Abstract
Introduction
Studies suggest that patients with cancer are more likely to experience severe outcomes from COVID-19. Therefore, cancer centres have undertaken efforts to care for patients with cancer in COVID-free units. Nevertheless, the frequency and relevance of nosocomial transmission of COVID-19 in patients with cancer remain unknown. The goal of this study was to determine the incidence and impact of hospital-acquired COVID-19 in this population and identify predictive factors for COVID-19 severity in patients with cancer.
Methods
Patients with cancer and a laboratory-confirmed diagnosis of COVID-19 were prospectively identified using provincial registries and hospital databases between March 3rd and May 23rd, 2020 in the provinces of Quebec and British Columbia in Canada. Patient's baseline characteristics including age, sex, comorbidities, cancer type and type of anticancer treatment were collected. The exposure of interest was incidence of hospital-acquired infection defined by diagnosis of SARS-CoV-2 ≥ 5 days after hospital admission for COVID-unrelated cause. Co-primary outcomes were death or composite outcomes of severe illness from COVID-19 such as hospitalisation, supplemental oxygen, intensive-care unit (ICU) admission and/or mechanical ventilation.
Results
A total of 252 patients (N = 249 adult and N = 3 paediatric) with COVID-19 and cancer were identified, and the majority were residents of Quebec (N = 233). One hundred and six patients (42.1%) received active anticancer treatment in the last 3 months before COVID-19 diagnosis. During a median follow-up of 25 days, 33 (13.1%) required admission to the ICU, and 71 (28.2%) died. Forty-seven (19.1%) had a diagnosis of hospital-acquired COVID-19. Median overall survival was shorter in those with hospital-acquired infection than that in a contemporary community-acquired population (27 days versus unreached, hazard ratio (HR) = 2.3, 95% CI: 1.2–4.4, p = 0.0006. Multivariate analysis demonstrated that hospital-acquired COVID-19, age, Eastern Cooperative Oncology Group status and advanced stage of cancer were independently associated with death.
Interpretation
Our study demonstrates a high rate of nosocomial transmission of COVID-19, associated with increased mortality in both univariate and multivariate analysis in the cancer population, reinforcing the importance of treating patients with cancer in COVID-free units. We also validated that age and advanced cancer were negative predictive factors for COVID-19 severity in patients with cancer.
Keywords: COVID-19, Cancer, Hospital-acquired, Nosocomial
1. Introduction
In December 2019, a cluster of pneumonias caused by a novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan, China [1]. As of July 15th, 2020, more than 13 382 020 million cases of COVID-19 have been declared worldwide, with more than 579 546 deaths (the case-fatality rate globally of ∼4%) [1,2]. Risk factors for severe disease include older age and underlying comorbidities, such as obesity and diabetes [3,4].
Patients with cancer are at particularly increased risk for severe COVID-19.[[4], [5], [6]] Indeed, Liang et al. [5] first described a nationwide analysis in China of COVID-19 in patients with cancer. In this study of 1590 patients, however, only 18 patients (1.1%) had a diagnosis of cancer. Compared with patients without cancer, these patients were more likely to experience severe COVID-19. In another retrospective analysis, Yu et al. [7] identified COVID-19 in 0.79% of their patients with cancer (12 of 1524 patients), which was higher than the cumulative incidence of all diagnosed COVID-19 cases that was reported in the city of Wuhan over the same time period (0.37%; 41 152 of 11 081 000 cases). Next, a report from Italy demonstrated that among 355 patients hospitalised for COVID-19, 20.3% of patients had active cancer [8]. To address the severity of COVID-19 among patients with cancer, Dai et al. [9] studied the outcomes of 105 patients with cancer with COVID-19 compared with 536 matched controls with COVID-19. Patients with cancer had higher risks in all severe outcomes, and patients with haematological malignancy, lung cancer, or with stage IV cancer were more likely to experience severe events [9]. More recent reports in a large cohort of 928 patients from the COVID-19 and Cancer Consortium (CCC19) identified that age, smoking status, male sex, Eastern Cooperative Oncology Group (ECOG) performance status and presence of comorbidities were independent risk factors for severe COVID-19 [6].
Despite these advances in the understanding of COVID-19 and cancer, the frequency of hospital-acquired infection in this population at a high risk for severe disease remains unknown. Data from Wuhan demonstrated a 7.1% rate of nosocomial transmission of COVID-19 among 918 patients with COVID-19 [10], while a study from the UK found a rate of 20% [11]. These alarming rates occurred in the absence of optimised targeted infection control and prevention (ICP) measures [12]. Because of these reports, as well as the high level of contact that patients with cancer have with the healthcare system (e.g. frequent blood tests, treatments and hospitalisations for treatment-related complications), cancer centres rapidly enforced ICP measures to limit exposure of these patients to COVID-19. There is therefore an urgent need to describe the impact of hospital-acquired COVID-19 infection in patients with cancer to reinforce stringent infection control strategies to protect this vulnerable population. The goal of the present study was to characterise the incidence of hospital-acquired transmission in Canadian cancer centres across two provinces and further define predictive factors for COVID-19 severity in the cancer patient population.
2. Methods
2.1. Patient population
We conducted an observational cohort study of patients with cancer and a laboratory-confirmed diagnosis of COVID-19. Patients were identified using provincial registries and hospital databases between March 3rd and May 23rd, 2020. Patients were also prospectively identified by their treating oncologist (or haematologist) and referred for study inclusion. This study was conducted across eight Canadian institutions in Quebec and British Columbia and was approved by the institutional ethics committee at each site (Ethics number: MP-02-2020-8911 and H20-00892).
The inclusion criteria for this study were patients with a laboratory-confirmed case of COVID-19 with any history of invasive malignancy (either solid or haematologic). All clinical data were extracted from chart review. Patients’ baseline characteristics were recorded and included age, gender, ECOG status before COVID-19 illness, smoking history, tumour type, presence of co-morbidities and concomitant medications, including active anticancer treatment at the time of COVID-19 illness.
The exposure of interest was incidence of hospital-acquired infection, defined by a diagnosis of COVID-19 ≥ 5 days after admission to the hospital for a COVID-unrelated cause in a non-COVID unit according to the national public health definition [13]. Acquisition in long-term care facilities were not considered hospital-acquired. Primary outcome was death from any cause or/and co-primary outcomes were composite outcomes of severe illness from COVID-19 such as supplemental oxygen, intensive-care unit (ICU) admission and/or mechanical ventilation. Clinical outcomes, including mortality, were ascertained through patient's medical records reviewing following hospital discharge. The index date was set from time of COVID-19 detection until the date of last follow-up or death.
2.2. Statistical analysis
Descriptive analyses were performed for age, sex, cancer type, stage, active cancer therapy and type of therapy, cancer status, smoking history and presence of co-morbidities in accordance with type of COVID-19 infection (hospital-acquired versus community-acquired) and baseline patient and tumour characteristics were compared using the Chi-squared test. There were no corrections for multiple comparisons. Active anticancer therapy was defined as either 1) cytotoxic chemotherapy or 2) all other therapies (targeted agents, endocrine therapy, immunotherapy, radiotherapy) received within 3 months of COVID-19 diagnosis. Survival curves were estimated using the Kaplan–Meier method and compared with the log-rank test (Mantel–Cox method) in a univariate analysis. Multivariate analysis was performed using multivariable Cox regression model to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for death adjusting for other clinicopathologic features. All tests were two-sided and statistical significance was set at a p-value <0.05. All statistical analyses were conducted using the GraphPad Prism and R softwares.
3. Results
A total of 252 patients (N = 249 adult and N = 3 paediatric) with COVID-19 and cancer were identified, and the majority were residents of the province of Quebec (N = 233). Patients’ baseline characteristics at time of COVID-19 diagnosis are presented in Table 1 .
Table 1.
Baseline characteristics.
| Patient characteristics | COVID-19 setting |
||||
|---|---|---|---|---|---|
| Total (N = 252) | Hospital-acquired (N = 47) | Community-acquired (N = 205) | p-value | ||
| Age (year) | Median age | 73 | 69 | 74 | – |
| Range | 4–95 | 36–89 | 4–95 | ||
| Gender - no. (%) | Male | 127 (50) | 26 (55) | 101 (49) | 0.45 |
| Female | 125 (50) | 21 (45) | 104 (50) | ||
| ECOG-PS - no. (%) | 0 | 49 (19) | 6 (13) | 43 (21) | 0.17 |
| 1 | 92 (37) | 22 (47) | 70 (34) | ||
| 2 | 50 (20) | 8 (17) | 42 (20) | ||
| 3 | 27 (11) | 9 (19) | 18 (9) | ||
| 4 | 8 (3) | 1 (2) | 7 (3) | ||
| Unknown | 26 (10) | 1 (2) | 25 (12) | ||
| Cancer type - no. (%) | Lung | 36 (14) | 12 (26) | 24 (12) | – |
| Breast | 34 (13) | 5 (11) | 29 (14) | ||
| Lymphoma | 28 (11) | 5 (11) | 23 (11) | ||
| Prostate | 22 (9) | 4 (9) | 18 (9) | ||
| Colon/colorectal | 20 (8) | 4 (9) | 16 (8) | ||
| Leukaemia | 20 (8) | 2 (4) | 18 (9) | ||
| Gynaecologic | 15 (6) | 3 (6) | 12 (6) | ||
| Multiple myeloma | 11 (4) | 0 | 11 (5) | ||
| Bladder | 8 (3) | 0 | 8 (4) | ||
| Renal cell cancer | 8 (3) | 0 | 8 (4) | ||
| Myeloproliferative neoplasm | 7 (3) | 0 | 7 (3) | ||
| Pancreas | 7 (3) | 2 (4) | 5 (2) | ||
| Esophageal/gastric | 6 (2) | 1 (2) | 5 (2) | ||
| Sarcoma | 5 (2) | 1 (2) | 4 (2) | ||
| Skin cancers | 4 (2) | 1 (2) | 3 (1) | ||
| CNS [2] | 4 (2) | 2 (4) | 2 (1) | ||
| Cholangiocarcinoma | 4 (2) | 2 (4) | 2 (1) | ||
| Thyroid | 4 (2) | 0 | 4 (2) | ||
| Head and neck | 4 (2) | 2 (4) | 2 (1) | ||
| Hepatocellular carcinoma | 2 (0.8) | 0 | 2 (1) | ||
| Mesothelioma | 1 (0.4) | 0 | 1 (0.5) | ||
| Unknown primary | 2 (0.8) | 1 (2) | 1 (0.5) | ||
| Stage - no. (%) | IV/advanced | 106 (42) | 24 (51) | 82 (40) | 0.38 |
| I-III/localized | 90 (36) | 14 (30) | 76 (37) | ||
| Unknown | 56 (22) | 9 (19) | 47 (23) | ||
| Active cancer therapy - no. (%) | No | 146 (58) | 30 (64) | 116 (57) | 0.36 |
| Yes | 106 (42) | 17 (36) | 89 (43) | ||
| Type of cancer therapy - no. (%) | Chemotherapy | 63 (59) | 12 (71) | 51 (57) | 0.44 |
| Targeted | 16 (15) | 1 (6) | 15 (17) | ||
| Anti–PD-1/PD-L1 | 13 (12) | 3 (18) | 10 (11) | ||
| Endocrine | 13 (12) | 1 (6) | 12 (13) | ||
| Smoking history - no. (%) | Active | 26 (10) | 6 (13) | 20 (10) | 0.06 |
| Past | 52 (21) | 5 (11) | 47 (23) | ||
| Never | 85 (34) | 6 (13) | 79 (39) | ||
| Unknown | 89 (35) | 30 (64) | 59 (29) | ||
| RECIST - no. (%) | PD | 34 (13) | 6 (13) | 28 (14) | 0.06 |
| SD | 20 (8) | 1 (2) | 19 (9) | ||
| PR | 13 (5) | 3 (6) | 10 (5) | ||
| CR | 49 (19) | 4 (9) | 45 (22) | ||
| NE [2] | 136 (54) | 33 (70) | 103 (50) | ||
| Comorbid conditions – no. (%) | Pulmonary | 80 (32) | 13 (28) | 67 (33) | 0.58 |
| Cardiac | 78 (31) | 17 (36) | 61 (30) | ||
| Diabetes | 63 (25) | 16 (34) | 47 (23) | ||
| Chronic kidney disease | 47 (19) | 11 (23) | 36 (18) | ||
ECOG-PS, Eastern Cooperative Oncology Group performance status; no., number; CNS, central nervous system.
The median age was 73 years, and 50% were male. The most frequent cancers were lung and breast cancers, occurring in 36 (14.3%) and 34 (13.5%) patients, respectively. One hundred and six patients (42.1%) had stage IV disease. Among the 106 patients (42.1%) receiving active anticancer therapy, 63 (59.4%) of patients were receiving cytotoxic chemotherapy, while targeted agents were used by 15.0% and immune checkpoint inhibition, or endocrine therapy were each used by 12.3% of patients. Hospital acquisition of COVID-19 occurred in 47 patients (19%). When comparing the hospital-acquired and community-acquired groups, baseline characteristics, such as age, sex, stage, active cancer therapy, cancer therapy type and presence of co-morbidities, were well balanced between the two groups (Table 1). Furthermore, routine blood tests were not statistically significant different between the nosocomial and non-nosocomial cases in terms of the neutrophil count (p = 0.08), lymphocyte count (p = 0.29), neutrophil-lymphocyte ratio (p = 0.23) or C-reactive protein (p = 0.48).
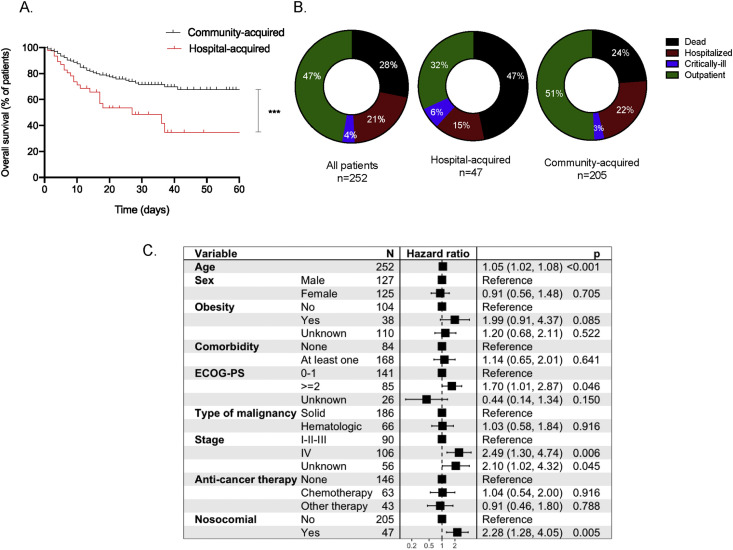
With a median follow-up of 25 days, 71 patients (28.2%) died at last follow-up. Thirty-three patients (13.1%) required ICU admission (Table 2 ).
Table 2.
Primary outcomes in patients with hospital-acquired and community-acquired COVID-19 infections.
| COVID-19 course of illness N = 252 | Hospital-acquired |
Community-acquired |
p-value | |
|---|---|---|---|---|
| N = 47 | N = 205 | |||
| Mortality - n. (%) | 71 (28) | 22 (47) | 49 (24) | <0.002 |
| O2 requirement - n. (%) | 118 (47) | 20 (43) | 98 (48) | <0.515 |
| ICU admission - n. (%) | 33 (13) | 6 (13) | 27 (13) | <0.941 |
| Ventilation - n. (%) | 15 (6) | 3 (6) | 12 (6) | <0.890 |
| Median hospital stay in days (range) | 11 (1–56) | 9.5 (1–22) | 8.5 (1–56) | <0.870 |
| Median ICU stay in days (range) | 8 (2–34) | 11 (1–34) | 7 (5–12) | <0.555 |
ICU, intensive-care unit; n., number.
The rate of death appeared higher in the hospital-acquired group than in the community-acquired group (49% versus 22%, p = 0.002), while the rates of oxygen use, ICU admission or ventilation requirement were similar between the two groups. The median length-of-stay in the hospital and ICU for the entire cohort was 11 days and 8 days, respectively. For the hospital-acquired group, the median length-of-stay, defined by time of positive SARS-CoV-2 test and discharge, was 9.5 days compared with 8.5 days in the community-acquired group (p = 0.870).
Given the higher rate of death observed in the hospital-acquired group, we performed Kaplan–Meier analysis in accordance with type of COVID-19 transmission. We found that patients who had hospital-acquired COVID-19 had shorter overall survival (OS) than those who had community-acquired infection (27 days versus not reached, HR = 2.32, 95% CI: 1.2–4.4, p < 0.001) (Fig. 1 A). At last follow-up, the rate of death remained higher for patients with hospital-acquired COVID-19 (p = 0.002) (Fig. 1B).
Fig. 1.
A. Overall survival of hospital-acquired COVID-19 versus community-acquired COVID-19. ∗∗∗p ≤ 0.001. Fig. 1B. Status of last follow-up in the whole cohort, hospital-acquired cohort and community-acquired cohort. Fig. 1C. Multivariate analysis for overall survival in all patients. ECOG-PS; Eastern Cooperative Oncology Group performance status.
Next, we performed multivariate analysis to determine independent negative risk factors for COVID-19 severity. We found that hospital-acquired COVID-19 was associated with an increased HR for death in this cohort (HR = 2.3, 95% CI: 1.3–4.0, p = 0.005). Age (HR = 1.1, 95% CI: 1.0–1.1, p < 0.001), ECOG status ≥ 2 (HR = 1.7, 95% CI: 1.0–2.9 p = 0.046) and stage IV disease (HR = 2.5, 95% CI: 1.3–4.7, p = 0.006) were also independently associated with poor outcome (Fig. 1C).
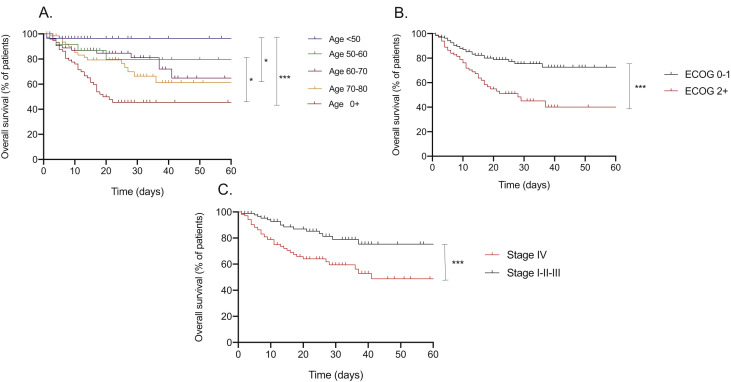
Of note, only 10 (4.0%) patients received treatment for COVID-19 (n = 8 with hydroxychloroquine, n = 1 with lopinavir/ritonavir and n = 1 with interleukin-6 inhibitor). We then performed Kaplan–Meier analyses for the other factors associated with mortality in the multivariate analysis. In keeping with the multivariate analysis, older age (Fig. 2 A), poor ECOG (Fig. 2B) and advanced stage IV disease (Fig. 2C) were all associated with significantly shorter OS.
Fig. 2.
A. Overall survival for all patients stratified in accordance with age. ∗∗∗p ≤ 0.001, ∗p ≤ 0.05, ∗p ≤ 0.05 Fig. 2B. Overall survival for all patients stratified in accordance with ECOG status. ∗∗∗p ≤ 0.001. Fig. 2C. Overall survival for all patients stratified in accordance with cancer stage. ∗∗∗p ≤ 0.001. ECOG; Eastern Cooperative Oncology Group.
4. Discussion
In our study of 252 patients with COVID-19 and cancer across the provinces of Quebec and British Columbia in Canada, we found a high mortality at 28% among adult patients, while none of the patients in the paediatric cohort experienced severe disease. Reflective of the higher incidence of COVID-19 in Quebec, the majority of patients in this cohort resided in the province of Quebec compared with British Columbia. Moreover, the vast majority of patients in our cohort were adults, reflecting the rarity of overt COVID-19 infections in the pediatric population. Most commonly affected cancers in this cohort were lung cancer and breast cancer, which aligns with previous publications describing the cancer type in relation to COVID-19 [6]. We found a high rate of hospital-acquired COVID-19 and this was associated with high mortality both in univariate and multivariate analyses.
Our study reinforces the importance of strategizing and further study of ICP to prevent transmission of COVID-19 to hospitalised patients [14,15]. For example, screening staff members and patients either for symptoms of COVID-19 or COVID-19 infection before entry in COVID-free units could be a potential method [16], as is suggested by several guidelines [17]. Designating COVID-free units and personnel including pharmacists, physicians, nurses and other house-staff also recommended by healthcare authorities [18]. Adequate personal protective equipment [19] and strict hand-hygiene protocols [20] are also of utmost importance for decreasing nosocomial infection.
Patients with cancer in our cohort experienced much higher mortality from COVID-19 than the Canadian average (28% versus 9.4% in Quebec, 6.3% in British Columbia) [2]. Moreover, we observed a discrepancy between this high mortality rate and the rate of ICU admission, which may reflect that patients with cancer are less likely to be admitted to the intensive care setting. This finding highlights the importance of healthcare providers discussing advance directives, especially among patients with cancer.
The largest study to date from the CCC19 consortium discovered negative prognostic factors associated COVID-19 severity in the cancer population. This study of 928 patients identified that age, male sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status and presence of comorbidities were independent risk factors for severe COVID-196. Our study not only re-identified these negative risk factors (i.e. age, poor ECOG status, advanced stage of cancer), but also identified hospital acquisition of COVID-19 as being an independent, negative factor. This novel finding clearly needs to be confirmed in other cancer centres, but identifies a patient-extrinsic factor to be considered in the fight against COVID-19.
Despite this novel finding, our study has several limitations. Firstly, over 80% of patients in our cohort were hospitalised, and this reflects a selection bias for more severely ill patients. Despite our effort to identify every patient with cancer and a COVID-19 diagnosis through collaboration with microbiology departments and provincial registries (Fonds de recherche en Santé du Québec - FRSQ) which provided us access to comprehensive lists of all tested outpatients, community-acquired cases were likely missed in this study. In addition, we could not detect any differences in groups who received treatment for COVID-19 given the small number of patients in this study who were treated with hydroxychloroquine or antiviral agents. Lastly, we did not correct for factors which could increase hospital-acquired transmission, such as availability of adequate protective personal equipment, ventilation systems, single-patient rooms and location of COVID-19 outbreaks.
5. Conclusion
Our study in patients with cancer demonstrates a high mortality from COVID-19 in the adult population. This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, and this was associated with high mortality in both univariate and multivariate analyses. Other independent negative risk factors for COVID-19 severity included age, ECOG status and advanced cancer stage. Our study reinforces the importance of adherence to stringent infection control guidelines to protect vulnerable patients such as those with cancer.
Author contribution statement
AE: Conceptualisation, Methodology, Analysis, Data acquisition, Writing- original draft preparation, supervision. AD: Analysis, Data acquisition, Writing- original draft preparation. NP: Analysis, Data acquisition, Writing- original draft preparation. BR: Conceptualisation, Methodology, Analysis, Writing- original draft preparation, supervision. CL: Analysis, Data acquisition, Supervision. All other authors contributed by coordinating data contributions at their respective sites. All authors contributed intellectual content during the drafting and revisioning of the work and approved the final version.
Funding
This work was partially supported by Astra Zeneca.
Conflict of interest statement
BR and AE declare grant support from Astra Zeneca (grant number: N/A).
Other authors have no conflict of interest to declare.
Acknowledgments
The authors wish to acknowledge the support of the FRSQ COVID-19 biobank as well as the CCC19 consortium. Patients from the Jewish General Hospital, McGill University Healthcare Centre, and the Centre hospitalier de l’Université de Montréal were included as part of the CCC19 consortium data set.
References
- 1.WHO) WHO Coronavirus situation report. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 2.Center JHUaMCR COVID-19 dashboard by the center for systems science and engineering (CSSE) at johns hopkins university (JHU) https://coronavirus.jhu.edu/map.html
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. Jama. Feb 7 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. Mar 27 2020 doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. Mar. 2020;21(3):335–337. doi: 10.1016/s1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. May 28 2020 doi: 10.1016/s0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in wuhan, China. JAMA Oncology. 2020 doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 9.Dai M., Liu D., Liu M., Zhou F., Li G., Zhen Z., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Canc Discov. Apr 28 2020 doi: 10.1158/2159-8290.Cd-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y., Li W., Wang Z., Chen H., Tian L., Liu D. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol. Apr 13 2020:1–2. doi: 10.1017/ice.2020.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobucci G. Covid-19: doctors sound alarm over hospital transmissions. BMJ. 2020;369 doi: 10.1136/bmj.m2013. m2013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H. Early lessons from the frontline of the 2019-nCoV outbreak. Lancet. Feb 29 2020;395(10225):687. doi: 10.1016/s0140-6736(20)30356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quebec INdSPd COVID-19 Mesures de prévention et contrôle des infections pour les milieux de soins aigus : recommandations intérimaires. June 6, 2020. https://www.inspq.qc.ca/sites/default/files/covid/2906-mesures-prevention-milieux-soins-aigus-covid19.pdf
- 14.Routy B., Derosa L., Zitvogel L., Kroemer G. COVID-19: a challenge for oncology services. OncoImmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1760686. 2020/01/01. 1760686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glauser W. Proposed protocol to keep COVID-19 out of hospitals. Can Med Assoc J. 2020;192(10):E264–E265. doi: 10.1503/cmaj.1095852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MoH Ontario. COVID-19 guidance: primary care providers in a community setting. June 6, 2020. http://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_primary_care_guidance.pdf
- 18.Canada Go Infection prevention and control for COVID-19: second interim guidance for acute healthcare settings. June 6, 2020. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/infection-prevention-control-covid-19-second-interim-guidance.html
- 19.WHO) WHO Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
- 20.Lotfinejad N., Peters A., Pittet D. Hand hygiene and the novel coronavirus pandemic: the role of healthcare workers. J Hosp Infect. Mar. 2020;19 doi: 10.1016/j.jhin.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]