Abstract
Ketamine has been reported to exert a prophylactic effect against stress-induced depressive-like behavior by modulating the guanosine-based purinergic system. However, the molecular pathways underlying its prophylactic effect and whether guanosine also elicits a similar effect remain to be determined. Here, we investigated the prophylactic effect of ketamine and guanosine against corticosterone (CORT – 20 mg/kg, p.o.)-induced depressive-like behavior in mice. Furthermore, we characterized if the prophylactic response may be associated with mTORC1-driven signaling in the hippocampus and prefrontal cortex. A single administration of ketamine (5 mg/kg, i.p.), but not guanosine (1 or 5 mg/kg, p.o.), given 1 week before the pharmacological stress prevented CORT-induced depressive-like behavior in the tail suspension test (TST) and splash test (SPT). Fluoxetine treatment for 3 weeks did not prevent CORT-induced behavioral effects. A single administration of subthreshold doses of ketamine (1 mg/kg, i.p.) plus guanosine (5 mg/kg, p.o.) partially prevented the CORT-induced depressive-like behavior in the SPT. Additionally, CORT reduced Akt (Ser473) and GSK-3β (Ser9) phosphorylation and PSD-95, GluA1, and synapsin immunocontent in the hippocampus, but not in the prefrontal cortex. No alterations on mTORC1/p70S6K immunocontent were found in both regions in any experimental group. CORT-induced reductions on PSD-95, GluA1, and synapsin immunocontent were prevented only by ketamine treatment. Collectively, these findings suggest that ketamine, but not guanosine, exerts a prophylactic effect against depressive-like behavior, an effect associated with the stimulation of long-lasting pro-synaptogenic signaling in the hippocampus.
Keywords: Corticosterone, Depression, Ketamine, Guanosine, Prophylactic effect
Abbreviations: Akt, Protein kinase B; AMPA, Alpha-amino-3-hydroxy-methyl-5-4-isoxazole propionic acid; ANOVA, Analysis of variance; CORT, Corticosterone; FDA, Food and Drug Administration; FST, Forced swimming test; GluA1, AMPA receptor subunits 1; GSK, Glycogen synthase kinase-3β; i.p., Intraperitoneal; MDD, Major depressive disorder; mTORC1, Mechanistic target of rapamycin protein complex 1; NMDA, N-Methyl-d-aspartate; NSF, Novelty-suppressed feeding test; OFT, Open-field test; p.o., Per oral; P70S6K, 70 kDa ribosomal protein S6 kinase; PSD-95, Postsynaptic density protein-95 kDa; SPT, Splash test; TST, Tail suspension test.
1. Introduction
Major depressive disorder (MDD), a prevalent and pervasive psychiatric condition affecting more than 300 million individuals, is the most cause of disability worldwide (World Health Organization, 2017). Importantly, stress exposure is the main risk factor for the onset of depressive symptoms (Otte et al., 2016) and several lines of evidence have shown that increased levels of cortisol are associated with depressive episodes (Goodyer et al., 2000; Harris et al., 2000). For instance, compelling evidence has revealed associations among subjective well-being, MDD, anxiety, and alexithymic traits during the COVID-19 pandemic, a high-stress condition that may increase suicide risk (De Berardis et al., 2017; Pappa et al., 2020; Tang et al., 2020). Of note, depressive-like behavior has been reported in rodents subjected to chronic unpredictable stress or repeated administration of corticosterone (Li et al., 2011; Moretti et al., 2012; Pazini et al., 2016; Zeni et al., 2019). However, current monoamine-based antidepressant drugs have reduced efficacy and large time-lag for exerting a therapeutic response, and these are significant limitations for patients with severe MDD and at risk of suicide (Kaster et al., 2016; Papakostas and Ionescu, 2015). Therefore, novel antidepressants with new mechanisms of action are a major focus of current drug development. Reinforcing this assumption, agomelatine that targets the melatonergic system has been reported to be effective as an antidepressant drug, which could be a novel intriguing antidepressant option (Pompili et al., 2013).
However, the most promising advance for the treatment of MDD emerged with the discovery of ketamine, an N-Methyl-d-aspartate (NMDA) receptor antagonist that produces fast and sustained antidepressant responses (Berman et al., 2000; Zarate et al., 2006). Ketamine can relieve depressive symptoms within hours after a single dose and its effects can last up to 2 weeks, even in treatment-resistant patients with suicidal ideation (DiazGranados et al., 2010; Price et al., 2009; Zarate et al., 2006). Compelling reports have shown that the antidepressant effect of ketamine is dependent on the mechanistic target of rapamycin protein complex 1 (mTORC1) signaling pathway and synaptic protein synthesis (Li et al., 2011; Li et al., 2010; Sarkar and Kabbaj, 2016). mTORC1 stimulation controls synaptic protein translation such as postsynaptic density protein-95 kDa (PSD-95), alpha-amino-3-hydroxy-methyl-5-4-isoxazole propionic acid (AMPA) receptor subunits 1 (GluA1), and synapsin, which are essential for spinogenesis and synaptogenesis (Abdallah et al., 2016). Notably, esketamine was approved for adults with treatment-resistant MDD (Wei et al., 2020). A study by Brachman et al. (2016) has provided groundbreaking evidence that ketamine is also effective as a prophylactic agent against stress-induced depressive-like behavior, opening novel perspectives for the design of strategies to prevent MDD. Of special interest, McGowan et al. (2018) showed that the prophylactic effect of ketamine against stress was associated with increased levels of guanosine precursors, guanosine's diphosphate and triphosphate, both in the hippocampus and prefrontal cortex, suggesting an interaction between ketamine and the purinergic system.
We previously reported that guanosine, an endogenous guanine-based purine, could be a novel agent to exert fast antidepressant-like responses or even potentiate the actions of ketamine (Almeida et al., 2020; Camargo et al., 2020a; Camargo and Rodrigues, 2019). This nucleoside is a neuromodulator with remarkable neurotrophic and neuroprotective effects (Bettio et al., 2016; Di Liberto et al., 2016; Tasca et al., 2018). Of note, guanosine administered systemically or centrally produces an antidepressant-like effect in the tail suspension test (TST) and forced swimming test (FST) through the modulation of NMDA receptors and the mTORC1 pathway (Bettio et al., 2012). Moreover, a single administration of guanosine exerted a fast onset antidepressant-like effect in the olfactory bulbectomy mice model (Almeida et al., 2020). The ability of guanosine to augment the antidepressant-like and pro-synaptogenic effects of subthreshold doses of ketamine in the novelty-suppressed feeding test (NSF) and TST was also demonstrated (Camargo et al., 2019; Camargo et al., 2020a). Noteworthy, a single combined administration with subthreshold doses of ketamine and guanosine was effective in counteracting corticosterone (CORT)-induced depressive-like behavior 24 h after treatment (Camargo et al., 2020b). However, it remains to be determined if guanosine could be effective as a prophylactic agent or even potentiate the prophylactic actions of ketamine against stress.
Considering that ketamine-induced mTORC1-driven pro-synaptogenic effect has a long-lasting window (Li et al., 2011; Moda-Sava et al., 2019), we hypothesized that this response could be associated with its prophylactic effect against CORT-induced depressive-like behavior. Moreover, taking into account that ketamine may modulate the purinergic system (McGowan et al., 2018) and guanosine shares some common molecular targets to ketamine (Camargo and Rodrigues, 2019), we also hypothesized that this nucleoside could elicit a prophylactic effect or would be able to potentiate the ketamine's effects. Therefore, the present study had three major purposes. First, we aimed to validate the prophylactic effect of ketamine against the depressive-like behavior elicited by the repeated administration of corticosterone (a protocol that mimics chronic stress) and characterize if this response may be associated with mTORC1-mediated signaling. Second, we investigated the possible prophylactic effect of guanosine against this same protocol of corticosterone administration and whether the modulation of mTORC1 signaling could be associated with this response. Third, we evaluated the ability of the combined administration of ketamine and guanosine to exert a prophylactic response and the role of mTORC1 signaling.
2. Material and methods
2.1. Animals
Male Swiss mice (55–60 days of age) were housed in groups of 8 in a cage (41 × 34 × 16 cm), under controlled temperature (21 ± 1 °C) and humidity (50 ± 20%) with a 12:12 h light/dark cycle (lights on at 7:00 a.m.), and with free access to food and water. The animals were used according to the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the experiments were performed after approval of the protocol by the Institutional Ethics Committee.
2.2. Drugs and treatments
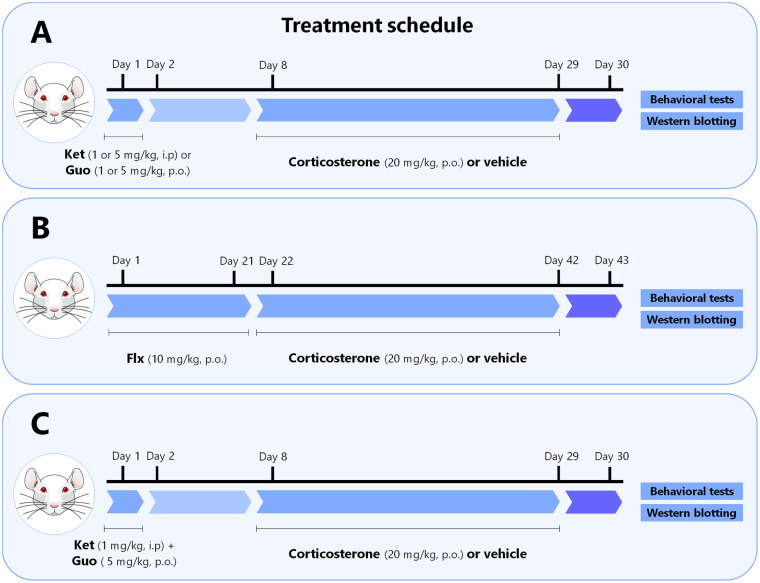
Mice received a single administration of ketamine (1 or 5 mg/kg, i.p., dissolved in 0.9% saline) or guanosine (1 or 5 mg/kg, p.o., dissolved in distilled water) 1 week prior to the administration of vehicle or CORT. Both drugs were administered in a volume of 10 ml/kg body weight. In another set of experiments, mice were daily administered with fluoxetine (10 mg/kg, p.o., dissolved in distilled water) for 3 weeks before the onset of the administration of vehicle or CORT. In the last set of experiments, mice received a single administration of ketamine (1 mg/kg, i.p.) plus guanosine (5 mg/kg, p.o.) 1 week before the administration of vehicle or CORT. Vehicle or CORT (20 mg/kg, dissolved in distilled water with 2% Tween 80 and 0.2% DMSO) was administered by gavage (p.o.) once a day (between 9:00 a.m. and 10 a.m.) for 21 days. On the 22nd day, 24 h after the last CORT administration, animals were subjected to the depression-related behavioral tests (10 min apart). Subsequently, mice were immediately euthanized by decapitation, and the hippocampus and prefrontal cortex were collected for western blotting analysis. The drugs (Sigma Chemical Co., St. Louis, USA) were freshly prepared before administration, and all doses and time points of administration were chosen based on previous studies (Bettio et al., 2012; Brachman et al., 2016; Camargo et al., 2020a; Pazini et al., 2016). A diagram of the treatment regimen, behavioral and neurochemical analyses is provided in Fig. 1 .
Fig. 1.
Experimental design and schedule of the treatment regimen, behavioral and neurochemical analyses. a Mice were treated with a single administration of ketamine (1 or 5 mg/kg, i.p.) or guanosine (1 or 5 mg/kg, p.o.) 1 week prior to the administration with vehicle or corticosterone. b Mice were daily administered with fluoxetine (10 mg/kg, p.o.) for 3 weeks before starting the chronic administration with vehicle or corticosterone. c Mice were treated with a single administration of a subthreshold dose of ketamine (1 mg/kg, i.p.) plus a high dose of guanosine (5 mg/kg, p.o.) 1 week before the administration with vehicle or corticosterone. a, b, c Vehicle or corticosterone (20 mg/kg) was administered orally (p.o.), once a day, for 21 consecutive days. On the 22nd day, 24 h after the last corticosterone administration, animals were subjected to the depression-related behavioral tests, namely tail suspension test, open-field test, and splash test (10 min apart). Subsequently, mice were immediately euthanized by decapitation and the hippocampus and prefrontal cortex were collected for western blotting analysis. Figure designed using images from Mind the Graph.
2.3. Tail suspension test (TST)
The total immobility time of mice suspended by the tail was measured as previously proposed (Steru et al., 1985). Visually isolated mice were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was recorded for 6 min by an experienced observer blind to the experimental groups. Mice were considered immobile only when they hung passively and completely motionless.
2.4. Open-field test (OFT)
Mice were individually subjected to the OFT as previously described (Rodrigues et al., 1996). The apparatus consisted of a wooden box measuring 40 × 60 × 50 cm high. The floor of the arena was divided into 12 equal squares. The number of squares crossed for 6 min was considered a parameter of locomotor activity.
2.5. Splash test (SPT)
The SPT consists of squirting a 10% sucrose solution (w/v) on the dorsal coat of mice placed in clear boxes (12 × 20 × 30 cm). Due to its viscosity, the sucrose solution dirties the mice which then initiate a grooming behavior. After applying the sucrose solution, the latency time to the first grooming and the total time spent grooming were recorded for 5 min, as an index of self-care and motivational behavior (Rosa et al., 2014; Willner, 2005).
2.6. Western blotting
Western blotting was conducted as previously described (Leal et al., 2020). The hippocampus and prefrontal cortex were mechanically homogenized in 400 μl of 50 mM TRIS pH 7.0, 1 mM EDTA, 100 mM NaF, 0.1 mM PMSF, 2 mM Na3VO4, 1%Triton X-100, 10% glycerol, and protease inhibitor cocktail. Lysates were centrifuged (10,000 g for 10 min, at 4 °C) and the supernatants were diluted 1/1 (v/v) in 100 mM TRIS pH 6.8, 4 mM EDTA, 8% SDS, and boiled for 5 min. Thereafter, sample dilution (40% glycerol, 100 mM TRIS, bromophenol blue, pH 6.8) in the ratio 25:100 (v/v) and β-mercaptoethanol (final concentration 8%) were added to the samples. Protein content was quantified using bovine serum albumin (BSA) as a standard (Peterson, 1977). The samples containing 60 μg protein/track were separated by SDS-PAGE using 7–10% gel and the proteins were transferred to nitrocellulose membranes using a semi-dry blotting apparatus (1.2 mA/cm2; 1.5 h). To verify the transfer efficiency process, membranes were stained with Ponceau and subsequently, the membranes were blocked with 5% BSA in TBS (10 mM Tris, 150 mM NaCl, pH 7.5). The immunocontent of total and phosphorylated forms of protein kinase B (Akt – Ser473, #9271), glycogen synthase kinase 3β (GSK-3β – Ser9, #9336), mTORC1 (Ser2448, #2971) and 70 kDa ribosomal protein S6 kinase (p70S6K – Thr389, #9205), as well as the total immunocontent of Akt (#9272), GSK-3β (#9315), mTORC1 (#2972), p70S6K (#9202), PSD-95 (#2507), GluA1 (#13185), synapsin (#2312), and β-actin (loading control, #4970) were detected using specific antibodies (Cell Signaling, 1:1000) diluted in TBS-T (10 mM Tris, 150 mM NaCl, 0.1% Tween-10, pH 7.5) containing 2.5% BSA and incubated overnight. Next, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Cell Signaling, 1:2500) for 60 min, and the immunoreactive bands were developed using a chemiluminescence kit (Amersham ECL Select, Piscataway, USA). The optical density (OD) of the bands was quantified using Image Lab Software® 4.1 (Bio-Rad Laboratories). The phosphorylation levels of Akt, GSK-3β, mTOR, and p70S6K were determined as a ratio of OD of the phosphorylated band over OD of the total band. The immunocontent of PSD-95, GluA1, and synapsin was determined as a ratio of the specific protein band over the OD of the β-actin band. Results are expressed as compared to the control group 100%.
2.7. Statistical analysis
The D'Agostino-Pearson test was used to assess data normality. The differences among experimental groups were determined by two-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test, when appropriate. Data are presented as mean ± standard error of mean (SEM). A value of p < .05 was considered significant.
3. Results
3.1. Ketamine prevents CORT-induced depressive-like behavior and hippocampal synaptogenic markers deficits
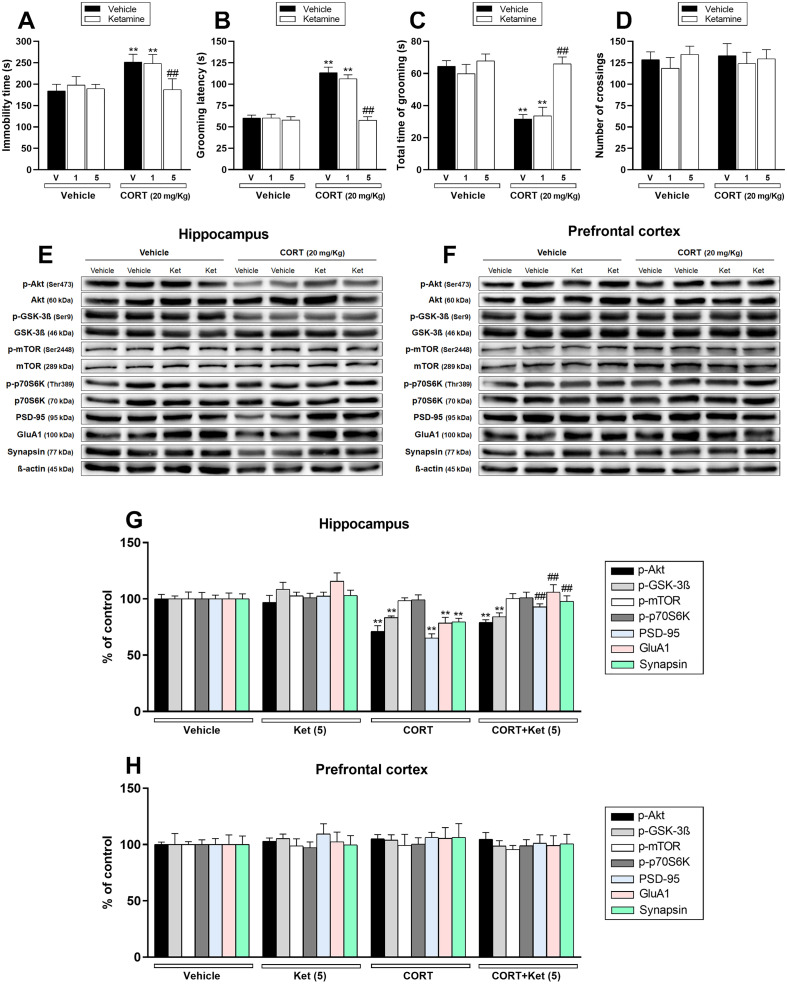
The first set of experiments was designed to validate herein the prophylactic effect of ketamine previously shown in C57BL/6NTac mice (Brachman et al., 2016). Chronic CORT administration significantly increased the immobility time (a behavioral despair behavior) in the TST (p < .01; Fig. 2a) and the latency to first grooming (indicative of impaired self-care behavior) in the SPT (p < .01; Fig. 2b). Additionally, CORT administration reduced the time spent grooming (an anhedonic marker) in the SPT (p < .01; Fig. 2c). No effect was observed in the number of crossings in the OFT (Fig. 2d). These results indicate that the CORT protocol induced a depressive-like phenotype. In contrast, a single administration of ketamine at dose 5 mg/kg, i.p., but not 1 mg/kg, i.p., significantly prevented the increase in immobility time and grooming latency (p < .01), and the reduced total time of grooming (p < .01) induced by CORT. These results suggest that ketamine was effective in producing a prophylactic effect against the depressive-like behavior elicited by CORT administration.
Fig. 2.
Effect of a single administration with ketamine (Ket – 1 or 5 mg/kg, i.p.) 1 week prior to the administration with vehicle or corticosterone (CORT – 20 mg/kg, p.o.) on depression-related behaviors and synaptic markers in the hippocampus and prefrontal cortex of mice. a Ketamine (5 mg/kg, i.p.) administration prevented CORT-induced increase in the immobility time in the TST (n = 8). b, c Ketamine (5 mg/kg, i.p.) administration prevented the increase in the grooming latency and reduction in the total time of grooming induced by CORT in the SPT (n = 8). d All groups of mice had comparable number of crossings in the OFT (n = 8). e, f Representative bands of phospho-Akt (Ser473), Akt, phospho-GSK-3β (Ser9), GSK-3β, phospho-mTOR (Ser2448), mTOR, phospho-p70S6K (Thr389), p70S6K, PSD-95, GluA1, synapsin, and β-actin in the (e) hippocampus and (f) prefrontal cortex of mice. g, h Quantification of these proteins in the (g) hippocampus and (h) prefrontal cortex. Values are expressed as means ± S.E.M (n = 7). ** p < .01 as compared with the vehicle-treated group; ## p < .01 as compared with the CORT-treated group (two-way ANOVA followed by Newman-Keuls post hoc test).
We next sought to investigate whether the mTORC1-mediated signaling pathway may be associated with the prophylactic effect of ketamine. Fig. 2 shows the influence of CORT administration and/or ketamine treatment on the phosphorylated forms of protein kinase B (Akt – Ser473), glycogen synthase kinase 3β (GSK-3β – Ser9), mTOR (Ser2448), and 70 kDa ribosomal protein S6 kinase (p70S6K – Thr389), as well as the immunocontent of PSD-95, GluA1, synapsin in the hippocampus (Fig. 2e, g) and prefrontal cortex (Fig. 2f, h) of mice. CORT administration significantly reduced Akt and GSK-3β phosphorylation and PSD-95, GluA1, and synapsin immunocontent in the hippocampus of mice (p < .01). No significant effects of any treatment were observed on mTOR and p70S6K phosphorylation in the hippocampus. However, a single administration of ketamine (5 mg/kg, i.p.) 1 week prior to the CORT administration significantly prevented the reduction on PSD-95, GluA1, and synapsin immunocontent induced by this hormone in the hippocampus (p < .01). Conversely, ketamine treatment was not effective in preventing CORT-induced decrease on hippocampal Akt and GSK-3β phosphorylation. Furthermore, no significant alterations on the phosphorylation levels of Akt, GSK-3β, mTOR, p70S6K, as well as on immunocontent of PSD-95, GluA1, and synapsin were observed in the prefrontal cortex after CORT and/or ketamine administration.
3.2. Fluoxetine is ineffective to prevent the depressive-like behavior and hippocampal synaptogenic markers deficits induced by CORT
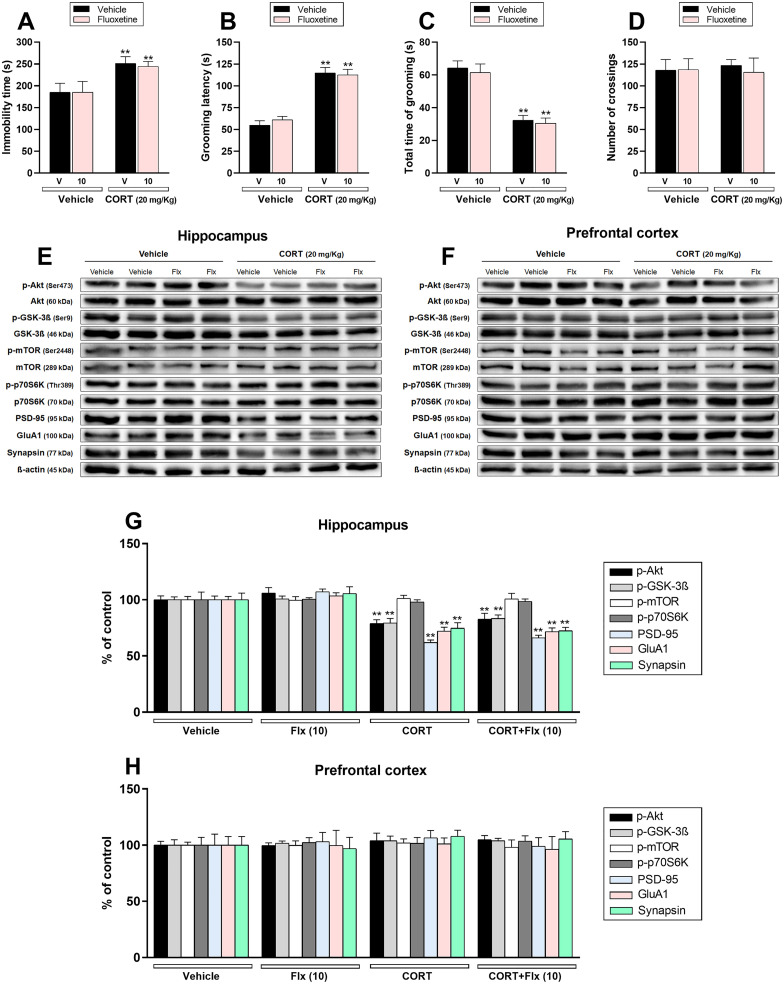
The second set of experiments was designed to validate whether fluoxetine fails to exert a prophylactic effect, as previously reported in C57BL/6NTac mice (Brachman et al., 2016). Chronic CORT administration raised the immobility time (p < .01; Fig. 3a) and the latency to first grooming (p < .01; Fig. 3b) in the TST and SPT, respectively. Furthermore, CORT reduced the time spent grooming (p < .01; Fig. 3c) in mice subjected to the SPT. No effect was observed in the number of crossings in the OFT (Fig. 3d). Importantly, repeated fluoxetine administration for 3 weeks before the CORT protocol was ineffective to prevent the depressive-like behavior.
Fig. 3.
Effect of repeated treatment with fluoxetine (Flx – 10 mg/kg, p.o.) before the chronic administration with vehicle or corticosterone (CORT – 20 mg/kg, p.o.) on depression-related behaviors and synaptic markers in the hippocampus and prefrontal cortex of mice. a Fluoxetine (10 mg/kg, p.o) administration did not prevent CORT-induced increase in the immobility time in the TST (n = 8). b, c Fluoxetine (10 mg/kg, p.o) administration did not prevent the increase in the grooming latency and reduction in the time spent grooming induced by CORT in the SPT (n = 8). d All groups of mice had comparable number of crossings in the OFT (n = 8). e, f Representative bands of phospho-Akt (Ser473), Akt, phospho-GSK-3β (Ser9), GSK-3β, phospho-mTOR (Ser2448), mTOR, phospho-p70S6K (Thr389), p70S6K, PSD-95, GluA1, synapsin, and β-actin in the (e) hippocampus and (f) prefrontal cortex of mice. g, h Quantification of these proteins in the (g) hippocampus and (h) prefrontal cortex. Values are expressed as means ± S.E.M (n = 7). ** p < .01 as compared with the vehicle-treated group (two-way ANOVA followed by Newman-Keuls post hoc test).
The influence of fluoxetine on mTORC1-mediated signaling was also investigated. Fig. 3 shows the influence of CORT and/or fluoxetine administration on the phosphorylated forms of Akt (Ser473), GSK-3β (Ser9), mTOR (Ser2448), and p70S6K (Thr389), as well as the immunocontent of PSD-95, GluA1, synapsin in the hippocampus (Fig. 3e, g) and prefrontal cortex (Fig. 3f, h). CORT significantly reduced Akt and GSK-3β phosphorylation as well as PSD-95, GluA1, and synapsin immunocontent in the hippocampus of mice (p < .01), but no effect was observed on mTOR and p70S6K phosphorylation in this brain structure. Fluoxetine treatment did not protect against CORT-induced reduction in any hippocampal protein. Additionally, no alterations were observed on mTORC1-related targets in the prefrontal cortex after CORT and/or fluoxetine administration.
3.3. Guanosine is unable to prevent CORT-induced depressive-like behavior and hippocampal synaptogenic markers deficits
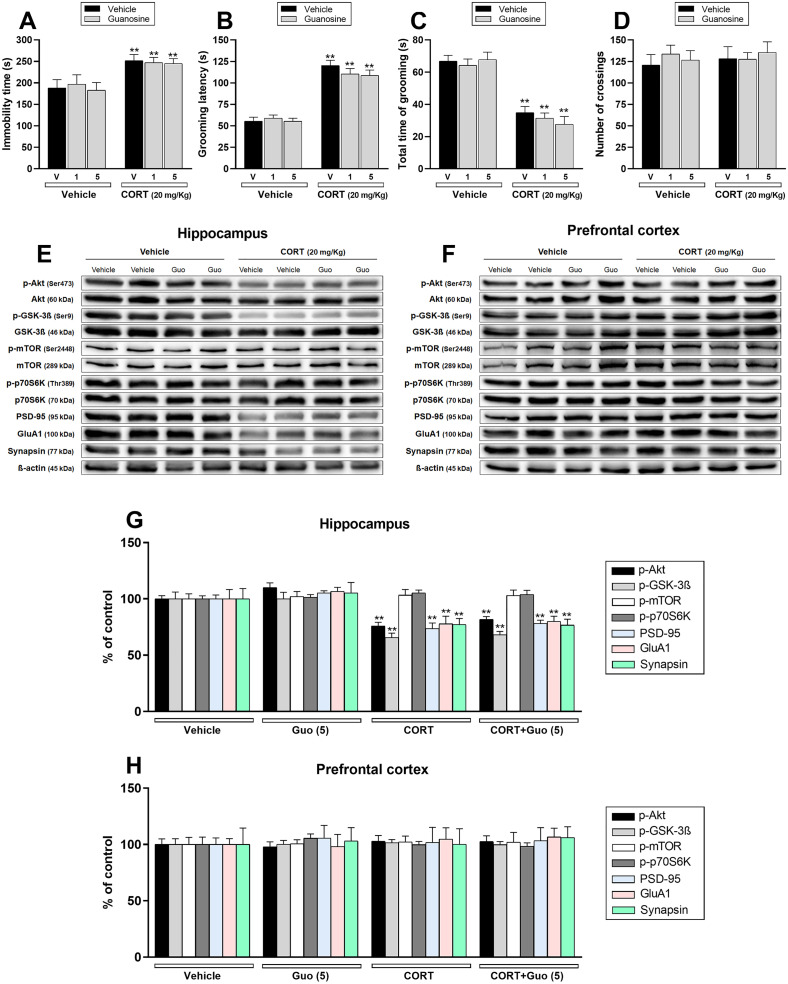
We next investigated the possible prophylactic effect of guanosine, a novel target postulated for exhibiting fast antidepressant-like responses (Camargo and Rodrigues, 2019). Chronic CORT administration enhanced the immobility time in the TST (p < .01; Fig. 4a) and latency to start the first grooming in the SPT (p < .01; Fig. 4b), as well as reduced the time spent grooming in the SPT (p < .01; Fig. 4c). No effect was observed in the OFT (Fig. 4d). As opposed to ketamine's results, guanosine was unable to prevent any behavioral alteration induced by the CORT.
Fig. 4.
Effect of a single administration with guanosine (Guo – 1 or 5 mg/kg, i.p.) 1 week prior to the administration with vehicle or corticosterone (CORT – 20 mg/kg, p.o.) on depression-related behaviors and synaptic markers in the hippocampus and prefrontal cortex of mice. a Guanosine (1 or 5 mg/kg, p.o) administration did not prevent CORT-induced increase in the immobility time in the TST (n = 8). b, c Guanosine (1 or 5 mg/kg, p.o) administration did not prevent the increase in the grooming latency and reduction in the total time of grooming induced by CORT in the SPT (n = 8). d All groups of mice had comparable number of crossings in the OFT (n = 8). e, f Representative bands of phospho-Akt (Ser473), Akt, phospho-GSK-3β (Ser9), GSK-3β, phospho-mTOR (Ser2448), mTOR, phospho-p70S6K (Thr389), p70S6K, PSD-95, GluA1, synapsin, and β-actin in the (e) hippocampus and (f) prefrontal cortex of mice. g, h Quantification of these proteins in the (g) hippocampus and (h) prefrontal cortex. Values are expressed as means ± S.E.M (n = 7). ** p < .01 as compared with the vehicle-treated group (two-way ANOVA followed by Newman-Keuls post hoc test).
The influence of guanosine on mTORC1-mediated signaling was subsequently investigated. Fig. 4 shows the influence of CORT administration and/or guanosine treatment on the phosphorylation levels of Akt (Ser473), GSK-3β (Ser9), mTOR (Ser2448), and p70S6K (Thr389), as well as the immunocontent of PSD-95, GluA1, synapsin in the hippocampus (Fig. 4e, g) and prefrontal cortex (Fig. 4f, h). CORT protocol significantly decreased Akt and GSK-3β phosphorylation and PSD-95, GluA1, and synapsin immunocontent in the hippocampus of mice (p < .01), although no alteration in any protein was observed in the prefrontal cortex. A single administration of guanosine given before the pharmacological stress failed to prevent the reduction induced by CORT on Akt, GSK-3β, PSD-95, GluA1, and synapsin immunocontent. No alterations on mTORC1-related targets were detected in the prefrontal cortex after CORT and/or guanosine administration.
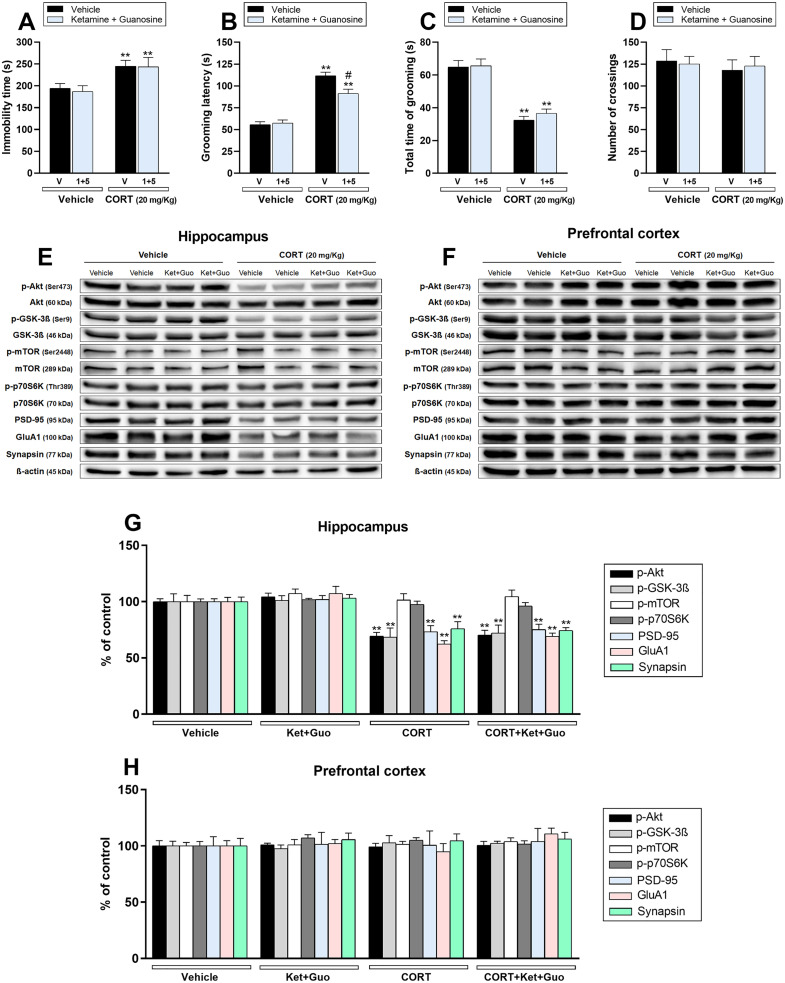
3.4. Ketamine plus guanosine partially prevented CORT-induced reduction on self-care behavior, without altering hippocampal synaptogenic markers deficits induced by CORT
Considering that guanosine potentiates the antidepressant-like of subthreshold doses of ketamine in CORT-administered mice (Camargo et al., 2020b), we next investigated whether this augmentation response could be effective in producing a prophylactic effect. Chronic CORT administration significantly increased immobility time in the TST (p < .01; Fig. 5a) and grooming latency in the SPT (p < .01; Fig. 5b), and reduced time spent grooming in the SPT (p < .01; Fig. 5c). Furthermore, no alteration was observed in the OFT in any group (Fig. 5d). A single coadministration of ketamine plus guanosine partially counteracted the CORT-induced increase in the grooming latency (p < .05). However, this combined administration did not prevent the other behavioral alterations induced by the CORT.
Fig. 5.
Effect of single administration with a subthreshold dose of ketamine (Ket – 1 mg/kg, i.p.) plus an ineffective dose of guanosine (Guo – 5 mg/kg, p.o.) 1 week before the chronic administration with vehicle or corticosterone (CORT – 20 mg/kg, p.o.) on depression-related behaviors and synaptic markers in the hippocampus and prefrontal cortex of mice. a Ketamine 1 mg/kg, i.p.) plus guanosine (5 mg/kg, p.o) administration did not prevent the increase in the immobility time in the TST induced by CORT (n = 8). b Ketamine 1 mg/kg, i.p.) plus guanosine (5 mg/kg, p.o) administration attenuated CORT-induced increase in the latency to first grooming in the SPT (n = 8). c Ketamine 1 mg/kg, i.p.) plus guanosine (5 mg/kg, p.o) administration did not prevent the reduction in the time spent grooming induced by CORT in the SPT (n = 8). d All groups of mice had comparable number of crossings in the OFT (n = 8). e, f Representative bands of phospho-Akt (Ser473), Akt, phospho-GSK-3β (Ser9), GSK-3β, phospho-mTOR (Ser2448), mTOR, phospho-p70S6K (Thr389), p70S6K, PSD-95, GluA1, synapsin, and β-actin in the (e) hippocampus and (f) prefrontal cortex of mice. g, h Quantification of these proteins in the (g) hippocampus and (h) prefrontal cortex. Values are expressed as means ± S.E.M (n = 7). ** p < .01 as compared with the vehicle-treated group; # p < .05 as compared with the CORT-treated group (two-way ANOVA followed by Newman-Keuls post hoc test).
The influence of ketamine plus guanosine treatment on mTORC1-mediated signaling was next evaluated. Fig. 5 shows the influence of CORT administration and/or ketamine plus guanosine treatment on the phosphorylation of Akt (Ser473), GSK-3β (Ser9), mTOR (Ser2448), and p70S6K (Thr389), as well as the immunocontent of PSD-95, GluA1, synapsin in the hippocampus (Fig. 5e, g) and prefrontal cortex (Fig. 5f, h). CORT administration significantly decreased Akt and GSK-3β phosphorylation and PSD-95, GluA1, and synapsin immunocontent in the hippocampus of mice (p < .01), while no alterations on these proteins were observed in the prefrontal cortex. A single administration of ketamine plus guanosine failed to prevent the reduction on Akt, GSK-3β, PSD-95, GluA1, and synapsin induced by CORT.
4. Discussion
This study reinforces and extends the notion that a single administration with a subanesthetic dose of ketamine given before the pharmacological stress protects mice against CORT-induced depressive-like behavior. Additionally, we provide evidence that ketamine's ability in preventing the CORT-induced impairments on synaptogenic markers occurs selectively in the hippocampus of mice, and these findings could be associated with its prophylactic response. We also confirm the previous findings that the repeated fluoxetine administration fails to exert a prophylactic response (Brachman et al., 2016; Chen et al., 2020), and we demonstrated that this drug is also unable to prevent CORT-induced reduction on hippocampal synaptogenic markers. In addition, we have shown that guanosine is not capable of preventing CORT-induced depressive-like behavior and hippocampal synaptic markers impairment, although the combined administration of subthreshold doses of ketamine plus guanosine attenuated the impaired self-care behavior induced by CORT.
The evidence that ketamine may have a prophylactic effect against stress besides producing rapid-onset and long-lasting antidepressant effects has emerged as a possible novel strategy to manage those patients at risk to develop severe MDD (Brachman et al., 2016). However, it remains to be fully resolved whether this prophylactic effect elicited by ketamine translate from mice to humans. In the present study, we extend the investigations regarding the prophylactic effect of ketamine in mice subjected to repeated administration of CORT. This experimental protocol has been postulated to be useful for the study of MDD since it induces behavioral and neurochemical alterations similar to those occurring in patients with this disorder (Sterner and Kalynchuk, 2010; Zhao et al., 2009). Here, we found that CORT administration for 21 days robustly induced a depressive-like behavior, as evidenced by the behavioral alterations in the TST and SPT, in agreement with previous studies (David et al., 2009; Moda-Sava et al., 2019; Rosa et al., 2014; Zhao et al., 2008). Importantly, we showed that a single administration of ketamine (5 mg/kg, i.p.) 1 week before starting the pharmacological stress was effective in preventing the depressive-like behavior, although a lower dose (1 mg/kg, i.p.) that exerts an antidepressant-like response in this model when administered following CORT administration (Neis et al., 2018; Pazini et al., 2016), failed to produce the same effect. The ketamine's prophylactic effect against the behavioral effects elicited by CORT is in agreement with a previous study, which indicated that a single administration of ketamine prevented CORT-induced increase in the grooming latency in the SPT (90 mg/kg, i.p.) and the latency to feed in the NSF test (10 and 90 mg/kg, i.p) in C57BL/6NTac mice (Brachman et al., 2016). Furthermore, the prophylactic efficacy of a low dose of ketamine (3 mg/kg, i.p.) against depressive-like behavior induced by chronic unpredictable stress (14 days) in C57BL/6 J mice subjected to the sucrose preference test was also reported (Krzystyniak et al., 2019). Here, we provide novel evidence that ketamine was effective in preventing the increase in the immobility time in the TST and reduction in the total time of grooming in the SPT induced by CORT administration in Swiss mice, reinforcing the notion that ketamine may be a useful prophylactic strategy in at-risk populations. The present study also provides additional evidence that fluoxetine (10 mg/kg, p.o.) administration for 3 weeks before stress did not protect against CORT-induced depressive-like behavior in Swiss mice subjected to the TST and SPT. These results are in agreement with previous studies reporting that repeated fluoxetine (18 mg/kg, p.o.) treatment was ineffective to elicit a prophylactic effect against CORT in the SPT and NSF test in C57BL/6NTac mice (Brachman et al., 2016; Chen et al., 2020).
Due to recent reports showing that ketamine may affect the purinergic system and that guanosine shares some common molecular targets with ketamine (Almeida et al., 2020; Camargo et al., 2019; McGowan et al., 2018), we next investigated whether guanosine would be able to afford prophylactic response against the CORT-induced depressive-like behavior. Guanosine serum levels were shown to be reduced in patients with MDD, reinforcing the notion that this nucleoside may play a role in the pathophysiology of this disorder (Ali-Sisto et al., 2016). However, as opposed to the actions displayed by ketamine, a single administration of guanosine (1 or 5 mg/kg, p.o.) given 1 week before the pharmacological stress was unable to prevent CORT-induced depressive-like behavior. Thus, one may speculate that this nucleoside could be only effective to produce fast (Almeida et al., 2020; Camargo and Rodrigues, 2019), but not long-term responses against stress. Our research group demonstrated that guanosine potentiates the antidepressant-like effect of a subthreshold dose of ketamine in the NSF test and TST in the vehicle- and CORT-administered mice (Camargo et al., 2019; Camargo et al., 2020a; Camargo et al., 2020b). Thus, we next investigated the ability of a single administration with an ineffective dose of guanosine (5 mg/kg, p.o.) in combination with a subthreshold dose of ketamine (1 mg/kg, p.o.) to elicit a prophylactic response. We found that ketamine plus guanosine treatment partially prevented the reduced self-care behavior evoked by CORT, although it did not prevent the CORT-induced behavioral despair and anhedonic-like behavior. However, additional studies are necessary to ascertain the long-lasting beneficial effects of this combined treatment in self-care-related responses. Furthermore, this combined strategy was not capable of exerting the full spectrum of ketamine's prophylactic actions, highlighting the necessity to understand the mechanisms of action associated with its long-lasting effect.
Despite the numerous studies investigating the mechanisms of ketamine's antidepressant effects, few studies have focused on the mechanisms underlying the prophylactic effect of this drug. The available mechanistic studies provide evidence that prophylactic ketamine treatment may act by modulating the neural activity (Dolzani et al., 2018; Mastrodonato et al., 2018) and promoting synaptic plasticity in the hippocampus and prefrontal cortex (Krzystyniak et al., 2019) as well as modulating the immune system (Mastrodonato et al., 2020), effects likely associated with its prophylactic response. Furthermore, the antidepressant-like effect, but not the prophylactic effect of ketamine was associated with its ability to increase pro-neurogenic markers (LaGamma et al., 2018). Regarding this issue, the ketamine's prophylactic effect in socially defeated 129S6/SvEvTac mice was associated with increased ∆FosB expression, a transcriptional regulator of synaptic plasticity, in the prefrontal cortex and hippocampus, whereas ∆FosB transcriptional silencing in the hippocampal CA3 area inhibited the ketamine's behavioral response (Mastrodonato et al., 2018). Additionally, ketamine's prophylactic effect against chronic unpredictable stress in C57BL/6 J mice was associated with an increase in dendritic spines density in the prefrontal cortex and hippocampal CA1 and CA3 areas (Krzystyniak et al., 2019), but the signaling pathways implicated in these effects remain to be determined.
To provide some insight into the molecular targets underpinning the prophylactic effect of ketamine, particularly whether these targets are similar or divergent from its antidepressant response, we investigate if mTORC1-driven long-lasting synaptogenic signaling could be associated with ketamine's prophylactic effect against CORT. Here, we found that CORT robustly reduced the phosphorylation of Akt (Ser473), and its downstream target GSK-3β (Ser9) in the hippocampus, but not in the prefrontal cortex, while no alterations were detected on mTOR (Ser2448)/p70S6K (Thr389) phosphorylation in both structures. Additionally, CORT downregulated PSD-95, GluA1, and synapsin immunocontent in the hippocampus, but not in the prefrontal cortex, suggesting a synaptogenic deficit. These results are partially in line with prior reports showing the distinct effects of CORT administration and chronic stress on mTORC1-related targets in the hippocampus and prefrontal cortex (Freitas et al., 2016; Li et al., 2011; Pazini et al., 2016; Zhu et al., 2018). Particularly, it has been shown that CORT protocol reduced mTOR phosphorylation and synaptic proteins content in the hippocampus of mice without affecting p70S6K (Thr389) (Freitas et al., 2016; Pazini et al., 2016), whereas chronic stress decreased Akt, mTOR, and p70S6K phosphorylation (Zhu et al., 2018) and synaptic proteins levels (Li et al., 2011) in the prefrontal cortex of mice and rats, respectively. The exact reasons for these discrepancies are unclear but considering that the hippocampus expresses a high density of glucocorticoid receptors, this aspect could make this brain region more vulnerable to the deleterious effects of CORT administration rather than the prefrontal cortex (Lee et al., 2002). In addition, these neurochemical divergences may also be attributable to mouse strains differences or testing conditions (Jacobson and Cryan, 2007; Lathe, 2004).
Our results unveil that CORT-induced reduction on PSD-95, GluA1, and synapsin immunocontent was completely prevented by a single administration of ketamine. These results suggest that ketamine is effective in preventing CORT-induced synaptic impairments in the hippocampus and this effect could be associated with its behavioral prophylactic effect. Reinforcing this assumption, repeated fluoxetine treatment or a single administration of guanosine, which had no behavioral prophylactic effect, was not capable of preventing CORT-induced synaptic markers impairment in the hippocampus, indicating that this effect was selectively exerted by ketamine. Therefore, one may speculate that ketamine-induced long-lasting pro-synaptogenic effect in the hippocampus could make this brain region less prone to CORT-induced synaptic impairment and could enhance the resilience of mice against the detrimental effects evoked by this hormone. However, no preventive effect was observed concerning the reduced Akt and GSK-3β phosphorylation in CORT-treated mice. Considering that ketamine affects rapidly and transiently the phosphorylation of Akt and GSK-3β (Beurel et al., 2011; Li et al., 2010; Liu et al., 2013), we cannot rule out that these targets may also be associated with its prophylactic effect in earlier time points. It is worth noting that the combined administration of subthreshold doses of ketamine and guanosine, which partially prevented the reduced self-care behavior, did not prevent CORT-induced detrimental effects on hippocampal PSD-95, GluA1, and synapsin immunocontent, possibly because of the subtle behavioral response, not enough to impact the pro-synaptogenic signaling. However, further investigations are necessary to understand the possible preventive effect triggered by ketamine plus guanosine treatment and the mechanisms associated with this response.
5. Conclusions
Taken together, our results reinforce the notion that a single administration of ketamine robustly exerts a prophylactic effect against CORT administration, and provides evidence that this behavioral response is associated with the stimulation of long-lasting pro-synaptogenic signaling pathway, specifically in the hippocampus. Moreover, we showed that a single administration with subthreshold doses of ketamine plus guanosine partially prevented CORT-induced reduction on self-care behavior possibly conferring a subtle stress resilience that deserves further investigation. The prophylactic effect of ketamine could have therapeutic relevance as a potential novel strategy to manage those patients at risk to develop severe MDD and suicide, i.e., individuals under high-stress conditions. Particularly in the COVID-19 pandemic, medical health workers or people in confinement with psychiatric disorders are good examples of a predictably at-risk patient population (De Berardis et al., 2017; Pappa et al., 2020; Tang et al., 2020).
Nonetheless, this research has important caveats that need to be considered. Taking into account that the causal relationship between mTORC1-driven signaling and behavioral/pro-synaptogenic outcomes elicited by ketamine's prophylactic treatment was not addressed herein, additional experiments using rapamycin (mTORC1 inhibitor) could be undertaken to ascertain this issue. Furthermore, considering that the stimulation of the pro-synaptogenic pathway may occur independently of mTORC1 stimulation, further experiments using general inhibitors of protein synthesis (such as anisomycin) would give some insight into this issue. Finally, although a single administration of ketamine upregulated the pro-synaptogenic markers (PSD-95, GluA1, and synapsin) in the hippocampus of CORT-treated mice after 4 weeks, the morphological evaluation of spinogenesis and synaptogenesis was not addressed in the present study. Despite these limitations, the data reported herein suggest that targeting the long-lasting pro-synaptogenic signaling pathway can induce a resilience-enhancing effect, protecting against stress-related disorders.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, #310113/2017-2) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Ana L. S. Rodrigues and Márcia M. de Souza are recipients of CNPq Research Productivity Fellowship.
Declaration of Competing Interest
All the authors declare that have no biomedical financial interests or conflict of interest in this study.
Acknowledgments
The authors thank funding agencies CNPq and CAPES by the financial support, and the Laboratório Multiusuário de Estudos em Biologia (LAMEB) by technical support.
References
- Abdallah C.G., Adams T.G., Kelmendi B., Esterlis I., Sanacora G., Krystal J.H. Ketamine's mechanism of action: a path to rapid-acting antidepressants. Depress. Anxiety. 2016;33:689–697. doi: 10.1002/da.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali-Sisto T., Tolmunen T., Toffol E., Viinamäki H., Mäntyselkä P., Valkonen-Korhonen M., Honkalampi K., Ruusunen A., Velagapudi V., Lehto S.M. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology. 2016;70:25–32. doi: 10.1016/j.psyneuen.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Almeida R.F., Pocharski C.B., Rodrigues A.L.S., Elisabetsky E., Souza D.O. Guanosine fast onset antidepressant-like effects in the olfactory bulbectomy mice model. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-65300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bettio L.E.B., Cunha M.P., Budni J., Pazini F.L., Oliveira Á., Colla A.R., Rodrigues A.L.S. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav. Brain Res. 2012;234:137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Bettio L.E.B., Gil-Mohapel J., Rodrigues A.L.S. Guanosine and its role in neuropathologies. Purinergic Signal. 2016;12:411–426. doi: 10.1007/s11302-016-9509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Song L., Jope R.S. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman R.A., McGowan J.C., Perusini J.N., Lim S.C., Pham T.H., Faye C., Gardier A.M., Mendez-David I., David D.J., Hen R., Denny C.A. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol. Psychiatry. 2016;79:776–786. doi: 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo A., Rodrigues A.L.S. Novel targets for fast antidepressant responses: possible role of endogenous neuromodulators. Chronic Stress. 2019;3 doi: 10.1177/2470547019858083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo A., Pazini F.L., Rosa J.M., Wolin I.A.V., Moretti M., Rosa P.B., Neis V.B., Rodrigues A.L.S. Augmentation effect of ketamine by guanosine in the novelty-suppressed feeding test is dependent on mTOR signaling pathway. J. Psychiatr. Res. 2019;115:103–112. doi: 10.1016/j.jpsychires.2019.05.017. [DOI] [PubMed] [Google Scholar]
- Camargo A., Dalmagro A.P., Zeni A.L.B., Rodrigues A.L.S. Guanosine potentiates the antidepressant-like effect of subthreshold doses of ketamine: possible role of pro-synaptogenic signaling pathway. J. Affect. Disord. 2020;271:100–108. doi: 10.1016/j.jad.2020.03.186. [DOI] [PubMed] [Google Scholar]
- Camargo A., Dalmagro A.P., Rosa J.M., Zeni A.L.B., Kaster M.P., Tasca C.I., Rodrigues A.L.S. Subthreshold doses of guanosine plus ketamine elicit antidepressant-like effect in a mouse model of depression induced by corticosterone: role of GR/NF-κB/IDO-1 signaling. Neurochem. Int. 2020;139 doi: 10.1016/j.neuint.2020.104797. [DOI] [PubMed] [Google Scholar]
- Chen B.K., Mendez-David I., Luna V.M., Faye C., Gardier A.M., David D.J., Denny C.A. Prophylactic efficacy of 5-HT4R agonists against stress. Neuropsychopharmacology. 2020;45:542–552. doi: 10.1038/s41386-019-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I., Drew M., Craig D.A., Guiard B.P., Guilloux J.P., Artymyshyn R.P., Gardier A.M., Gerald C., Antonijevic I.A., Leonardo E.D., Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D., Fornaro M., Orsolini L., Valchera A., Carano A., Vellante F., Perna G., Serafini G., Gonda X., Pompili M., Martinotti G., Di Giannantonio M. Alexithymia and suicide risk in psychiatric disorders: a mini-review. Front. Psychiatry. 2017;14:1–6. doi: 10.3389/fpsyt.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Liberto V., Mudò G., Garozzo R., Frinchi M., Fernandez-Dueñas V., Di Iorio P., Ciccarelli R., Caciagli F., Condorelli D.F., Ciruela F., Belluardo N. The guanine-based purinergic system: the tale of an orphan neuromodulation. Front. Pharmacol. 2016;7:1–15. doi: 10.3389/fphar.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N., Ibrahim L., Brutsche N., Ameli R., Henter L., Luckenbaugh D., Machado-Vieira R., Zarate C.A. Rapid resolution of suicidal ideation after a single infusion of an NMDA antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzani S.D., Baratta M.V., Moss J.M., Leslie N.L., Tilden S.G., Sørensen A.T., Watkins L.R., Lin Y., Maier S.F. Inhibition of a descending prefrontal circuit prevents ketamine-induced stress resilience in females. eNeuro. 2018;5:1–18. doi: 10.1523/ENEURO.0025-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A.E., Egea J., Buendia I., Gómez-Rangel V., Parada E., Navarro E., Casas A.I., Wojnicz A., Ortiz J.A., Cuadrado A., Ruiz-Nuño A., Rodrigues A.L.S., Lopez M.G. Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol. Neurobiol. 2016;53:3030–3045. doi: 10.1007/s12035-015-9182-6. [DOI] [PubMed] [Google Scholar]
- Goodyer I.M., Herbert J., Tamplin A., Altham P.M.E. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br. J. Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Harris T.O., Borsanyi S., Messari S., Stanford K., Cleary S.E., Shiers H.M., Brown G.W., Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br. J. Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Jacobson L.H., Cryan J.F. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav. Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Kaster M.P., Moretti M., Cunha M.P., Rodrigues A.L.S. Novel approaches for the management of depressive disorders. Eur. J. Pharmacol. 2016;771:236–240. doi: 10.1016/j.ejphar.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Krzystyniak A., Baczynska E., Magnowska M., Antoniuk S., Roszkowska M., Zareba-Koziol M., Das N., Basu S., Pikula M., Wlodarczyk J. Prophylactic ketamine treatment promotes resilience to chronic stress and accelerates recovery: correlation with changes in synaptic plasticity in the CA3 subregion of the hippocampus. Int. J. Mol. Sci. 2019;20:1–15. doi: 10.3390/ijms20071726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGamma C.T., Tang W.W., Morgan A.A., McGowan J.C., Brachman R.A., Denny C.A. Antidepressant but not prophylactic ketamine administration alters calretinin and calbindin expression in the ventral hippocampus. Front. Mol. Neurosci. 2018;11:1–14. doi: 10.3389/fnmol.2018.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. The individuality of mice. Genes Brain Behav. 2004;3:317–327. doi: 10.1111/j.1601-183X.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- Leal R.B., Lopes M.W., Formolo D.A., de Carvalho C.R., Hoeller A.A., Latini A., Sousa D.S., Wolf P., Prediger R.D., Bortolotto Z.A., Linhares M.N., Lin K., Walz R. Amygdala levels of the GluA1 subunit of glutamate receptors and its phosphorylation state at serine 845 in the anterior hippocampus are biomarkers of ictal fear but not anxiety. Mol. Psychiatry. 2020;25:655–665. doi: 10.1038/s41380-018-0084-7. [DOI] [PubMed] [Google Scholar]
- Lee A.L., Ogle W.O., Sapolsky R.M. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R.-J., Banasr M., Dwyer J.M., Iwata M., Li X.-Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–965. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu R.J., Dwyer J.M., Banasr M., Lee B., Son H., Li X.Y., Aghajanian G., Duman R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.J., Fuchikami M., Dwyer J.M., Lepack A.E., Duman R.S., Aghajanian G.K. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrodonato A., Martinez R., Pavlova I.P., LaGamma C.T., Brachman R.A., Robison A.J., Denny C.A. Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol. Psychiatry. 2018;84:846–856. doi: 10.1016/j.biopsych.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrodonato A., Cohensedgh O., LaGamma C.T., McGowan J.C., Hunsberger H.C., Denny C.A. Prophylactic (R,S)-ketamine selectively protects against inflammatory stressors. Behav. Brain Res. 2020;378 doi: 10.1016/j.bbr.2019.112238. [DOI] [PubMed] [Google Scholar]
- McGowan J.C., Hill C., Mastrodonato A., Lagamma C.T., Kitayev A., Brachman R.A., Narain N.R., Kiebish M.A., Denny C.A. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology. 2018;43:1813–1821. doi: 10.1038/s41386-018-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava R.N., Murdock M.H., Parekh P.K., Fetcho R.N., Huang B.S., Huynh T.N., Witztum J., Shaver D.C., Rosenthal D.L., Alway E.J., Lopez K., Meng Y., Nellissen L., Grosenick L., Milner T.A., Deisseroth K., Bito H., Kasai H., Liston C. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:1–11. doi: 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M., Colla A., De Oliveira Balen G., Dos Santos D.B., Budni J., De Freitas A.E., Farina M., Rodrigues A.L.S. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J. Psychiatr. Res. 2012;46:331–340. doi: 10.1016/j.jpsychires.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Neis V.B., Bettio L.B., Moretti M., Rosa P.B., Olescowicz G., Fraga D.B., Goncalves F.M., Freitas A.E., Heinrich I.A., Lopes M.W., Leal R.B., Rodrigues A.L.S. Single administration of agmatine reverses the depressive-like behavior induced by corticosterone in mice: comparison with ketamine and fluoxetine. Pharmacol. Biochem. Behav. 2018;173:44–50. doi: 10.1016/j.pbb.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Otte C., Gold S., Penninx B., Pariante C., Etkin A., Fava M., Mohr D., Schatzberg A. Major depressive disorder. Nat. Rev. Dis. Primers. 2016;2:1–20. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- Papakostas G.I., Ionescu D.F. Towards new mechanisms: an update on therapeutics for treatment-resistant major depressive disorder. Mol. Psychiatry. 2015;20:1142–1150. doi: 10.1038/mp.2015.92. [DOI] [PubMed] [Google Scholar]
- Pappa S., Ntella V., Giannakas T., Giannakoulis V.G., Papoutsi E., Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav. Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazini F.L., Cunha M.P., Rosa J.M., Colla A.R.S., Lieberknecht V., Oliveira Á., Rodrigues A.L.S. Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR pathway. Mol. Neurobiol. 2016;53:6818–6834. doi: 10.1007/s12035-015-9580-9. [DOI] [PubMed] [Google Scholar]
- Peterson G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pompili M., Serafini G., Innamorati M., Venturini P., Fusar-Poli P., Sher L., Amore M., Girardi P. Agomelatine, a novel intriguing antidepressant option enhancing neuroplasticity: a critical review. World J. Biol. Psychiatry. 2013;14:412–431. doi: 10.3109/15622975.2013.765593. [DOI] [PubMed] [Google Scholar]
- Price R.B., Nock M.K., Charney D.S., Mathew S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A.L.S., Rocha J.B.T., Mello C.F., Souza D.O. Effect of perinatal lead exposure on rat behaviour in open-field and two-way avoidance tasks. Pharmacol. Toxicol. 1996;79:150–156. doi: 10.1111/j.1600-0773.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Rosa P.B., Ribeiro C.M., Bettio L.E.B., Colla A., Lieberknecht V., Moretti M., Rodrigues A.L.S. Folic acid prevents depressive-like behavior induced by chronic corticosterone treatment in mice. Pharmacol. Biochem. Behav. 2014;127:1–6. doi: 10.1016/j.pbb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Sarkar A., Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol. Psychiatry. 2016;80:448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner E.Y., Kalynchuk L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry. 2010;34:777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tang W., Hu T., Yang L., Xu J. The role of alexithymia in the mental health problems of home-quarantined university students during the COVID-19 pandemic in China. Personal. Individ. Differ. 2020;165 doi: 10.1016/j.paid.2020.110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca C.I., Lanznaster D., Oliveira K.A., Fernández-Dueñas V., Ciruela F. Neuromodulatory effects of guanine-based purines in health and disease. Front. Cell. Neurosci. 2018;12:1–14. doi: 10.3389/fncel.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Chang L., Hashimoto K. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol. Biochem. Behav. 2020;190 doi: 10.1016/j.pbb.2020.172870. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural- neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2017. Depression and Other Common Mental Disorders: Global Health Estimates; pp. 1–24. (doi:CC BY-NC-SA 3.0 IGO) [Google Scholar]
- Zarate C.A., Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zeni A.L.B., Camargo A., Dalmagro A.P. Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol. Biochem. Behav. 2019;179:63–72. doi: 10.1016/j.pbb.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Ma R., Shen J., Su H., Xing D., Du L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008;581:113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xie W., Dai J., Wang Z., Huang Y. The varying effects of short-term and long-term corticosterone injections on depression-like behavior in mice. Brain Res. 2009;1261:82–90. doi: 10.1016/j.brainres.2008.12.083. [DOI] [PubMed] [Google Scholar]
- Zhu J.-X., Shan J.-L., Hu W.-Q., Zeng J.-X., Shu J.-C. Gallic acid activates hippocampal BDNF-Akt-mTOR signaling in chronic mild stress. Metab. Brain Dis. 2018;34:93–101. doi: 10.1007/s11011-018-0328-x. [DOI] [PubMed] [Google Scholar]