Abstract
A 62-year-old man with metastatic hepatocellular carcinoma presented with ST elevation myocardial infarction had received one dose of nivolumab 3 weeks prior. Cardiac catheterisation was negative for obstructive coronary artery disease. He was transferred to the cardiac intensive care unit due to ventricular arrhythmias and markedly elevated troponin T levels. Transthoracic echocardiogram showed severely depressed left ventricular ejection fraction of 18% (normal 55%–70%) with mid and apical ballooning consistent with takotsubo syndrome (TTS). Intravenous glucocorticoids were administered due to suspicion for superimposed myocarditis. Cardiac MRI 3 days later showed mid-myocardial and subepicardial delayed enhancement in the inferior and lateral walls as well as apex indicative of myopericarditis. He clinically improved on steroids and was discharged with outpatient follow-up. This case highlights major cardiac complications that may arise with immune checkpoint inhibitor therapy. In addition, it emphasises the importance of assessing for concomitant myocarditis even when initial imaging suggests TTS.
Keywords: heart failure, cardiovascular system, immunological products and vaccines, chemotherapy
Background
Immune checkpoint inhibitor (ICI) therapies have revolutionised the landscape of cancer treatment and outcomes in recent years. Conceptually, tumour cells have the ability to evade detection by the immune system and suppress T-cell mediated responses against them. ICI therapies act by releasing the brakes on pathways that typically restrain T-cell function, thereby enhancing their cytotoxic activity on tumour cells.1 First used clinically in the treatment of advanced melanoma,2 ICI therapies have since been used in an ever-growing range of malignancies that include non-small cell lung cancer,3 renal cell carcinoma4 and Hodgkin’s lymphoma.5 Currently available therapies target cytotoxic T-lymphocyte antigen-4 as well as the programmed death-1 protein, such as nivolumab and its ligand programmed death ligand 1 (PDL1).1 However, as the indications and use of ICI therapies continue to expand, so has the medical community’s awareness of immune-related adverse events (IrAEs) in multiple organ systems, including the heart.6
Cardiovascular IrAEs have a wide spectrum of clinical presentations, including asymptomatic troponinemia, arrhythmias, takotsubo syndrome (TTS) and heart failure, and myocarditis.6 Although rare, ICI-related myocarditis can be life-threatening;7 hence, swift recognition is imperative in stemming its fulminant course. In this case report, we describe the workup and management of suspected cardiovascular IrAE post-ICI administration. Additionally, we highlight the presence of concomitant myopericarditis and TTS, a phenomenon not well defined in literature to date.
Case presentation
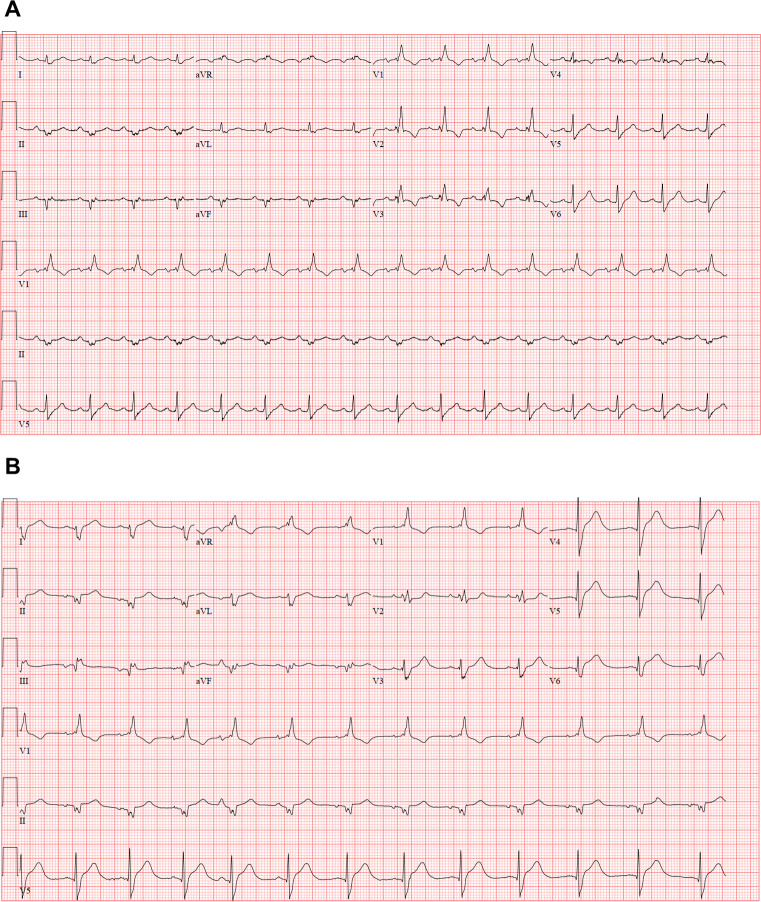
A 62-year-old man presented to the emergency department with 12 hours of mid-sternal chest pain following 3 days of nausea and vomiting. His medical history was significant for ongoing tobacco use and newly diagnosed metastatic hepatocellular carcinoma in the context of chronic, untreated hepatitis C with associated cirrhosis (Child-Pugh B). He met with an oncologist 3 weeks prior and received his first dose of nivolumab at the time. He was afebrile on presentation; blood pressure was 132/95 mm Hg and heart rate was 69 beats/min. Initial laboratory workup was remarkable for a high-sensitivity troponin T level of 408 ng/L (normal ≤15 ng/L). ECG showed sinus rhythm with right bundle branch block and left anterior fascicular block, similar to his baseline from 3 weeks ago; however, there were new ST elevations in leads V5–V6 as well as II–III (figure 1). Given concern for acute ST elevation myocardial infarction (STEMI), he was taken emergently to the cardiac catheterisation laboratory. Coronary angiography revealed mild non-obstructive coronary artery disease (CAD); however, during an attempted left ventriculogram, he went into sustained monomorphic ventricular tachycardia (VT) that terminated just before electrical cardioversion was instituted. He was subsequently transferred to the coronary intensive care unit for closer monitoring.
Figure 1.
ECG at baseline (A) and during initial presentation (B).
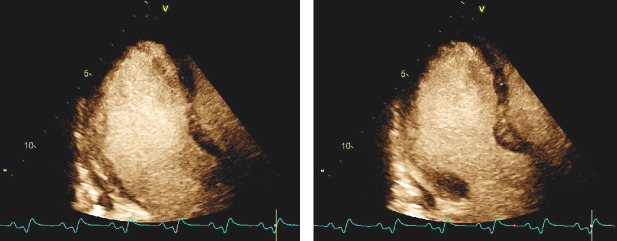
Repeat troponin T levels 2 hours and 6 hours post-presentation were 611 ng/L and 1005 ng/L respectively. N-terminal pro-B-type natriuretic peptide (NT-proBNP) was elevated at 1424 pg/mL as well (normal 5–82 pg/mL). An echocardiogram was performed at the bedside (video 1), with a representative still image during myocardial enhancement agent administration shown (figure 2). This revealed a severely depressed left ventricular ejection fraction (LVEF) of 18% (normal 55%–70%) with substantial hypokinesis of the mid and apical walls alongside relative preservation of basal contractile function. Interestingly, decreased myocardial uptake of the ultrasound enhancement agent in the apical segments was noted, which suggested possible microvascular dysfunction.8 During the first 24 hours of hospitalisation, he continued to manifest frequent ventricular ectopy and non-sustained VT.
Video 1.
Figure 2.
Apical three-chamber view with ultrasound enhancement agent administration in diastole (left) and systole (right). There is mid and apical hypokinesis with relatively preserved basal contractility. There appears to be decreased myocardial uptake of the ultrasound enhancement agent in the apical segments which may be due to microvascular dysfunction.
Although the echocardiogram appeared characteristic of TTS, the markedly elevated cardiac biomarkers in combination with severely depressed LVEF and recurrent ventricular arrhythmias raised concern for acute myocarditis. Intravenous methylprednisolone (1 mg/kg every 12 hours) was initiated and given for over 3 days for presumed ICI-related myocarditis. There was quiescence of the VT following methylprednisolone administration; additionally, his chest pain and nausea improved. Cardiac MRI (CMRI) 3 days later demonstrated mid-myocardial to subepicardial myocardial delayed enhancement (MDE) in the inferior lateral walls as well as the apex consistent with myopericarditis; LVEF was improved at 41% although an apical ballooning pattern was still present (figure 3). He was dismissed home on a prednisone taper with close outpatient follow-up.
Figure 3.
Cardiac MRI, delayed post contrast imaging in a short axis imaging plane of the heart from base (A) to apex (F). There is mid-myocardial to subepicardial myocardial delayed enhancement in the inferior and lateral walls as well as the apex. A tiny pericardial effusion is also present.
Differential diagnosis
In the presence of cardiovascular risk factors, typical chest pain and evidence of STEMI on initial testing, acute coronary occlusion is paramount on the list of differential diagnoses to assess for, ideally with coronary angiography.9 Once obstructive CAD is ruled out, possibilities like myocarditis (viral or drug-related) or a stress-mediated cardiomyopathy such as TTS should come to mind, especially when NT-proBNP levels are high and troponin levels are relatively milder.10 Additional considerations may include left ventricular (LV) aneurysm, electrolyte disturbances (hyperkalemia, hypercalcemia) and pericarditis.
Outcome and follow-up
The patient underwent repeat echocardiogram 6 weeks following initial presentation; this showed normalisation of LVEF to 56% and no regional wall motion abnormalities. However, he was noted to have a productive cough and was admitted for further management and workup. Chest CT imaging showed bilateral patchy opacities concerning for pneumonia versus ICI-related pneumonitis. He was started on broad spectrum antibiotics and his steroid therapy was intensified. Blood and respiratory cultures (from bronchoalveolar lavage) were unrevealing. His symptoms improved with treatment and he was discharged on oral antibiotics and prednisone. No further cancer-directed treatment was pursued following discussion with oncology and palliative care. He was able to travel with a friend to several states for a month thereafter but was unfortunately readmitted to the hospital for abdominal pain control. After readjustment of his oral pain regimen, he is making plans to travel again before eventually transitioning fully to hospice care.
Discussion
In a pivotal publication, Johnson et al described two cases of fulminant myocarditis that occurred after treatment with combination of nivolumab and ipilimumab.11 Both patients developed significant conduction abnormalities as well as sustained ventricular arrhythmias leading to cardiac arrest. Postmortem histopathology revealed lymphocytic infiltration of the myocardium and conduction system. At the time, there were 18 drug-related myocarditis events reported in the Bristol-Myers Squibb Corporate Safety Database among 20,594 patients who received ICI therapy (0.09%). More recent observational studies suggested a higher incidence of approximately 1%, with a significant proportion who suffered cardiogenic shock, life-threatening arrhythmias and death.7 12 TTS has also been reported in limited series. In one case, a patient with metastatic melanoma developed apical ballooning and dynamic LV outflow obstruction after four doses of ipilimumab. A restaging positron emission tomography CT scan incidentally revealed fludeoxyglucose uptake in the apex suggestive of active inflammation, although this was not protocoled as a cardiac study.13 Conversely, Ederhy et al presented two cases of TTS visualised on C-MRI; no MDE was seen in both patients.14 A retrospective analysis of the WHO Pharmacovigilance Database showed increased odds of TTS associated with ICI therapy;15 however, the true incidence of this remains unclear.
In our case report, the mid-to-apical regional wall motion abnormalities identified on initial echocardiographic imaging and high NT-proBNP levels were suggestive of TTS. However, it is important to note that this pattern is non-specific and acute myocarditis can have similar findings.16 In this case, the significantly elevated troponins and recurrent ventricular arrhythmias pointed towards a malignant course reminiscent of acute myocarditis. The patient’s positive response to empiric high-dose steroids and the CMRI findings lent further credence towards this hypothesis. To our knowledge, the present case report is the first to demonstrate evidence of acute myopericarditis in tandem with a TTS-like appearance on cardiovascular imaging. This holds significant interest not only from an imaging standpoint but has important clinical implications as well. Although TTS may not necessarily have a benign long-term prognosis,17 myocarditis is more likely to be fatal especially if not recognised early. Once ICI-related myocarditis is suspected, ICI therapy should be withheld and high-dose glucocorticoids should be given empirically before further diagnostic testing is pursued.6 Additional immunosuppression treatments like plasmapheresis and mycophenolate mofetil may be considered if patients remain refractory to steroids.18
As above, echocardiographic imaging may prove non-specific, and so advanced cardiovascular imaging can be helpful in differentiating between TTS and myocarditis. CMRI provides detailed characterisation of cardiac tissue as well as its structure and function. In the appropriate clinical setting, the presence of MDE following gadolinium administration in a pattern not consistent with a coronary artery distribution is strongly supportive of myocarditis.19 In contrast, mid-to-apical hypokinesis alongside myocardial oedema (visualised on T2-weighted imaging) is more commonly seen with TTS.20 It should be noted that MDE and microvascular obstruction in the context of TTS have also been described in limited series.20 Hence, if the diagnosis remains in doubt despite laboratory and imaging testing, endomyocardial biopsy should be considered. In this case, the patient’s clinical improvement on immunosuppression in combination with imaging data were judged to be concordant with ICI-related myocarditis; hence, the potential risk associated with endomyocardial biopsy was not felt to be warranted.
Learning points.
Regardless of other differential diagnoses, acute coronary syndrome should be emergently ruled in/out when a patient with cardiovascular risk factors presents with typical chest pain, elevated cardiac biomarkers and new ST elevations on ECG.
Immune checkpoint inhibitor therapy is associated with an increasingly recognised spectrum of cardiac complications, among which myocarditis harbours significant mortality risk.
Although takotsubo syndrome can occur in the setting of immune checkpoint inhibitor administration, clinical suspicion for concomitant myocarditis should be maintained especially in unstable patients.
If endomyocardial biopsy is unfeasible, advanced imaging modalities such as cardiac MRI can be helpful in the recognition of myocarditis.
Footnotes
Contributors: NT was responsible for compiling the case report material and writing the first draft of the manuscript. NA and BW provided critical input into the choice of images to be included in the case report. NA and BW also made important edits to the manuscript text. All authors approved of the manuscript’s final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Darvin P, Toor SM, Sasidharan Nair V, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:1–11. 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–30. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Clark JI, Quinn DI. Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Ann Oncol 2017;28:1484–94. 10.1093/annonc/mdx151 [DOI] [PubMed] [Google Scholar]
- 5.Ramchandren R, Domingo-Domènech E, Rueda A, et al. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 study. J Clin Oncol 2019;37:1997–2007. 10.1200/JCO.19.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol 2019;74:1714–27. 10.1016/j.jacc.2019.07.079 [DOI] [PubMed] [Google Scholar]
- 7.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelmoneim SS, Mankad SV, Bernier M, et al. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr 2009;22:1249–55. 10.1016/j.echo.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 9.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;127:529–55. 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 10.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on takotsubo syndrome: a position statement from the Taskforce on takotsubo syndrome of the heart failure association of the European Society of cardiology. Eur J Heart Fail 2016;18:8–27. 10.1002/ejhf.424 [DOI] [PubMed] [Google Scholar]
- 11.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210–25. 10.1016/j.ejca.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 13.Geisler BP, Raad RA, Esaian D, et al. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer 2015;3:4. 10.1186/s40425-015-0048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ederhy S, Cautela J, Ancedy Y, et al. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging 2018;11:1187–90. 10.1016/j.jcmg.2017.11.036 [DOI] [PubMed] [Google Scholar]
- 15.Ederhy S, Dolladille C, Thuny F, et al. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: a new adverse cardiac complication. Eur J Heart Fail 2019;21:945–7. 10.1002/ejhf.1497 [DOI] [PubMed] [Google Scholar]
- 16.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955–71. 10.1016/j.jacc.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghadri JR, Kato K, Cammann VL, et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol 2018;72:874–82. 10.1016/j.jacc.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 18.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging 2013;6:833–9. 10.1161/CIRCIMAGING.113.000416 [DOI] [PubMed] [Google Scholar]
- 20.Bratis K. Cardiac magnetic resonance in takotsubo syndrome. Eur Cardiol 2017;12:58–62. 10.15420/ecr.2017:7:2 [DOI] [PMC free article] [PubMed] [Google Scholar]