Abstract
We present this case of human herpes virus 8-positive germinotropic lymphoproliferative disorder in a 20-year-old woman seen in the surgical oncology clinic for localised lymphadenopathy. This is the first case to be reported in the UK, and we discuss it along with a literature review including investigations and treatment options. This will demonstrate the importance of preoperative workup and multidisciplinary teamwork in deciding management plans and serve as a guide for future encounters of this rare condition in clinical practice.
Keywords: surgical oncology, pathology
Background
Human herpes virus 8 (HHV-8), also known as Kaposi sarcoma human virus, has been described to cause germinotropic lymphoproliferative disorder (GLPD),1 as well as other lymphoproliferative disorders including; primary effusion lymphoma, multicentric Castleman disease (MCD) and MCD-associated plasmablastic lymphoma.2 HHV 8+ve GLPD is a rare condition, with 20 cases reported in the literature,3 4 which typically presents as localised lymphadenopathy in immunocompetent otherwise asymptomatic patients;4 however, in a few HIV-positive cases, it has been also reported to present as generalised lymphadenopathy,2 4 B symptoms and even progression to high-grade lymphoma5 6 which has raised questions about the overlap between HHV-8 +ve GLPD and MCD-associated plasmablastic lymphoma as parts of the spectrum of lymphoproliferative diseases rather than separate entities.2 5 When it presents in a typical manner, HHV-8 +ve GLPD is reported to have good outcomes when treated by surgery with or without radiotherapy or chemotherapy.3 4
Case presentation
A 20-year-old woman presented to the surgical clinic with a 1-year history of a lump in the left groin, which was increasing in size and causing occasional discomfort but not associated with any symptoms of lethargy, night sweats or weight loss. She had a medical history of transposition of the great arteries as an infant for which she had surgery at 6 weeks old and has been followed up by the cardiologists since but was not on any regular medication. She was a non-smoker, did not drink alcohol and reported no history of drug abuse. There was no significant family history of note.
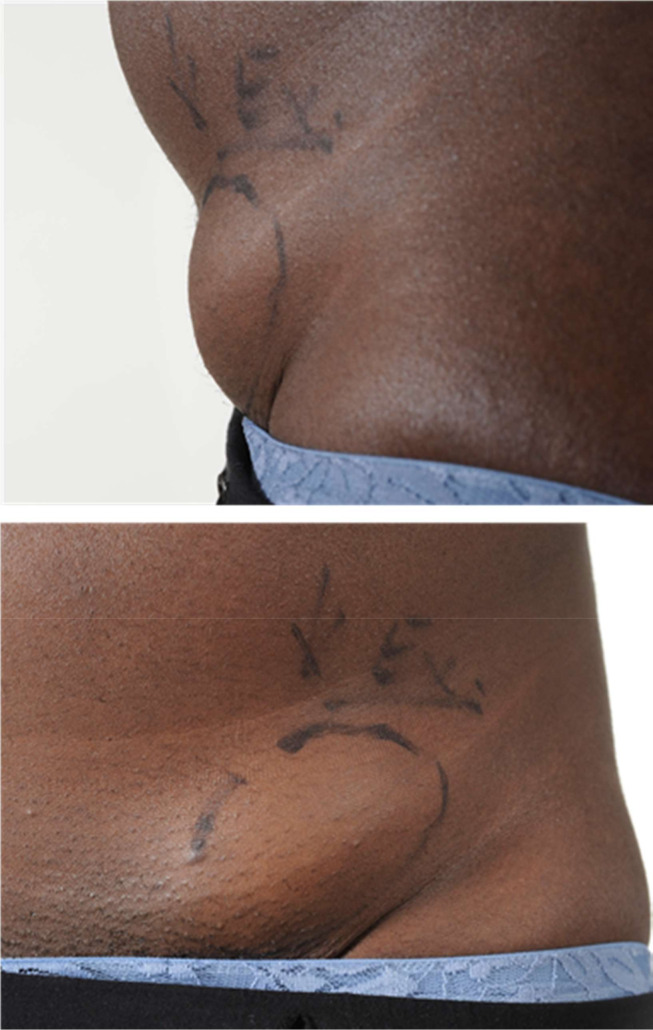
Physical examination (figure 1) revealed a 7×4 cm partly mobile mass lesion overlying the medial one-third of the inguinal ligament but no associated lymphadenopathy and apart from some healing skin lesions at the back of her neck, which she attributed to a recent episode of eczema exacerbation and chest scars compatible with her surgical history the rest of her examination was unremarkable, she was apyrexial, and her vital signs were all within the normal range.
Figure 1.
Physical examination.
Investigations
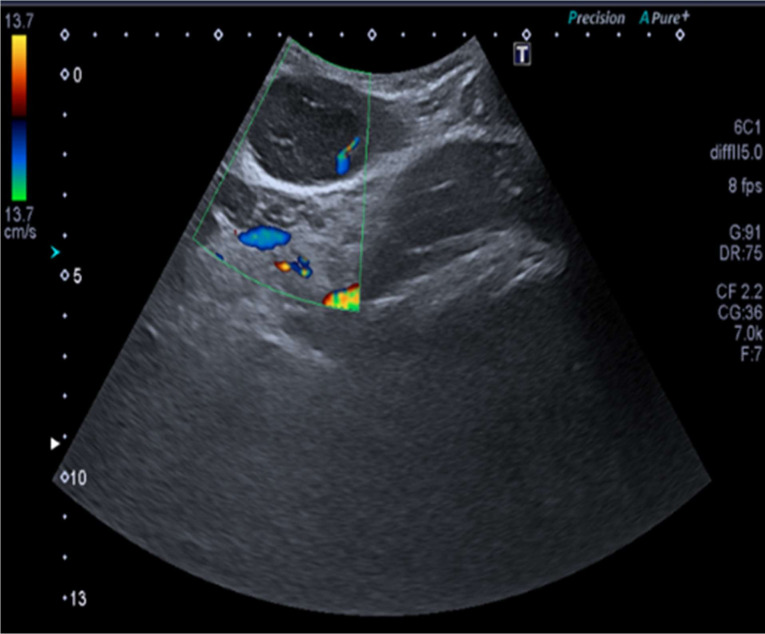
Basic blood tests, including a full blood count and biochemistry showed red blood cell (RBC) microcytosis (79.0 fL) and lymphopenia (1.2×109/L) but were otherwise within the normal range. The initial ultrasound scan (figure 2) reported a 7.1×3×4.2 cm indeterminate superficial, swelling with no pelvic connection and underwent a diagnostic core biopsy. This reported HHV-8+ve GLPD. Subsequent blood tests (table 1) revealed detection of HHV-8 nucleic acid in the plasma, an Epstein-Barr Virus (EBV) viral load log value of <2.50 whereas Human Immunodeficiency Virus (HIV), Hepatitis B surface (HBs) antigen and Hepatitis C Virus (HCV) antibody were negative.
Figure 2.
Ultrasound scan.
Table 1.
Virology and serological markers
| Parameter | Result |
| HIV | Not detected |
| HepB sAg | Not detected |
| HepC Ig | Not detected |
| CMV IgM | Not detected |
| EBV nuclear IgG | Equivocal |
| EBV capsid IgG | Detected |
| EBV VCA IgM | Not detected |
| EBV nucleic acid | Detected, <316 copies/mL |
| EBV log viral load | <2.5 |
| HHV-8 DNA | Detected,<100 copies/mL |
CMV IgM, CytomegaloVirus IgM Antibody; EBV capsid IgG, Epstein-Barr Virus capsid IgG Antibody; EBV log viral load, Epstein-Barr Virus viral load; EBV nuclear IgG, Epstein-Barr Virus nuclear IgG Antibody; EBV nucleic acid, Epstein-Barr nucleic acid; EBV VCA IgM, Epstein-Barr Virus Viral Capsid Antibody IgM; HepB sAg, Hepatitis B surface Antigen; HepC Ig, Hepatitis C Ig Antibody; HHV-8, human herpes virus 8; HIV, Human Immunodeficiency Virus.
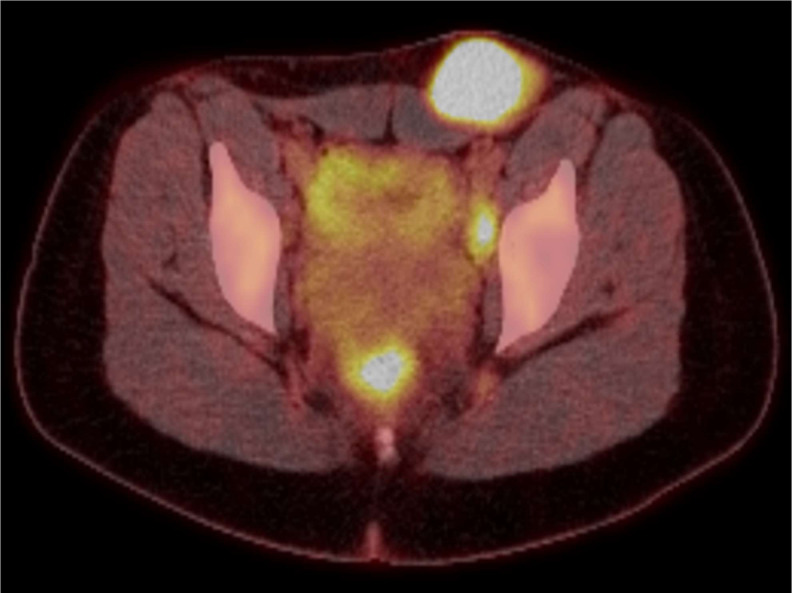
A staging F-18-FDG PET CT (Flourine 18 Fluorodeoxyglucose Positron Emission Tomography) scan (figure 3) from skull base to mid-thighs reported a number of enlarged metabolically active left groin and obturator lymph nodes in keeping with the known lymphoma but no other metabolically active retroperitoneal, mesenteric, intrathoracic or cervical adenopathy and the scan was otherwise unremarkable.
Figure 3.
Staging F-18-FDG PET (Flourine 18 Fluorodeoxyglucose Positron Emission Tomography) scan.
Treatment
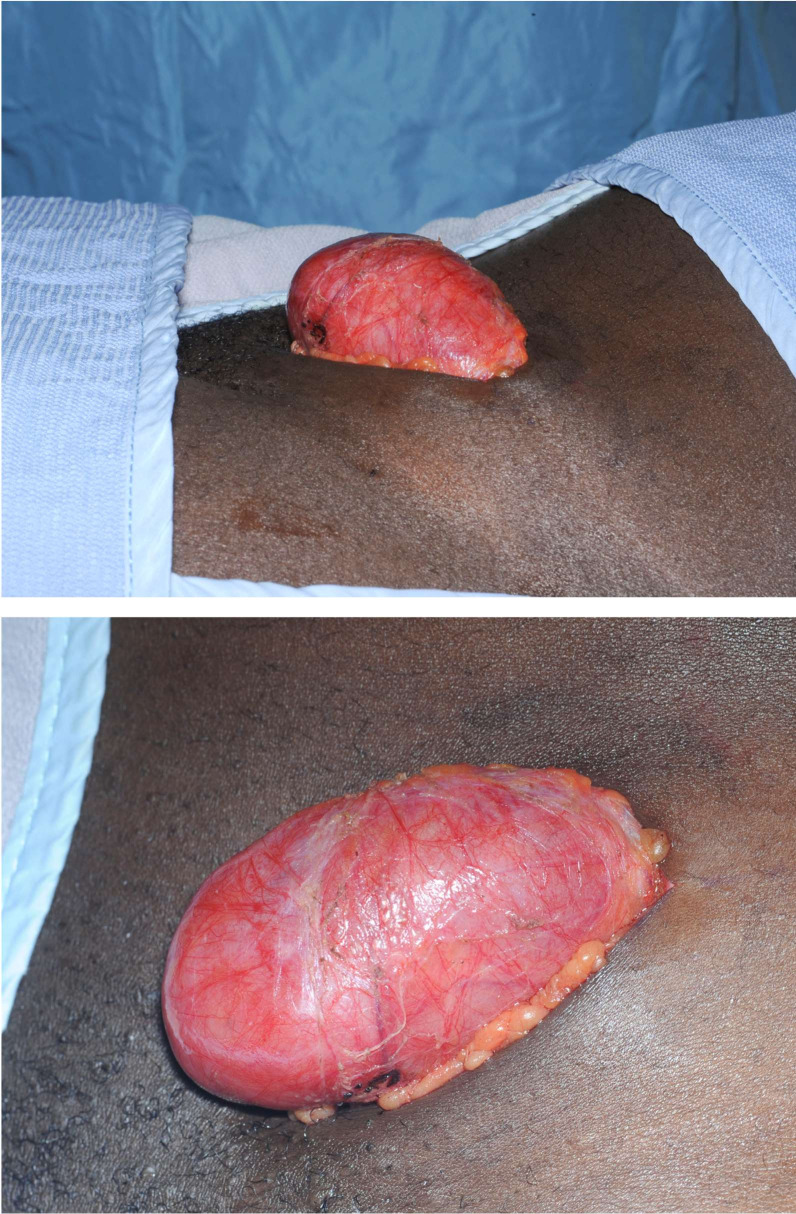
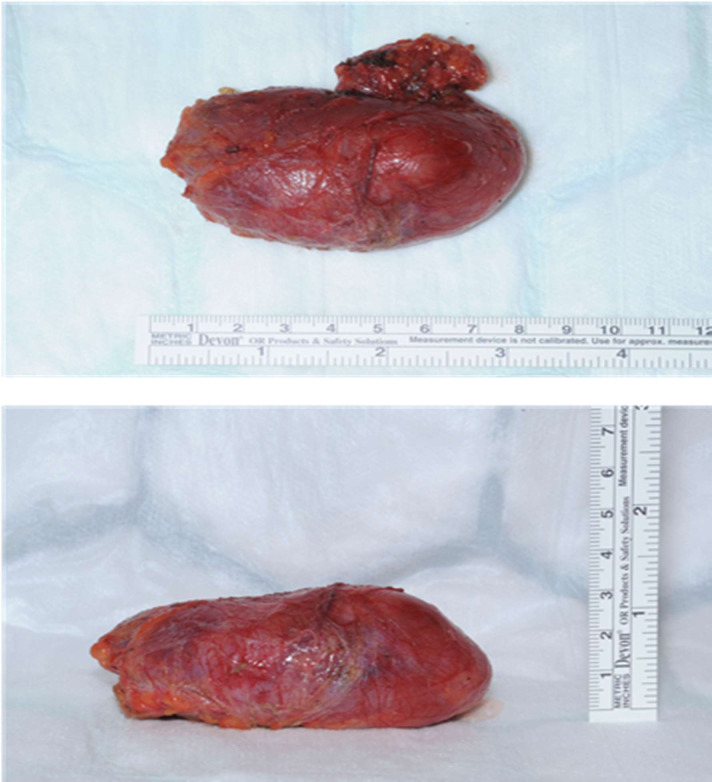
This young woman was initially seen in the surgical oncology clinic, and her subsequent management was coordinated by the lymphoma multidisciplinary team. Her case was discussed with the regional teenage/young adults lymphoma and cancer team, and given the rarity of her disease, input was requested from the sarcoma, melanoma and rare tumours multidisciplinary team. She underwent excision biopsy (figures 4 and 5) of the mass lesion by the surgical team which was sent for full histological examination, and she received postoperative involved-field radiotherapy to the left pelvic area under the oncology and haematology teams.
Figure 4.
Intraoperative findings.
Figure 5.
Postoperative/excision findings.
Outcome and follow-up
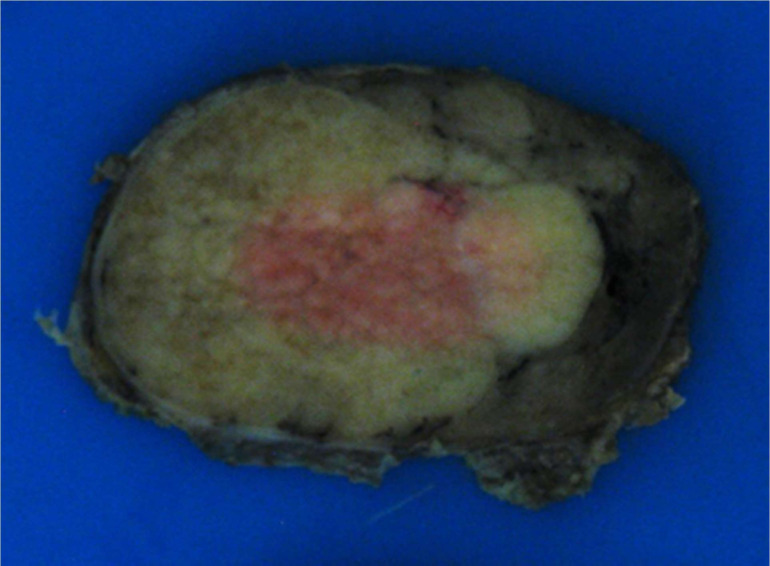
Histopathological examination macroscopically (figure 6) revealed a smooth nodule of tissue measuring 77×61×41 mm with shiny surface capsule and some attached fatty tissue weighing 61.5 g. Cut-surface showed a pale confluent multinodular area confined to the parenchyma and not infiltrating beyond the capsule.
Figure 6.
Histology, macroscopic examination.
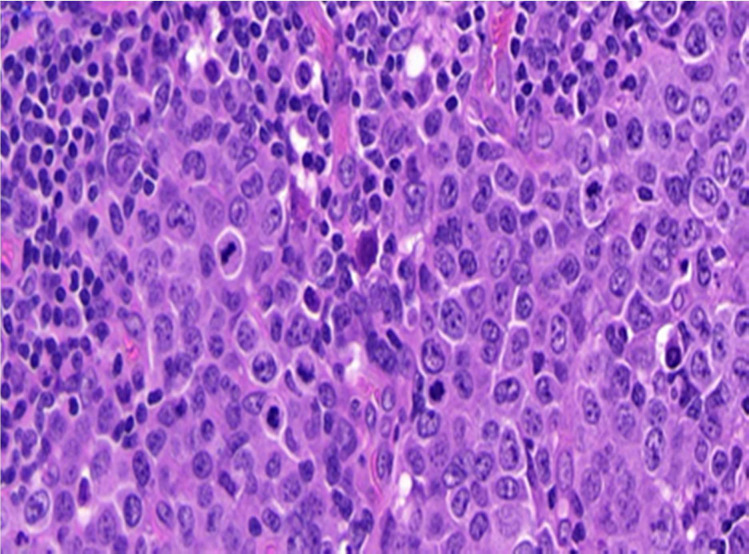
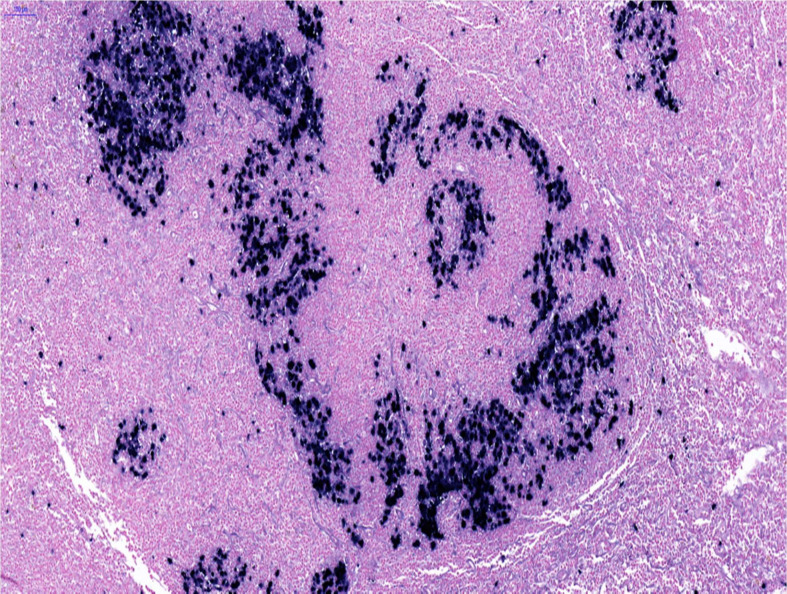
Microscopically (figure 7), sections showed a lymph node with preserved architecture and patent subcapsular sinus. Within the node, numerous distorted follicles expanded by large atypical cells were seen. The atypical cells were present in clusters centred on disrupted germinal centres and showed plasmablastic morphology comprising a smooth nuclear contour, vesicular eccentrically placed nuclei, multiple nucleoli and amphophilic cytoplasm. Frequent mitoses and apoptotic debris were seen associated with these cells.
Figure 7.
Histology, microscopic examination.
Occasional single atypical cells are also seen in the interfollicular areas. The paracortex showed mixed medium-sized lymphocytes and numerous plasma cells.
The large atypical cells infiltrating germinal centres were negative to CD45, CD79a and CD20. They were strongly positive for MUM1 (Multiple Myeloma 1 protein) and showed variable positivity for CD138. CD15, CD3, BCL2 (B Cell Lymphoma) and BCL6 showed focal patchy positivity. CD5 and CD30 were negative. CD23 and CD21 highlighted expanded dendritic cell meshworks in association with the follicles. CD10 showed some expression between the atypical cells confirming these are present expanding germinal centres.
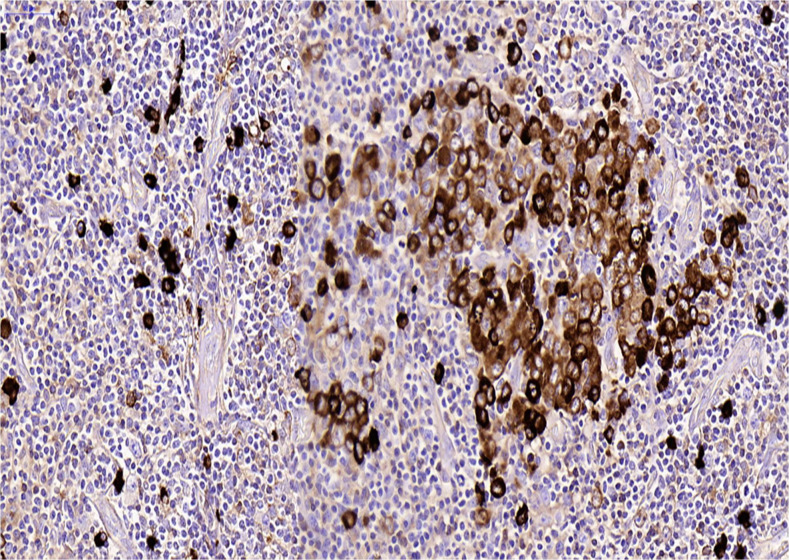
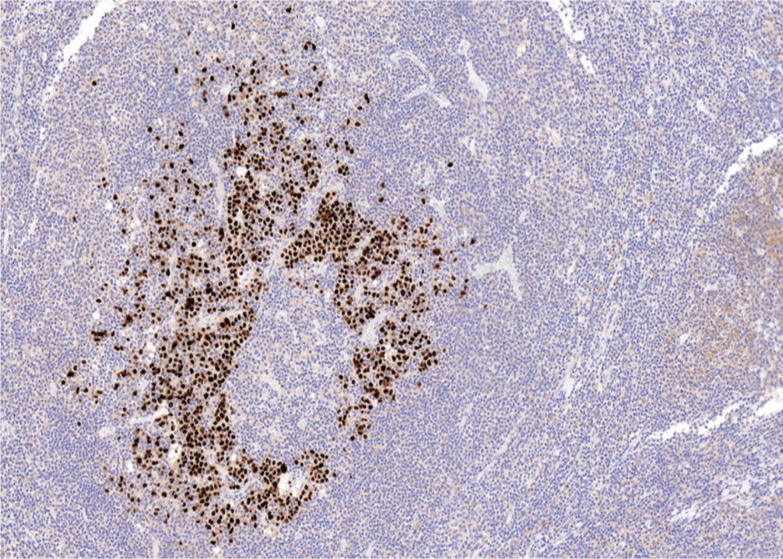
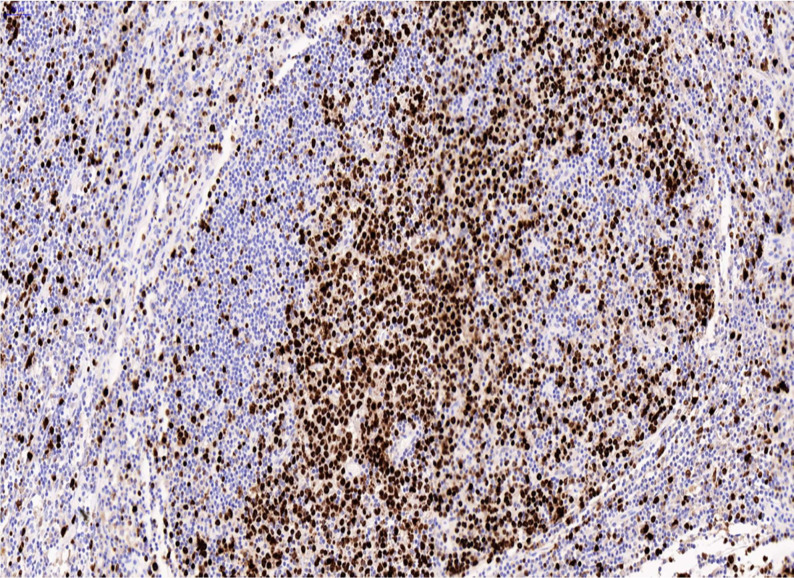
Lambda light chain restriction was seen within the large atypical cells (figure 8). There was strong dual positivity for EBV-encoded RNA (detected by in-situ hybridisation) (figure 9) and HHV-8 (detected by immunohistochemistry) in the blast-like cells (figure 10). Ki-67 showed a high proliferation rate among the atypical cells (figure 11).
Figure 8.
Lambda light chain restriction.
Figure 9.
EBV (in situ hybridisation).
Figure 10.
Human herpes virus 8 (immunohistochemistry).
Figure 11.
Ki-67.
The diagnosis was confirmed as HHV-8 and EBV-positive GLPD.
She made an excellent postoperative recovery and her wounds healed nicely and during follow-up by the oncology and haematology teams after radiotherapy her CT scans showed complete response to treatment.
Discussion
HHV-8 and EBV-associated GLPD is characterised by plasmablasts that are co-infected by HHV-8 and EBV and that preferentially involve the germinal centres of lymphoid follicles in lymph nodes.6 It is such a rare condition that only been 20 cases have been reported (see table 2).3 4 Although typically it presents as localised lymphadenopathy with no other symptoms in immunocompetent individuals4 as in our case, occasionally it has also been reported to present with generalised lymphadenopathy with B symptoms (weakness, dyspnoea, anorexia, fevers and chills),2 particularly in HIV positive patients with suggestions of its overlap with other lymphoproliferative conditions such as MCD-associated plasmablastic lymphoma.2 4 5 There are no set guidelines for its management and information regarding treatment is restricted to the few published case reports where the successful options were chemotherapy, surgical excision or surgical excision followed by radiotherapy that mostly had favourable outcomes and resulted in complete remission especially in typical presentation with localised disease.
Table 2.
Showing reported cases of HHV-8 Positive GPLD in English literature arranged chronologically with clinical details, management and outcomes3 4
| Study/ year |
Age in years /sex | Primary site | Systemic/ other features |
HIV | Treatment | Follow-up in months | Response |
| Du et al/20021 | 41/M | Axillary and cervical LN | Perianal lymphadenopathy | −ve | Six cycles of CHOP | 84 | Complete remission |
| Du et al/20021 | 61/M | Submandibular and inguinal LN | Slightly enlarged spleen | −ve | Surgical excision and radiotherapy | 180 | Complete remission |
| Du et al/20021 | 63/F | Paraaortic LN | No | Not available | Not available | – | Not available |
| Seliem et al/20072 | 45/M | Generalised LN | B symptoms, effusion and SMG | +ve | Prednisolone, rituximab and cyclophosphamide | 2 | Deterioration and death |
| D’antonio et al/200710 | 60/M | Cervical LN | B symptoms | −ve | Surgery | 24 | Complete remission |
| Oh et al/20117 | 75/M | Left submandibular LN | No | −ve | Four cycles of CHOP | 19 | Complete remission |
| D’antonio et al/201110 | 65/M | Right cervical LN | No | −ve | Surgery | 84 | Complete remission |
| Taris et al/201411 | 49/F | Right cervical LN | No | −ve | Surgical excision and radiotherapy | >12 | Complete remission |
| Courville et al/20145 | 63/F | Mesenteric LN | Warm autoimmune haemolytic anaemia, HBV and HCV | −ve | EPOCH | 13 | Progression to high-grade lymphoma |
| Papoudou-Bai et al/201513 | 53/M | Right cervical and supraclavicular LN | No | −ve | Not available | – | Complete remission |
| Gonzalez-Farre et al/20174 | 86/M | Submental LN | No | −ve | No treatment | 3 | Stable disease |
| Gonzalez-Farre et al/20174 | 52/M | Inguinal LN | No | −ve | CHOP | – | Not available |
| Gonzalez-Farre et al/20174 | 47/M | Generalised LN | B symptoms, effusion and SMG | +ve | CHOP | 11 | Complete remission |
| Gonzalez-Farre et al/20174 | 27/M | Generalised LN | B symptoms | +ve | Rituximab | 10 | Partial response, disease progression and chemotherapy |
| Gonzalez-Farre et al/20174 | 30/M | Generalised LN | B symptoms | +ve | R-DA-EPOCH | 46 | Stable and persistent disease |
| Gonzalez-Farre et al/20174 | 42/M | Generalised LN | B symptoms, effusion and SMG |
+ve | R-EPOCH | 4 | Complete remission |
| Ronaghy et al/20173 | 73/M | Paraaortic LN | No | −ve | Surgery | 16 | Complete remission |
| Bacha et al/201712 | 73/F | Inguinal LN | Weight loss | −ve | No treatment | – | No Follow-up |
| Bhavsar et al/20176 | 84/F | Cervical LN | No | −ve | CHOP | 18 | Complete remission |
| Bhavsar et al/20176 | 58/M | Right axillary LN | No | +ve | CHOP | 12 | Initial remision then progression to high-grade lymphoma |
CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; EPOCH, doxorubicin, etoposide, vincristine, cyclophosphamide and prednisolone; GLPD, germinotropic lymphoproliferative disorder; HHV, human herpes virus; LN, Lymphadenopathy.
Chemotherapy and medical treatment
The preferred regimen of chemotherapy is cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 and prednisone 100 mg. Of the 11 patients treated with chemotherapy from the cases mentioned, 6 patients received this treatment, 4 of which achieved remission and remained disease-free throughout periods of follow-up ranging from 11 to 84 months1 4 6 7 whereas 1 patient progressed to high-grade lymphoma then passed away6 and 1 patient had no information on follow-up after receiving the treatment. Other regimens of chemotherapy include; EPOCH which was used for a 63-year-old woman with mesenteric lymphadenopathy who progressed to high-grade lymphoma and continued management as such5 and R-EPOCH (rituximab (rituxan; Genentech) 375 as 3-hour infusion day 1; doxorubicin (generic) 10, etoposide (generic) 50 and vincristine (generic) 0.4 (no cap) as a continuous infusion on days 1, 2, 3 and 4 (96 hours total); cyclophosphamide (generic) 750 as a 2-hour infusion on day 5; and prednisone (generic) 60 mg two times per day (120 mg/m2/day) on days 1, 2, 3, 4 and 5)8 which was used for two patients; one went to full remission maintained for 4 months of follow-up whereas the other continued to have the persistent disease for 46 months of follow-up.4 The remaining two patients from the medical treatment group were a 27-year-old man who was treated for generalised lymphadenopathy with only rituximab initially which only achieved a partial response and he went on to have the progressive disease which was only cleared using further chemotherapy and remained in remission at 10 months of follow-up4 and a 45-year-old man who received prednisolone, rituximab and cyclophosphamide for generalised lymphadenopathy before quickly deteriorating and dying within 2 months of being diagnosed.2
Surgical treatment and radiotherapy
Surgery was the treatment of choice in five cases, all presenting with localised lymphadenopathy, two of which also had postoperative radiotherapy. Within the group treated with surgery only the remission rate was 100% and that state was maintained for follow-up periods ranging from 16 to 84 months.3 9 10 The patients who received postoperative radiotherapy also had a favourable outcome that was maintained throughout one for a year11 and the other almost 14 years of follow-up.1
No treatment
In the reviewed literature two cases did not receive any treatment; one was an 86-year-old man was managed conservatively with a ‘wait and see’ approach and the disease remained stable up to 3 months of follow-up with no progression4 and a 73-year-old woman with inguinal lymphadenopathy associated with weight loss that was lost to follow-up and therefore never received any treatment.12
The data for treatment and follow-up for the remaining two cases reported in the literature is not available one was a 63-year-old woman only mentioned to have had para-aortic lymphadenopathy1 and the other was a 53-year-old man who presented with cervical lymphadenopathy and although no details of his treatment were provided, it was reported that he had a good outcome.13
The clinical presentation of the case we report was typical of HHV-8+ve GLPD with localised lymphadenopathy in an immune-competent and HIV negative patient that generally has good outcomes,3 although some cases have deteriorated and had unfavourable outcomes especially in HIV positive patients and with a crossover between GLPD and MCD as closely related conditions on the same spectrum of lymphoproliferative diseases.2 4 The treatment of choice was surgical excision which, given her localised disease and the accessibility of the affected lymph nodes as well as providing symptomatic relief and further material for pathological and immunohistochemical testing, was the reasonable option. This was followed by radiotherapy to insure the complete clearance of the disease from the remaining lymph nodes in the pelvic area. So far, she has made good progress, and we are hoping that she remains well throughout the follow-up after completion of treatment.
Learning points.
Human herpes virus 8-positive germinotropic lymphoproliferative disorder should be considered in the differential diagnosis of unusual causes of lymphadenopathy
Preoperative investigations, such as core biopsy are key to establishing diagnosis and deciding the best method of treatment.
Multidisciplinary teams and collaboration of different specialties and institutions allow formulating the best possible management plan.
Due to the variety of treatment options for this poorly understood and rarely encountered condition, treatment plans have to be dicided on a case by case basis taking in consideration of accessibility of lymph nodes, extent of spread and immune status.
Good documentation and reporting of outcomes and follow-up can drive further research to obtain evidence based, effective, standardised treatment.
Acknowledgments
There has been major role in the diagnosis of the case from Dr Deirdre McCormick and Dr Christopher Moffat (Consultants at the Pathology Department in Portsmouth Hospitals NHS Trust, UK).
Footnotes
Contributors: AG the main and corresponding author of this case report including the literature search and discussion. JC provided the histopathology reports, images and contributed to the editing of the manuscript. ES made the diagnosis and provided support from a pathology perspective and AA was the operating surgeon and contributed to the editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Du M-Q, Diss TC, Liu H, et al. . KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 2002;100:3415–8. 10.1182/blood-2002-02-0487 [DOI] [PubMed] [Google Scholar]
- 2.Seliem RM, Griffith RC, Harris NL, et al. . HHV-8+, EBV+ multicentric plasmablastic microlymphoma in an HIV+ man: the spectrum of HHV-8+ lymphoproliferative disorders expands. Am J Surg Pathol 2007;31:1439–45. 10.1097/PAS.0b013e31804d43d8 [DOI] [PubMed] [Google Scholar]
- 3.Ronaghy A, Wang H-Y, Thorson JA, et al. . PD-L1 and Notch1 expression in KSHV/HHV-8 and EBV associated germinotropic lymphoproliferative disorder: case report and review of the literature. Pathology 2017;49:430–5. 10.1016/j.pathol.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Farre B, Martinez D, Lopez-Guerra M, et al. . HHV8-related lymphoid proliferations: a broad spectrum of lesions from reactive lymphoid hyperplasia to overt lymphoma. Mod Pathol 2017;30:745–60. 10.1038/modpathol.2016.233 [DOI] [PubMed] [Google Scholar]
- 5.Courville EL, Sohani AR, Hasserjian RP, et al. . Diverse clinicopathologic features in human herpesvirus 8-associated lymphomas lead to diagnostic problems. Am J Clin Pathol 2014;142:816–29. 10.1309/AJCPULI3W6WUGGPY [DOI] [PubMed] [Google Scholar]
- 6.Bhavsar T, Lee JC, Perner Y, et al. . KSHV-associated and EBV-associated Germinotropic lymphoproliferative disorder: new findings and review of the literature. Am J Surg Pathol 2017;41:795–800. 10.1097/PAS.0000000000000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh J, Yoon H, Shin DK, et al. . A case of successful management of HHV-8⁺, EBV⁺ germinotropic lymphoproliferative disorder (gld). Int J Hematol 2012;95:107–11. 10.1007/s12185-011-0975-8 [DOI] [PubMed] [Google Scholar]
- 8.Dunleavy K, Pittaluga S, Shovlin M, et al. . Low-Intensity therapy in adults with Burkitt's lymphoma. N Engl J Med 2013;369:1915–25. 10.1056/NEJMoa1308392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Antonio A, Antonio D'Antonio, Boscaino A, et al. . KSHV- and EBV-associated germinotropic lymphoproliferative disorder: a rare lymphoproliferative disease of HIV patient with plasmablastic morphology, indolent course and favourable response to therapy. Leuk Lymphoma 2007;48:1444–7. 10.1080/10428190701387039 [DOI] [PubMed] [Google Scholar]
- 10.D'Antonio A, Addesso M, Memoli D, et al. . Lymph node-based disease and HHV-8/KSHV infection in HIV seronegative patients: report of three new cases of a heterogeneous group of diseases. Int J Hematol 2011;93:795–801. 10.1007/s12185-011-0849-0 [DOI] [PubMed] [Google Scholar]
- 11.Taris M, de Mascarel A, Riols M, et al. . [KHSV/EBV associated germinotropic lymphoproliferative disorder: a rare entity, case report and review of the literature]. Ann Pathol 2014;34:373–7. 10.1016/j.annpat.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 12.Bacha D, Chelly B, Kilani H, et al. . HHV8/EBV coinfection lymphoproliferative disorder: rare entity with a favorable outcome. Case Rep Hematol 2017;2017:1–8. 10.1155/2017/1578429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papoudou-Bai A, Hatzimichael E, Kyriazopoulou L, et al. . Rare variants in the spectrum of human herpesvirus 8/Epstein-Barr virus-copositive lymphoproliferations. Hum Pathol 2015;46:1566–71. 10.1016/j.humpath.2015.06.020 [DOI] [PubMed] [Google Scholar]