This commentaryrefers to ‘Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors’, by I.E. Sama et al., doi:10.1093/eurheartj/ehaa373.
Sama et al.1 have recently reported that angiotensin-converting enzyme inhibitor (ACE-I) and angiotensin receptor blocker (ARB) usage was not associated with higher levels of plasma angiotensin-converting enzyme 2 (ACE2) in two cohorts of patients with heart failure. Membrane-bound ACE2 is the receptor for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) viral entry and also catalyses the conversion of angiotensin II to angiotensin 1–7, a vasodilator that is protective in the cardiovascular system. Although the relationship between plasma and membrane-bound ACE2 has not been elucidated—either with or without COVID-19 infection—the report by Sama et al. provides some reassurance that ACE-I and ARB usages do not appear to influence plasma ACE2 levels and may not be expected to increase the risk or severity of COVID-19 infection. This has also been in a study by Mancia et al.2 that demonstrated no association between ACE-I/ARB usage and mortality in COVID-19 infection.Sama et al. also found that men had higher plasma ACE2 levels than women. They reason that increased plasma ACE2 levels in men may be related to the observation that men are at higher risk of mortality from COVID-19 infection.
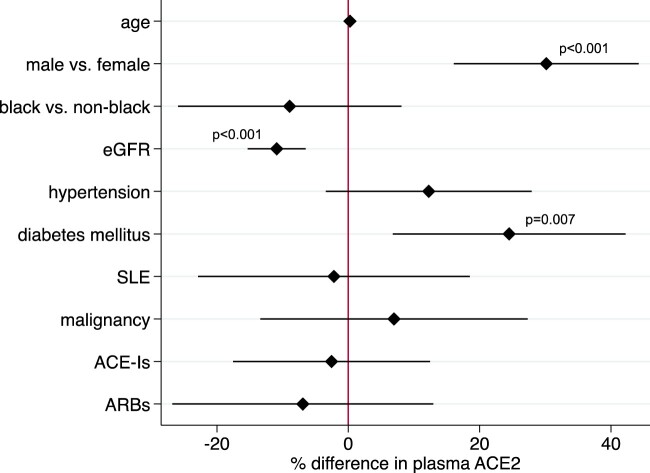
The angiotensin system is important in kidney physiology as well as in cardiovascular physiology. Angiotensin-converting enzyme 2 is also expressed in kidney tubules and may account for the finding that COVID-19 infection carries a high risk of acute kidney injury. To extend the observations of Sama et al. to kidney disease, we measured levels of plasma ACE2 in the Boston Kidney Biopsy Cohort, a prospective cohort study of patients undergoing native kidney biopsy.3 Angiotensin-converting enzyme 2 was measured using the same platform as Sama et al. (the proximity extension assay by Olink) in 551 participants with glomerulonephritis (30%), non-proliferative glomerulopathy (18%), vascular diseases (10%), diabetic nephropathy (12%), and other diagnoses (30%). In linear regression models adjusted for age, sex, race, estimated glomerular filtration rate (eGFR), and clinical comorbidities, plasma ACE2 levels were significantly higher in men, those with diabetes, and those with lower eGFR (Figure 1). We found no significant association between the usage of ACE-Is/ARBs and levels of plasma ACE2.
Figure 1.
Determinants of plasma angiotensin-converting enzyme 2 in chronic kidney disease. Percent differences are derived from multivariable linear regression models using log2-plasma angiotensin-converting enzyme 2 as the dependent variable and age, sex, race, estimated glomerular filtration rate per 20 mL/min/1.73 m2, and clinical comorbidities, including hypertension, diabetes, systemic lupus erythematosus, and malignancy as predictor variables. ACE-I, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; SLE, systemic lupus erythematosus.
The predominant organs affected by SARS-CoV-2 are also the tissues with the highest expression of the virus’ receptor, ACE2. Similarly, plasma levels of ACE2 appear to correlate with clinical variables that are risk factors for greater COVID-19 disease severity.In both the report by Sama et al. and ours, higher plasma ACE2 is observed in men than women and in those with diabetes, consistent with the higher risk of death from COVID-19 infection in men and in diabetes.4 Finally, plasma ACE2 levels do not appear to be influenced by ACE-I/ARB usage, which also does not appear to confer an increased risk of death in COVID-19. Our results suggest that chronic kidney disease may also emerge as a major risk factor for COVID-19 mortality.5 The variables that do or do not associate with plasma ACE2 appear to be the same variables that are risk factors for severity of COVID-19 infection, suggesting that plasma ACE2 may merit testing as a prognostic biomarker in COVID-19 infection.
Contributor Information
Insa M Schmidt, Department of Medicine, Section of Nephrology, Boston University Medical Center, 650 Albany Street, Boston, MA 02118, USA; Department of Medicine, Renal Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02115, USA.
Ashish Verma, Department of Medicine, Renal Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02115, USA.
Sushrut S Waikar, Department of Medicine, Section of Nephrology, Boston University Medical Center, 650 Albany Street, Boston, MA 02118, USA; Department of Medicine, Renal Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02115, USA.
Funding
This study was supported by National Institutes of Health (NIH) grant R01DK093574 (S.S.W.). I.M.S. is supported by the American Philosophical Society Daland Fellowship in Clinical Investigation.
Conflict of interest: S.S.W. reports personal fees from Public Health Advocacy Institute, CVS, Roth Capital Partners, Kantum Pharma, Mallinckrodt, Wolters Kluewer, GE Health Care, GSK, Mass Medical International, Barron and Budd (vs. Fresenius), JNJ, Venbio, Strataca, Takeda, Cerus, Pfizer, Bunch and James, Harvard Clinical Research Institute (aka Baim), and grants and personal fees from Allena Pharmaceuticals. I.M.S. and A.V. have nothing to disclose.
References
- 1. Sama IE, Ravera A, Santema Bt van Goor H, Ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020;doi:10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the risk of Covid-19. N Engl J Med 2020. 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava A, Palsson R, Kaze AD, Chen ME, Palacios P, Sabbisetti V, Betensky RA, Steinman TI, Thadhani RI, McMahon GM, Stillman IE, Rennke HG, Waikar SS. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 2018;29:2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 2020;14:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020;52:1193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]