Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic is an unprecedented challenge. Different models of reorganization have been described aiming to preserve resources and ensure optimal medical care. Limited clinical neurosurgical experience with patients with COVID-19 has been reported. We share organizational experience, attitudes, and preliminary data of patients treated at our institution.
Methods
Institutional guidelines and patient workflow are described and visualized. A cohort of all neurosurgical patients managed during the lockdown period is presented and analyzed, assessing suspected nosocomial infection risk factors. A comparative surgical subcohort from the previous year was used to investigate the impact on surgical activity.
Results
A total of 176 patients were admitted in 66 days, 20 of whom tested positive for COVID-19. Patients initially admitted to the neurosurgical ward were less likely to be suspected for a COVID-19 infection compared with patients admitted for critical emergencies, particularly with neurovascular and stroke-related diseases. The mortality of patients with COVID-19 was remarkably high (45%), and even higher in patients who underwent surgical intervention (77%). In addition to the expected decrease in surgical activity (–53%), a decrease in traumatic emergencies was noted.
Conclusions
By applying infection prevention and resource-sparing logistics measures shared by the international medical community, we were able to maintain essential neurosurgical care in a pandemic with controlled nosocomial infection risk. Special consideration should be given to medical management and surgical indications in patients infected with severe acute respiratory syndrome coronavirus 2, because they seem to show a problematic hemostatic profile that might result in an unfavorable clinical and surgical outcome.
Key words: COVID-19, Lockdown, Neurosurgery, SARS-CoV-2
Abbreviations and Acronyms: COVID-19, Coronavirus disease 2019; ICU, Intensive care unit; OR, Operating room; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SRS, Stereotactic radiosurgery
Introduction
In December 2019, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan, China. Coronavirus disease 2019 (COVID-19) has since spread throughout China, and then worldwide. COVID-19 was declared as a pandemic by the World Health Organization on March 11, 2020. By mid-March, Europe had become the new epicenter of the outbreak. In Belgium, patient zero was reported on February 4, the first COVID-19-related death was confirmed on March 11, and the country was soon put on lockdown. According to the latest estimates,1 Belgium's health care system capacity disposed of about 576 hospital beds and 15.9 intensive care unit (ICU) beds per 100,000 citizens, with an undetermined number of available ventilators. On May 21, 2020, 55,983 reported cases, and >9150 deaths had been attributed to COVID-19, resulting in one of Europe's highest deaths per one million inhabitants ratio (801/1 million), and a case/fatality ratio of 16.3%. Despite being a developed country, Belgium is not able to acquire or produce enough personal protective equipment to ensure the optimal safety of health care providers, in addition to the lack of reagents and logistical resources to proceed with mass testing. Accordingly, the health ministry guidelines reserved biochemical tests of nasopharyngeal samples only for patients with suspected COVID-19 requiring hospital admission, and no targeted screening strategy was defined for health care providers. Since March 12, 2020, all medical activities of our academic hospital have been reorganized. Nonurgent elective surgeries and outpatient activities were suspended. Wards, resources, and teams were redeployed to anticipate any potential congestion according to the latest national and institutional forecasts.2 To our knowledge, no specific neurosurgical practice recommendations anticipating such situations were available at the onset of the outbreak in Belgium, and there are still only limited clinical experiences reported to support recently published recommendations.3, 4 We therefore share our lockdown experience, attitude, and measures taken from the frontlines, substantiated by preliminary data of treated patients, outlining some features of patients with COVID-19.
Methods
Data Collection
After the approval of the institutional ethical committee, data were collected to form 2 cohorts: a crisis cohort, which included all patients requiring neurosurgical management during the lockdown period (March 6–May 10, 2020), and a surgical subcohort of patients who underwent any neurosurgical intervention during this period. Neurosurgical intervention was defined as any therapeutic procedure planned and applied by a neurosurgeon taking place in an operating room (OR) or stereotactic radiosurgery (SRS) room. A third comparative surgical cohort that included all patients who underwent any neurosurgical intervention at the same center, between March 1 and May 5, 2019, was used to investigate the impact of the crisis on our surgical activity. The hospital electronic health record was reviewed to identify all eligible patients for the crisis cohort. The extracted data consisted of demographic features, potential risk factors, and comorbidities (e.g., cigarette smoking, chronic obstructive pulmonary disease, diabetes, arterial hypertension, and obesity), clinical suspicion of COVID-19, SARS-CoV-2 infection status (if available), and neurosurgical indication and care. All data needed for the comparative surgical cohort were retrieved from the OR records.
Statistical Analysis
All descriptive statistics and statistical analyses were performed using R version 3.6.1 within the RStudio software version 1.2.1335 (R Foundation for Statistical Computing, Vienna, Austria). A χ2 test was used to investigate independence for categorical data; a Fisher exact test was applied when sample size consisted of occurrences <5 and a Welch t test was used as a location test when applicable. The predefined statistical significance level was assumed when P was <0.05.
Results
Institutional Guidelines
Our ad hoc institutional guidelines (Table 1 ) and departmental workflow were defined at the local onset of the outbreak in the absence of agreed guidelines. Our goal was to maintain optimal patient neurosurgical care and tackle modifiable risk factors for nosocomial SARS-CoV-2 infection and reduce the burden of the medical teams directly involved in the COVID-19 crisis. Therefore, and taking into consideration the recommendations of colleagues struck by the crisis in an earlier timeline,5, 6, 7 all elective clinical and surgical activities were suspended and systematic screening and testing were deployed as soon as possible. Moreover, workforce and resources were redistributed, and telemedicine was used, serving many patients. To protect health care staff, a social distancing policy was applied for all group-based activities, including case discussion meetings, nurse staff meetings, and seminars. All necessary meetings were replaced by video teleconferences or webinars, and the same tools facilitated resident education. Aiming to avoid cross-infection, small versatile teams dedicated to specific wards or units were constructed, back-up teams were provided, and shift organization was carefully planned.
Table 1.
Summary of Measures Applied in Our Neurosurgical Practice
| Measures to avoid the risk of hospital congestion | Stop all elective clinical and surgical activities to redeploy wards to COVID-19 outbreak Triage case by phone and implementation of telemedicine |
| Outpatient management | Use telemedicine for consulting and screening |
| Clinical scheduling | Online preoperative visits Elective clinic visits canceled |
| Surgical scheduling | Only emergent and semiurgency were scheduled Elective surgeries canceled |
| Inpatient measures | Separate neurosurgical units (clean areas) and COVID units Avoid all crossing between patients infected and noninfected |
| Emergency | Cerebral hemorrhages (subarachnoid and intracerebral hemorrhages) Acute hydrocephalus Tumors at risk of intracranial hypertension Spinal cord compressions with neurologic deficit Cranial and spinal trauma emergencies Spine oncology, epidural abscess, cauda equina or severe root compression |
| Screening related with COVID-19 | Reviews list of symptoms and exposure history Throat swab and chest computed tomography for all admission if testing available. Two swabs at a distance of 2–4 days (to minimize false-negatives possibility) Diagnosis confirmed by 1) positive nucleic acid test of SARS-CoV-2 detected reverse transcription-quantitative polymerase chain reaction, 2) highly homologous genome sequencing to SARS-CoV-2, and 3) positive serologic testing of SARS-CoV-2-specific immunoglobulin G and immunoglobulin M antibodies |
| Intraoperative measures if COVID-19-positive patient | Patient transportation on a closed circuit to a small negative-pressure suction room Respect airway management protocols for intubation/extubation (minimal personnel in the room, using contained air purifying respirators, out-of-room waiting time) Limit unnecessary personnel Avoid endonasal surgeries Decrease speed of bone drilling to reduce spread of bone dust Optimize surgical team to shorten duration of surgeries |
| Appropriate PPE | Disposable FFP2/N95 mask, water-resistant gown, gloves, goggles, cap, and full-face visor shield For COVID-19 positive patients, FFP3 mask and/or powered air purifying respirators |
| Specific surgical management | Endonasal surgery: manage patient as suspected case - nasal irrigation with povidone-iodine solution, caution with dural handling, minimize drilling and prefer osteotomes Spine surgery: favor prone position, minimally invasive approach, reduce suction and splatter Brain surgery: avoid awake strategies and biopsy rather than surgical resection if possible |
| ICU | Manage positive patients with COVID-19 to a separated COVID ICU unit Postoperative care for uncomplicated surgery includes craniotomies cases in a medium care unit rather than ICU |
| Postoperative management | Emphasize rapid discharge with close telemedicine follow-up |
| Measures for clinical team | Follow universal precautions and PPE guidelines Social distancing for all group-based activities Reduce the number of health care staff on clinical duty Clinical team-bases rotations to reduce virus exposure Social distancing for all group-based activities |
| Conference and education | All in-person conferences were canceled and replaced by seminars or webinars through video teleconferences |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PPE, personal protection equipment, ICU, intensive care unit.
Institutional guidelines defined clinical suspicion of COVID-19 as an influenzalike illness with potentially more specific symptoms (e.g., anosmia) or contact with a confirmed individual; tracing considerations were not required but sufficient. A chest computed tomography (CT) scan was performed for all patients consulting the emergency department and requiring admission. The CT scan was interpreted by a radiologist as negative (normal), indeterminate (abnormal), or positive (abnormal and suggestive of COVID-19). The patient's destination was determined depending on this result (i.e., in a classic ward [normal test] or a dedicated COVID-19 unit [abnormal test]). Chest CT was performed for any clinically suspected inpatient in addition to a nasopharyngeal swab for a polymerase chain reaction and antigen detection tests and the patient was directly admitted to a COVID-19 unit, regardless of the chest CT scan interpretation. The suspicion of COVID-19 was a 3-tiered scale (low, moderate, or high) based on the subsequent detection tests and workup results and was updated in real time in the electronic health record. These units were formed by multidisciplinary teams led by attending hospitalists. Positive SARS-CoV-2 infection status was defined as any positive result of the biochemical detection tests. Any transfer from a COVID-19 to non-COVID-19 ward required ≥2 consecutive repeated negative biochemical tests on nasopharyngeal and/or bronchoalveolar samples and multidisciplinary consensus.
To maintain isolation of patients with COVID-19 from noninfected inpatients, new dedicated tracks for in-hospital mobility were made, dedicated postoperative units were created avoiding a stay in the ICU, and visits were strictly prohibited. Moreover, and to prevent potential superspreaders, systematic and repeated testing of personnel was made mandatory as well as soon as available.
Neurosurgical Patient Flow and Management
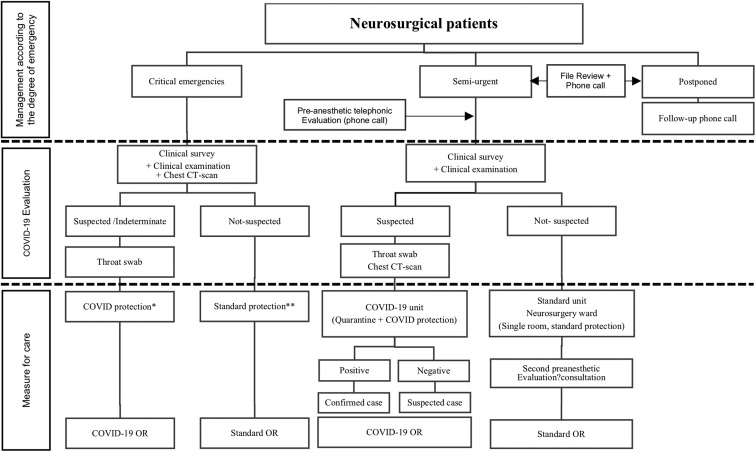
All critical emergencies in the absence of a chest CT scan were considered and managed as suspected for COVID-19. In the cases of patients with semi-urgent pathologies, the absence of a surgical treatment could be life-threatening or would result in worsening the prognosis (e.g., in oncologic diseases or refractory pain); a surgical treatment was scheduled in the shortest delay. When admitted, the patients were routed to the neurosurgery ward or the SRS facility through the dedicated lane. If eligible, the patients were routed to the neurosurgery ward or the SRS facility through the dedicated lane. On any suspicion of COVID-19 contact or infection, patients were transferred to a COVID-19 unit. Figure 1 shows the admission workflow of our institution.
Figure 1.
The admission workflow of our institution. ∗COVID protection: FFP2 medical masks, protective goggles, and suit; ∗∗Standard protection: surgical mask for patient and nursing staff, on a gown. CT, computed tomography; OR, operating room.
Two ORs were provided for patients with suspected or confirmed COVID-19. Specific protocols were applied to minimize the team's transmission risk: the minimum number of personnel was allowed in the OR and specific personal protective equipment was used for aerosol-generating procedures and high-risk surgeries such as endonasal and transsinusal approaches. In the ordinary OR, common measures were applied: intubation with the minimum personnel in the room, the use of self-contained closed-circuit respirators while avoiding positive-pressure ventilation, and prevention of pipe disconnection (Figure 1).
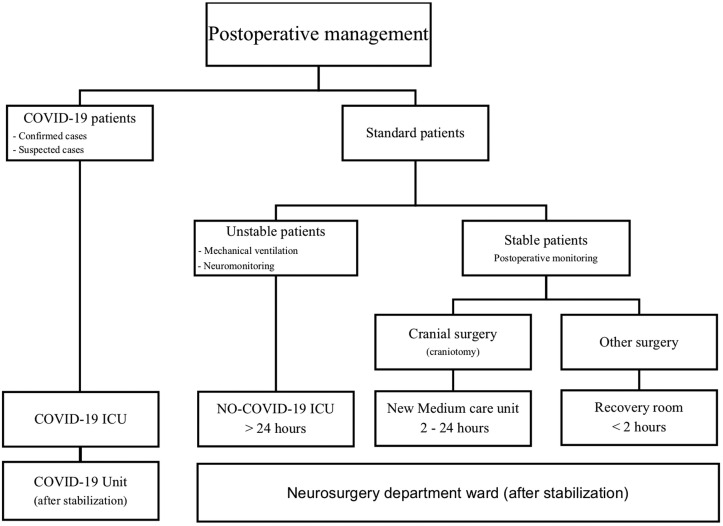
Four of 5 hospital ICUs were converted for COVID-19. One postanesthesia care unit was transformed into a medium care unit. Patients were admitted to these units according to their COVID-19 status, their need for mechanical ventilation, and the degree of required surveillance (Figure 2 ).
Figure 2.
The postoperative workflow of our institution. ICU, intensive care unit.
Crisis Cohort and COVID-19 Aspects
The retrospective review resulted in a crisis cohort of 176 inpatients (Table 2 ). The mean age was 52 years (range, 0–97 years), 83 patients were female (47%), and 20 patients were children (11%). Concerning the point of admission, 108 patients were admitted directly to the neurosurgical ward for semiurgent surgery or conservative treatment and 68 patients to the ICU or stroke unit for critical management. Regarding the care received, 22 patients received conservative treatment, 21 endovascular treatment, 132 cranial surgery, 44 spinal surgery, 3 surgical site infection surgery, and 12 SRS. The first postoperative location of surgical patients was the ICU for 54 patients, the stroke unit for 11 patients, the medium care unit for 66 patients, the neurosurgical ward for 22 patients, and 1 pediatric patient was transferred to the neonatal ICU. Of the included patients, 20 tested positive for SARS-CoV-2, 15 of whom were positive at the time of admission and only 5 of whom were found to be positive during their hospitalization. One patient tested positive during the first 24 hours of admission, 2 within 15 days, and all by 30 days. Our analysis showed a significant difference between patients who were initially admitted to the neurosurgical ward and others suspected of COVID-19 who subsequently tested positive for SARS-CoV-2 (Fisher exact test of independence, P = 0.003389).
Table 2.
Demographics and Clinical Characteristics of Study Cohort
| Characteristic | Global Cohort (n = 176) | COVID-19-Positive Patients (n = 20) | COVID-19-Negative Patients (n = 156) | P Value |
|---|---|---|---|---|
| Age (years), (standard deviation) | 52 (22) | 67 (14, 77) | 50 (22, 32) | 3.17683e−05 |
| Sex | 0.358 | |||
| Female | 83 (47) | 7 (35) | 76 (49) | |
| Male | 93 (53) | 13 (65) | 80 (51) | |
| Pediatric | 20 (11) | 0 (0) | 20 (13) | |
| Comorbidity factors | ||||
| Current smoker | 52 (30) | 6 (30) | 46 (29) | |
| Arterial hypertension | 54 (31) | 10 (50) | 44 (28) | |
| Diabetes | 24 (14) | 8 (40) | 16 (10) | |
| Coronary heart disease | 21 (12) | 0 (0) | 21 (13) | |
| Chronic obstructive pulmonary disease | 8 (5) | 1 (5) | 7 (4) | |
| Chronic kidney disease | 1 (1) | 0 (0) | 1 (1) | |
| Obesity | 24 (14) | 3 (15) | 21 (13) | |
| Number of comorbidity factors | 0.2464 | |||
| <2 | 129 (73) | 12 (60) | 107 (75) | |
| ≥2 | 47 (27) | 8 (40) | 39 (25) | |
| Preoperative location | 7.542e−10 | |||
| ICU | 53 (30) | 13 (65) | 40 (25) | |
| Stroke | 9 (5) | 2 (10) | 7 (4) | |
| Ward | 108 (61) | 0 (0) | 108 (69) | |
| COVID-19 unit | 6 (3) | 5 (25) | 1 (1) | |
| Admission indication | 0.02882 | |||
| Cranial indications | 132 (75) | 19 (95) | 113 (72) | |
| Trauma | 23 (13) | 3 (15) | 20 (13) | |
| Vascular | 39 (22) | 13 (65) | 26 (17) | |
| Hydrocephalus | 11 (6) | 0 (0) | 11 (7) | |
| Infection | 3 (2) | 0 (0) | 3 (2) | |
| Oncology | 43 (24) | 3 (15) | 40 (26) | |
| SRS, tumor | 11 (6) | 0 (0) | 11 (7) | |
| SRS, arteriovenous malformation | 1 (1) | 0 (0) | 1 (1) | |
| Parkinson disease | 1 (1) | 0 (0) | 1 (1) | |
| Spinal indications | 44 (25) | 1 (5) | 43 (27) | |
| Trauma | 6 (3) | 0 (0) | 6 (4) | |
| Infection | 2 (1) | 0 (0) | 2 (1) | |
| Neurologic deficit/refractory pain | 27 (15) | 0 (0) | 27 (17) | |
| Tumor | 9 (5) | 1 (5) | 8 (5) | |
| Conservative treatment | 22 (13) | 6 (30) | 16 (10) | 0.02294 |
| Surgical procedure | 154 (88) | 14 (70) | 140 (90) | |
| Craniectomy | 65 (42) | 8 (62) | 57 (41) | |
| Cranial endoscopy | 6 (4) | 0 (0) | 6 (4) | |
| Spine | 42 (27) | 1 (8) | 41 (29) | |
| SRS | 16 (10) | 0 (0) | 16 (11) | |
| Others | 3 (2) | 0 (0) | 3 (2) | |
| Embolization | 6 (4) | 1 (8) | 5 (4) | |
| Thrombectomy | 15 (10) | 4 (31) | 11 (8) | |
| Postoperative location | ||||
| ICU | 54 (35) | 10 (77) | 44 (31) | |
| Neonatal ICU | 1 (1) | 0 (0) | 1 (1) | |
| Stroke unit | 11 (7) | 3 (23) | 8 (6) | |
| Medium care unit | 66 (43) | 1 (8) | 65 (46) | |
| Ward | 22 (14) | 0 (0) | 22 (16) | |
| ICU length of stay (days) | 4.201e−04 | |||
| <2 | 33 (61) | 1 (10) | 32 (73) | |
| ≥2 | 21 (39) | 9 (90) | 12 (27) | |
| Deaths | 16 (9) | 9 (45) | 7 (4) | |
Values are number (%) except where indicated otherwise.
P values were calculated with the χ2 test, Fisher exact test, or Student t test.
SRS, stereotactic radiosurgery; ICU, intensive care unit.
Patients with COVID-19
Thirteen patients were admitted for an acute cerebrovascular event; their mean age was 64 years (range, 41–88 years). Two of them were younger than 45 years, both of whom showed an unusual large-vessel stroke. Three required a decompressive craniectomy and 3 died. Moreover, 3 other patients were admitted for head trauma, 2 required a craniectomy, and 1 died. One patient was treated for a metastatic spinal cord lesion and died. Three patients were admitted for a diagnostic biopsy of neoplastic lesions; all 3 developed a critical intracranial hemorrhage after these minimally invasive procedures (1 brain biopsies and 1 dural biopsy). The mortality in the group with COVID-19 was remarkably high (45%), and even higher in patients who underwent surgical intervention (77%; 7 of 9 patients). All but 1 patient with COVID-19 (95%) presented with a COVID-19 infection status that did not necessitate respiratory support and their neurologic condition was the main reason for their hospitalization.
Impact on Surgical Activity
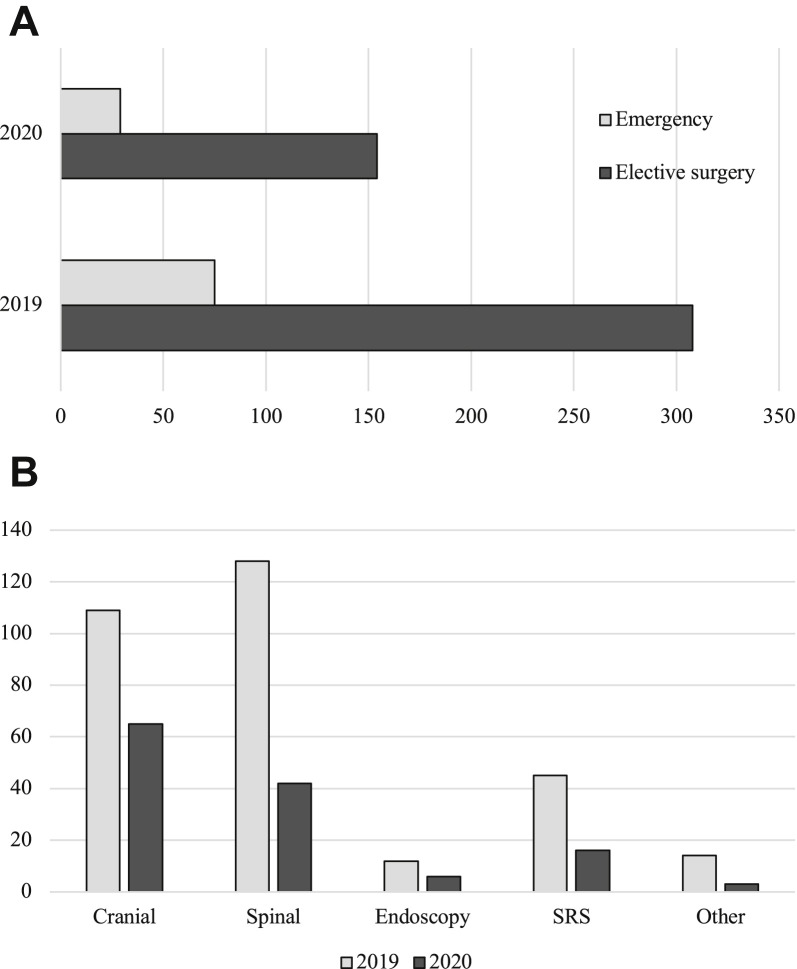
As shown in Figure 3 , a decrease was observed in the number of patients operated on, because surgical activity was reserved for critical and semiurgent surgery. The total number of patients operated on decreased from 308 to 154 (–50%), and emergent surgeries showed a decrease from 75 to 29 compared with a similar period in 2019.
Figure 3.
(A) Decrease in urgent and nonurgent surgical activity of our neurosurgical department during the lockdown period (March 6–May 10, 2020), compared with the same period in 2019. (B) Decrease in surgical activities by type of procedure. SRS, stereotactic radiosurgery.
Discussion
Measures and Practice Adaptation
The coronavirus pandemic as a global and unprecedented crisis generated a variety of containment measures defined on a multilevel basis by local, regional, national, and institutional actors. These measures differed largely in their radicality and span among countries and even regions and cities, because they were defined by resources, caseload, and experts' forecasting. In Northern Italy, a hub-and-spoke system8, 9, 10 was used to separate the treatment of patients in different hospitals depending on their infectious status of COVID-19. In the United States, Burke et al.11 proposed an algorithm aiming for dynamic resource allocation (a volume-limiting approach that was based on a 3-tiered system of viral surge quantification). In China, Tan et al.12 proposed that management measures and practical patient workflow should be based on conventional treatment guidelines and clinical experiences; restrictive scheduling of neurosurgical activities on emergency and semiurgent indications was implemented. In Singapore, outpatient mobility between hospitals was restricted and neurosurgical care was distributed across tertiary hospitals for subspecialty coverage (neurovascular, skull base, and neuro-oncology), and regional hospitals focused primarily on trauma and spine.
Three studies3, 4, 13 have accompanied their guidelines with clinical data. Sun et al.4 reported a cohort of 122 emergent surgeries, Agosti et al.3 collected data from 1 of the 4 Lombard hub hospitals in Varese and reported the clinical outcome of 34 emergencies, and Giorgi et al.13 reported a series of 19 patients admitted for spinal emergencies. None of these studies reported extensive data regarding the clinical outcome of their populations. including their COVID-19 status.
Because of the lack of relevant reported data and the heterogeneity of pandemic spread and impact, comparing the efficacy of taken measures on preventing COVID-19 transmission and their effect on the management of neurosurgical patients is not feasible and subject to misleading interpretations.
Cohort Results
Impact on Surgical Activity
Essential surgical activity was maintained; all urgent and semiurgent traumatic, vascular, oncologic, and spinal diseases were treated. For neoplastic diseases, SRS when indicated was preferred. As a 1-day procedure, it provided a potential alternative to low-risk surgery, especially for infected patients who needed oncologic management. Recent editorials have given some recommendations14 , 15 regarding the use of SRS during the crisis in patients with COVID-19, but feedback on its application to infected patients is required for a more thorough assessment.
The surgical activity, as expected, decreased, and aside from the cancelation of all postponable elective surgeries, a dramatic decrease in trauma-related surgeries such as traumatic brain injury and spinal trauma was observed, probably because of the decreased incidence of traffic and work accidents during the lockdown. This indirect effect of the lockdown (mainly promoted to the population as a measure to contain viral transmission) reduced the burden on our critical care teams and released resources for the COVID-19 crisis. More data are needed to have a clear insight into the changing landscape of neurosurgical emergencies during the outbreak. Moreover, concern about a potentially delayed aftermath as a result of postponed surgery and the population's anxiety in consulting should be anticipated and overridden by appropriate public health communication.
Observations on COVID-19 Cases
Testing Positive
Patients admitted for critical emergencies, particularly with neurovascular and stroke-related diseases, passing through ICUs and stroke units were more likely to be suspected for COVID-19. No correlation between the nature of the reported disease and the likelihood of testing positive for COVID-19 was observed. To explain this situation, we impute the higher frequency of multiple risk factors and comorbidities in this population requiring specific management with numerous intrahospital transfers from different locations comprising standard but also COVID-19 units, ICUs, neurovascular wards, stroke units, and radiology rooms and the need of closer and longer medical care, implying contact with multiple health care providers, which increases contamination risk.
Effects on Central Nervous System
The effect of SARS-CoV-2 on the central nervous system is still not well defined; emerging studies have reported neurologic manifestations in 36.4% of patients with confirmed COVID-19 most frequently attributed to vascular events. Because patients infected with the SARS-CoV-2 virus have been reported to present with hemostatic disorders, abnormal coagulation findings, including thrombocytopenia, increased levels of D-dimer, prolonged prothrombin time, and disseminated intravascular coagulation and vascular endothelial dysfunction, have been described.16 Klok et al.17 reported that 31% of critically ill patients with COVID-19 develop thrombotic complications that were linked to the severity of the infection. Mao et al.18 reported that 5.7% of the severely infected patients with COVID-19 developed cerebrovascular disease in the course of their illness. Major hemorrhagic complications with spontaneous intraparenchymal bleeding in severely infected patients are starting to emerge in the literature.19, 20, 21, 22 Panciani et al.23 described 4 patients with COVID-19 who underwent surgery or embolization for chronic subdural hematomas, with a mortality of 100%.
Sharifi-Razavi et al.21 hypothesized that the angiotensin-converting-enzyme II receptor, used by the SARS-CoV-2 virus for cell entry, is responsible for dysfunction of the cerebrovascular endothelial cells and may lead to disruption of autoregulation as well as blood pressure spikes as a result of arterial wall rupture. In most cited studies,17 , 18 a link is shown between cerebrovascular complications and the severity of the infection.
Despite their infection status, 12 of the 20 patients in our cohort developed a cerebrovascular event after testing positive for the virus, implementing that patients with nonsevere infection might develop coagulopathy as well. Moreover, 3 patients who were admitted for brain lesions that necessitated a biopsy for an anatomopathologic diagnosis were all complicated by a fatal intraparenchymal hemorrhage 24–72 hours postoperatively. Even if a coincidental occurrence of these events cannot be excluded, or if the neovascularization is to be blamed, the role of the infection should be considered.
Nevertheless, by May 18, 2020, SARS-CoV-2 had infected 4,862,273 patients worldwide, some of whom required surgery during their infection; based on reported experience, attention should be given to surgical indications and timing in these patients. As advocated by neurosurgical and neurocritical care societies, a unified approach to reporting experience from patients with COVID-19 is required, because the acquisition of such data would lead to an agreed approach to patient management.
Study Limitations
In addition to the flaws of a retrospective observational study, which lacked a control group, the early data collection and the time frame posed did not permit a long follow-up period of admitted patients, because they might have presented symptoms after their release or might have died. Moreover, a cautionary statement regarding the interpretation of our statistical analysis is due. The statistical power of our data analysis and subsequent significance of statistical inference is limited, because of its categorical nature, small sample size, and the low number of events in this monocentric data set, when calculating.
The rapidity with which studies and reviews are published does not permit a comprehensive review of existing literature and reported cohorts.
Conclusions
The COVID-19 outbreak has had a major impact on neurosurgical practice. Each neurosurgical department has reacted independently, following governmental and institutional guidelines. Our experience may show a temporary ability to maintain adequate essential neurosurgical care in a pandemic with controlled nosocomial infection risk by applying well-known measures, but collaboration and experience sharing will pave the way toward a collaborative response to this crisis.
Special consideration should be given when treating patients infected with SARS-CoV-2 because they may show a problematic hemostatic profile, which might result in an unfavorable clinical and surgical outcome, according to our experience.
CRediT authorship contribution statement
Alphonse Lubansu: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Mouhssine Assamadi: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Sami Barrit: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Victoria Dembour: Data curation, Writing - original draft. Gedeon Yao: Data curation. Salim El Hadwe: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Olivier De Witte: Writing - original draft.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.World Health Organization COVID-19 situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at:
- 2.Scienseano Epistat–Covid19 Monitoring. https://epistat.wiv-isp.be/covid/ Available at:
- 3.Agosti E., Giorgianni A., Pradella R., Locatelli D. COVID-19 outbreak: a single-center experience in neurosurgical and neuroradiological emergency network tailoring. World Neurosurg. 2020;138:548–550. doi: 10.1016/j.wneu.2020.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y., Mao Y. Editorial. Response to COVID-19 in Chinese neurosurgery and beyond. https://doi.org/10.3171/2020.3.JNS20929 [e-pub ahead of print]. J Neurosurg. [DOI] [PMC free article] [PubMed]
- 5.Amin-Hanjani S., Bambakidis N.C., Barker F.G. Editorial. COVID-19 and neurosurgical practice: an interim report. J Neurosurg. 2020;133:3–4. doi: 10.3171/2020.4.JNS201099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichberg D.G., Shah A.H., Luther E.M. Letter: Academic Neurosurgery Department Response to COVID-19 Pandemic: The University of Miami/Jackson Memorial Hospital Model. Neurosurgery. 2020;87:E63–E65. doi: 10.1093/neuros/nyaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Z.D., Chyi Yeu D.L., Ang B.T., Ng W.H., Seow W.T. Editorial. COVID-19 and its impact on neurosurgery: our early experience in Singapore. J Neurosurg. 2020;133:24–25. doi: 10.3171/2020.4.JNS201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernucci C., Brembilla C., Veiceschi P. Effects of the COVID-19 Outbreak in Northern Italy: Perspectives from the Bergamo Neurosurgery Department. World Neurosurg. 2020;137:465–468.e1. doi: 10.1016/j.wneu.2020.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cenzato M., DiMeco F., Fontanella M., Locatelli D., Servadei F. Editorial. Neurosurgery in the storm of COVID-19: suggestions from the Lombardy region, Italy (ex malobonum) J Neurosurg. 2020;133:33–34. doi: 10.3171/2020.3.JNS20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G., Pesenti A., Cecconi M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA. 2020;323:1545. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 11.Burke J.F., Chan A.K., Mummaneni V. Letter: The Coronavirus Disease 2019 Global Pandemic: A Neurosurgical Treatment Algorithm. Neurosurgery. 2020;87:E50–E56. doi: 10.1093/neuros/nyaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan Y., Wang J., Zhao K. Preliminary Recommendations for Surgical Practice of Neurosurgery Department in the Central Epidemic Area of 2019 Coronavirus Infection. Curr Med Sci. 2020;40:281–284. doi: 10.1007/s11596-020-2173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgi P.D., Villa F., Gallazzi E. The management of emergency spinal surgery during the COVID-19 pandemic in Italy: a preliminary report. Bone Jt J. 2020;102:671–676. doi: 10.1302/0301-620X.102B6.BJJ-2020-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liaw J., Patel V.A., Bann D.V. Letter: COVID-19 Pandemic: Safety Precautions for Stereotactic Radiosurgery. Neurosurgery. 2020;87:E201–E202. doi: 10.1093/neuros/nyaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franzin A., Spatola G., Giudice L., Migliorati K., Vivaldi O., Giorgi C. Maintaining stereotactic radiosurgical treatments during Covid-19 outbreak: the case of the Gamma Knife Unit in Brescia – Italy. Br J Neurosurg. 2020;34:353–354. doi: 10.1080/02688697.2020.1758297. [DOI] [PubMed] [Google Scholar]
- 16.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV, and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao L., Jin H., Wang M. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti C.B., Henchi S., Coppeta G.P., Testa S., Grassia R. Bleeding in COVID-19 severe pneumonia: The other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147–149. doi: 10.1016/j.ejim.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral hemorrhage: causative or coincidental? New Microbes and New Infections. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vu D., Ruggiero M., Choi W.S. Three unsuspected CT diagnoses of COVID-19. Emerg Radiol. 2020;27:229–232. doi: 10.1007/s10140-020-01775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panciani P.P., Saraceno G., Zanin L., Renisi G., Signorini L., Fontanella M.M. Letter: COVID-19 Infection Affects Surgical Outcome of Chronic Subdural Hematoma. Neurosurgery. 2020;87:E167–E171. doi: 10.1093/neuros/nyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]