Abstract
The SARS-CoV-2 pandemic has posed a significant challenge for risk evaluation and mitigation among cancer patients. Susceptibility to and severity of COVID-19 in cancer patients has not been studied in a prospective and broadly applicable manner. CAPTURE is a pan-cancer, longitudinal immune profiling study designed to address this knowledge gap.
The SARS-CoV-2 pandemic has posed a significant challenge for risk evaluation and mitigation among cancer patients. Susceptibility to and severity of COVID-19 in cancer patients has not been studied in a prospective and broadly applicable manner. CAPTURE is a pan-cancer, longitudinal immune profiling study designed to address this knowledge gap.
Main Text
Introduction
The global oncology community is faced with unprecedented challenges in the management of cancer patients in the context of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. In order to safeguard cancer patients, broad “self-shielding” policies and deviations from standard of care cancer management were implemented early in the pandemic to reduce the risk of viral transmission and severe COVID-19 respectively. Further, there was limited evidence to guide—or feasibility to implement—harm-minimization strategies that account for the heterogeneity of the cancer population, the spectrum of cancer states (remission or progressive) and interventions (surgery; chemo-, immuno-, radio-, targeted, hormonal, and cellular therapies; and bone marrow transplantation). These measures inevitably will have an impact on long-term cancer outcomes and are not sustainable. As such measures are eased nationally and globally (e.g., the recent blanket halting of self-shielding in the UK), there remains significant uncertainty regarding the ongoing risk of infection and severe disease in cancer patients given the risk of recurrent outbreaks of SARS-CoV-2 and uncertain vaccine effectiveness. This applies to both those who have not yet been exposed, who are likely to be in the majority due to self-shielding practices, and those who have been infected, given the uncertainty around long-term immunity to SARS-CoV-2 and the possibility of re-infection. Therefore, questions on appropriate management of cancer patients will persist well into the future.
Another risk is born out of cancer patients’ regular access to hospital services, close interactions with healthcare workers (HCWs) and increased hospital-acquired (termed “nosocomial”) transmission. Difficulties in decision making therefore lie in balancing potential nosocomial and community exposure risks (e.g., regular travel) with anti-cancer therapy benefits. Data on quantified risks of SARS-CoV-2 infection and adverse COVID-19 outcomes associated with regular clinic attendances and treatment scheduling for patients is urgently needed to weigh against treatment benefit. This includes varied scenarios, such as commencing immune checkpoint blockade (CPI) for stage IV melanoma, with prospects of durable disease control or even cure, or a 6-month adjuvant chemotherapy regimen for resected breast cancer with moderate relapse risks. As such, an epidemiological and immunological understanding of the interaction between the virus, the host, the cancer, and its management is needed across the clinical spectrum. Such understanding can then be placed in the context of the wider healthcare and public policy, to inform tailored harm-minimization strategies of SARS-CoV-2 and maximize cancer-specific survival.
Additionally, cancer populations are a crucial group of patients to inform a broader understanding of the immune response to SARS-CoV-2. Inherent perturbations on cell subsets (e.g., lymphoid and myeloid malignancies), or therapy-induced impact on immune states (e.g., immune checkpoint blockade) may provide opportunities to understand contributions of distinct immune compartments and key regulators of the anti-SARS-CoV-2 response. To date, the extent to which humoral or cellular responses contribute to effective immune protection, and how durable that immunity to SARS-CoV-2 is, remains unknown.
Herein, we aim to provide an overview of knowledge to date of the clinical features of COVID-19 observed in cancer patients, as well as potential impact of cancer and anti-cancer interventions on the immune response to SARS-CoV-2. We propose a comprehensive, longitudinal clinical outcomes and immune profiling program—the CAPTURE study—designed to rapidly accumulate data that will contribute to our understanding of immunopathology and immune protection in cancer patients, placed in the context of an evolving pandemic.
Clinical Studies of SARS-CoV-2 in Cancer Patients to Date
Early reports of SARS-CoV-2 outcomes in cancer patients have largely come from piecemeal, retrospective cohorts of infected patients (Table S1). Conclusions on subgroups were drawn from small numbers of patients—for example, increased risk of death on immunotherapy and chemotherapy was extrapolated from 6 and 17 patients, respectively (Zhang et al., 2020). Lung cancer was highlighted as a particular risk in early studies, but death rates from acute severe infections varied widely.
Large registry studies have provided a more granular understanding of clinical features associated with the risk of adverse COVID-19 outcomes. The UK Coronavirus Cancer Monitoring Project (UKCCMP) (n = 800) demonstrated mortality from COVID-19 was associated with general risk factors of age, gender, and comorbidities in patients with active cancer (Lee et al., 2020). Importantly, there was no significant impact on mortality for patients who were treated with chemo-, immuno-, hormonal, targeted, and radiotherapy within 4 weeks of COVID-19 diagnosis. The COVID-19 and Cancer Consortium (CCC19) (n = 925) reported on cancer patients with mostly symptomatic COVID-19 disease (Kuderer et al., 2020). The overall mortality rate was 13%. Interestingly, a leading factor for elevated mortality risk was progressing cancer, defined as progressive disease compared with those in remission (no measurable disease). Additionally, the COVID-Surg Collaborative registry reported a 27.6% mortality rate among COVID-19-positive patients undergoing cancer surgery compared with non-cancer surgery (COVIDSurg Collaborative, 2020). The TERAVOLT study (thoracic cancers international COVID-19 collaboration registry for thoracic cancers) focused on COVID-19-positive patients with thoracic cancers (non-small cell and small cell lung cancers, mesothelioma, thymic epithelial tumors, and carcinoid/neuroendocrine tumors of thoracic origin) and observed a high death rate of 33% (n = 200) (Garassino et al., 2020) (Table S1). A single-center study in New York specifically on patients with lung cancer reported smoking status as a correlate of severe COVID-19 (Luo et al., 2020b), and anti-PD1 treatment did not impact COVID-19 severity (Luo et al., 2020a). The association of smoking and poor outcomes was also reflected in a large pan-cancer analysis of 423 patients with symptomatic COVID-19 (Robilotti et al., 2020). In contrast, treatment with CPI was a significant risk factor for severe outcomes of COVID-19 in this study. The varied conclusions drawn to date on CPI risks further highlight the need to account for tumor types and other variables when considering potential impact of anti-cancer interventions on COVID-19 outcomes.
Collectively, these studies have begun to shed light on the epidemiological and clinical vulnerabilities in cancer patients who succumb to or endure poor outcomes of SARS-CoV-2. However, what has been critically missing in cohort and registry reports to date are data on (1) the true prevalence of SARS-CoV-2 infection in the cancer population, given population screening has not been widely implemented, and (2) the experience of those who remain well (uninfected, asymptomatic, or subclinically affected), to determine the drivers of mortality and the absolute risks of severe adverse events within the cancer community as a whole. True clinical status (infected, negative, convalescent) for the great majority of cancer patients has not been established, and hence, true population prevalence is unknown. Tracking of consecutive patients is also needed, to provide insights beyond a snapshot, as infection status for some will change over time. Addressing these knowledge gaps requires a comprehensive, longitudinal study, designed to contemporaneously capture data of both infected (encompassing all phenotypes) and non-infected patients and followed over time, to reflect the experience of all patients with cancer (the “denominator”). Further, assessment of immune response features in response to SARS-CoV-2 in both these patient groups can inform potential mechanisms that may underpin the observed COVID-19 clinical outcomes and correlations to specific features.
Immune Response to SARS-CoV-2 in the Context of Cancer
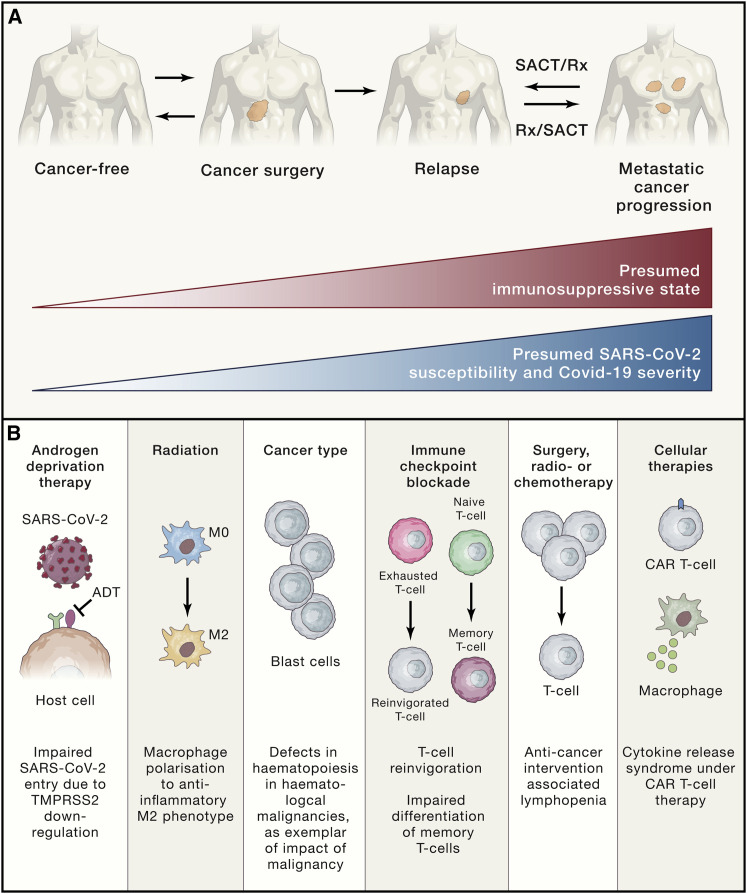
There are multiple, varied mechanisms by which cancer, anti-cancer therapies, and other immune-modulating agents commonly used in cancer patients (e.g., steroids) modulate host immunity (Figures 1A and 1B). The extent to which this impacts anti-SARS-CoV-2 immune responses (innate or adaptive) and the clinical course of COVID-19 in cancer patients warrant dedicated evaluation. Montopoli and colleagues have observed that prostate cancer patients receiving androgen deprivation therapy (ADT) may be protected against SARS-CoV-2 infections (Montopoli et al., 2020). Indeed, ADT used in prostate cancer downregulates TMPRSS2, the serine protease for S protein priming for cell entry by SARS-CoV-2. This is of particular importance, given this population generally associates with high-risk demographic features (i.e., male, advanced age) for adverse events with COVID-19.
Figure 1.
Schematic Representation of Immune Changes in Cancer Patients that Can Impact Immune Response during a SARS-CoV-2 Infection
(A) Cancer type, stage, and therapy (including surgery, systemic anti-cancer therapy, and other anti-cancer interventions) can result in varying degrees of immune suppression, which conceivably may lead to increased clinical susceptibility to SARS-CoV-2 infection and COVID-19 illness.
(B) Examples of the varied mechanisms by which host immunity (innate or humoral/cellular adaptive immunity) is altered, with potential influence on the immune response as well as immunopathologic hyperinflammation of COVID-19. SACT, systemic anti-cancer therapy; Rx, other anti-cancer treatments including radio-, hormonal, targeted, and cellular therapy; ADT, androgen deprivation therapy; CAR-T, chimeric antigen receptor T cell.
Preclinical studies suggest low-dose radiation can induce polarization of macrophages toward the M-2 anti-inflammatory phenotype and reduce IL-1 and TNF-alpha target cells producing IL-6 (Lara et al., 2020). As such, thoracic radiation therapy may potentially attenuate pulmonary hyperinflammation characteristic of severe SARS-CoV-2 infections, where there is increase of inflammatory monocyte-derived macrophages, accompanied by elevated levels of CCL2 and CCL7, both critical for monocyte recruitment. Peripheral antiviral response to SARS-CoV-2 in cancer patients may be compromised due to their malignancy or as a result of anti-cancer treatments. Hematological malignancies can have specific defects in lymphoid (B and T cells) and myeloid lineages. Patients with advanced, solid malignancies are characterized by persistent systemic hyperinflammation with elevated cytokines by virtual of uncontrolled disease, and cytotoxic chemotherapy and surgery are associated with lymphopenia and general immunosuppressive states. In COVID-19, lymphopenia, elevated levels of circulating cyto/chemokines, and inflammatory markers (CRP, D-Dimers) are characteristic peripheral features. In particular, lymphopenia is a hallmark of COVID-19 especially in severe disease, with reduced levels of circulating NK cells, CD8+ T cells, and to a lesser extent, CD4+ cells.
Immune modulating agents, whether as adjunct or directed treatments in cancer, may have distinct implications in COVID-19. Granulocyte colony-stimulating factor (G-CSF) is commonly used in patients receiving myelosuppressive chemotherapy regimens to minimize risks of bacterial infections associated with neutropenia. However, the potential risks of hyper-neutrophilic states in the context of SARS-CoV-2 infections are unknown, especially where respiratory compromise is mediated via tissue hyperinflammation. Anti-CD20 antibodies are used in a variety of hematological malignancies to achieve B cell depletion, potentially impacting generation of humoral immunity to SARS-CoV-2. Glucocorticoids are often used for cancer patients, as adjunct treatment (i.e., premedication for chemotherapy), treatment for oncological emergencies (i.e., spinal cord compression), or directed toward the malignancy itself (i.e., for leukemias and lymphomas). Preliminary data from the RECOVERY (randomized evaluation of COVID-19 therapy) trial has demonstrated mortality benefits for administration of dexamethasone in patients requiring mechanical ventilation with established hyperinflammation (University of Oxford, 2020). In light of this, a pertinent question would be whether steroid exposure earlier in the disease course blunts viral clearance or impairs mounting of an effective immune response, portending a worse outcome for patients with cancer. A longitudinal understanding of the degree to which the immunocompromised states of cancer patients impact infection, viral clearance, clinical course of COVID-19, and subsequent generation of long-term immunity is needed. Additionally, de novo viral mutational changes within the host are a pertinent feature of RNA viruses, and evaluation of viral evolution in infected patients who may not mount an optimal immune response will be of importance.
The intersection of the PD1/PD-L1 axis with viral response is of exceptional relevance in the cancer population, given CPIs are associated with durable responses or even cure in multiple metastatic tumor types and are used in the adjuvant setting with curative intent. Is checkpoint blockade a friend or foe in the context of COVID-19? CPI reverses immunosuppressive states by reinvigoration of exhausted T cells for antigen recognition. Conceivably anti-PD1 therapy could reinvigorate exhausted T cells during viral infection to suppress viral dissemination. Conversely, it is the overactivation of T cells contributing to hyperinflammation and tissue damage that underpins the lung pathology and morbidity in COVID-19, and in this phase CPI could potentially be harmful. Additionally, NK and T cells in COVID-19 patients show increased activation and exhaustion markers (PD1 and TIM-3), and T cell activation and antibody response with increased titers of neutralizing antibodies are closely related (Grifoni et al., 2020). Lastly, little is known about acute viral infections while on anti-PD1 therapy. Memory T cells, which are also pivotal in recognition of infection, may clonally expand intratumorally, but differentiation from naive to memory T cells seems to be reduced with anti-PD1 blockade (Wei et al., 2019). This may impair the adaptive response to SARS-CoV-2 infection.
Patients receiving cellular therapeutics (stem cell transplantation, chimeric antigen receptor T cells, or tumor-infiltrating lymphocytes) represent a clinically distinct patient group that potentially renders them most vulnerable to SARS-CoV-2. These patients are faced with both secondary immunodeficiencies due to high-dose cytotoxic and immunosuppressive regimens and also treatment complications related to immune dysregulation or hyperinflammation including graft-versus-host disease and cytokine-release syndromes. Further factors to consider are superimposed nosocomial and opportunistic infection risks from extended hospital admissions, not limited to COVID-19.
Finally, it is equally important to understand how COVID-19 impacts anti-cancer immune response, to consider both detrimental and beneficial effects. The concept of oncolytic virotherapy was born out of serendipitous observations between cancer regression and viral infections or immunizations, leading to treatments such as talimogene laherparepvec (T-VEC) as a genetically modified herpes simplex virus used for management of malignant melanoma today.
The CAPTURE Study
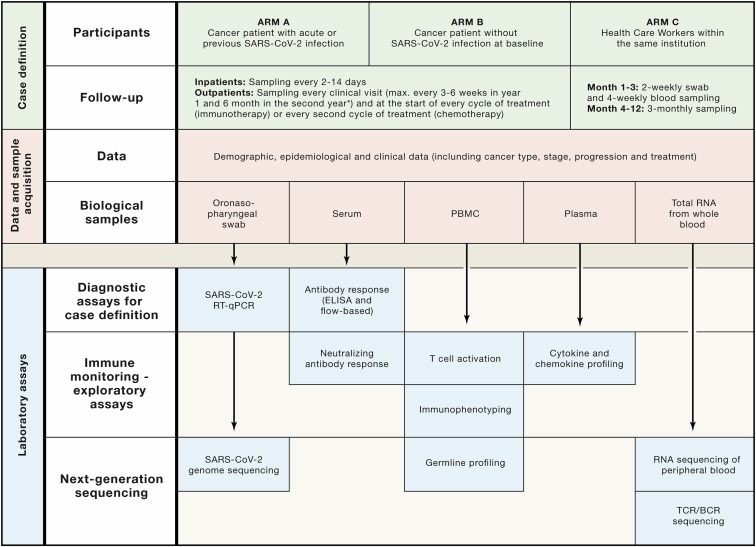
CAPTURE (COVID-19 antiviral response in a pan-tumor immune monitoring study) is a prospective, longitudinal study of cancer patients and HCWs, established in response to the unique challenges of the SARS-CoV-2 pandemic for the care of cancer patients (https://www.royalmarsden.nhs.uk/capture-covid-19-antiviral-response-pan-tumour-immune-study). The overarching aim is to establish a prospective and unbiased understanding of the susceptibility to and morbidity of SARS-CoV-2/COVID-19 in cancer patients and the patterns of nosocomial viral transmission. Such understanding could inform clinical decision making and healthcare policy, especially advice on self-shielding, safe delivery of cancer therapy and reduction of transmission. First, to achieve an accurate picture of the true incidence and prevalence of SARS-CoV-2, we screen all patients longitudinally, irrespective of their clinical presentation: symptomatic, asymptomatic, and convalescent. Second, to facilitate a broad understanding of the impact of cancer and anti-cancer therapies on the course of SARS-CoV-2, we include patients from across a range of cancer types and cancer interventions. Third, we follow the patients long term to understand the extent and duration of immunity, the impact of immune-modulating therapies (including antivirals and vaccines), incidence of re-infection, and long-term sequelae of SARS-CoV-2, including the impact on cancer outcomes. Potential re-infections will be defined as testing positive for SARS-CoV-2 following convalescence and prior evidence of viral clearance. Through comprehensive blood sampling, we aim to understand the correlates of immune pathology and protection and if and how these are impacted by inherent or iatrogenic immune defects in cancer patients and the host characteristics such as HLA haplotype and germline variation (Figure 2 ). We seek to elucidate differences in immune response among cancer patients, incorporating comparative analyses between infected and uninfected patients, and also compared to non-cancer controls within the study and with published data in non-cancer patients. Where measures of incidence and prevalence will reflect evolving public health plans, the immunological findings will be generalizable with respect to impact of cancer and therapy on COVID-19 outcomes. Finally, while we are aiming to detect accurate biological signals through detailed profiling, no single institution will be able to accrue sufficient patient numbers for detailed subgroup analyses. Therefore, we are expanding to include other centers in the UK and would welcome other centers internationally to join this effort.
Figure 2.
Overview of CAPTURE Study Procedures and Analysis Plans
Patients are recruited into study arms A or B, and follow-up schedules are bespoke to their COVID-19 status and accounts for their clinical visit/treatment requirements; while healthcare workers are recruited and followed-up according to schedule as outlined. Comprehensive demographic, epidemiological, and clinical data will be collected along with swabs and blood samples. Diagnostic assays will be performed for all participants and will inform selection of samples for detailed immune monitoring and next-generation sequencing. Standard operating procedure documents (for local sample collection, processing, and storage) will be implemented in additional institutions, and sample analyses will be centralized to ensure standardization.
An important question with regard to SARS-CoV-2 infections in cancer patients is the extent to which it is due to nosocomial exposure, either from other patients or from HCWs, and the extent to which this affects outcomes. Related recruitment within CAPTURE of HCWs will achieve an accurate picture of incidence/prevalence as well as long-term immunity and re-infection in this group. Critically, through viral lineage studies of patient and associated HCWs, we aim to understand nosocomial infection and place it in the wider context of community and hospital patterns of transmission.
A key issue in study design in the context of a rapidly evolving pandemic is that pertinent research questions and hypotheses will emerge over time. An important element of the design of CAPTURE, therefore, has been to collect data and biological samples that both address immediate questions around safe provision of cancer care and inform an understanding of antiviral responses in the context of cancer, and also futureproof for additional opportunities, analyses, and collaborations. A pertinent example will be for monitoring of immune responses to future SARS-CoV-2 vaccines in patients with cancer within this adaptable, longitudinal framework.
We commenced recruitment for CAPTURE on May 4, 2020, at the Royal Marsden NHS Foundation Trust and are expanding recruitment to include other sites in the UK. 3 months since study opening, 227 patients and HCWs have been enrolled, with 536 swab and 448 blood specimens collected longitudinally. The CAPTURE Study Management Group is composed of a broad team of multidisciplinary clinicians with detailed input from Patient Representatives Working Group about the overall approach, and the merit of research to cancer patients. The laboratory studies will take place at the Francis Crick Institute, utilizing a clinically accredited SARS-CoV-2 diagnostic pipeline and leveraging significant infection and immunity expertise and Crick’s status as a Worldwide Influenza Centre with current active COVID-19 research (https://www.crick.ac.uk/research/covid-19).
Data Collection
An extensive clinical case report form (CRF) is used covering 34 categories including demographic data; data on concurrent medication and co-morbidities; details of the clinical course and outcome of SARS-CoV-2 (including need for hospital admission, respiratory and other organ support, and full range of laboratory investigations); and cancer-specific data, including staging, prognostic information, previous and current anti-cancer interventions and outcomes, and to facilitate analysis of interactions between the susceptibility to and severity of SARS-CoV-2 infection and the presence of malignancy and cancer treatments. A quality-of-life assessment is included, which will shed light on the ongoing complications of COVID-19. In order to capture more nuanced details regarding lifestyle and environmental factors and household risks, self-reported data are collected through a secure online questionnaire. Given the widespread modification to cancer care through reduced hospital visits, frequency of blood test and imaging, and most critically, modification of standard of care therapies, we also aim to collect data on the impact of these measures on diagnosis, management, and outcomes of cancer. Specifically, our quality-of-life questionnaires and CRF generally were designed to allow integrative analyses with other studies, including local and international efforts such as the Cancer Research UK COVID-19 survey, UKCCMP, CCC19, and the European Society of Medical Oncology COVID-19 Registry for patients with cancer (ESMO-CoCARE).
A critical feature of CAPTURE is that it will continue follow-up as well as recruitment of new patients over the next 24 months on the back of changing risk of infection in the community and hospital and ongoing changes in public health policy. Self-shielding guidance in the UK is now lifted across the board in the same way that it was applied, and yet this is a very heterogeneous group of patients with different sets of vulnerabilities and in whom risk assessment will need to be individualized. The current policies also mean that viral prevalence in cancer patients is likely to be lower than in the general population, and thus a greater number of cancer patients will be at risk in the coming months. An important risk is the reliance of cancer patients on ongoing access to healthcare provision and frequent hospital attendance. For patients undergoing complex cancer surgery, high-dose chemotherapy, and adoptive cellular therapy, the length of hospital stay can run into many weeks, while for others frequent administration of intravenous drugs may mandate weekly visits to the hospital. HCWs caring for cancer patients are a key group in which we need an understanding of the incidence of asymptomatic infection, “silent” transmission, and the risk of re-infection following initial exposure. Large-scale study of closely related and co-located HCWs and patients participating in this study will help to define transmission patterns across the hospital, highlighting the opportunities for interventions and policy change.
Laboratory Analyses
Longitudinal collections of viral swabs paired with comprehensive blood sampling (for analyses including multi-assay serology, cyto/chemokine profiling, immunophenotyping on peripheral blood mononuclear cells, TCR/BCR sequencing, and germline analyses) will give maximal scope for detailed mapping and monitoring of immune responses to SARS-CoV-2. The longitudinal collection of viral swabs in CAPTURE will enable determination of the duration of infection in those testing positive. PCR analysis for additional viruses (including other human coronaviruses [HCoVs] and non-corona viruses) in symptomatic patients testing negative for SARS-CoV-2 coupled with serological analysis will allow evaluation for cross-reactivity and/or cross-immunity with non-SARS-CoV-2 viruses. Viral genome sequencing and phylogenetic analysis in both cancer patients and HCWs, analyzed against the background of genetic drift in the virus, will define the nosocomial transmission patterns, which has been reported to define transmission clusters even in a single institution (Rockett et al., 2020).
Early reports posit that cancer patients have lower seroconversion rates—that is, decreased ability to generate detectable levels of specific anti-SARS-CoV-2 antibodies following viral exposure—than controls (Solodky et al., 2020). Additionally, durability of immunity is entirely unexplored. Extended follow-up and longitudinal sampling within CAPTURE afford the opportunity to evaluate both sufficiency and durability of immune responses in cancer patients and in the context of a potential future vaccine. Antibody responses will be monitored for S-binding IgG, IgM, and IgA, which will also help to investigate the cross-reactivity of antibodies raised in response to other HCoVs. Results will be supplemented by ELISA against viral S1 subunit. Seroconverted patients will further be monitored by neutralizing antibody assays to obtain a precise measure of the biological activity of detected antibodies. We will analyze T cell activation in SARS-CoV-2-positive and negative patients and compare those with the responses in control groups to identify impairments in adaptive immunity related to the underlying cancer and associated anti-cancer therapies. This will allow us to explore T cell activation and antibody response relationships, which will be supplemented by detailed immunophenotyping and RNA sequencing of peripheral blood as well as TCR and BCR sequencing to draw a detailed picture of both innate and adaptive immune response in cancer patients. Germline profiling, including HLA typing and the detection of polymorphisms in immune-related genes, will reveal genetic susceptibilities to severe COVID-19 and will supplement the detailed analysis of immune response to obtain a comprehensive picture of susceptibility for each participant.
The detailed analyses of the immune system in response to SARS-CoV-2 allows scope for biomarker discovery, with the aim of identifying cancer patients most vulnerable to infection. While it is clear that the increase of cyto/chemokines is a hallmark of a severe COVID-19 late in the course of infection, what is needed are early indicators prior to clinical progression or infection for those destined for maladaptive hyperinflammation. Prospective monitoring of cytokine kinetics in the general cancer population may allow detection of differences, which will likely be a multi-parametric set of indices, that may serve as baseline predictors for their response to SARS-CoV-2.
Conclusion
SARS-CoV-2 exposure, infection, and cancer will continue to coincide in the foreseeable future, even if not on a pandemic scale, pending widespread implementation of a successful vaccine or pharmacotherapy. There is also a need to learn lessons for future similar pandemics. Clinicians and policy makers alike need to be armed with a fundamental understanding of the interaction between host immunity, the virus, cancer, and anti-cancer treatments placed in the wider healthcare context in order to minimize harm and optimize cancer outcomes. Prospective data on immune responses derived from large numbers of patients representative of the entire clinical spectrum, with a relevant denominator population, are needed. In the same way that we have evolved to personalized medicine in the management of cancer, we must also adopt a bespoke approach to the management of SARS-CoV-2 in cancer patients, extending into the post-pandemic era and grounded in evidence-based practice.
Consortia
The members of the CAPTURE consortium include Lewis Au, Susana Banerjee, Katie Bentley, Shree Bhide, Laura Amanda Boos, Fiona Byrne, The Crick COVID19 Consortium, Ian Chau, David Cunningham, Joanne Droney, Annika Fendler, Andrew Furness, Camille Gerard, Firza Gronthud, Kevin Harrington, Adrian Hayday, Shaman Jhanji, Robin Jones, George Kassiotis, Yasir Khan, Sacheen Kumar, James Larkin, Steve K.W. Leung, Paula Lorgelly, Ethna McFerran, Christina Messiou, Emma Nicholson, Alicia Okines, Clare Peckitt, Lisa Pickering, Karin Purshouse, Alison Reid, Jennifer Rusby, Scott S.T. Shepherd, Ben Shum, Tim Slattery, Naureen Starling, Anthony Swerdlow, Stefan Symeonides, Kate Tatham, Samra Turajlic, Nicholas Turner, Liam Welsh, Katalin Wilkinson, Robert Wilkinson, Matthew Wheater, and Nadia Yousaf.
Acknowledgments
We thank the CAPTURE trial team, including Eleanor Carlyle, Kim Edmonds, and Lyra Del Rosario, as well as Somya Agarwal, Hamid Ahmod, Ravinder Dhaliwal, Lauren Dowdie, Lucy Holt, Justine Korteweg, Charlotte Lewis, Karla Lingard, Mary Mangwende, Aida Murra, Kema Peat, Sarah Sarker, Nahid Shaikh, Sarah Vaughan, and Fiona Williams. We also thank Mike Gavrielides for infomatics support, the volunteer staff at the Francis Crick Institute, Antonia Toncheva, Karolina Rzeniewicz, and Nicole Neuman for editorial assistance. Due to limitations on cited references and the pace at which the field is evolving, we acknowledge researchers in COVID-19, particularly in furthering our understanding of clinical correlates and immune responses in patients with cancer. The CAPTURE study is sponsored by the Royal Marsden NHS Foundation Trust and funded from a grant from the Royal Marsden Cancer Charity (RMCC) Programme (Ref. RMCC32).
Declaration of Interests
S.T. reports grants and speaker fees from Roche Tissue Diagnostics. L.A., L.B., F.B., S.S., A.F., A.S. have no conflicts of interest to declare.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.09.005.
Supporting Citations
The following references appear in the Supplemental Information: Dai et al. (2020); Liang et al. (2020); Mehta et al. (2020).
Supplemental Information
Reporting date, brief study design, patient characteristics, and summary statistics of key results are shown.
References
- COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., Zhang Z., You H., Wu M., Zheng Q., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., Baena J., Banna G., Berardi R., Bettini A.C., et al. TERAVOLT investigators COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.Y., Desai A., de Lima Lopes G., Jr., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara P.C., Burgos J., Macias D. Low dose lung radiotherapy for COVID-19 pneumonia. The rationale for a cost-effective anti-inflammatory treatment. Clin Transl Radiat Oncol. 2020;23:27–29. doi: 10.1016/j.ctro.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y.W., Cazier J.B., Angelis V., Arnold R., Bisht V., Campton N.A., Chackathayil J., Cheng V.W., Curley H.M., Fittall M.W., et al. UK Coronavirus Monitoring Project Team COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Rizvi H., Preeshagul I.R., Egger J.V., Hoyos D., Bandlamudi C., McCarthy C.G., Falcon C.J., Schoenfeld A.J., Arbour K.C., et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.06.007. Published online June 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., Pradhan K., Thota R., Reissman S., Sparano J.A., et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., Carbone G.M., Cavalli A., Pagano F., Ragazzi E., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., Bogler Y., Caldararo M., Figueroa C.J., Glickman M.S., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett R.J., Arnott A., Lam C., Sadsad R., Timms V., Gray K.A., Eden J.S., Chang S., Gall M., Draper J., et al. Revealing COVID-19 transmission in Australia by SARS-CoV-2 genome sequencing and agent-based modeling. Nat Med. 2020;26:1398–1404. doi: 10.1038/s41591-020-1000-7. [DOI] [PubMed] [Google Scholar]
- Solodky M.L., Galvez C., Russias B., Detourbet P., N’Guyen-Bonin V., Herr A.L., Zrounba P., Blay J.Y. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31:1087–1088. doi: 10.1016/j.annonc.2020.04.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Oxford . 2020. Dexamethasone reduces death in hospitalised patients with severe respiratory complications of COVID-19.https://www.ox.ac.uk/news/2020-06-16-dexamethasone-reduces-death-hospitalised-patients-severe-respiratory-complications#:∼:text=Dexamethasone%20reduced%20deaths%20by%20one,0.96%5D%3B%20p%3D0.0021 [Google Scholar]
- Wei J., Luo C., Wang Y., Guo Y., Dai H., Tong C., Ti D., Wu Z., Han W. PD-1 silencing impairs the anti-tumor function of chimeric antigen receptor modified T cells by inhibiting proliferation activity. J Immunother Cancer. 2019;7:209. doi: 10.1186/s40425-019-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., Jia P., Guan H.Q., Peng L., Chen Y., et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reporting date, brief study design, patient characteristics, and summary statistics of key results are shown.