, Singh B, Kochupillai N. Adrenal tumour, congestive heart failure and hemiparesis in an 18-year-old male. A clinical-pathological conference Int J Cardiol 1995;49:233–238. [DOI] [PubMed] [Google Scholar]
- 44.Lin PC, Hsu JT, Chung CM, Chang ST. Pheochromocytoma underlying hypertension, stroke, and dilated cardiomyopathy. Tex Heart Inst J 2007;34:244–246. [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiwara M, Imachi H, Murao K, et al. Improvement in renal dysfunction and symptoms after laparoscopic adrenalectomy in a patient with pheochromocytoma complicated by renal dysfunction. Endocrine. 2009;35:57–62. [DOI] [PubMed] [Google Scholar]
- 46.Petkou D, Petropoulos IK, Kordelou A, Katsimpris JM. Severe bilateral hypertensive retinopathy and optic neuropathy in a patient with pheochromocytoma. Klin Monbl Augenheilkd. 2008;225:500–503. [DOI] [PubMed] [Google Scholar]
- 47.Brouwers FM, Lenders JW, Eisenhofer G, Pacak K. Pheochromocytoma as an endocrine emergency. Rev Endocr Metab Disord. 2003;4:121–128. [DOI] [PubMed] [Google Scholar]
- 48.Karri V, Khan SL, Wilson Y. Bowel perforation as a presenting feature of pheochromocytoma: case report and literature review. Endocr Pract. 2005;11:385–388. [DOI] [PubMed] [Google Scholar]
- 49.Thosani S, Ayala-Ramirez M, Román-González A, et al. Constipation: an overlooked, unmanaged symptom of patients with pheochromocytoma and sympathetic paraganglioma. Eur J Endocrinol. 2015;173:377–387. [DOI] [PubMed] [Google Scholar]
- 50.Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999;340:1872–1879. [DOI] [PubMed] [Google Scholar]
- 51.Pacak K, Jochmanova I, Prodanov T, et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31:1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnichon N, Brière JJ, Libé R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korpershoek E, Favier J, Gaal J, et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96:E1472–E1476. [DOI] [PubMed] [Google Scholar]
- 55.Bickmann JK, Sollfrank S, Schad A, et al. Phenotypic variability and risk of malignancy in SDHC-linked paragangliomas: lessons from three unrelated cases with an identical germline mutation (p.Arg133*). J Clin Endocrinol Metab. 2014;99:E489–E496. [DOI] [PubMed] [Google Scholar]
- 56.Bourdeau I, Grunenwald S, Burnichon N, et al. A SDHC founder mutation causes paragangliomas (PGLs) in the French Canadians: new insights on the SDHC-related PGL. J Clin Endocrinol Metab. 2016;101:4710–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Else T, Marvin ML, Everett JN, et al. The clinical phenotype of SDHC-associated hereditary paraganglioma syndrome (PGL3). J Clin Endocrinol Metab. 2014;99:E1482–E1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isaacson B, Bullova P, Frone M, et al. An aggressive temporal bone SDHC paraganglioma associated with increased HIF-2alpha signaling. Endocr Pract 2016;22:190–195. [DOI] [PubMed] [Google Scholar]
- 59.Millar AC, Mete O, Cusimano RJ, et al. Functional cardiac paraganglioma associated with a rare SDHC mutation. Endocr Pathol. 2014;25:315–320. [DOI] [PubMed] [Google Scholar]
- 60.Zbuk KM, Patocs A, Shealy A, Sylvester H, Miesfeldt S, Eng C. Germline mutations in PTEN and SDHC in a woman with epithelial thyroid cancer and carotid paraganglioma. Nat Clin Pract Oncol. 2007;4:608–612. [DOI] [PubMed] [Google Scholar]
- 61.Timmers HJ, Kozupa A, Eisenhofer G, et al. Clinical presentations, biochemical phenotypes, and genotypephenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–786. [DOI] [PubMed] [Google Scholar]
- 62.Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23:2440–2446. [DOI] [PubMed] [Google Scholar]
- 63.Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bayley JP, Kunst HP, Cascon A, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–372. [DOI] [PubMed] [Google Scholar]
- 65.Kunst HP, Rutten MH, de Mönnink JP, et al. SDHAF2 (PGL2-SDH5) and hereditary head and neck paraganglioma. Clin Cancer Res. 2011;17:247–254. [DOI] [PubMed] [Google Scholar]
- 66.Kaelin WG Jr. Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutaratedependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castro-Vega LJ, Letouzé E, Burnichon N, et al. Multiomics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown ST, Kelly KF, Daniel JM, Nurse CA. Hypoxia inducible factor (HIF)-2 alpha is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem. 2009;110:622–630. [DOI] [PubMed] [Google Scholar]
- 69.Wiesener MS, Jürgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. [DOI] [PubMed] [Google Scholar]
- 70.Eisenhofer G, Pacak K, Huynh TT, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. [DOI] [PubMed] [Google Scholar]
- 72.Grouzmann E, Tschopp O, Triponez F, et al. Catecholamine metabolism in paraganglioma and pheochromocytoma: similar tumors in different sites? PLoS One. 2015;10:e0125426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cascón A, Comino-Méndez I, Currás-Freixes M, et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst. 2015;107. [DOI] [PubMed] [Google Scholar]
- 74.Rustin P, Munnich A, Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10:289–291. [DOI] [PubMed] [Google Scholar]
- 75.Havekes B, van der Klaauw AA, Weiss MM, et al. Pheochromocytomas and extra-adrenal paragangliomas detected by screening in patients with SDHD-associated head-and-neck paragangliomas. Endocr Relat Cancer. 2009;16:527–536. [DOI] [PubMed] [Google Scholar]
- 76.Buffet A, Smati S, Mansuy L, et al. Mosaicism in HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab. 2014;99:E369–E373. [DOI] [PubMed] [Google Scholar]
- 77.Lorenzo FR, Yang C, Ng Tang Fui M, et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 2013;91:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clark GR, Sciacovelli M, Gaude E, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014;99:E2046–E2050. [DOI] [PubMed] [Google Scholar]
- 79.Casey R, Garrahy A, Tuthill A, et al. Universal genetic screening uncovers a novel presentation of an SDHAF2 mutation. J Clin Endocrinol Metab. 2014;99:E1392–E1396. [DOI] [PubMed] [Google Scholar]
- 80.Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf ). 1992;37:304–306. [DOI] [PubMed] [Google Scholar]
- 81.Olson SW, Deal LE, Piesman M. Epinephrine-secreting pheochromocytoma presenting with cardiogenic shock and profound hypocalcemia. Ann Intern Med. 2004;140: 849–851. [DOI] [PubMed] [Google Scholar]
- 82.van der Harst E, de Herder WW, de Krijger RR, et al. The value of plasma markers for the clinical behaviour of phaeochromocytomas. Eur J Endocrinol. 2002;147:85–94. [DOI] [PubMed] [Google Scholar]
- 83.Carballeira A, Fishman LM. Steroid enzyme activities in extraadrenal pheochromocytomas. J Clin Endocrinol Metab. 1982;54:619–624. [DOI] [PubMed] [Google Scholar]
- 84.Isobe K, Nakai T, Yashiro T, et al. Enhanced expression of mRNA coding for the adrenaline-synthesizing enzyme phenylethanolamine-N-methyl transferase in adrenaline-secreting pheochromocytomas. J Urol. 2000;163:357–362. [PubMed] [Google Scholar]
- 85.Nölting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23:21–33. [DOI] [PubMed] [Google Scholar]
- 86.Bausch B, Koschker AC, Fassnacht M, et al. Comprehensive mutation scanning of NF1 in apparently sporadic cases of pheochromocytoma. J Clin Endocrinol Metab. 2006;91:3478–3481. [DOI] [PubMed] [Google Scholar]
- 87.Eisenhofer G, Timmers HJ, Lenders JW, et al. Age at diagnosis of pheochromocytoma differs according to catecholamine phenotype and tumor location. J Clin Endocrinol Metab. 2011;96:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin Y, Yao L, King EE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burnichon N, Vescovo L, Amar L, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20:3974–3985. [DOI] [PubMed] [Google Scholar]
- 90.Abermil N, Guillaud-Bataille M, Burnichon N, et al. TMEM127 screening in a large cohort of patients with pheochromocytoma and/or paraganglioma. J Clin Endocrinol Metab. 2012;97:E805–E809. [DOI] [PubMed] [Google Scholar]
- 91.Neumann HP, Sullivan M, Winter A, et al. Germline mutations of the TMEM127 gene in patients with paraganglioma of head and neck and extraadrenal abdominal sites. J Clin Endocrinol Metab. 2011;96:E1279–E1282. [DOI] [PubMed] [Google Scholar]
- 92.Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43: 663–667. [DOI] [PubMed] [Google Scholar]
- 93.Burnichon N, Cascón A, Schiavi F, et al. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18:2828–2837. [DOI] [PubMed] [Google Scholar]
- 94.Korpershoek E, Koffy D, Eussen BH, et al. Complex MAX rearrangement in a family with malignant pheochromocytoma, renal oncocytoma, and erythrocytosis. J Clin Endocrinol Metab. 2016;101:453–460. [DOI] [PubMed] [Google Scholar]
- 95.Qin N, de Cubas AA, Garcia-Martin R, et al. Opposing effects of HIF1alpha and HIF2alpha on chromaffin cell phenotypic features and tumor cell proliferation: insights from MYC-associated factor X. Int J Cancer. 2014;135:2054–2064. [DOI] [PubMed] [Google Scholar]
- 96.Marmon LM, Albrecht F, Canessa LM, Hoy GR, Jose PA. Identification of dopamine1A receptors in the rat small intestine. J Surg Res. 1993;54:616–620. [DOI] [PubMed] [Google Scholar]
- 97.Foo SH, Chan SP, Ananda V, Rajasingam V. Dopamine-secreting phaeochromocytomas and paragangliomas: clinical features and management. Singapore Med J. 2010;51:e89–e93. [PubMed] [Google Scholar]
- 98.Schlumberger M, Gicquel C, Lumbroso J, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest. 1992;15:631–642. [DOI] [PubMed] [Google Scholar]
- 99.Figueroa SC, Khan U, Kurukulasuriya LR, Gardner D, Sowers JR. Surgical cure of hypertension in a patient with MEN 2A syndrome and mixed dopamine, metanephrine pheochromocytoma. J Clin Hypertens (Greenwich) 2010;12:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakazawa S, Kishikawa H, Akiyama K, et al. Dopamine-secreting pheochromocytoma with neurofibromatosis type 1: a case report [in Japanese]. Hinyokika Kiyo. 2012;58: 543–547. [PubMed] [Google Scholar]
- 101.Palmar I, Vircburger M, Manojlović D, et al. [The von Hippel-Lindau syndrome with pheochromocytoma]. Srp Arh Celok Lek. 2002;130(suppl 2):43–46. [PubMed] [Google Scholar]
- 102.Teasdale S, Reda E. Neurofibromatosis-related phaeochromocytoma: two cases with large tumours and elevated plasma methoxytyramine. Endocrinol Diabetes Metab Case Rep. 2015;2015:150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Timmers HJ, Pacak K, Huynh TT, et al. Biochemically silent abdominal paragangliomas in patients with mutations in the succinate dehydrogenase subunit B gene. J Clin Endocrinol Metab. 2008;93:4826–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piccini V, Rapizzi E, Bacca A, et al. Head and neck paragangliomas: genetic spectrum and clinical variability in 79 consecutive patients. Endocr Relat Cancer. 2012;19: 149–155. [DOI] [PubMed] [Google Scholar]
- 105.Boedeker CC, Erlic Z, Richard S, et al. Head and neck paragangliomas in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2009;94:1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeAngelis LM, Kelleher MB, Post KD, Fetell MR. Multiple paragangliomas in neurofibromatosis: a new neuroendocrine neoplasia. Neurology. 1987;37:129–133. [DOI] [PubMed] [Google Scholar]
- 107.Gaal J, van Nederveen FH, Erlic Z, et al. Parasympathetic paragangliomas are part of the Von Hippel-Lindau syndrome. J Clin Endocrinol Metab. 2009;94:4367–4371. [DOI] [PubMed] [Google Scholar]
- 108.Maier W, Marangos N, Laszig R. Paraganglioma as a systemic syndrome: pitfalls and strategies. J Laryngol Otol 1999;113:978–982. [DOI] [PubMed] [Google Scholar]
- 109.Kota SK, Kota SK, Panda S, Modi KD. Pheochromocytoma: an uncommon presentation of an asymptomatic and biochemically silent adrenal incidentaloma. Malays J Med Sci. 2012;19:86–91. [PMC free article] [PubMed] [Google Scholar]