Abstract
Cannabidiol (CBD) is the major non-psychotropic phytocannabinoid present in Cannabis sativa. In 2018, Congress designated certain C. sativa plant material as “hemp”, thus removing it from the DEA’s list of controlled substances. As a result, CBD-containing hemp extracts and other CBD products are now widely available and heavily marketed, yet their FDA regulatory status is still hotly debated. The goal of this study was to investigate the effects of a cannabidiol-rich cannabis extract (CRCE) on the gut microbiome and associated histomorphological and molecular changes in the mouse gut mucosa. Male C57BL6/J mice were gavaged with either 0, 61.5, 184.5, or 615 mg/kg/bw of CRCE in sesame oil for 2 weeks (Mon-Fri). Substantial CRCE-induced increases in the relative abundance of A. muciniphila, a bacterial species currently accepted as probiotic, was observed in fecal samples at all doses. This was paralleled by decreases in the relative abundance of other gut bacterial species. Coincident with the observed changes in gut ecology were multiple pro-inflammatory responses, including increased expression of cytokines and chemokines – Il1ß, Cxcl1, and Cxcl2 in the colon tissue. Furthermore, dramatic increases in the relative abundance of A. muciniphila significantly decreased expression of Muc2 – a gene intimately associated with gut integrity. Taken together, these findings raise concerns about the safety of long-term CBD usage and underline the need for additional well-designed studies into its tolerability and efficacy.
Keywords: Akkermansia muciniphila, Cannabidiol, dietary supplement, gut inflammation, microbiome, probiotics
Introduction
Cannabidiol (CBD), the major non-psychotropic (that is, it does not cause a high after ingestion) phytocannabinoid present in Cannabis sativa, has long been touted for its medicinal properties. Recently, an FDA-approved, purified CBD drug product (EPIDIOLEX®) was indicated for the treatment of rare, drug-resistant pediatric epilepsies (i.e., Dravet’s Syndrome and Lennox-Gastaut Syndrome) (Devinsky et al. 2016; Devinsky et al. 2017). Until 2018, CBD, like the psychoactive phytocannabinoid Δ9-tetrahydrocannabinol (THC), was classified as a Schedule I narcotic, thus legally restricting its wide-spread medical use. In December 2018, however, CBD’s regulatory status changed dramatically when the U.S. Congress designated C. sativa containing less than 0.3% THC by dry weight, and produced under a qualifying program, as “hemp,” thus removing it from the DEA’s list of controlled substances (Agriculture Improvement Act of 2018). As a result, CBD-containing hemp extracts and other CBD products are now widely available and heavily marketed with a host of health-related and medical claims, though they still fall under FDA restrictions, not being recognized as an allowed food ingredient or dietary supplement. However, the supplement market is presently flooded – at retail stores of all kinds and online - with a plethora of products claiming to contain CBD, in spite of the FDA position.
It appears likely that, regardless of uncertainties as to regulation, CBD-containing consumable health products will be widely used, and therefore, their effects on the digestive system merit investigation. In earlier studies, we demonstrated the hepatotoxic potential of high doses of cannabidiol-rich cannabis extracts (CRCE) with an evident CBD/drug interaction potential and lower tolerance to CRCE after repetitive dosing (Ewing et al. 2019a; Ewing et al. 2019b). This raises serious concerns regarding CBD’s impact on other parts of the gastrointestinal tract. Of particular interest is the potential interaction between CBD and the gut microbiome, as links between gut ecology and the tight balance between health and disease are being increasingly recognized. For instance, numerous studies, including those from our own laboratories indicate that diet and dietary supplements can modulate the gut microbiome resulting in both beneficial (De Vadder et al. 2016; Gurley et al. 2019) and deleterious (Suez et al. 2014; Miousse et al. 2017; Kumagai et al. 2018; Miousse et al. 2020) effects.
Currently, two major categories of CBD-containing products are available to consumers; some are essentially purified CBD, usually prepared in an oil vehicle, and others are marketed as “full spectrum hemp extracts” (FSHEs), and contain a range of other cannabinoids, terpenes, and other constituents. The latter often are touted to display an “entourage effect” of multiple phytochemicals attendant to CBD in FSHEs. To what extent this mixture of phytoconstituents plays in the safety and efficacy of CBD-rich FSHEs, particularly with regard to effects on the gut microbiome remains to be determined. This is important because multiple phytocannabinoids, including CBD, exhibit potent antibacterial activity in several models (Appendino et al. 2008; Hernández-Cervantes et al. 2017), and their impact on the gut microbiome, whether positive or negative, could have significant health consequences.
Furthermore, recent studies indicate a high level of discrepancy between label claims and actual CBD content in commercially available products (Bonn-Miller et al. 2017). While the majority of noncompliant products contained less CBD than claimed on the label, a number of products exhibited substantially higher quantities. Given the rapid dissemination of non-regulated CBD-containing products throughout the U.S. market, including various edibles and drinks, a scenario where high doses of CBD are consumed within a short period of time is quite likely. Therefore, the goal of this study was to investigate the effects of CRCE on the mouse gut microbiome and any associated histomorphological and molecular alterations.
Materials and Methods
CRCE extract characterization, dosing solution, and dose calculations
The product used in this study was prepared by extracting CBD-rich, THC-poor C. sativa chemovar (commonly referred to as hemp) (5.61% of CBD and 0.2% THC w/w) using hexane as a solvent. The ratio of CBD to THC in the extract was the same as in the plant material (28:1). The extract was evaporated to dryness followed by temperature elevation at 80°C for several hours in order to effect complete decarboxylation of cannabinoids. Prior to use, the final extract was analyzed for its cannabinoid content, solvent residue, heavy metals, bacterial/fungal counts, and aflatoxin content.
Following phytochemical characterization, CRCE doses were calculated to deliver the required amount of CBD. CRCE was diluted in sesame seed oil to prepare the final oral gavage solution. Allometric scaling for CBD mouse equivalent doses (MED) was determined per the recommendation of Wojcikowski and Gobe (2014), which, in turn, is based upon an FDA Industry Guidance (U.S. Food and Drug Administration, 2005). MED were based upon the maximum recommended human maintenance dose (20 mg/kg) of purified CBD (EPIDIOLEX®) as well as our previous experience with CRCE in other mouse strains (Ewing et al. 2019a; Ewing et al. 2019b). The quantity of CBD administered to a 25 g mouse (average animal weight in this study) was 5 mg/kg (typical Epidiolex® starting dose in humans) (Silvestro et al. 2019) × 12.3 (mouse allometric scaling factor) to yield a total CBD dose of 61.5 mg/kg delivered in sesame seed oil at a total volume of 300 μL. This initial dose was designated 1X MED. Consequently, the doses of 184.5 mg/kg and 615 mg/kg were designated 3X and 10X, respectively. Control mice received 300 μL of sesame seed oil.
Animals
Male C57BL6/J mice (7-weeks of age) were purchased from Jackson Labs (Bar Harbor, MA). Animals were acclimated for 1 week prior to study initiation. Mice were gavaged with CRCE for 10 days (Mon-Fri) with 61.5, 184.5, or 615 mg/kg (MED of 1X, 3X, and 10X, respectively). Each group consisted of 6 mice except for the 10X group, which contained 8 animals. Mice were housed 3 per cage (4 animals per cage for 10X group) in polycarbonate cages in an appropriate animal room at UAMS from arrival until euthanasia. Room temperature was maintained at 19–22 °C with a relative humidity of 55–70%. Automatic light controls were set to provide fluorescent lighting for a 12 h photoperiod (07:00–19:00 for light phase).
Animals were scheduled to be terminated 6 hours after the last gavage. Colon fecal samples were collected using aseptic techniques, placed into sterile 1.5 ml Eppendorf tubes and flash frozen in liquid nitrogen. Jejunum and colon samples were harvested immediately after fecal samples and flash frozen in liquid nitrogen as well. Proximal jejunum samples were also fixed in Carnoy’s solution (60:30:10 methanol:chloroform:acetic acid) for further immunohistochemical analysis.
To avoid any potential fasting-exacerbated toxicity, food and water were provided ad libitum. Each animal was individually identified with an ear tag. All procedures were approved by the UAMS Institutional Animal Care and Use Committee (protocol number: AUP #3902), and all personnel followed appropriate safety precautions.
Analysis of gut microbiome
Fecal samples from individual mice were kept in collection tubes containing a nucleic acid stabilizer (Zymo Research, Irvine, CA, USA). Microbiome DNA extraction was performed using ZymoBIOMICS DNA Kits (Zymo Research) to obtain DNA derived from bacterial communities. The purified DNA samples were sent to Novogene (CA, USA) for DNA deep sequencing of the whole metagenome using Illumina technology to generate sequencing library by Nextera XT library prep kit (Illumina). The individual sample libraries were indexed and sequenced to generate 150 bp paired-end reads over 6 GB per sample using the Illumina High-seq 4000 machine (Illumina). All of the data was deposited to SRA database and is publicly available under bioproject PRJNA604262.
Raw Illumina fastq files were preprocessed to ensure that only the high-quality reads will be used for further bioinformatics analysis; adapters trimming and quality filtering were performed using Trimmomatic software version 0.36 with default parameters (Bolger et al. 2014). Additional read quality filtering and mouse (mm10) contamination removal were done by the metaWRAP Read qc module (default parameters) (Uritskiy et al. 2018). High quality fastqs were further used as the inputs for reference taxonomic classification and quantification using Centrifuge software version 1.0.4 with default parameters to generate species profile (Kim et al. 2016). The taxonomic profile results generated by the Centrifuge software were further analyzed for taxonomic diversity and plots using R suite software.
Histomorphology
Proximal jejunum samples were collected immediately after termination and fixed in Carnoy’s solution (60:30:10 methanol:chloroform:acetic acid) for histological analyses. The latter was performed independently by two gastrointestinal pathology fellowship-trained pathologists with over 15 years combined experience in a blinded fashion.
Gene expression
Total RNA was extracted from flash frozen jejunum and colon tissues using the RNeasy Mini Kit (QIAGEN, Germantown, MD, USA) according to the manufacturer’s protocol. Following purification, 1000 ng were reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher, Waltham, MA, USA). Primers that were validated and used by our laboratory in several other studies (Ewing et al. 2020; Miousse et al. 2020), were added at a final concentration of 5 μM (Supplementary Table S1). Gene expression values were normalized to the internal control genes TBP and Ywhaz (jejunum samples) or 18S rDNA (colon samples) and expressed as fold change according to the ΔΔCT method.
Statistical analysis
All statistical analyses were performed with the Graphpad Prism 6 software (Graphpad Software. San Diego, CA). Treatment groups were compared with their respective untreated group using ANOVA followed by a Bonferroni multiple comparison test. In cases where the data was not normally distributed, a Kruskal-Wallis test followed by a Dunn’s multiple comparisons test was used instead. Groups were also compared to vehicle with a student’s t-test with Welch’s correction if there were unequal variances.
Results
Phytochemical characterization of the used CRCE product
Results of the phytochemical analysis are shown in Table 1. Other measurements were as follows: loss on drying – 0.32%; heavy metals: lead, mercury, cadmium, and arsenic – not detected; aflatoxins: AFB1, AFB2, AGF1, AFG2 – not detected; Escherichia coli – absent; Salmonella – absent; Total Aerobic Microbial Count (TAMC) – <10 cfu/g; Total Yeast & Mold Count (TYMC) – <10 cfu/g; all of which are below the USP acceptable levels for non-sterile oral preparations.
Table 1.
Phytoconstituents of the Cannabidiol-rich cannabis extract used in this study.
| Constituent | Levels, % |
|---|---|
| Cannabidiol | 57.9 |
| Cannabichromene | 2.03 |
| Δ9-tetrahydrocannabinol | 1.69 |
| Cannabigerol | 1.07 |
| Δ8-tetrahydrocannabinol | <0.01 |
| Tetrahydrocannabivarin | <0.01 |
| Residual Solvent | <0.05 |
Toxicological implications of CRCE administration
Administration of CRCE via gavage was well-tolerated at the two lower doses, with all mice completing the study and no adverse effects detected. However, the highest administered dose (615 mg/kg, 10X) was associated with decreased locomotor activity and food intake, reduced body weight, and lethargy beginning on day 3. By day 5, four out of eight experimental animals in the 10X group were moribund and had to be euthanized. Consequently, the four remaining mice were euthanized at day 5 as well. Detailed toxicological assessments stemming from the histopathology of specific organs will be published elsewhere.
Analysis of the gut microbiome
Fecal samples were collected from each mouse at study termination. For control animals as well as those receiving the two lower doses (1X and 3X), termination occurred at day 12; for the 10X dose, termination occurred between days 3 and 5.
A decrease in the microbial diversity index was observed at 1X CRCE, with nearly the same decrease observed in the 3X group (Figure 1A). Administration of the 10X dose resulted in a profound, up to35%, decrease in the diversity index for all mice (Figure 1A). Principal component analysis of beta diversity index (Bray Curtis method), based upon the community structure as determined from multi-dimensional scaling plots, demonstrated a small but significant discrimination between the control and 1X and 3X groups (Figure 1B). Furthermore, a very strong discrimination was observed between the mice gavaged with 10X CRCE and the other groups (Figure 1B).
Figure 1.
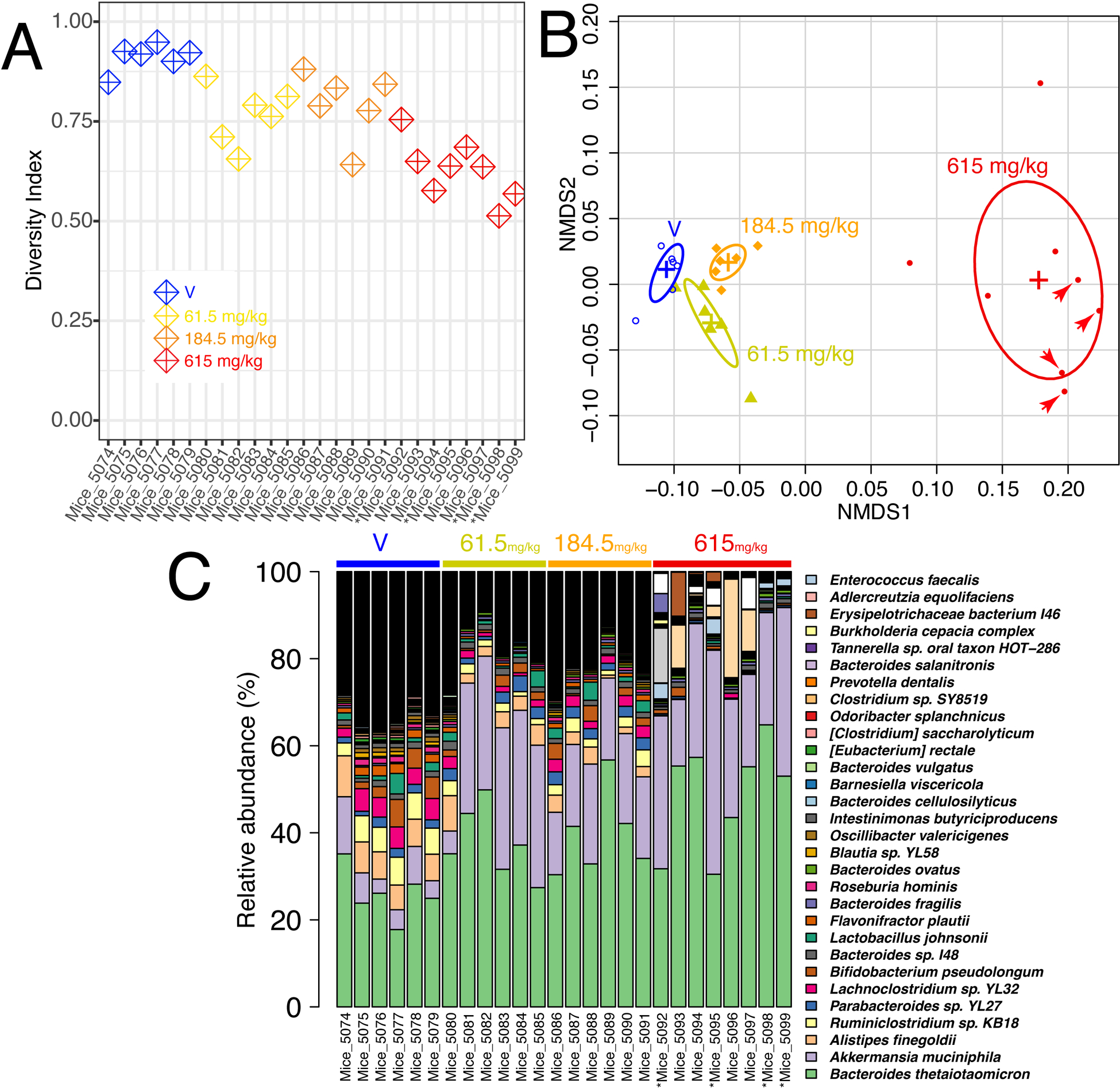
Effects of the Cannabidiol-rich cannabis extract on the gut microbiome. (A) Alpha diversity (Simpson’s diversity Index). (B) Beta diversity plot based on Bray Curtis diversity of gut microbiome species abundance. (C) Relative abundance of 30 most represented bacterial species. The raw count of the taxa that have relative abundance > 1% is provided in the supplementary table 2.
The most significant decline was observed in Alistipes finegoldii, Ruminiclostridium sp. KB18, and Lachnoclostridium sp. YL32. However, an overall decrease in the microbial diversity associated with CRCE was paralleled by robust increases in the relative abundance of select bacterial species, with an evident increase in Bacteroides (Figure 1C and D). Of particular interest was a substantial increase in Akkermansia muciniphila, a mucin-degrading bacterium that belongs to the phylum Verrucomicrobia, among all treatment groups. Significant increases in A. muciniphila relative abundance were observed in all treatment groups and ranged from 3–6% in control mice and up to 40% in mice gavaged with 10X CRCE. (Figure 1C and Figure 2).
Figure 2.
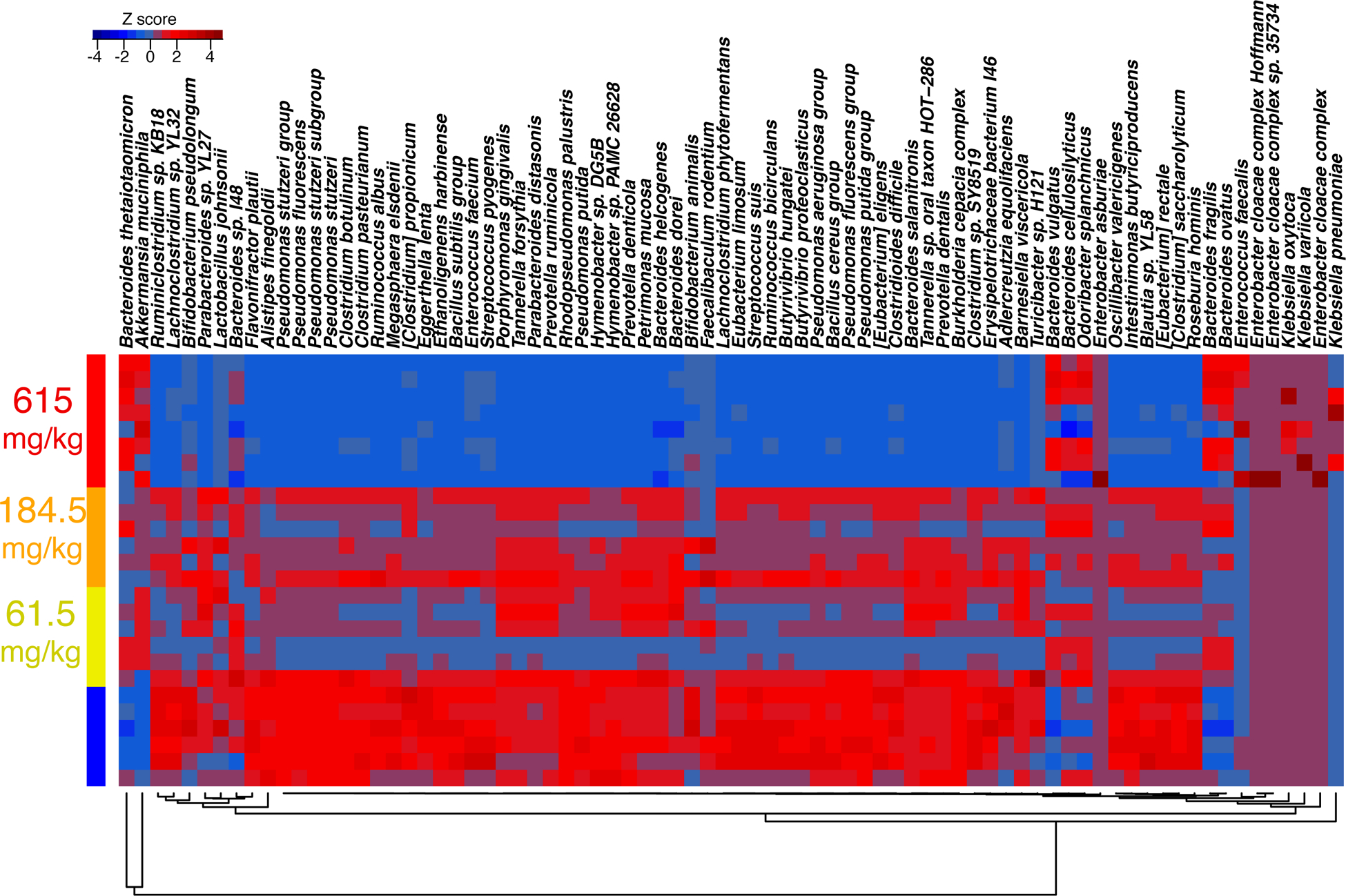
Heat map of the Cannabidiol-rich cannabis extract-induced changes based on standard Z-score of most abundant bacterial species.
Histomorphological analysis
Histomorphological evaluation of the mouse jejunum samples revealed no evidence of epithelial injury, intraepithelial inflammation, or increase in stromal inflammation, nor submucosal edema in any of the control or treated mice (Figure 3). Epithelial vacuoles were present in both vehicle and treated mice, most likely related to the lipid content of the vehicle (sesame seed oil).
Figure 3.
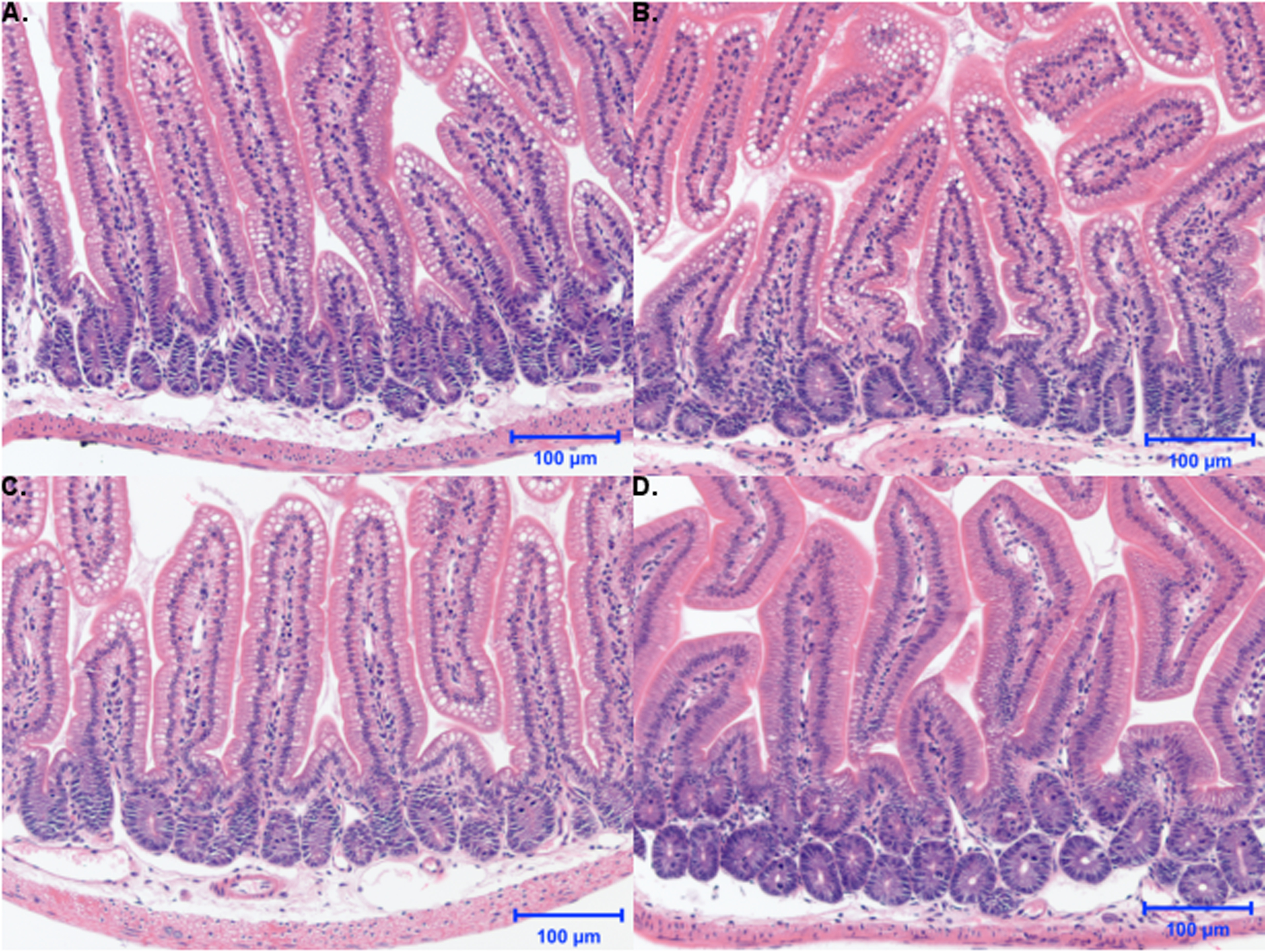
Effects of 2-week administration of Cannabidiol-rich cannabis extract (CRCE) on jejunum histomorphology. Stained jejunum sections from (A) vehicle mice or those gavaged with (B) 61.5 mg/kg, (C) 184.5 mg/kg, or (D) 615 mg/kg CRCE in sesame oil for 10 days (200x magnification). Note that 615 mg/kg group was terminated after 2–3 doses due to overt toxicity elicited by CRCE.
Gene expression analysis confirms CRCE-induced pro-inflammatory responses in the jejunum and colon tissues
Because of the observed significant shifts in gut microbiome structure, we examined the pro-inflammatory potential of CRCE administration. This was performed via gene expression profiling for genes reportedly involved in inflammatory responses to exogenous gut stimuli (Masterson et al. 2015; Ewing et al. 2020).
In the jejunum, 8 out of the 15 investigated genes were significantly (p < 0.05) differentially expressed in at least one experimental group, with most of the genes being up-regulated, except for tumor necrosis factor (Tnf), which exhibited a 5-fold reduction after administration of the 10X dose (Table 2). Of particular interest was the marked increase (3.56-fold, p < 0.05) in Toll-like receptor 4 (Tlr4) expression that was observed even at the 1X dose. Subsequently, MYD88 innate immune signal transduction adaptor (Myd88), the gene involved in the same regulatory loop as Tlr4, was also up-regulated, albeit to a lower extent. Additionally, small but significant increases in the expression of Icam1, an adhesion molecule necessary for immune cell transmigration, was noted.
Table 2.
Gene expression in jejunum after 2 weeks of gavage with CRCE.
| Jejunum 2-weeks | ||||||
|---|---|---|---|---|---|---|
| Mouse | Human | Function | Vehicle | 61.5 mg/kg | 184.5 mg/kg | 615 mg/kg |
| Ccl1 | CCL1 | C-C motif chemokine ligand 1 | 1.00 ± 0.18 | 1.69 ± 0.34 | 1.04 ± 0.23 | 1.34 ± 0.32 |
| Cxcl1 | CXCL1 | C-X-C motif chemokine ligand 1 | 1.00 ± 0.16 | 2.25 ± 0.85 | 1.28 ± 0.20 | 4.69 ± 2.10 |
| Cd14 | CD14 | Monocyte differentiation antigen CD14 | 1.00 ± 0.18 | 1.52 ± 0.35 | 1.63 ± 0.13A | 2.43 ± 0.75 |
| Cxcl2 | CXCL2 | C-X-C motif chemokine ligand 2 | 1.00 ± 0.08 | 0.87 ± 0.16 | 0.79 ± 0.10 | 1.30 ± 0.29 |
| Fas** | FAS | Fas cell surface death receptor | 1.00 ± 0.03 | 1.11 ± 0.07 | 1.27 ± 0.09 A | 0.92 ± 0.09 |
| Fasl | FASLG | Fas ligand | 1.00 ± 0.14 | 1.73 ± 0.49 | 2.63 ± 0.49 A | 2.03 ± 0.58 |
| Icam1 | ICAM1 | Intercellular adhesion molecule 1 | 1.00 ± 0.05 | 1.20 ± 0.18 | 1.48 ± 0.06 A | 1.90 ± 0.35 AB |
| Il1a | IL1A | Interleukin 1 alpha | 1.00 ± 0.12 | 1.11 ± 0.13 | 1.02 ± 0.07 | 1.11 ± 0.38 |
| Il1b | IL1B | Interleukin 1 beta | 1.00 ± 0.13 | 1.21 ± 0.27 | 1.13 ± 0.15 | 0.52 ± 0.21 |
| Il2 | IL2 | Interleukin 2 | 1.00 ± 0.10 | 0.75 ± 0.42 | 1.02 ± 0.30 | 1.10 ± 0.38 |
| Il4 | IL4 | Interleukin 4 | 1.00 ± 0.24 | 1.82 ± 0.79 | 4.21 ± 1.89 | 0.70 ± 0.23 |
| Myd88 | MYD88 | MYD88 innate immune signal transduction adaptor | 1.00 ± 0.09 | 1.12 ± 0.11 | 1.57 ± 0.15AB | 1.67 ± 0.09AB |
| Tlr2 | TLR2 | Toll like receptor 2 | 1.00 ± 0.11 | 0.98 ± 0.11 | 0.82 ± 0.03 | 0.92 ± 0.18 |
| Tlr4 | TLR4 | Toll like receptor 4 | 1.00 ± 0.18 | 3.56 ± 0.43 A | 4.77 ± 0.54 AB | 7.03 ± 1.12 AB |
| Tnf | TNF | Tumor necrosis factor | 1.00 ± 0.13 | 1.00 ± 0.43 | 0.88 ± 0.17 | 0.19 ± 0.08 A |
Data presented at mean fold change from respective vehicle ± SEM (n = 3–6).. Gene expression values between vehicle and each dose were compared with a t-test with Welch’s correction, if needed for unequal variances (A, significant comparison), and a one-way ANOVA or Kruskal-Wallis test (if failed equal variance test), followed by a multiple comparisons test, using a Bonferroni or Dunn’s correction, of each dose versus vehicle (**, significant ANOVA or Kruskall-Wallis without significant comparison; B, significant comparison).
In the colon, 10 out of 15 genes were significantly differentially expressed in at least one experimental group (Table 3). Interestingly, the C-X-C motif chemokine ligand 2 gene (Cxcl2), whose expression, while amplified at the 1X dose, increased by 275-fold in mice gavaged with 10X CRCE (Table 3). Similar to jejunum, Icam1 was found up-regulated at two higher doses of CRCE.
Table 3.
Gene expression in colon after 2 weeks of gavage with CRCE.
| Colon 2-weeks | ||||||
|---|---|---|---|---|---|---|
| Mouse | Human | Function | Vehicle | 61.5 mg/kg | 184.5 mg/kg | 615 mg/kg |
| Ccl1 | CCL1 | C-C motif chemokine ligand 1 | 1.00 ± 0.16 | 1.69 ± 0.27 | 4.47 ± 1.17A | 3.61 ± 1.34 |
| Cd14 | CXCL1 | C-X-C motif chemokine ligand 1 | 1.00 ± 0.07 | 1.26 ± 0.17 | 1.23 ± 0.11 | 2.81 ± 0.28AB |
| Cxcl1 | CD14 | Monocyte differentiation antigen CD14 | 1.00 ± 0.21 | 2.80 ± 0.62A | 2.67 ± 0.54A | 5.57 ± 1.60AB |
| Cxcl2** | CXCL2 | C-X-C motif chemokine ligand 2 | 1.00 ± 0.01 | 7.32 ± 1.23A | 97.33 ± 34.23 | 275.7 ± 110.5 |
| Fas | FAS | Fas cell surface death receptor | 1.00 ± 0.12 | 1.06 ± 0.12 | 1.46 ± 0.08AB | 0.97 ± 0.09 |
| Fasl* | FASLG | Fas ligand | ||||
| Icam1 | ICAM1 | Intercellular adhesion molecule 1 | 1.00 ± 0.08 | 1.35 ± 0.09A | 2.29 ± 0.31 AB | 2.76 ± 0.33 AB |
| Il1a | IL1A | Interleukin 1 alpha | 1.00 ± 0.25 | 2.14 ± 0.40 | 4.64 ± 0.74AB | 3.51 ± 0.55A |
| Il1b | IL1B | Interleukin 1 beta | 1.00 ± 0.14 | 1.57 ± 0.31 | 3.34 ± 0.94 | 3.31 ± 0.95 |
| Il2 | IL2 | Interleukin 2 | 1.00 ± 0.11 | 1.25 ± 0.19 | 1.62 ± 0.14A | 1.29 ± 0.27 |
| Il4* | IL4 | Interleukin 4 | ||||
| Myd88 | MYD88 | MYD88 innate immune signal transduction adaptor | 1.00 ± 0.13 | 1.05 ± 0.04 | 1.11 ± 0.03 | 1.09 ± 0.04 |
| Tlr2 | TLR2 | Toll like receptor 2 | 1.00 ± 0.13 | 0.90 ± 0.15 | 1.57 ± 0.16A | 0.94 ± 0.24 |
| Tlr4 | TLR4 | Toll like receptor 4 | 1.00 ± 0.18 | 1.12 ± 0.15 | 0.98 ± 0.09 | 1.03 ± 0.11 |
| Tnf | TNF | Tumor necrosis factor | 1.00 ± 0.13 | 2.22 ± 0.45A | 2.38 ± 0.39A | 1.80 ± 0.40 |
Data presented at mean fold change from respective vehicle ± SEM (n = 3–6). *, not expressed (cells empty). Gene expression values between vehicle and each dose were compared with a t-test with Welch’s correction, if needed for unequal variances(A, significant comparison), and a one-way ANOVA or Kruskal-Wallis test (if failed equal variance test), followed by a multiple comparisons test, using a Bonferroni or Dunn’s correction, of each dose versus vehicle (**, significant ANOVA or Kruskall-Wallis without significant comparison; B, significant comparison).
Given the observed increase in A. muciniphila among mice administered CRCE and this bacteria’s role in regulating gut mucus levels (Derrien et al. 2017), we investigated Muc2, a gene whose function is tightly associated with mucus production in the gut. While there were no significant differences in Muc2 between controls and animals gavaged with 1X and 3X CRCE, a 2-fold decrease in colon Muc2 expression was observed in mice receiving the 10X dose. Muc2 expression in the jejunum, however, remained unaffected in all groups (Figure 4).
Figure 4.
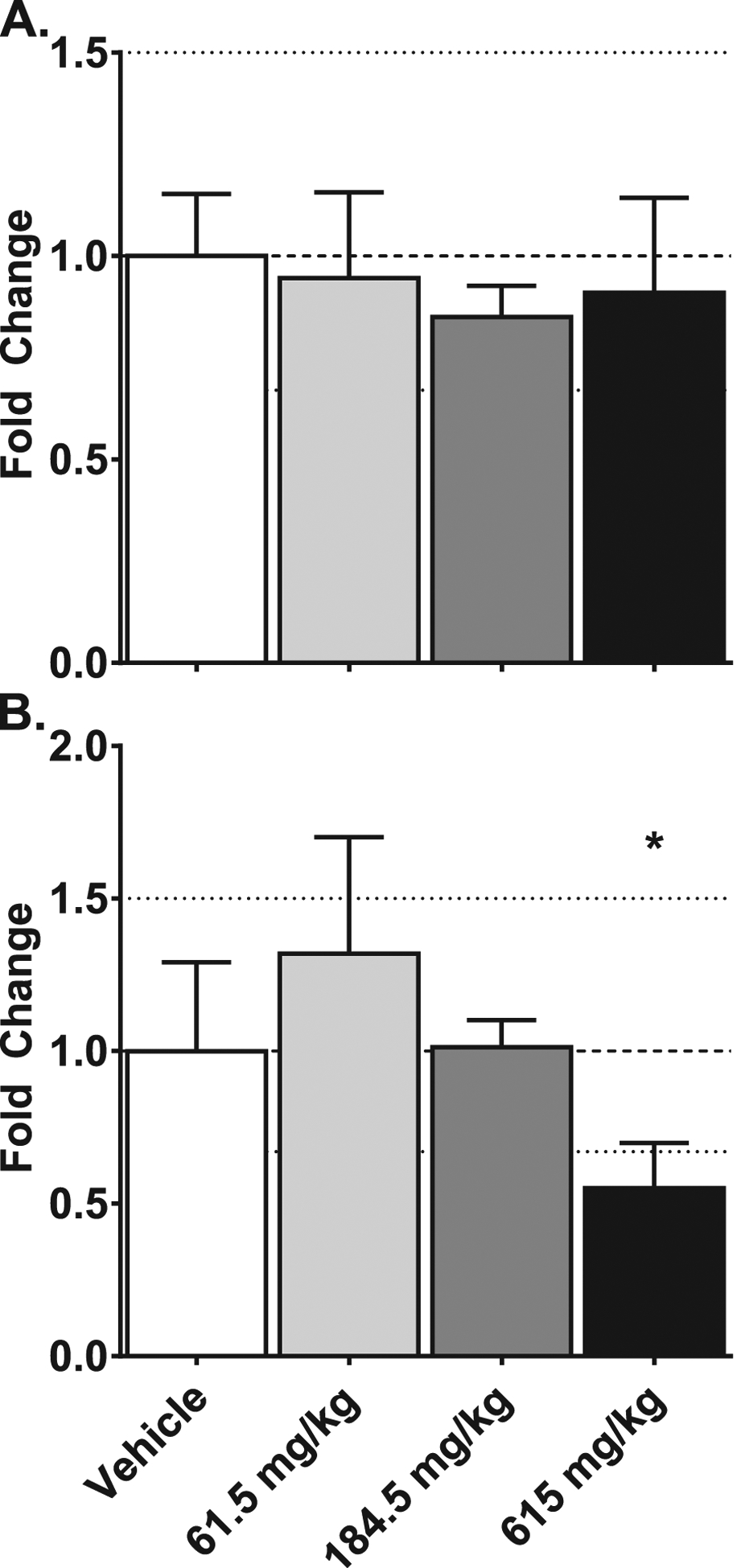
Effects of gavaging mice with Cannabidiol-rich cannabis extract on the expression of Muc2 gene in the jejunum (A) and colon (B) tissues. Gene expression was measured using the quantitative real-time (qRT) PCR. Data are presented as mean ± SEM fold changed from vehicle (n = 6), asterisk (*) denotes p < .05.
Discussion
CBD in its various forms – from purified CBD to “full spectrum hemp extracts” – has made significant inroads into the health care arena, with further push for expansion into the food and cosmetic markets. In fact, sales of CBD-containing products are predicted to exceed $1B in 2020 (Borchardt 2017). Because of its considerable market presence, the probability of long-term CBD exposure among consumers is high. Surprisingly, despite CBD’s commercial popularity, very little research has been conducted on the safety of prolonged CBD ingestion. Recent product quality surveys imply that CBD exposure from commercially available dosage forms may either be enhanced or lessened due to discrepancies between product label claims and actual CBD content. In addition, several phytocannabinoids have been shown to possess potent antibacterial properties (Appendino et al. 2008; Hernández-Cervantes et al. 2017). Taken together, these elements prompted us to investigate the effects of prolonged CRCE administration on gut toxicology and ecology.
The past decade has seen a dramatic increase in research into factors that influence gut microbiome homeostasis. During that time, much attention has been paid to the role diet and other exogenous factors (e.g., drugs, dietary supplements, probiotics, etc.) play in modulating gut ecology and their subsequent impact on human health. Of particular interest are weight loss products that are believed to exert their beneficial effects via interactions with and modulation of the gut microbiome. Green tea extract (GTE) is one such product (i.e., botanical dietary supplement) that has been widely demonstrated, in both experimental and clinical settings, to affect gut ecology. For instance, GTE has been shown to effectively shift the balance between Bacteroidetes and Firmicutes towards the former (Henning et al. 2018; Zhang et al. 2018; Gurley et al. 2019). Interestingly, the relative proportion of Bacteroidetes to Firmicutes is markedly decreased in both obese humans and obese mice, and GTE-modulated restoration of the Bacteroidetes to Firmicutes ratio is believed to be a central mechanism underlying GTE-induced weight loss (Ley et al. 2006; Henning et al. 2018; Gurley et al. 2019). Green tea and GTE are among the most popular botanical dietary ingredients in the world. As mentioned above, another botanical ingredient rapidly gaining in global popularity is CBD. At present, CBD’s mechanism of action is poorly understood, but its effect on the gut microbiome may provide insight into its efficacy and safety.
Here, we observed that oral administration of CRCE in sesame oil (61.5 – 615 mg/kg) markedly affected mouse gut ecology. These effects were manifested as a decrease in the diversity index, exemplified by a measurable decline in bacterial species, the most significant of which was for A. finegoldii, Ruminiclostridium sp. KB18, and Lachnoclostridium sp. YL32. A. finegoldii is a strictly anaerobic Gram-negative rod isolated from appendicular tissue samples of patients with acute appendicitis as well as from perirectal abscess materials and in colon cancer patients with bacteremia following surgical resection (Rautio et al. 1997; Rautio et al. 2000; Fenner et al. 2007; Mavromatis et al. 2013). Experimental evidence suggests that A. finegoldii is involved in numerous fermentative processes in the gut, including the synthesis of alkaline phosphatases, esterase and esterase lipase, galactosidases and glucosidase, with succinic acid considered as a major end product (Mavromatis et al. 2013). Limited knowledge exists regarding the specific functions of two Firmicutes whose abundance was decreased by CRCE – Ruminiclostridium sp. KB18 (also known as Hungateiclostridiaceae bacterium KB18) and Lachnoclostridium sp. YL32. Similar to our previous studies with decaffeinated GTE (dGTE) in B6C3F1 mice (performed in a separate animal facility at a different time), Ruminiclostridium sp. KB18 and Lachnoclostridium sp. YL32 were among the most abundant bacterial species, signifying them as common bacterial residents, at least in the mouse gut. However, contrary to CRCE, dGTE did not reduce their abundance, even at a dose of 200 mg/kg (Gurley et al. 2019).
Decreases in relative abundance of resident bacterial flora among all treatment groups raises concerns regarding the untoward effects CBD may have on the gut microbiome. This is especially true for patients receiving CBD as a treatment modality, as clinically relevant doses of 20 mg/kg are four-fold higher (allometrically speaking) than the lowest dose used in this study. Additional dose ranging studies of CBD/CRCE are clearly needed in order to further clarify the impact of phytocannabinoids on gut microbiome diversity. Examination of fecal samples from patients currently undergoing therapy with EPIDIOLEX® may provide further insight into the translational validity of our findings.
Reductions in gut microflora diversity coupled with significant increases in A. muciniphila may portend negative gut health repercussions. A. muciniphila is a mucin-degrading bacterium commonly found in the human gut at ~3–5% abundance, a value similar to that observed in our mouse model. This is particularly important since declines in A. muciniphila abundance have been reported in patients with metabolic syndrome, obesity, and diabetes (Png et al. 2010; Roopchand et al. 2015; Brahe et al. 2015; PMID: 20648002). Furthermore, an accumulating body of evidence suggests that administration of A. muciniphila as a probiotic may mitigate obesity and obesity-associated disease (Zhou et al. 2017). In our earlier dGTE study of similar duration (2 weeks), a significant increase in A. muciniphila abundance was noted, suggesting a potential link towards GTE-associated weight management benefits (Gurley et al. 2019). However, the extent of this increase in A. muciniphilia abundance, while statistically significant, was not as dramatic as that observed with CRCE and did not occur at the expense of other common resident gut bacteria. Thus, while an increase in A. muciniphila may be considered positive for gut ecology, the extent of this increase coupled to the marked decline in relative abundance of other common resident gut bacterial species is concerning. Given A. muciniphilia’s propensity for mucin degradation, over time this combined effect may lead to compromises in gut integrity. While there were no significant histomorphological changes in the mouse jejunum after 2 weeks of CRCE exposure, we cannot vouch for the colon as tissue samples from this section of the gut were not available for histological analysis.
The observed shifts in gut ecology were associated with numerous pro-inflammatory responses that were prevalent in the colon as revealed by overexpression of various cytokines (i.e., Il1ß) and chemokines (i.e., Cxcl1, Cxcl2). Both Cxcl1 and Cxcl2 are chemokines produced by activated residential tissue macrophages possessing both neutrophil-chemoattracting and neutrophil-activating properties. Research indicates that Cxcl1 and Cxcl2 overexpression is often associated with gastrointestinal toxicity following exposure to noxious chemicals (e.g., 5-fluorouracil) or pathogenic bacterial species (Sakai et al. 2013; Masterson et al. 2015). Furthermore, probably the most common resident in this study, Bacteroides thetaiotaomicron, was also significantly increased at the highest dose of CRCE. This increase in relative abundance may relate directly to changes in gene expression profiles in both the jejunum and colon. Endotoxins secreted by B. thetaiotaomicron have been shown to stimulate adhesion molecules in endothelial cells, specifically Icam1 (Rokosz et al., 1999), the gene whose expression was found increased in both jejunum and colon tissues.
Coincident with the upregulation of Icam1, Il1b, Cxcl1 and Cxcl2, significant downregulation in colonic Muc2 gene expression is suggestive of potential damage to the gut epithelium. Mucin production by goblet cells is regulated, in part, by Muc2. This high molecular weight, heavily glycosylated protein protects the mucosal surface from infection by limiting tissue damage and bacterial translocation across the intestinal epithelium. Thus, reduced Muc2 expression may be in response to uncontrolled expansion of mucus-digesting A. muciniphila. Collectively, alterations in gut bacterial diversity, Muc2 expression, and inflammatory protein secretion may ultimately disrupt intestinal tight junctions and increase the likelihood of sepsis (Shukla et al. 2018). Future studies investigating intestinal barrier function and bacterial translocation to the liver in response to CBD/CRCE exposure are clearly needed. Furthermore, such studies may shed light on mechanisms of CBD/CRCE-induced liver injury as gut barrier dysfunction and endotoxemia have become increasingly recognized in the pathogenesis of liver disease (Rao 2009).
While pro-inflammatory responses in the jejunum were not as pronounced as those in the colon, significant induction of Tlr4 and Myd88, suggests that release of inflammatory cytokines and reactive oxygen species in this gut region are also stimulated by phytoconstituents within CRCE. Finally, the presence of substantial quantities of epithelial vacuoles within jejunal tissue of both control and CRCE-gavaged mice appear attributable to the sesame seed oil vehicle, a finding which warrants further study into the prolonged effects of CBD carrier oils on gut health.
Conclusions
From the results of this study, CBD/CRCE induce complex responses in the gut microbiome, with consequences that may be favorable or detrimental. On one hand, CRCE-mediated increases in the relative abundance of A. muciniphila, a bacterial species considered as probiotic, can be considered beneficial. On the other hand, the seemingly opportunistic increase in A. muciniphila is at the expense of other bacterial species, while paralleled by numerous pro-inflammatory responses, raises concerns about the potential long-term effects of CBD ingestion.
Supplementary Material
Acknowledgements
The authors would like to thank Robin Mulkey and Bianca Schutte for providing excellent animal care at the UAMS Animal Facility.
Funding
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers 1P20GM109005 and P20GM125503; the National Institute of General Medical Sciences grant number T32GM106999; Clinical and Translational Science Awards UL1TR000039 and KL2TR000063; and the Arkansas Biosciences Institute.
About the authors
Charles M. Skinner is a Senior Research Associate at the Center for Dietary Supplements Research, Department of Environmental and Occupational Health, University of Arkansas for Medical Sciences. His research has been mainly focused on the field of dietary supplements and the effects of cannabidiol-rich cannabis extracts on the tissues and organs of mice and humans. The recent addition of Emulate’s organ-on-chip technology to the Koturbash lab, allows him to look at the effects of dietary supplements and CBD on human tissues.
Intawat Nookaew is an Associate Professor in the Department of Biomedical Informatics at the College of Medicine, University of Arkansas for Medical Sciences. He received his PhD degree from King’s Mongkut’s University of Technology Thonburi, Thailand. (2008). Intawat completed his training as of postdoctoral fellow in Chalmers University of Technology in Sweden in the area of systems biology. He has published over 115 peer-reviewed articles and book chapters. Intawat’s research has been focus on development bioinformatics & computational biology frameworks to uncover secrets of life from large-scale data in the area of biotechnology and clinical/biomedical research. His research is also focused on microbiome analysis using whole genome shotgun sequencing to understand impact of microbiome on health and diseases.
Laura E. Ewing is a senior PhD student at the Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences. She received a MS degree in zoology from the University of Oklahoma. Her thesis involves the evaluation of the combinatorial effects of methionine and radiation exposure on gastrointestinal health and the microbiome. During her tenure with Dr. Koturbash, Ms. Ewing was also highly involved in studies on the cannabidiol-rich cannabis extract-induced liver toxicity.
Thidathip Wongsurawat is an Instructor in the Department of Biomedical Informatics (DBMI), University of Arkansas for Medical Sciences (UAMS). She received her PhD degree from Nanyang Technological University, Singapore (2015). Thidathip completed her training as postdoctoral fellow at Bioinformatics Institute, A*STAR, Singapore and also DBMI, UAMS, USA. Thidathip focuses on utilizing next-generation sequencing and long-read sequencing technology to answer biological questions faster and better than previous. Thidathip implements sequencing technology across a number of projects, including microbial genomes, metagenome, human genomics, cancer research and environmental research.
Piroon Jenjaroenpun is an Instructor in the Department of Biomedical Informatics at the University of Arkansas for Medical Sciences College of Medicine. He is a bioinformatician with more than ten years of experience working in Next-generation sequencing (NGS) data analysis at the Bioinformatic Institute of Singapore. He completed his training as a postdoctoral fellow at UAMS in Third-generation sequencing data analysis, such as Pacific Biosciences and Oxford Nanopore Technology. He has published more than 30 peer-reviewed articles related to NGS. Piroon’s research has been focused on developing and applying bioinformatics pipelines and tools to integrate and make sense of biological data, for example, microbial genomes, transcriptome, metagenome, human genomics, and cancer research
Charles M. Quick is an Associate Professor of Pathology at the University of Arkansas for Medical Sciences. He has completed fellowships in both Surgical / Gastrointestinal Pathology and Women’s and Perinatal Pathology and serves as the Director of Anatomic Pathology Subspecialty Practice and Director of Gynecologic Pathology for the Department of Pathology at UAMS.
Eric U. Yee is an Assistant Professor of Pathology at the University of Arkansas for Medical Sciences. He has completed a fellowship in Gastrointestinal and Hepatobiliary Pathology and serves as the Director of Gastrointestinal Pathology Subspecialty Service at UAMS.
Brian D. Piccolo is an Assistant Professor at the USDA-ARS-Arkansas Children’s Nutrition Center (ACNC), located at the Arkansas Children’s Hospital in Little Rock, AR. He also has a joint appointment in the Department of Pediatrics at the University of Arkansas for Medical Sciences. His research is focused on exploring host-microbe intestinal cross-talk mechanisms associated with host energy regulation.
Mahmoud A. ElSohly is a Research Professor at The National Center for Natural Products Research, and Professor of Pharmaceutics and Drug Delivery, School of Pharmacy, University of Mississippi (UM) and is the Director of the National Institute on Drug Abuse (NIDA) Marijuana Project at UM. He is also the President and Laboratory Director of ElSohly Laboratories Incorporated, an analytical forensic drug testing and product development laboratory. He received his undergraduate and Masters from Cairo University, Cairo, Egypt and his Ph.D. in 1975 from the University of Pittsburgh, School of Pharmacy, Pittsburgh, PA. He has been with the University of Mississippi since 1975 and has been Director of the NIDA Marijuana Project since 1981. He has over 40 years’ experience working with the isolation of natural products (notably cannabis secondary metabolites), synthetic, analytical and forensic chemistry. He has more than 30 patents and over 300 publications in these areas of science. Dr. ElSohly is also a member of many professional organizations, such as American Society of Pharmacognosy, American Chemical Society, American Academy of Forensic Sciences, Society of Forensic Toxicology, International Cannabinoids Research Society, International Association of Cannabinoid Medicines, to name a few and have received numerous awards.
Larry A. Walker is Director Emeritus of the National Center for Natural Products Research (NCNPR) at the University of Mississippi. He was trained in pharmacy and pharmacology, and has worked for much of his 38 yr career on natural products drug discovery, pharmacology, toxicology and metabolism. He has been integrally involved in the design and execution of preclinical and early clinical development studies on drugs and natural products.
Bill J. Gurley is a Principal Scientist at the National Center for Natural Products Research at the University of Mississippi, Oxford. His research interests include mechanisms of herb-drug interactions, toxicity of multiple-component herbal dietary supplements, phytochemical modulation of human drug-metabolizing enzymes and drug transport proteins, pharmacokinetics of phytochemicals in humans and botanical supplement use in special populations. Gurley has been conducting pre-clinical and clinical research on botanical dietary supplements for more than 20 years.
Igor Koturbash is an Associate Professor at the Department of Environmental and Occupational Health and Co-Director of the Center for Dietary Supplements Research at the University of Arkansas for Medical Sciences (UAMS) Fay W. Boozman College of Public Health. Being both MD and PhD, Igor has a long-lasting interest in diet and dietary supplements and their impact on human health. Therefore, the major focus of his research is safety, efficacy and mechanisms of action of dietary supplements and understanding how diet and dietary supplements can modulate tissue response to cancer therapy. Igor is heavily involved in a number of safety and efficacy studies on various dietary supplements and herbs, including methionine supplementation, green tea extract and cannabidiol (CBD), to name a few. Igor has published 90+ peer-reviewed articles and book chapters and his research has received uninterrupted extramural funding from various sources since the beginning of his independent career.
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman MM. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008. August;71(8):1427–30. doi: 10.1021/np8002673. Epub 2008 Aug 6. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA. 2017. November 7;318(17):1708–1709. doi: 10.1001/jama.2017.11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt D Hemp cannabis product sales projected to hit $1 billion in 3 years. Forbes 2017, August, 23. [Google Scholar]
- Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Hansen T, Pedersen O, Astrup A, Ehrlich SD, Larsen LH. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr Diabetes. 2015. June 15;5:e159. doi: 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017. May;106:171–181. doi: 10.1016/j.micpath.2016.02.005. Epub 2016 Feb 11. Review. [DOI] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016. July 12;24(1):151–7. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, Wong M, Tilton N, Bruno P, Bluvstein J, Hedlund J, Kamens R, Maclean J, Nangia S, Singhal NS, Wilson CA, Patel A, Cilio MR. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016. March;15(3):270–8. doi: 10.1016/S1474-4422(15)00379-8. Epub 2015 Dec 24. Erratum in: Lancet Neurol. 2016 Apr;15(4):352. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Wright S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med. 2017. August 17;377(7):699–700. doi: 10.1056/NEJMc1708349. [DOI] [PubMed] [Google Scholar]
- Ewing LE, Skinner CM, Quick CM, Kennon-McGill S, McGill MR, Walker LA, ElSohly MA, Gurley BJ, Koturbash I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules. 2019a. April 30;24(9). pii: E1694. doi: 10.3390/molecules24091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing LE, McGill MR, Yee EU, Quick CM, Skinner CM, Kennon-McGill S, Clemens M, Vazquez JH, McCullough SS, Williams DK, Kutanzi KR, Walker LA, ElSohly MA, James LP, Gurley BJ, Koturbash I. Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules. 2019b. June 17;24(12). pii: E2256. doi: 10.3390/molecules24122256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing LE, Miousse IR, Pathak R, Skinner CM, Kosanke S, Boerma M, Hauer-Jensen M, Koturbash I. NZO/HlLtJ as a novel model for the studies on the role of metabolic syndrome in acute radiation toxicity. Int J Radiat Biol. 2020. January;96(1):93–99. doi: 10.1080/09553002.2018.1547437. Epub 2019 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner L, Roux V, Ananian P, Raoult D. Alistipes finegoldii in blood cultures from colon cancer patients. Emerg Infect Dis. 2007. August;13(8):1260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Miousse IR, Nookaew I, Ewing LE, Skinner CM, Jenjaroenpun P, Wongsurawat T, Kennon-McGill S, Avula B, Bae JY, McGill MR, Ussery D, Khan IA, Koturbash I. Decaffeinated Green Tea Extract Does Not Elicit Hepatotoxic Effects and Modulates the Gut Microbiome in Lean B6C3F₁ Mice. Nutrients. 2019. April 3;11(4). pii: E776. doi: 10.3390/nu11040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning SM, Yang J, Hsu M, Lee RP, Grojean EM, Ly A, Tseng CH, Heber D, Li Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur J Nutr. 2018. December;57(8):2759–2769. doi: 10.1007/s00394-017-1542-8. Epub 2017 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cervantes R, Méndez-Díaz M, Prospéro-García Ó, Morales-Montor J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation. 2017;24(4–5):183–199. doi: 10.1159/000481824. Epub 2017 Nov 18. Review. [DOI] [PubMed] [Google Scholar]
- H.R.2 – Agriculture Improvement Act of 2018, Public Law No: 115–334, December 20, 2018.
- Kim D, Song L, Breitwieser FP, Salzberg SL. Centrifuge: Rapid and sensitive classification of metagenomic sequences. Genome Res. 2016, 26, 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai T, Rahman F, Smith AM. The Microbiome and Radiation Induced-Bowel Injury: Evidence for Potential Mechanistic Role in Disease Pathogenesis. Nutrients. 2018. October 2;10(10). pii: E1405. doi: 10.3390/nu10101405. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006. December 21;444(7122):1022–3. [DOI] [PubMed] [Google Scholar]
- Masterson JC, McNamee EN, Fillon SA, Hosford L, Harris R, Fernando SD, Jedlicka P, Iwamoto R, Jacobsen E, Protheroe C, Eltzschig HK, Colgan SP, Arita M, Lee JJ, Furuta GT. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2015. August;64(8):1236–47. doi: 10.1136/gutjnl-2014-306998. Epub 2014 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromatis K, Stackebrandt E, Munk C, Lapidus A, Nolan M, Lucas S, Hammon N, Deshpande S, Cheng JF, Tapia R, Goodwin LA, Pitluck S, Liolios K, Pagani I, Ivanova N, Mikhailova N, Huntemann M, Pati A, Chen A, Palaniappan K, Land M, Hauser L, Rohde M, Gronow S, Göker M, Detter JC, Bristow J, Eisen JA, Markowitz V, Hugenholtz P, Kyrpides NC, Klenk HP, Woyke T. Complete genome sequence of the bile-resistant pigment-producing anaerobe Alistipes finegoldii type strain (AHN2437(T)). Stand Genomic Sci. 2013. April 15;8(1):26–36. doi: 10.4056/sigs.3527032. eCollection 2013 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Pathak R, Garg S, Skinner CM, Melnyk S, Pavliv O, Hendrickson H, Landes RD, Lumen A, Tackett AJ, Deutz NEP, Hauer-Jensen M, Koturbash I. Short-term dietary methionine supplementation affects one-carbon metabolism and DNA methylation in the mouse gut and leads to altered microbiome profiles, barrier function, gene expression and histomorphology. Genes Nutr. 2017. September 6;12:22. doi: 10.1186/s12263-017-0576-0. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Ewing LE, Skinner CM, Pathak R, Garg S, Kutanzi KR, Melnyk S, Hauer-Jensen M, Koturbash I. Methionine dietary supplementation potentiates ionizing radiation-induced gastrointestinal syndrome. Am J Physiol Gastrointest Liver Physiol. 2020. March 1;318(3):G439–G450. doi: 10.1152/ajpgi.00351.2019. Epub 2020 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010. November;105(11):2420–8. doi: 10.1038/ajg.2010.281. Epub 2010 Jul 20. [DOI] [PubMed] [Google Scholar]
- Rao R Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009. August;50(2):638–44. doi: 10.1002/hep.23009. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio M, Lönnroth M, Saxén H, Nikku R, Väisänen ML, Finegold SM, Jousimies-Somer H. Characteristics of an unusual anaerobic pigmented gram-negative rod isolated from normal and inflamed appendices. Clin Infect Dis. 1997. September;25 Suppl 2:S107–10. [DOI] [PubMed] [Google Scholar]
- Rautio M, Saxén H, Siitonen A, Nikku R, Jousimies-Somer H. Bacteriology of histopathologically defined appendicitis in children. Pediatr Infect Dis J. 2000. November;19(11):1078–83. [DOI] [PubMed] [Google Scholar]
- Rokosz A, Meisel-Mikołajczyk F, Malchar C, Nowaczyk M, Górski A. Adhesion molecule expression stimulated by Bacteroides thetaiotaomicron cell-surface antigens. Arch Immunol Ther Exp (Warsz). 1999;47(3):169–78. [PubMed] [Google Scholar]
- Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes. 2015. August;64(8):2847–58. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Sagara A, Matsumoto K, Hasegawa S, Sato K, Nishizaki M, Shoji T, Horie S, Nakagawa T, Tokuyama S, Narita M. 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS One. 2013;8(1):e54788. doi: 10.1371/journal.pone.0054788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Meena AS, Manda B, Gomes-Solecki M, Dietrich P, Dragatsis I, Rao R. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor-dependent mechanism. FASEB J. 2018. June 18:fj201800351R. doi: 10.1096/fj.201800351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro S, Mammana S, Cavalli E, Bramanti P, Mazzon E. Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules. 2019; 24:1459. doi: 10.3390/molecules24081459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014. October 9;514(7521):181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- Uritskiy GV, DiRuggiero J, Taylor J. MetaWRAP-a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome. 2018. September 15;6(1):158. doi: 10.1186/s40168-018-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2005. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Http://www.fda.gov/cder/guidance/index.htm. (Accessed 29 January 2020).
- Wojcikowski K, Gobe G. Animal studies on medicinal herbs: predictability, dose conversion and potential value. Phytother Res. 2014. January;28(1):22–7. doi: 10.1002/ptr.4966. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Zhu J, Zhang M, Ho CT, Huang Q, Cao J. Metagenomics Analysis of Gut Microbiota in a High Fat Diet-Induced Obesity Mouse Model Fed with (−)-Epigallocatechin 3-O-(3-O-Methyl) Gallate (EGCG3″Me). Mol Nutr Food Res. 2018. July;62(13):e1800274. doi: 10.1002/mnfr.201800274. [DOI] [PubMed] [Google Scholar]
- Zhou K Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. 2017. June;33:194–201. doi: 10.1016/j.jff.2017.03.045. Epub 2017 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


