Abstract
Purpose
The aim of this study was to investigate potential markers of coagulopathy and the effects of thromboprophylaxis with low-molecular-weight heparin (LMWH) on thromboelastography (TEG) and anti-factor Xa in critically ill COVID-19 patients.
Material and Methods
We conducted a prospective study in 31 consecutive adult intensive care unit (ICU) patients. TEG with and without heparinase and anti-factor Xa analysis were performed. Standard thromboprophylaxis was given with dalteparin (75–100 IU/kg subcutaneously).
Results
Five patients (16%) had symptomatic thromboembolic events. All patients had a maximum amplitude (MA) > 65 mm and 13 (42%) had MA > 72 mm at some point during ICU stay. Anti-factor Xa activity were below the target range in 23% of the patients and above target range in 46% of patients. There was no significant correlation between dalteparin dose and anti-factor Xa activity.
Conclusions
Patients with COVID-19 have hypercoagulability with high MA on TEG. The effect of LMWH on thromboembolic disease, anti-factor Xa activity and TEG was variable and could not be reliably predicted. This indicates that standard prophylactic doses of LMWH may be insufficient. Monitoring coagulation and the LMWH effect is important in patients with COVID-19 but interpreting the results in relation to risk of thromboembolic disease poses difficulties.
Keywords: COVID-19, Intensive care, Thrombelastography, Low-molecular-weight heparin, Thromboembolism
1. Introduction
Patients with Corona virus disease 2019 (COVID-19) have been reported prone to develop thrombotic and thromboembolic disease [[1], [2], [3], [4]]. Microthrombosis has been suggested to contribute to both respiratory failure and neurological complications [5,6] and emerging data describe coagulation abnormalities as a marker of poor prognosis [[7], [8], [9]].
Low molecular weight heparins (LMWH) are often prescribed to decrease the risk of thromboembolic complications in hospitalized patients and treatment with LMWH was recently shown to reduce mortality in high-risk patients with COVID-19 [10]. However, data is limited on the mechanisms underlying the coagulation deficits associated with COVID-19 and how they are affected by LMWH.
Thromboelastography (TEG) offers a comprehensive assessment of coagulation in whole blood samples, describing plasmatic coagulation as well as platelet function and fibrinolysis. Furthermore, the addition of heparinase to the sample eliminates the iatrogenic heparin effect in blood samples thus allowing estimation of the effect of LMWH.
The aim was to characterize coagulation abnormalities and the effect of LMWH with respect to TEG profile with and without heparinase, anti-factor Xa activity and clinical manifestations (i.e. bleeding and thrombosis) in 31 COVID-19 patients admitted to intensive care units at Uppsala University Hospital, Uppsala, Sweden.
2. Materials and methods
The study was approved by the National Ethical Review Agency (EPM; No. 2020–01623). Informed consent was obtained from the patient, or next of kin if the patient was unable give consent. The Declaration of Helsinki and its subsequent revisions were followed. The protocol of the study was registered a priori (ClinicalTrials ID: NCT04316884). STROBE guidelines were followed for reporting.
This prospective observational study was performed at a mixed surgical/medical intensive care unit (ICU) at Uppsala University Hospital, a tertiary care hospital in Sweden.
2.1. Data collection and patient groups
All adult patients with COVID-19 consecutively admitted to the ICU March 13th to April 14th 2020 with informed consent and routinely performed TEG results accessible were included in the study. COVID-19 was diagnosed with positive reverse-transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swabs.
Dalteparin (Fragmin®, Pfizer AB, Sollentuna, Sweden) subcutaneously was used for thrombosis prophylaxis. COVID-19 patients with body weight below 70 kg were given 5000 IU dalteparin, those with body weight 70–90 kg were given 7500 IU dalteparin and those above 90 kg were given 10,000 IU dalteparin. The anti-factor Xa target range for thrombosis prophylaxis was 0.2–0.4 kIU/L. [11]
Clinical data were recorded prospectively daily. Simplified acute physiology score 3 (SAPS3) [12], renal function, circulatory support and respiratory support data were collected as reported in the results. Blood samples were collected on ICU admission and daily during the ICU stay. Blood count (CBC), high sensitivity C-reactive protein (CRP), kidney and liver function tests were performed. CBC was analyzed on a Sysmex XN™ instrument (Sysmex, Kobe, Japan) while hsCRP, ferritin, kidney and liver markers were analyzed on an Architect ci16200 (Abbott Laboratories, Abbott Park, IL, US).
2.2. Coagulation tests
The viscoelastic properties were analyzed using the TEG 6 s® platform (Haemonetics, Boston, MA), [13] the citrated multichannel cartridge facilitates the simultaneous performance of the citrated kaolin (CK), citrated kaolin with heparinase (CKH), citrated rapidTEG® (CRT), and citrated functional fibrinogen (CFF) assays. R-time (R), angle (A), maximal amplitude (MA), and lysis at 30 min (Lys30) from the standard (kaolin) and heparinase curves were analyzed.
International Normalized Ratio (INR), activated partial thromboplastin time, anti-factor Xa and Fibrin D-dimer were all analyzed on a STA-R Max2 (Stago, Asnières-sur-Seine, France). The reagent used for the PK/INR method was STA SPA+ from Stago, Catalogue number 00330 STA. The reagent is used for determination of the combined Factors II, VII and X. Coagulation samples were taken every morning except anti-factor Xa which was taken approximately 4 h after subcutaneous injection of dalteparin. The limit of quantification for the anti-factor Xa assay was 0.1 kIU/L and the total coefficient of variation was 4.5% at 0.56 kIU/L. The limits of quantification for the D-dimer assay was 0.27 mg/L and the total coefficient of variation was 4.2% at 2.16 mg/L.
2.3. Statistical analysis
Continuous variables are presented as median (IQR, interquartile range) and categorical variables as number of observations (percent of total number of observations). Mann-Whitney U test was used for group comparisons and Spearman Rank to assess correlation between variables. Repeated tests were compared using Skillings-Mack test. A two-sided p < .05 was considered significant. Calculations were performed using Statistica (version 13.2, Stat. Soft. Inc. Tulsa, OK, USA) and Stata (version 15.1, StataCorp, College Station, TX, USA).
3. Results
A total of 31 patients diagnosed with COVID-19 treated in ICU were enrolled in the study. Characteristics of patients with and without thromboembolic events in the study cohort are listed in Table 1 . Most patients were male and the median BMI in the cohort was 30. One fourth of the cohort had a history of smoking, but ongoing smoking was unusual. The most common comorbidities were hypertension, diabetes mellitus and chronic lung disease.
Table 1.
Patient demographic characteristics and comorbidities.
| All participants |
No TE |
Any TE |
|
|---|---|---|---|
| n = 31 | n = 26 | n = 5 | |
| Women, n (%) | 6 (19) | 6 (23) | 0 (0) |
| Age | 65 (51–70) | 65 (51–71) | 51 (34–65) |
| Body weight, kg | 85 (80–94) | 85 (80–94) | 84 (81–89) |
| Height, cm | 173 (165–177) | 173 (165–177) | 171 (163–176) |
| BMI, kg/m2 | 30 (27–33) | 30 (26–33) | 30 (28–35) |
| SAPS3 | 53 (48–60) | 55 (49–60) | 49 (48–50) |
| COVID-19 day on arrival | 10 (8–12) | 10 (8–12) | 10 (9–10) |
| Comorbidities, n (%) | |||
| Hypertension | 18 (58) | 16 (62) | 2 (40) |
| Heart failure | 1 (3) | 1 (4) | 0 (0) |
| Peripheral vessel disease | 4 (13) | 4 (15) | 0 (0) |
| Previous thromboembolic event | 1 (3) | 1 (4) | 0 (0) |
| Pulmonary disease | 8 (26) | 8 (31) | 0 (0) |
| Diabetes mellitus | 9 (29) | 9 (35) | 0 (0) |
| Malignancy | 1 (3) | 1 (4) | 0 (0) |
BMI: Body mass index, COVID-19: Coronavirus disease 2019, IQR: Interquartile range, SAPS3: Simplified Acute Physiology Score, TE: thromboembolism.
Median (IQR) or n (%).
The median ICU admission was 10 days after the initial COVID-19 symptoms. During their ICU stay 24 patients (77%) were subjected to invasive ventilation and 25 patients (81%) were treated with vasopressors (noradrenalin) at some point.
Five patients developed clinical symptomatic thromboembolic events, (four with pulmonary embolism and one with deep vein thrombosis), all thromboembolic events diagnosed day 11–16 after onset of COVID-19 symptoms. No clinically significant bleeding was registered. Patients developing thromboembolism had a shorter activated partial thromboplastin time (APTT) and higher D-dimer compared to patients not developing thromboembolism. There were no differences in INR or platelet count. Comparison of coagulation markers are listed in Table 2 . Out of 31 patients included in the study, 20 had elevated MA on TEG. There were no differences in TEG variables in patients with or without thromboembolic events. All patients had a MA > 65 mm, while 23 patients (74%) had MA > 69 mm and 13 (42%) had MA > 72 mm at some point during ICU stay. No signs of increased thrombolysis were seen.
Table 2.
Inflammation and coagulation tests of patients at ICU admission.
| No TE |
Any TE |
p-value | Reference range | |
|---|---|---|---|---|
| n | 26 | 5 | ||
| CRP (mg/L) | 207 (117–269) | 143 (136–144) | .271 | <5 |
| Ferritin, (μg/L) | 1159 (514–2607) | 1152 (676–1930) | .830 | 25–310 |
| APTT (s) | 39 (35–48) | 33 (32–35) | .001 | 30–42 |
| D-dimer (mg/L) | 1.1 (0.7–2.8) | 3.7 (2.0–6.6) | .039 | <0.67 |
| INR | 1.1 (1.0–1.2) | 1.1 (1.0–1.1) | .318 | 0.9–1.2 |
| Platelet count (x109) | 196 (143–241) | 220 (157–224) | .830 | 150–350 |
| TEG | ||||
| R (s) | 7.2 (6.4–8.2) | 6.2 (5.3–7.7) | .347 | 4.6–9.1 |
| RKCH (s) | 7.0 (6.2–7.7) | 6.5 (5.4–8.5) | .628 | 4.3–8.0 |
| Angle (deg) | 77 (76–79) | 76 (74–77) | .216 | 63–78 |
| MA (mm) | 70 (69–73) | 70 (68–70) | .519 | 52–69 |
| Lys30 (%) | 0.0 (0.0–0.2) | 0.0 (0.0–0.0) | .106 | 0–2.6 |
| MA outside reference range (>69), n | 17 | 3 | ||
APTT: Activated partial thromboplastin time, CRP: C-reactive protein, INR: international normalized ratio, IQR: Interquartile range, KCH: kaolin with heparinase, Lys30: Clot lysis at 30 min, MA: maximum amplitude, R: reaction time, TE: thromboembolism, TEG: Thromboelastography.
Median (IQR). p-value from Mann-Whitney U test.
No major changes were seen in laboratory variables during the ICU stay, except for decreasing CRP (Table 3 ).
Table 3.
The evolution of inflammation and coagulation tests of patients during ICU stay.
| Day <4 |
Day 4–7 |
Day > 7 |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | ||
| CRP (mg/L) | 214 (152–294) | 31 | 252 (207–344) | 27 | 100 (48–230) | 20 | <.001 |
| Ferritin (μg/L) | 1257 (520–2645) | 30 | 1898 (794–2832) | 25 | 1559 (856–2974) | 17 | .001 |
| APTT (s) | 37 (34–41) | 19 | 44 (42–48) | 15 | 40 (37–49) | 11 | .068 |
| D-dimer (mg/L) | 2.1 (0.9–3.2) | 31 | 3.3 (1.9–4.3) | 27 | 3.0 (2.0–5.8) | 17 | .001 |
| INR | 1.1 (1.0–1.2) | 20 | 1.1 (1.0–1.1) | 13 | 1.1 (1.1–1.2) | 12 | .869 |
| Platelet count (x109) | 227 (163–248) | 31 | 358 (278–421) | 27 | 457 (328–528) | 20 | <.001 |
| Anti-factor Xa (kIE/L) | 0.2 (0.2–0.3) | 9 | 0.4 (0.3–0.4) | 13 | 0.4 (0.3–0.6) | 13 | .344 |
| TEG | |||||||
| R (s) | 7.3 (6.7–8.2) | 21 | 8.9 (7.3–10.6) | 11 | 8.1 (6.1–9.5) | 11 | .288 |
| RKCH (s) | 6.9 (6.2–7.7) | 21 | 8.3 (6.8–9.4) | 11 | 7.6 (5.7–10.1) | 11 | .580 |
| Angle (deg) | 76 (75–78) | 21 | 76 (69–80) | 11 | 79 (77–81) | 11 | .326 |
| MA (mm) | 69 (68–71) | 21 | 71 (69–72) | 11 | 71 (70–73) | 11 | .603 |
| Lys30 | 0.0 (0.0–0.2) | 21 | 0.0 (0.0–0.0) | 11 | 0.0 (0.0–0.1) | 11 | 1.0 |
APTT: Activated partial thromboplastin time, CRP: C-reactive protein, INR: international normalized ratio, IQR: Interquartile range, KCH: kaolin with heparinase, Lys30: Clot lysis at 30 min, MA: maximum amplitude, R: reaction time, TEG: Thromboelastography.
Median (IQR). p-value from Skillings-Mack test.
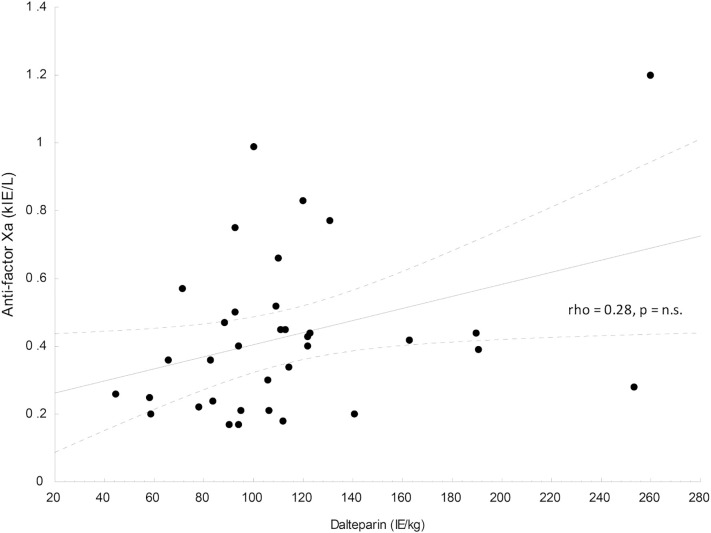
In the patients without thromboembolic events, i.e. with prophylaxis dose of dalteparin, 6 of the 26 (23%) had an anti-factor Xa at least once below the target range, while 12 of 26 (46%) had and anti-factor Xa at least once above the target range. There was no correlation between dalteparin dose and anti-factor Xa (Fig. 1 ).
Fig. 1.
The association between dalteparin dose and anti-factor Xa in the cohort with corresponding Spearman rank correlation coefficient.
4. Discussion
In this cohort study of 31 COVID-19 patients admitted to intensive care, a majority of patients show a hyper-coagulopathic phenotype during the course of illness. Further, standard prophylactic doses of LMWH may be insufficient to protect patients from thromboembolic complications. Analysis of anti-factor Xa activity and TEG appear inadequate to monitor the effect of LMWH in these patients.
The increased risk of thromboembolic complications in COVID-19 has been widely discussed, and early reports have indicated an associated coagulation abnormality predisposing thromboembolic disease. Standard coagulation parameters classically show marked elevation of fibrin-D-dimer, marginal increases in prothrombin time and slight thrombocytopenia [7]. In addition, Ranucci et al. recently demonstrated increased clot strength with significant platelet and fibrinogen contribution [14].
The present study reports the results of 31 patients using TEG, showing that hypercoagulability is a general feature across this population. This was seen in the form of increased maximum amplitude (MA) but not as a decreased R-time. This is in partial agreement with previous studies that have shown both decreased clot formation time and increased maximum amplitude [15,16]. A greater than normal MA has been able to predict thromboembolism in trauma and postoperatively [17]. In orthopedic trauma patients the risk of developing venous thromboembolism increases already when MA ≥ 65 mm, and further doubles if MA ≥ 72 mm [18]. This can be compared to our results where all patients had MA ≥ 65 mm and 42% had MA ≥ 72 mm. However, MA was not different in patients with or without thromboembolic events. Our results also indicate a higher than usual incidence of thromboembolic disease compared to the standard ICU population [19]. Still, it is not in the range of early clinical reports describing patients with COVID-19 [1]. This may reflect rapid implementation of supra normal prophylactic dosing of LMWH in our ICU.
COVID-19 patients have several factors predisposing to thrombotic disease, including immobilization, inflammation, venous catheterization and sometimes complex underlying illness. LMWH reduces the risk of lower extremity deep vein thrombosis and pulmonary embolism in critically ill patients [19] and the International Society on Thrombosis and Haemostasis recommends the use of LMWH for all COVID-19 patients admitted to hospital [20]. LMWH exert their effect by augmenting the inhibiting impact on activated factor X and thrombin by antitrombin III. This can be monitored by measuring plasma levels of anti-factor Xa activity, as recommended when dosing may be difficult [21]. A substantial number of patients in the present cohort had sub-prophylactic LMWH activity compared to current recommendations [11] despite normal dosing. This may lead to the conclusion that measuring anti-factor X activation may be useful. However, our results indicate that COVID-19 may be associated with thromboembolic complications even with anti-factor Xa activation in the accepted prophylactic range. This prompts for vigilance as it suggests that it may be difficult to predict the risk of thromboembolic complications using standard tests, and equally hard to safely conclude that a patient has adequate treatment with LMWH.
Strengths of this study includes data from TEG with and without heparinase, allowing assessment of heparin effects. Weaknesses include the relatively low number of patients and events and the fact that a number of patients in the study still receive critical care.
5. Conclusion
COVID-19 patients have hypercoagulability with high maximum amplitude (MA) on TEG, and often show insufficient effect of standard doses of LMWH. Our results suggest that neither anti-factor Xa activity nor TEG can reliably determine the effect of LMWH in patients with COVID-19. Further studies are needed to investigate the optimal dose of LMWH in these patients as well as potential alternative thrombosis prophylaxis.
Sources of funding
The study was funded by SciLifeLab, the Knut and Alice Wallenberg Foundation and in part by the Swedish Research Council (grant no 2014-02569 and 2014-07606). The funders had no part in study design; data collection, analysis or interpretation; writing of the report or the decision to submit for publication.
Declaration of Competing Interest
None.
Acknowledgements
We thank Research nurses Joanna Wessbergh and Elin Söderman for their expertise in compiling the study.
References
- 1.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. (10.1016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniel E.L., Clifford S.D., Matthieu L. Facing COVID-19 in ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46(6):1105–1108. doi: 10.1007/s00134-020-06059-6. (10.1007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B., Wang Y., Jimenez D., Ross J., Desai M., Parikh S., et al. Pulmonary embolism hospitalizations and use of inferior vena caval filters in older adults: national study of medicare beneficiaries. J Am Coll Cardiol. 2019;73:9. (10.1016) [Google Scholar]
- 4.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. (10.1016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. (10.1164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. (10.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. (10.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei M.Y., Ward S.M. The anti-factor Xa range for low molecular weight heparin Thromboprophylaxis. Hematol Rep. 2015;7(4):5844. doi: 10.4081/hr.2015.5844. (10.4081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno R.P., Metnitz P.G., Almeida E., Jordan B., Bauer P., Campos R.A., et al. SAPS 3--from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias J.D., Haney E.I., Mathew B.A., Lopez-Espina C.G., Orr A.W., Popovsky M.A. New-generation thromboelastography: comprehensive evaluation of citrated and heparinized blood sample storage effect on clot-forming variables. Arch Pathol Lab Med. 2017;141(4):569–577. doi: 10.5858/arpa.2016-0088-OA. [DOI] [PubMed] [Google Scholar]
- 14.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. (10.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. (10.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. (10.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown W., Lunati M., Maceroli M., Ernst A., Staley C., Johnson R., et al. The ability of thromboelastography to detect hypercoagulability. J Orthop Trauma. 2019;34(6):278–286. doi: 10.1097/BOT.0000000000001714. (10.1097) [DOI] [PubMed] [Google Scholar]
- 18.Gary J., Schneider P., Galpin M., Radwan Z., Munz J., Achor T., et al. Can Thrombelastography predict venous thromboembolic events in patients with severe extremity trauma? J Orthop Trauma. 2016;30:294–298. doi: 10.1097/BOT.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 19.Cook D., Meade M., Guyatt G., Walter S., Heels-Ansdell D., Warkentin T.E., et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305–1314. doi: 10.1056/NEJMoa1014475. [DOI] [PubMed] [Google Scholar]
- 20.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. (10.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia D.A., Baglin T.P., Weitz J.I., Samama M.M. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S–e43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]