Highlights
-
•
Exposure to disinfecting chemicals is highly dependent on physicochemical property.
-
•
Children are at elevated exposure due to more mouthing-mediated ingestion.
-
•
Some disinfecting chemicals may pose health risks for certain modeled individuals.
-
•
Estimated risks differ greatly between the uses of in vivo and in vitro toxicity endpoints.
Keywords: COVID-19, Disinfecting chemicals, Fate and transport, Uptake dose, Blood concentration, Risk assessment
Abstract
Disinfection of surfaces has been recommended as one of the most effective ways to combat the spread of novel coronavirus (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19). However, overexposure to disinfecting chemicals may lead to unintended human health risks. Here, using an indoor fate and chemical exposure model, we estimate human exposure to 22 disinfecting chemicals on the lists recommended by various governmental agencies against COVID-19, resulting from contact with disinfected surfaces and handwashing. Three near-field exposure routes, i.e., mouthing-mediated oral ingestion, inhalation, and dermal absorption, are considered to calculate the whole-body uptake doses and blood concentrations caused by single use per day for three age groups (3, 14, and 24-year-old). We also assess the health risks by comparing the predicted whole-body uptake doses with in vivo toxicological data and the predicted blood concentrations with in vitro bioactivity data. Our results indicate that both the total exposure and relative contribution of each exposure route vary considerably among the disinfecting chemicals due to their diverse physicochemical properties. 3-year-old children have consistent higher exposure than other age groups, especially in the scenario of contact with disinfected surfaces, due to their more frequent hand contact and mouthing activities. Due to the short duration of handwashing, we do not expect any health risk from the use of disinfecting chemicals in handwashing. In contrast, exposure from contact with disinfected surfaces may result in health risks for certain age groups especially children, even the surfaces are disinfected once a day. Interestingly, risk assessments based on whole-body uptake doses and in vivo toxicological data tend to give higher risk estimates than do those based on blood concentrations and in vitro bioactivity data. Our results reveal the most important exposure routes for disinfecting chemicals used in the indoor environment; they also highlight the need for more accurate data for both chemical properties and toxicity to better understand the risks associated with the increased use of disinfecting chemicals in the pandemic.
1. Introduction
Disinfection is recommended as a best practice measure to prevent the transmission of the novel coronavirus (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) through close contact with fomites (U.S. Centers for Disease Control and Prevention, 2020b). This is because SARS-CoV-2 can remain viable on hard surfaces such as plastic, stainless steel, and cardboard for hours and even days (Van Doremalen et al., 2020). To this end, health and environmental authorities in the United States, China, Canada, Singapore, and many other countries have published a series of guidance documents or official lists to recommend disinfecting products with possible or proven viricidal efficacy. Typical viricidal ingredients in these products include alcohols, quaternary ammonium salts, phenolic compounds, diols, and biguanides. While all offer viricidal efficacy, these disinfecting chemicals differ substantially in structure, properties, and environmental behavior: for instance, quaternary ammonium salts are permanently charged and thus involatile, whereas phenolic compounds are generally volatile and more hydrophilic. These disinfecting chemicals can be added into rinse-off liquid hand soaps or rinse-free hand sanitizers for hand sanitization; they can also be used in pre-saturated wipes or sprays for disinfecting the impervious surfaces of furniture or high-touch objects in homes or offices, such as tables, countertops, desks, sinks, toys, and keyboards (Chen, 2020).
While accidental exposure to disinfecting chemicals because of misuse or improper use is of increasing concern since the outbreak of COVID-19 (Chang et al., 2020), it should be noted that humans can also be exposed to disinfecting chemicals during and post proper disinfectant use. During handwashing, disinfecting chemicals penetrate the hand epidermis and enter the circulatory system. The exposure is limited if hand soaps are soon washed off, because of short contact time, or more considerable if the rinse-free hand sanitizers are left over. More often, without being wiped off, disinfectants remain on the disinfected hard surfaces or objects all day long, either as surface residues or bound to settled dust. Touching or rubbing the treated hard surfaces dislodges disinfecting chemicals to hands (surface-to-hand transfer); subsequent mouthing, licking, or biting of hands further transfers these compounds to mouth (hand-to-mouth transfer). Direct mouthing of disinfected objects such as toys also results in the oral ingestion of disinfecting chemicals (object-to-mouth transfer). Such mouthing-mediated exposure can be of greater concern for infants and toddlers as they mouth almost everything when exploring the environment. Exposure to disinfecting chemicals may be associated with adverse health outcomes. For example, a class of quaternary ammonium salts named benzalkonium chlorides have been found to be irritant to animals (Choi et al., 2018); the Veterinary Poisons Information Service in London has received 20 reports on benzalkonium chlorides per year since 1992, concerning the buccal irritation of cats caused by their grooming after they “accidentally walk across treated surfaces” (Campbell and Chapman, 2000). Earlier epidemiological evidence has also suggested possible links between human disease with the use of disinfecting products. For instance, Weinmann et al. (2017) showed that compared with no use, high use of disinfectants was associated with a more than twofold increased odds of incident asthma, and low/medium use of disinfectants was associated with remittent asthma. In addition, the health risk of disinfecting chemicals can be disputable, because a chemical recommended by one authority may be viewed as poisonous in another country. For example, while antiseptic hand soaps containing triclocarban and triclosan have been removed from the list of ingredients “generally recognized as safe (GRAS)” by the U.S. Food and Drug Administration (U.S. Food and Drug Administration, 2016), they are authorized by Health Canada’s list of hand sanitizers against SARS-CoV-2 because they “meet Health Canada’s requirements for safety, effectiveness and quality” (Health Canada, 2020b). For these reasons, it is imperative to thoroughly understand the magnitude of human exposure to disinfecting chemicals during and post the application and potential adverse health outcomes associated with the exposure, if we want to take preventive measures to avoid the secondary health risks arising from the reduction of pandemic risk. This understanding is exceptionally important since COVID-19 is not likely to be eradicated in a short time and regular household disinfection is believed to be a new normal in the post COVID-19 world.
In this work, we evaluate human exposure and health risks associated with the proper use of 22 active ingredients in recommended disinfecting products against SARS-CoV-2. Specifically, we estimate the use rates of disinfecting chemicals in two application scenarios, one describing the disinfection of indoor surfaces and objects (“surface application”) and the other describing handwashing (“hand hygiene”). For the “surface application” scenario, we simulate the fate and transport of 22 disinfecting chemicals between indoor compartments, human post-application uptake doses, as well as resulting blood concentrations, using a published process-based model named PROduction-To-EXposure (PROTEX) (Li et al., 2018a, Li et al., 2018b). For the “hand hygiene” scenario, we simulate dermal uptake during handwashing and resulting blood concentrations. We then evaluate exposure-related health risks by comparing the modeled uptake doses with in vivo toxicological data, and the modeled blood concentrations with in vitro bioactivity data.
2. Material and methods
2.1. Modeling indoor fate and human exposure
The PROTEX model (Li et al., 2018a, Li et al., 2018b) contains (i) an indoor chemical mass balance module, describing the fate and distribution of applied disinfecting chemicals between five indoor compartments (indoor air, carpet, flooring, hard surfaces, and walls and ceilings) and resultant chemical loadings on indoor surface compartments, and (ii) a human exposure and toxicokinetic module, describing the entry of disinfecting chemicals into the human body through three routes, i.e., mouthing-mediated route (hand-to-mouth and object-to-mouth contact), inhalation of indoor air, and dermal absorption, and resultant body concentrations. In the PROTEX model, “hard surfaces” encompass the impervious surfaces of both furniture (e.g., tables, countertops, desks, and sinks) and high-touch objects (e.g., such as toys, and keyboards).
In this work, we consider two scenarios of disinfectant application. First, a “surface application” scenario means the application of disinfecting products, e.g., diluted solutions or disposable pre-saturated wipes, to the “hard surfaces” compartment through broadcasting, spraying, or wiping. Earlier work estimated that disinfecting 1 m2 of hard surfaces consumes on average 8.7 g (following a normal distribution) of disinfecting products (supplementary material, Text S1) (Weerdesteijn et al., 1999). The disinfected hard surfaces are often air-dried, with the disinfecting products not being wiped off. Active ingredients in disinfecting products remain on the disinfected surfaces for a long time, which thus causes the potential for continuous exposure post the application. We assume the disinfecting chemicals first enter the “hard surfaces” compartment of the indoor chemical mass balance module and then undergo multicompartmental transport and distribution. PROTEX assumes that the modeled individual touches a different fresh location of indoor surfaces during each surface-to-hand contact. In this scenario, human exposure is an aggregation of chemical uptake through a mouthing-mediated route (surface-to-hand-to-mouth and object-to-mouth transfer of both dust-bound chemicals and surface residue), inhalation of indoor air, and dermal absorption. We do not consider the dermal absorption of disinfecting chemicals during disinfection, because wearing gloves is a recommended best practice for disinfection (U.S. Centers for Disease Control and Prevention, 2020a) and, therefore, adherence of disinfecting chemicals to the hand skin is minimal (Popendorf and Selim, 1995). We do not consider inhalation exposure during the spraying of disinfecting products because the investigated disinfecting chemicals are mostly minimally volatile and the inhalation exposure is small compared with the post-application exposure (supplementary material, Text S2).
Second, a “hand hygiene” scenario describes the cleaning of hands with a rinse-off hand washer. Surveyed data shows that on average 1.6–1.7 g (following a uniform distribution) of hand washers are used in each handwashing (Sanderson et al., 2006) (supplementary material, Text S1). We assume the disinfecting chemicals are directly applied to the “skin” compartment of the human exposure and toxicokinetic module. In this scenario, we consider human exposure through dermal absorption, as inhalation is minimal given that evaporation of disinfecting chemicals is suppressed in when chemicals are dissolved in aqueous solutions (i.e., hand washer). The U.S. Centers for Disease Control and Prevention recommends scrubbing hands for 20 s before rinse under running water. We assume that the residue of disinfecting chemicals on hand skin is negligible after rinsing, i.e., dermal absorption discontinues at the end of the hand scrubbing.
In this work, the PROTEX model is parameterized to represent the exposure of a typical female white American in an “average” home in the U.S. Specifically, the modeled home holds a family of 3.14 persons (the average U.S. family size) and has a floor area of 164 m2 (the average U.S. home size) (U.S. Environmental Protection Agency, 2019), with 60% of the floor area covered by carpet and the rest 40% by flooring (defaults in PROTEX). Hard surfaces, including furniture and objects, account for 24% of the total floor area (~40 m2), in which on average 3 m2 (~10%) needs to be disinfected (McCready et al., 2013). The hard surfaces and walls and ceiling are coated with a 20-nm layer of organic film (Weschler and Nazaroff, 2017). The home is ventilated with a U.S. average air exchange rate of 0.45 h−1 (U.S. Environmental Protection Agency, 2019). We evaluate the exposure of the modeled individual at the ages of 3 (young childhood), 14 (adolescence), and 25 (adulthood) because our earlier work shows that chemical exposure varies marginally between ages throughout adulthood (Li et al., 2018a). U.S.-specific anthropometric and behavioral parameters of the modeled individual are age-dependent. For instance, as shown in supplementary material Fig. S1, at ages 3, 14 and 25, the modeled individual weighs 8.3, 52, and 71 kg, possesses a skin surface of 0.5, 1.4, and 1.8 m2, mouths hands (fingers and palms) 16.9, 5.2, and 1 time per hour, and mouths various objects 10.1, 3.2, and 1 time per hour. Since the disinfected area is ~10% of the total area of the “hard surfaces” compartment, we assume that only one-tenth of surface-to-hand and object-to-mouth contacts contribute to mouthing-mediated exposure. For other unspecified parameters, we use default values built in the original PROTEX model for calculation. Underlying this practice is an assumption that human exposure factors (e.g., the human activity pattern) remain the same as normal during the COVID19 pandemic, as there is presently no information available on the change in human activity pattern during the pandemic time. For instance, the original PROTEX model adopts a U.S. average scenario that the carpet and flooring are cleaned up twice a month and hard surfaces are cleaned ten times a month (Li et al., 2018b), in which cleanup means to remove settled dust, dirt, and impurities from the room but not to use any chemicals to kill pathogens, according to the U.S. CDC’s definition (U.S. Centers for Disease Control and Prevention, 2020a). We assume that during the COVID19 pandemic, the frequencies of cleanup remain the same, but additional disinfection is introduced, which aims to kill pathogens but not necessarily to clean dirty surfaces (U.S. Centers for Disease Control and Prevention, 2020a). The original PROTEX model also adopts a normal case that individuals spend 90% of their time indoors; it is currently unclear the extent to which Americans’ indoor stay would be extended due to the “stay-at-home” order in each state. Since the PROTEX model is mechanistic and process-based, it is possible to remediate the potential issues arising from these assumptions if more realistic data become available in the future.
2.2. Disinfecting chemicals and exposure characterization
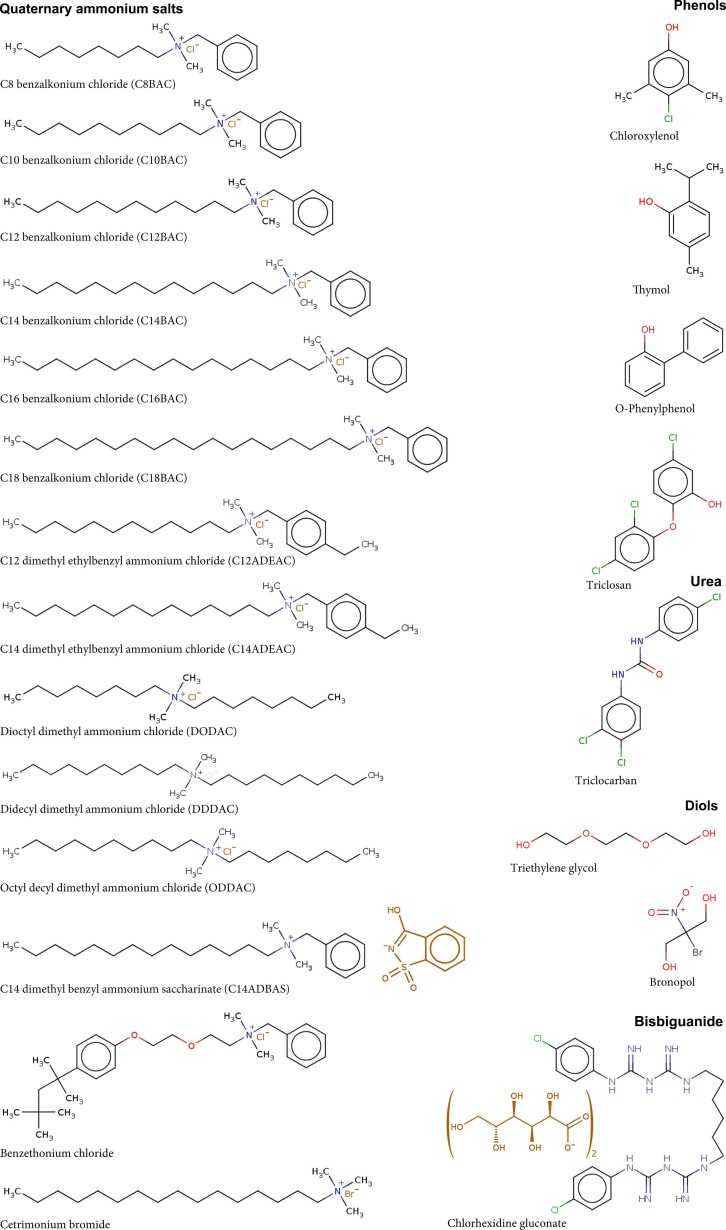
We select 22 chemicals used as active ingredients in disinfectants (for both surface and hand sanitization) recommended by the health and environmental authorities in the U.S. (U.S. Environmental Protection Agency, 2020b), China (China's National Health Commission, 2020), Canada (Health Canada, 2020a, Health Canada, 2020b), and Singapore (National Environment Agency, 2020). Their molecular structures, names, and abbreviations are shown in Fig. 1 . These chemicals include 14 quaternary ammonium salts, four phenolic compounds (chloroxylenol, thymol, O-phenylphenol, triclosan), a urea (triclocarban), two diols (Triethylene glycol, and bronopol), and a bisbiguanide (chlorhexidine gluconate). Note that not all chemicals are recommended to be used in all countries. For instance, chlorhexidine gluconate is judged to be “not effective” against SARS-CoV-2 and thus not recommended by China’s guidance (China's National Health Commission, 2020), whereas triclocarban and triclosan are not permitted being used in disinfecting products sold in the U.S.
Fig. 1.
Disinfecting chemicals investigated in this study. Functional groups are colored blue, green, and red. Parts in dark brown are anions (normally without viricidal efficacy). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Text S1 in the supplementary material also derives the weight fractions of disinfecting chemicals in disinfectant products (Isaacs et al., 2018), which follows a triangular distribution and ranges from the 5th percentile of 0.0071% to the 95th percentile of 9.3%, with a median of 1.8%. In each scenario, we calculate the use rates of the investigated disinfecting chemicals (medians and 95th percentiles) as the product of the total amount of disinfecting products used in each disinfection (see Section 2.1) and corresponding weight fractions. We do not consider the dilution of disinfectants during use.
The PROTEX model requires inputs of a chemical’s molar mass, partition coefficients between water, octanol, and air (K OW, K OA, and K AW), the reaction rate constant with [OH] in the indoor air, reaction half-lives in indoor surfaces (carpet, flooring, hard surfaces, and the organic film), and biotransformation half-life in the human body. Supplementary material Table S1 shows the values of these parameters. Specifically, when measured values (as cited in Table S1) are unavailable, the octanol–water partition coefficients (K OW) and pKa are computed using ACD/Labs software (Advanced Chemistry Development Inc., Canada), as Hodges et al. (2019) indicated that ACD/Labs predictions for cationic chemicals are in slightly better agreement with measured values than other quantitative structure-property relationship (QSPR) models are. The air-water partition coefficients (K AW) are estimated using the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) models (Zang et al., 2017) and Open Structure-activity/property Relationship App (OPERA) (Mansouri et al., 2018) when measured values are unavailable. For ionogenic organic chemicals such as quaternary ammonium salts, the model calculation builds on “distribution ratios” (D OW, D OA, and D AW), i.e., combined partition coefficients of the neutral and ionized species of an ionogenic organic chemical, rather than partition coefficients. We derive the octanol-water (D OW) and air-water (D AW) distribution ratios based on pKa of ionogenic organic chemicals using the Henderson–Hasselbalch equation (Schwarzenbach et al., 2003), whereas the octanol-air (D OA) distribution ratios are calculated as the ratios between D OW and D AW based on the air–water–octanol triangle (Mackay et al., 2016). The reaction rate constants with [OH] are consensus values of predictions of AOPwin (built-in in EPISuite™) and OPERA. Presently, there is no experimental evidence indicating the reaction of the investigated disinfecting chemicals on indoor surfaces. As such, we adopt an assumption frequently used in earlier modeling studies (Bennett and Furtaw, 2004, Zhang et al., 2014) that the reaction of chemicals on indoor surfaces is negligible. While this assumption seems plausible because a recent study shows that atmospheric reaction of chemicals on surfaces can be “entirely suppressed” by a layer of organic film thicker than 10 nm (Zhou et al., 2012), we conduct a sensitivity analysis later this work to illustrate the response of modeling results to this assumption. The model uses default values of internal energies and activation energies to adjust the partition coefficients and reaction rate constant to a certain temperature. In addition, human biotransformation-halves are computed using the human biotransformation QSAR models by Papa et al. (2018) implemented in the QSARINS-Chem module (Gramatica et al., 2014) of the software QSAR-INSubria (QSARINS) (Gramatica et al., 2013). Since the training set in Papa et al. models contains few ionogenic organic chemicals, we calculate both the best estimates and 95th percentiles to characterize the uncertainty associated with the predicted biotransformation halves.
We characterize human exposure using PROTEX-predicted (i) whole-body uptake doses (in μgchem/kgbodyweight/d), i.e., the amount of chemical crossing the inner absorption barrier (e.g., the epithelial layers in the gastrointestinal tract and pulmonary alveoli) and entering the circulatory system (Zartarian et al., 2005), and (ii) blood concentrations (in μM), of disinfecting chemicals resulting from a single disinfection event each day. Uptake doses are presented in the form of (i) best estimates calculated using the medians of the use rates, and (ii) 95th percentiles calculated using the 95th percentiles of the use rates. Blood concentrations are presented in the form of (i) best estimates calculated using the respective medians of the use rates and human biotransformation half-lives, and (ii) 95th percentiles calculated using the respective 95th percentiles of the use rates and human biotransformation half-lives. For cases that hard surfaces or hands are disinfected more than once a day, our “unit” predictions can be scaled directly to the realistic frequency of disinfection due to the linearity of the PROTEX model. Note that blood concentration can be time-variant in the case of intermittent exposure; the blood concentrations calculated here are averages over the entire day.
2.3. Toxicological data and risk assessment
We evaluate the health risks of the investigated disinfecting chemicals by (i) comparing the PROTEX-predicted uptake doses with maximum acceptable doses derived from in vivo animal-based toxicological data, and (ii) comparing the PROTEX-predicted blood concentrations with in vitro bioactivity thresholds. If the predicted exposure is lower than the corresponding effect threshold then we consider there is no risk associated with the use of a disinfecting chemical.
For the maximum acceptable doses, we use the Reference Doses (RfD) curated by the U.S. EPA’s CompTox Chemistry Dashboard (U.S. Environmental Protection Agency, 2020a) and the Derived No-Effect Levels (DNEL) curated by European Chemical Agency (European Chemical Agency, 2020). Both the RfD and DNEL are derived from available no-effect threshold exposure level observed in animal studies and account for the uncertainty rooted in possible intraspecies differences, interspecies variation, duration of the study, and original data quality (ECETOC, 2010, U.S. Environmental Protection Agency, 2014). Due to the high similarity in the designs and definitions of RfD and DNEL, we consider them equivalent in this study. In addition, the European Chemicals Agency reports DNELs for both workers and the general population when the DNEL for workers are consistently higher than that for the general population. We consider both types of DNELs, as several investigated disinfecting chemicals only have worker DNELs. Multiple reported RfD values in the U.S. EPA’s CompTox Chemistry Dashboard are also compiled. In this work, the maximum acceptable dose of each disinfecting chemical is depicted as a range to allow for the variability in multiple estimates, with the lowest RfD or DNEL being the lower estimate and the highest RfD or DNEL being the upper estimate.
For in vitro bioactivity thresholds, assay-specific ToxCast data (version 4) for the disinfectants are downloaded from the U.S. EPA’s CompTox Chemistry Dashboard (U.S. Environmental Protection Agency, 2020a) and subsequently processed in a way similar to a previous study (Turley et al., 2019). In brief, ToxCast records cell line responses to chemicals assessed by bioassays and report the AC50 values (µM, representing the concentration at which half of the maximal activity is achieved) for each assay. The active assays indicate the adverse effects posed by a tested chemical on the cells, such as cytotoxicity, disruption to transporter function, interference with DNA binding (Supplementary material Table S4). We first remove all background and control assays from the retrieved data. Then a cytotoxicity center is calculated by taking the median of the AC50 values of all active cytotoxicity assays (cytotoxicity as the intended target sub-family) as the non-specific and least sensitive internal toxicity endpoint. The cytotoxicity limit reported in ToxCast is used as the non-specific but more sensitive internal toxicity endpoint. For more specific and sensitive toxicity responses, active assays with AC50 values lower than the cytotoxicity limit are collected and the 5thpercentile values are used subsequently to represent the most conservative endpoint. Such a filter of active assays by the cytotoxicity limit is necessary since cytotoxicity can confound the results: when cells are stressed to trigger cytotoxicity, the observed response may not be specific for the investigated effect (Judson et al., 2016). The 5th percentile AC50 is the lower estimate and the cytotoxicity center is the upper estimate for no-effect threshold.
3. Results and discussion
3.1. Evaluation of model performance
Before presenting and interpreting the modeling results, we first evaluate the performance of the PROTEX model. Since monitoring or biomonitoring data are not available for most disinfecting chemicals investigated here, we compare our predictions with those from a commonly used consumer exposure model called ConsExpo (web version 1.0.7, last update: March 31, 2020) (Delmaar et al., 2005). ConsExpo supports simulating human exposure to chemicals used in consumer products through inhalation, dermal permeation, and direct oral ingestion (e.g., chemicals migrating from packaging materials) under a range of consumer use scenarios.
For the “hand hygiene” scenario, we compare the PROTEX and ConsExpo predictions of the uptake dose after a single handwashing event (supplementary material, Fig. S2). In addition to the physicochemical properties of each disinfecting chemical, ConsExpo requires user-defined skin permeability (in m/h), which is predicted using the approach outlined by Weschler and Nazaroff (2012). Fig. S2 shows that the PROTEX and ConsExpo predictions are in general agreement for the 22 investigated chemicals, with a difference within an order of magnitude for 11 chemicals and two orders of magnitude for 20 chemicals. The two model predictions are closest to each other for triclosan (a factor of 1.2), DODAC (a factor of 1.8), and C10BAC (a factor of 1.9). However, the discrepancy between two model predictions is most prominent for diols (triethylene glycol and bronopol), where ConsExpo gives 1300- and 1100-times higher estimates, respectively, than PROTEX.
For the “surface application” scenario, we compare the PROTEX and ConsExpo predictions of chemical loadings adhering to the hand skin after hand rubbing (supplementary material, Fig. S3). Here, the chemical loadings instead of uptake dose are used for comparison because ConsExpo does not support the prediction of oral ingestion associated with hand-to-mouth contact. Chemicals other than quaternary ammonium salts are excluded for comparison, as they are somewhat volatile and can evaporate from the hard surfaces, whereas ConsExpo does not consider the evaporation loss of chemicals post the application. As shown in Fig. S3, PROTEX predicts chemical-specific dermal loadings, ranging from 0.14 to 0.70 mg/cm2. This range overlaps with the estimate by ConsExpo, i.e., a mean of 0.24 mg/cm2 with a 95% confidence interval between 0.03 and 0.63 mg/cm2, which is a “generic” value for all chemicals rubbed from a chemical-treated surface.
In sum, PROTEX gives estimates comparable to those from ConsExpo. In addition, our PROTEX modeling predicts that the use of a disinfecting hand washer for 20 s once a day leads to dermal absorption of 2.2 × 10−4 and 2.1 × 10−4 mg/kg/d (medians) of triclosan by a 14- and 25-year-old females, respectively. These values are in the same order of magnitude as the daily exposure of the general Americans to triclosan (medians of 1.4 × 10−4 mg/kg/d for ages 12–19 and 2.3 × 10−4 mg/kg/d for ages 20–65), which were back-calculated from biomonitoring data in the National Health and Nutrition Examination Survey (NHANES) (Wambaugh et al., 2014). While this agreement suggests the fidelity of PROTEX to realistic human exposure, it should be noted that sources other than handwashing may also contribute to human triclosan exposure. Given that monitoring and biomonitoring data are lacking for a more thorough evaluation of the model, measuring the occurrence of disinfecting chemicals in various indoor compartments and human tissues are warranted in the future.
3.2. Indoor fate of chemicals post surface application
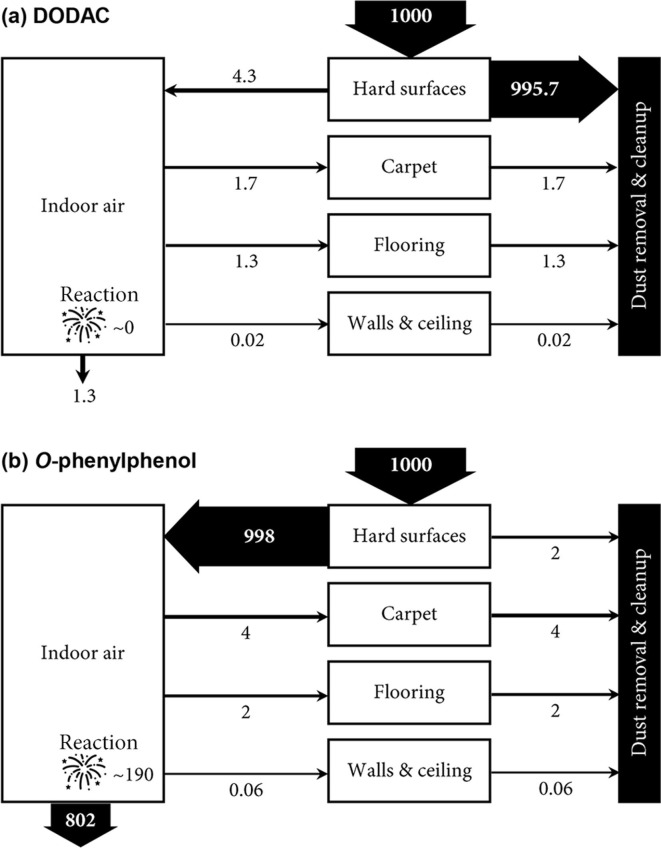
As the first step toward an understanding of human exposure to disinfecting chemicals, we simulate the indoor fate and distribution of these chemicals. Since the volatility of chemicals is the dominant factor governing chemicals’ indoor fate and distribution, for illustration we select two chemicals: the permanently charged and hence involatile DODAC (i.e., with an infinite D OA), and the moderately volatile O-phenylphenol (i.e., with a D OA of 107.5 at pH = 7).
Fig. 2 a depicts the transport of DODAC between different indoor compartments. Over 99% of DODAC remains on the hard surface compartment where it is first applied, which can be either present as surface residues or absorbed onto the settled dust on the hard surfaces. While this fraction can be efficiently removed through regular surface cleanup (10 times a month as assumed in PROTEX), it is available for mouthing-mediated human exposure between the cleanup events. Approximately 0.5% of DODAC, most of which is bound to resuspended dust, enters the indoor air compartment and is subsequently deposited on other indoor surfaces such as carpet, flooring, and the organic film on walls and ceiling. Therefore, while the application of DODAC targets the hard surface compartment, it is not impossible to observe its occurrence in other nontargeted indoor compartments. Yet, DODAC’s low volatility leads its concentration in the nontargeted indoor compartments to be orders of magnitude lower than that on hard surfaces. For instance, our PROTEX modeling shows that DODAC concentration on hard surfaces is ~3000 and 107 times higher than that in the organic film and flooring, respectively. Such inter-compartmental migration has also been found for other indoors-used chemicals. A similar instance is that the broadcast application of chlorpyrifos (K OA = 1010.6 according to the OPERA model) resulted in a detectable level of this compound on nontargeted furniture surfaces, which was 5200 times lower than that on the targeted carpet surfaces (Lu and Fenske, 1998).
Fig. 2.
Mass balance of dioctyl dimethyl ammonium chloride (DODAC, Panel a) and O-phenylphenol (Panel b) in the modeled indoor environment. The total applied amount is assumed to be 1000 units.
Note that the above calculation assumes that DODAC is permanently charged given its application in the form of a water solution. However, the dissociation of DODAC might be suppressed as the disinfected hard surface dries. It remains unknown the minimum level of humidity that maintains the dissociation of DODAC. To explore whether our above calculation is still valid if the indoor environment is completely dry, we perform an additional simulation by assuming an extreme situation where neutral DODAC does not dissociate at all. Supplementary material, Fig. S4 shows that despite being more volatile, neutral DODAC behaves similarly as the DODAC cation in the indoor environment, with the dominant share (95%) of the applied amount remaining on the disinfected hard surfaces and a small fraction (5%) migrating between indoor compartments. Likewise, surface cleanup is the dominant mechanism whereby neutral DODAC is eliminated from the room. As such, while the assumption of the reduced dissociation is uncertain, the main conclusions present in this work do not change.
For comparison, Fig. 2b depicts the transport of O-phenylphenol between different indoor compartments. Unlike DODAC, more than 99% of the applied O-phenylphenol readily evaporates from the disinfected hard surfaces and enters the indoor air, with 0.2% remaining on the hard surfaces and available for mouthing-mediated exposure. Compared with DODAC, O-phenylphenol is more abundant in the indoor air. Over 80% of the evaporated O-phenylphenol will be ventilated out from the room, whereas the rest 20% will be degraded through radical reactions. In this case, we can see relatively rapid dissipation, or reduced persistence, of O-phenylphenol in the indoor environment. Furthermore, the higher volatility of O-phenylphenol results in its relatively equal distribution among indoor compartments. For example, our PROTEX modeling indicates that O-phenylphenol concentration on the treated hard surface is merely 1.5 and 114 times higher than that in the untreated organic film and flooring, respectively.
3.3. Human exposure to disinfecting chemicals
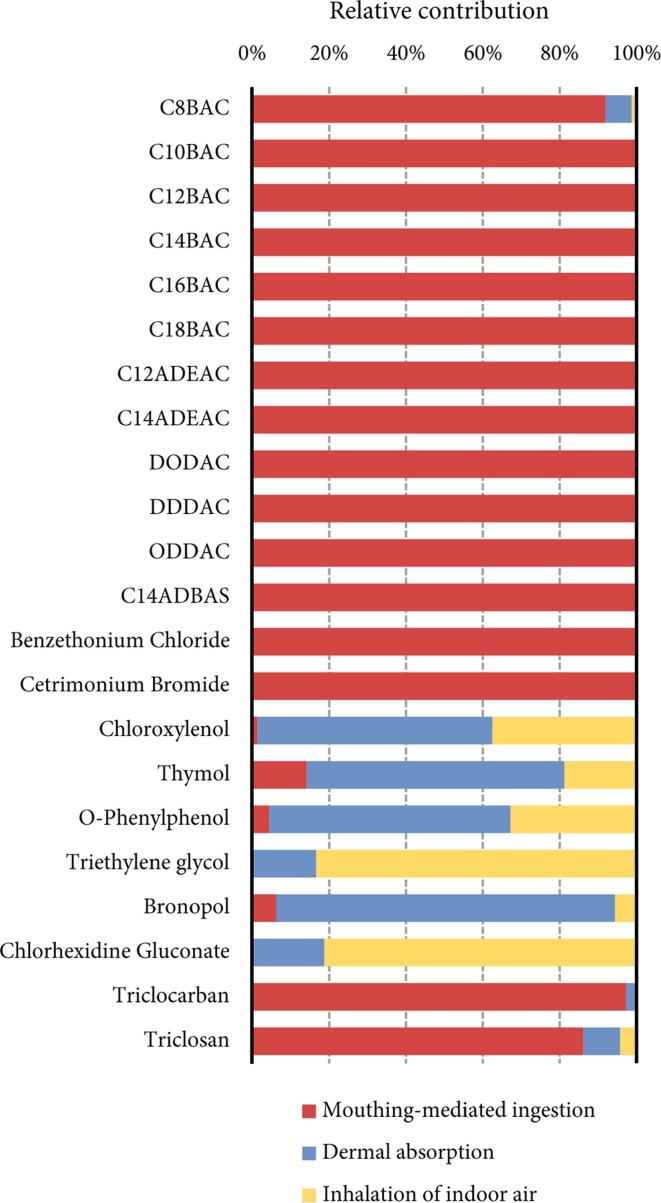
PROTEX predicted the relative contribution of three routes of entry (mouthing-mediated ingestion, dermal absorption, and inhalation of indoor air) to the aggregate exposure of a 3-year-old child to various disinfecting chemicals in the “surface application” scenario (Fig. 3 ). For comparison, results for a 25-year-old adult are presented in the supplementary material, Fig. S5. The relative contribution is chemical-specific and thus a reflection of their partition properties. Fig. 3 shows that mouthing-mediated ingestion dominates the aggregate exposure of the modeled child to quaternary ammonium salts, triclocarban, and triclosan, which is also the case for the modeled adult (Fig. S5). These chemicals are either involatile (the permanently charged quaternary ammonium salts) or minimally volatile (with a D OA of 1012.4 and 1010.7 for triclocarban and triclosan, respectively, at pH = 7). As discussed in Section 3.2, these high D OA chemicals tend to adhere to the hard surfaces after surface application. Furthermore, their low volatility favors their transfer from the treated hard surface to hand skin during surface-hand contact. We can, therefore, anticipate that mouthing is a remarkable contributor to human exposure to these chemicals. This anticipation echoes an earlier general conclusion that “indoor exposure of chemicals with high K OA is predominantly the result of nondietary ingestion of dust and surface contact” (Zhang et al., 2014).
Fig. 3.
Relative contribution of mouthing-mediated ingestion of dust-bound chemicals and surface residues, dermal absorption, and inhalation of indoor air to aggregate exposure of the modeled 3-year-old child to disinfecting chemicals.
By contrast, the rest of the investigated disinfecting chemicals are moderately volatile (with K OA ranging from 107 to 109) and thus have a limited potential for migration from treated hard surface to hands. Fig. 3 and Fig. S5 in supplementary material show that dermal absorption dominates human exposure to phenolic chemicals and bronopol, which partition almost equally between water and organic phases (with a D OW ranging from 1 to 10 at pH = 7, based on the neutral values present in Table S1), whereas inhalation contributes most to exposure to chlorhexidine gluconate and triethylene glycol, which are extremely hydrophilic (with a D OW lower than 10−6 at pH = 7). In general, the anatomical structure of hand skin poses permeation resistance to both highly hydrophobic and hydrophilic chemicals: the lipid matrix of stratum corneum retards the permeation of highly hydrophobic chemicals, whereas the aqueous intracellular fluid in the viable epidermis retards the permeation of highly hydrophilic chemicals (Brown et al., 2016). As such, chemicals with a moderate K OW or D OW, neither too large nor too small, have the highest capability of permeation through hand skin. For instance, Li et al. (2019) found that when chemicals are released exclusively into the indoor air, dermal absorption from indoor air contributes to up to 30% of aggregate human exposure to chemicals with a K OA from 107 and 109 and a K OW from 102 to 104; however, the contribution is minimal for other chemicals.
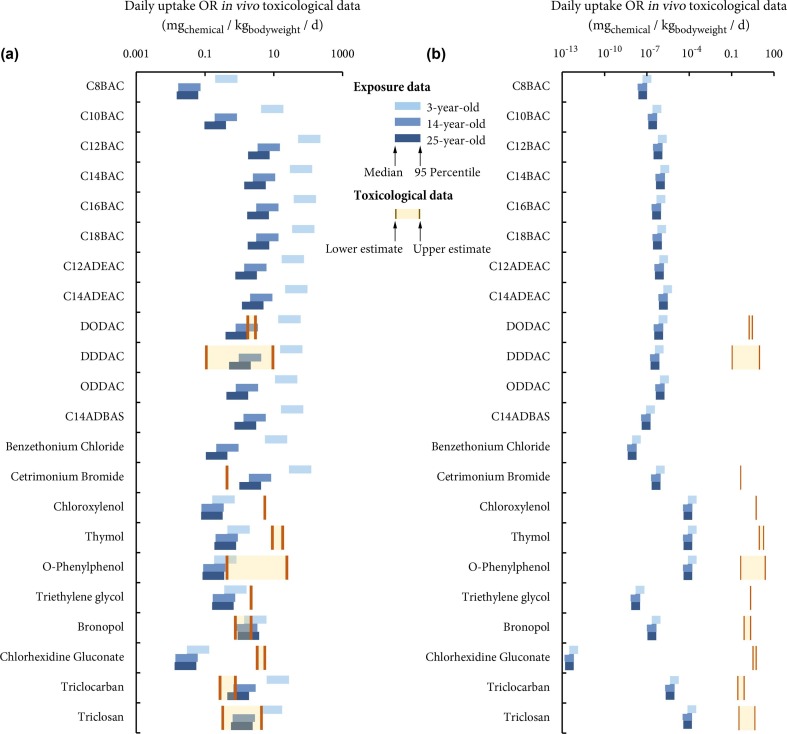
We then calculate the uptake doses of the investigated disinfecting chemicals after a single use per day in the “surface application” (Fig. 4 a) and “hand hygiene” (Fig. 4b) scenarios. In both scenarios, the predicted uptake doses vary by around one order of magnitude from the medians to 95th percentiles (Fig. 4). In the “surface application” scenario (Fig. 4a), the modeled individuals are exposed to quaternary ammonium salts (notably C12 to C18BAC) the highest, regardless of age group, because these chemicals are most strongly retained on the treated hard surfaces. By contrast, in the “hand hygiene” scenario (Fig. 4a), the modeled individuals are exposed to phenolic compounds the highest, regardless of age group, as these chemicals are neither too hydrophobic nor too hydrophilic and thus demonstrate a strong dermal absorption capability. In both scenarios, exposure to chlorhexidine gluconate is the lowest among all disinfecting chemicals. A comparison between Fig. 4a and b indicates that for the single use of all the investigated disinfecting chemicals, exposure after surface application outweighs that through handwashing. The reason behind this contrast is intuitive: washing hands under running water rinses the disinfecting chemicals off, hence limiting the duration of dermal exposure (20 s), whereas disinfecting chemicals can reside on the treated hard surfaces all day long, having time” for humans to touch, before they are removed by the regular cleanup. The difference in exposure between two scenarios is most prominent for chlorhexidine gluconate (10 orders of magnitude), followed by quaternary ammonium salts such as benzethonium chloride and C14ADBAS (7 orders of magnitude), whereas it is moderate for phenolic chemicals such as chloroxylenol and O-phenylphenol (~2000 times). We can, therefore, conclude that the post-application exposure is more remarkable than instant exposure during handwashing when the investigated disinfecting chemicals are employed to cope with the SARS-CoV-2 virus.
Fig. 4.
Modeled uptake of disinfecting chemicals by a 3-year-old child, a 14-year-old teenager, and a 25-year-old adult in the “surface application” (Panel a) and “hand hygiene” (Panel b) scenarios, and the comparison with in vivo toxicological data.
In both scenarios, the modeled 3-year-old child has generally higher uptake doses than the 14-year-old teenager and the 25-year-old adult (Fig. 4). In the “surface application” scenario (Fig. 4a), the age-specific difference in uptake is most remarkable for quaternary ammonium salts, by a factor ranging from 14 for C8BAC to 55 for benzethonium chloride between ages 3 and 25, but smaller for other compounds, especially phenolic chemicals. As discussed above, human exposure to quaternary ammonium salts mostly come from mouthing-mediated ingestion. Compared with teenagers and adults, children have much more frequent surface-to-hand and hand-to-mouth contacts, which results in an elevated contribution of mouthing-mediated ingestion. As such, we can expect an order of magnitude higher uptake of quaternary ammonium salts by children than teenagers and adults. By contrast, in the “hand hygiene” scenario (Fig. 4b), human uptake is more consistent among age groups, with a difference generally within a factor of 3. This difference reflects mainly age-dependent differences in bodyweight and the area of hand surface (different masses of handwasher used). In sum, our modeling results demonstrate that children tend to be exposed to higher doses of disinfecting chemicals than teenagers and adults; the higher contribution of mouthing-mediated ingestion to the aggregate exposure, the larger such an age-dependent difference can be.
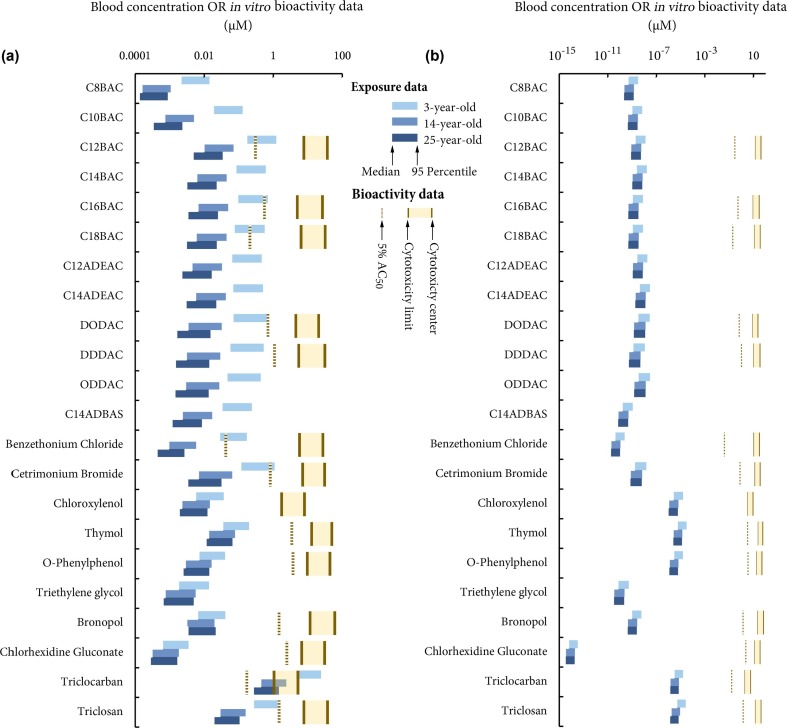
We also predict the blood concentration of the investigated disinfecting chemicals after a single use per day (Fig. 5 ). The blood concentration reflects the combined effects of uptake of disinfecting chemicals and elimination (e.g., biotransformation, renal clearance, and fecal egestion). In both scenarios, the predicted blood concentrations vary by around one order of magnitude from the medians to 95th percentiles (Fig. 5). In the “surface application” scenario (Fig. 5a), triclocarban and triclosan have the highest concentrations (respective best estimates of 0.27 and 0.02 μM for the 25-year-old adult) despite their modest uptake (ranking 12 and 10, respectively; Fig. 4a), as they are an order of magnitude more resistant to biotransformation than the other disinfecting chemicals (Table S1 in supplementary material). In comparison, while the modeled individuals have the highest uptake of C12 to C18 BACs, the blood concentrations of these compounds are not in top rank (ranking 4 to 9) because they are predicted to be relatively rapidly eliminated from the human body (Table S1). Here, we should confess that QSAR prediction of biotransformation half-lives of quaternary ammonium salts is challenging and uncertain because they are seldom included in the training sets for developing the currently used QSAR tools. While the QSAR used in this work (Papa et al., 2018) recognizes that C12BAC and C14BAC fall within its applicability domain and warns that C16BAC and C18BAC exceed it, readers should be with caution when interpreting the predicted blood concentrations of quaternary ammonium salts. Nevertheless, our predicted relative order of elimination rates of the investigated disinfecting chemicals seems to agree with experimental studies and thus be somewhat reasonable. For instance, the half-life of total elimination of BACs was found to be 1.30–1.56 h in rats (Xue et al., 2004), which is an order of magnitude faster than that of triclosan of 9–14 h in rats, as reviewed in Arnot et al. (2018). In the “hand hygiene” scenario (Fig. 5b), the relative order of predicted blood concentrations of the investigated disinfecting chemicals more closely mimics that of the predicted uptakes.
Fig. 5.
Modeled blood concentrations of disinfecting chemicals in a 3-year-old child, a 14-year-old teenager, and a 25-year-old adult in the “surface application” (Panel a) and “hand hygiene” (Panel b) scenarios, and the comparison with in vitro bioactivity data (In vitro bioactivity data for triethylene glycol are not displayed in this logarithmic plot because its cytotoxicity limit is 0 μM).
Note that the above conclusion builds on an assumption that the disinfecting chemicals staying on indoor surfaces are not degradable. Earlier experimental studies have demonstrated that the organic film on indoor surfaces can protect chemicals therein from atmospheric oxidation (Zhou et al., 2019, Zhou et al., 2012). Since [OH] radicals and other oxidants must first oxidize the organic layer before reacting with the chemicals “dissolved” in it, the rate of oxidation becomes the limiting step of the reaction of chemicals (Alwarda et al., 2018). The latest estimate (Alwarda et al., 2018) indicates that oxidizing a 1-nm-thick organic layer in indoor conditions would take 12–460 days, which means the disinfecting chemicals in the typical 20-nm thick organic film assumed in PROTEX would be preserved for 240 (lower estimate) to 9200 (higher estimate) days. To illustrate the influence of the potential degradation of the disinfecting chemicals on modeled exposure, we conduct a sensitivity analysis, using DODAC (involatile) and O-phenylphenol (volatile) as the example, as shown in the supplementary material, Table S2. Should the lower estimate be used, the estimated exposures to DODAC and O-phenylphenol would drop by 84% and 7%, respectively, relative to the calculation based on default assumption, whereas should the higher estimate be used, the estimated exposures are almost unchanged. That is, in the worst case, our assumption of no degradation on indoor surface compartments leads to an overestimation of exposure to highly involatile chemicals by a factor of five.
3.4. Health risk of exposure to disinfecting chemicals
Not all disinfecting chemicals investigated have effect thresholds publicly available from the sources used in this study (Tables S3 and S4 in supplementary material). Out of the 22 disinfecting chemicals, 11 have RfD and/or DNEL data (Fig. 4); in addition, the European Chemicals Agency database indicates that there is “no hazard identified”, instead of simply without any information, for C12BAC, C14BAC, C18BAC, and triethylene glycol. Irritation is the most common hazard identified for these chemicals while skeletal toxicity, hepatoxicity, and disruption to homeostasis are possible for some chemicals (Table S3 in supplementary material). In comparison, in vitro bioactivity thresholds from ToxCast provides wider coverage with AC50 values available for all the 11 chemicals mentioned above and another four chemicals (Fig. 5). It should be noted that among these 15 chemicals, chloroxylenol and triethylene glycol have no active assays with AC50 lower than their cytotoxicity limits. Triethylene glycol also has a very low cytotoxicity center (0.00129 µM) and its cytotoxicity limit is 0 µM, according to ToxCast. A total of 20 targets on the macromolecular and cellular level are found among active assays with AC50 below cytotoxicity limit for these chemicals (Table S4 in supplementary material).
Comparing the estimated exposure with these effect thresholds enables risk assessment for these chemicals in the studied scenarios. Regardless of the age group and effect thresholds, the “hand hygiene” scenario is found to pose no risks to human health. As shown in Figs. 4b and 5b, the predicted exposure is three to twelve orders of magnitude lower than even the lowest level that can potentially cause adverse effects.
Comparing the uptake doses with maximum acceptable doses (Fig. 4a), we can find that, in the “surface application” scenario, all three age groups are at risk for cetrimonium bromide after a single application, while they are not at risk for chloroxylenol, thymol, triethylene glycol, and chlorhexidine gluconate despite variations in exposures and maximum acceptable doses. If the lower estimate for maximum acceptable doses and the 95th percentile of exposure are used for a more conservative risk assessment, DODAC, DDDAC, bronopol, triclocarban, and triclosan pose risk for all age groups. For O-phenylphenol, the 25-year-old adult would have little risk, while the 14-year-old teenager at the 95th percentile of exposure could experience some risk if the lower estimate for maximum acceptable doses is used, and the 3-old-year young child could be more likely to be at risk due to the higher exposure. On the other hand, if the upper estimate for maximum acceptable doses is used, in addition to cetrimonium bromide, only bronopol and triclocarban will pose risk to teenagers and adults except for those with lower exposure. However, risk assessment results for young children stay generally the same (except for O-phenylphenol for which young children will not experience risk) due to their much higher exposure levels.
By contrast, comparing the predicted exposures with in vitro bioactivity thresholds gives a substantially different profile of risks (Fig. 5a). Our results indicate there is little risk for all chemicals for teenagers and adults, except for triclocarban when the lower estimate for bioactivity threshold is used and the modeled individuals are in the higher range of exposure estimates. The 3-year-old child is at risk for triclocarban no matter the variation in exposure or bioactivity threshold. By using the most sensitive bioactivity threshold (5th percentile of AC50), many children could be at risk for C12BAC, C18BAC, benzethonium chloride. Only children with 95th percentile of exposure may be at risk for C16BAC, DODAC, cetrimonium bromide, and triclosan. We should again emphasize that the above results are based on a single disinfection event a day, and that the actual human exposure can be proportional to the frequency of disinfection. For instance, if we evaluate the risk based on 95th percentile of exposure and the lower estimate for bioactivity threshold, the exposed individual may be at risk because of exposure to triclosan if the hard surface is disinfected five times a day, and because of exposures to C12BAC and cetrimonium bromide if the hard surface is disinfected six times a day.
High-throughput in vitro toxicity testing like ToxCast creates a new pathway for bridging the gap between the availably of human-relevant toxicity data and the ever-growing number of chemicals circulating in commerce to better inform regulatory decisions (Judson et al., 2014, National Research Council, 2007, Punt et al., 2020). Since its creation, ToxCast has been mainly used as a screening level tool to identify “hot spots” where chemical exposure and toxicity may overlap (Wetmore et al., 2015), rank chemicals of concerns based on potential hazards (Tilley et al., 2017), and prioritize the further analysis of chemicals exhibiting in vitro bioactivity for certain health outcomes (Auerbach et al., 2016, Karmaus et al., 2016). To our knowledge, there is still a void for side-by-side quantitative examination of in vitro bioactivity data-based risk assessment and in vivo toxicological data-based, or traditional, risk assessment. By such an examination, our study offers insights into the application of high-throughput in vitro bioactivity data in risk assessment. Ideally, a comparison between blood concentration with in vitro bioactivity data should yield similar results from the comparison between the uptake doses with in vivo toxicological data if both the exposure and toxicity are accurately reflecting the underlying biomechanisms. However, the risk assessment results from in vivo and in vitro in this study are not in good agreement. Results from comparing daily uptake and in vivo toxicological data show higher risks in contrast with the comparison of blood concentration and in vitro bioactivity data in the “surface application” scenario. Even for the “hand washing” scenario, the gap between exposure and effect thresholds indicated by in vitro bioactivity data is several orders of magnitude lower than that indicated by in vivo toxicological data.
This disagreement underscores two aspects of high uncertainty in the field of risk assessment. First, there is a need for a better understanding of the biodistribution of disinfecting chemicals within the body. Despite recent developments in estimating key biodistribution determinants, a lack of accurate data for such determinants remains as one of the most challenging issues in the in vitro-in vivo extrapolation (IVIVE) for exposure (Wambaugh et al., 2018, Wambaugh et al., 2015). Indeed, the biotransformation data used in this study is derived from QSAR models and carries corresponding uncertainty, especially for the quaternary ammonium salts that fall outside the applicability domain. An overestimate of biotransformation could then result in an underestimate of blood concentration, which would lead to a lower assessment of risk, everything else being equal. Second, there is currently a lack of conciliation between traditional toxicity endpoints (i.e., RfD and DNEL) and in vitro bioactivity endpoints to achieve more consistent results in the context of risk assessment. For traditional toxicity endpoints that have been extrapolated from animal studies, the point of departure is usually divided by an arbitrary factor of ten to account for interspecies extrapolation as a conservative approach (ECETOC, 2010, U.S. Environmental Protection Agency, 2014). While investigations on a few selected chemicals showed inconsistency between oral equivalent dose extrapolated from bioactivity data in human cell lines and the point of departure observed from animal studies (Silva et al., 2015, Turley et al., 2019), a recent examination on approximately 200 chemicals indicated that there may not be systematic differences between these two metrics (Wang, 2018). If one is to believe human cell line results are more relevant to human health, this could suggest that the approach for deriving traditional toxicity endpoints may be overly cautious and overestimating the risks from the same exposure. Nevertheless, in vitro bioactivity data is not without its own shortcomings. Cross examinations between ToxCast test results with in vivo toxicity data show that chemicals with assay results in ToxCast reporting inactive for specific outcomes may indeed induce the corresponding outcomes in vivo (Pham et al., 2016, Silva et al., 2015).
4. Conclusions and looking forward
In this work, we present the first thorough overview of human exposure and health risk for disinfecting chemicals recommended to prevent the spread of SARS-CoV-2 in scenarios of surface application and hand hygiene. Our study shows that the wide range of physicochemical properties results in vastly different exposure patterns for different disinfecting chemicals and different age groups. While no health risks are identified with the hand hygiene scenario for any investigated disinfecting chemicals, some may pose risks with the surface application, especially for young children. As such, it is of potential health concern to leave disinfecting chemicals on high touch hard surfaces without wiping off, although the residuals of some disinfectants are believed to provide continued efficacy of suppressing the replication or survival of viruses, even after dryness (Brown et al., 2020). This health concern can be viewed as a secondary risk created by the response to the risk of the COVID-19 pandemic. Although our assessment indicates health risks arising from the use of disinfectants, this should not be taken as a recommendation of not using disinfectants to contain the threat of COVID-19 pandemic. It should also be emphasized that our assessment reflects the maximum health risks associated with the proper use of disinfecting chemicals, as we assume that disinfectants are used at full strength without dilution, the modeled individual is exposed to disinfecting chemicals through direct hand contact with disinfected surfaces and objects, and that disinfectants are not wiped off after surface application.
While it for the first time informs us of the potential health outcomes of the consumer use of disinfecting chemicals, conclusions present in this work can be preliminary because of insufficient information and methodological limitation. Therefore, we encourage future work to further elucidate the fate, exposure, and toxicity of disinfecting chemicals.
First, since most disinfecting chemicals are ionogenic organic chemicals (IOCs), and that the indoor fate and the human biodistribution processes of IOCs are less understood at the current moment, future work is encouraged to investigate in depth the behavior of IOCs in the environment and human body. For instance, it remains unclear whether, in what condition, and to what extent, IOCs can ionize on indoor surfaces and the human skin, and whether the proportionate combination of neutral and charged forms of IOCs can really represent the overall behavior of the partially ionized IOCs. It also remains unknown whether the application of IOCs, e.g., the disinfectants investigated in this work, can disturb the integrity of the stratum corneum (e.g., hyperkeratosis) and hence influences the mechanisms of absorption and toxicity. In addition, we have little information on whether the transporter-facilitated “active” absorption contributes to the uptake of mouthed IOCs in the gastrointestinal tract. There is currently a lack of QSAR tools for IOCs. With emerging information being available, modification to PROTEX and other existing models to accommodate the possible unique behavior of IOCs is warranted. Previous reviews have provided a pioneering guide on possible ways of expanding models towards this direction (Armitage et al., 2017, Bonnell et al., 2018).
Second, since toxicological data are either missing or associated with uncertainties, advancement in high-throughput toxicity testing is still in great need to close the gap between the number of chemicals with available data and the number of chemicals that humans are potentially exposed. In addition, it is important to develop new knowledge and best practice of how to use this high-throughput toxicity data in risk assessment, given the inconsistent results from traditional risk assessment methods using animal-based toxicity data. Despite pioneering works utilizing high-throughput in vitro bioactivity data in risk prioritization (Shin et al., 2015, Wegner et al., 2020, Wetmore et al., 2015), the value from the rapidly increasing amount of high-throughput toxicity data is unlikely to be fully realized without accompanying methodologies of its application in human health risk assessment that is recognized by a wide range of stakeholders.
Third, there is a need for a more thorough assessment of possible environmental and health impacts of disinfecting chemicals to avoid possible risk-risk trade-off, i.e., reducing the risk of the pandemic by creating new risks of consumer and ecological exposure. For instance, while our work recognizes that handwashing causes low human exposures, the large fraction of disinfectants washed off can be a relevant source to its presence in municipal wastewater, and by extension, the aquatic environment. Monitoring studies have reported surprisingly high levels of total BAC concentrations (C12 to C18) in sewage sludge in China (on average 3.6 μg/g) (Li et al., 2014) and surficial sediments in urban estuarine in the United States (on average 1.5 μg/g in New Jessy and New York) (Li and Brownawell, 2010). It remains unknown whether these levels can lead to significant adverse ecological impacts, and to what extent the levels surge after the COVID19 pandemic given that New Jessy and New York are the epicenters of the United States. Future monitoring work is also encouraged to determine the occurrence of disinfecting chemicals in various indoor compartments (e.g., settled dust, hard surfaces, objects) and human body tissues. Joint efforts between (bio)monitoring, modeling, and policy analysis may bring about best practice of disinfectant use to balance their benefits and impacts on human health and prepare us to better respond to public health emergencies in the future.
Lastly, it is worth investigating further on the viricidal efficacy of the disinfectants (Rabenau et al., 2005) and considering this information in determining the safety of disinfectants. In this study, we assumed the same usage for all disinfectants, which may not be true. It is possible that while some disinfectants are shown to pose lower risks to human health in our assessment, their health risks could be high in reality because their lower viricidal efficacy requires more frequent uses or uses in a larger quantity to achieve the same viricidal performance as others. Therefore, a more comprehensive evaluation should be done based on the desired function and the varying amount of disinfectants associated with this function. There is also a need for a more thorough examination of the risk-benefit trade-off, by considering a broader range of medical and health evidence such as the probability of contracting COVID-19 from hard surfaces across different population without the use of disinfectants, the reduction of this probability with different use patterns of various disinfectants, and individual health outcome and impact from COVID-19.
CRediT authorship contribution statement
Dingsheng Li: Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Alessandro Sangion: Methodology, Software, Writing - original draft, Writing - review & editing. Li Li: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank Yuchao Lu for data collection and Jon A. Arnot for assistance with pKa calculation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Handling Editor: Marti Nadal
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106108.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Alwarda R., Zhou S., Abbatt J.P. Heterogeneous oxidation of indoor surfaces by gas-phase hydroxyl radicals. Indoor Air. 2018;28:655–664. doi: 10.1111/ina.12476. [DOI] [PubMed] [Google Scholar]
- Armitage J.M., Erickson R.J., Luckenbach T., Ng C.A., Prosser R.S., Arnot J.A., Schirmer K., Nichols J.W. Assessing the bioaccumulation potential of ionizable organic compounds: current knowledge and research priorities. Environ. Toxicol. Chem. 2017;36:882–897. doi: 10.1002/etc.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot J.A., Pawlowski S., Champ S. A weight-of-evidence approach for the bioaccumulation assessment of triclosan in aquatic species. Sci. Total Environ. 2018;618:1506–1518. doi: 10.1016/j.scitotenv.2017.09.322. [DOI] [PubMed] [Google Scholar]
- Auerbach S., Filer D., Reif D., Walker V., Holloway Alison C., Schlezinger J., Srinivasan S., Svoboda D., Judson R., Bucher John R., Thayer Kristina A. Prioritizing environmental chemicals for obesity and diabetes outcomes research: a screening approach using ToxCast™ high-throughput data. Environ. Health Perspect. 2016;124:1141–1154. doi: 10.1289/ehp.1510456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D.H., Furtaw E.J. Fugacity-based indoor residential pesticide fate model. Environ. Sci. Technol. 2004;38:2142–2152. doi: 10.1021/es034287m. [DOI] [PubMed] [Google Scholar]
- Bonnell M.A., Zidek A., Griffiths A., Gutzman D. Fate and exposure modeling in regulatory chemical evaluation: new directions from retrospection. Environ. Sci. Processes Impacts. 2018;20:20–31. doi: 10.1039/c7em00510e. [DOI] [PubMed] [Google Scholar]
- Brown E., Dhanireddy K., Teska P., Eifert J., Williams R.C., Boyer R. Influence of drying time on prewetted disinfectant towelettes to disinfect glass surfaces. Am. J. Infect. Control. 2020;48:846–848. doi: 10.1016/j.ajic.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Brown T.N., Armitage J.M., Egeghy P., Kircanski I., Arnot J.A. Dermal permeation data and models for the prioritization and screening-level exposure assessment of organic chemicals. Environ Int. 2016;94:424–435. doi: 10.1016/j.envint.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Campbell A., Chapman M. Benzalkonium chloride. In: Campbell A., Chapman M., editors. Handbook of Poisoning in Dogs and Cats. Blackwell Science Ltd; London: 2000. [Google Scholar]
- Chang A., Schnall A.H., Law R., Bronstein A.C., Marraffa J.M., Spiller H.A., Hays H.L., Funk A.R., Mercurio-Zappala M., Calello D.P., Aleguas A., Borys D.J., Boehmer T., Svendsen E. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19 - National Poison Data System, United States, January 1, 2020-March 31, 2020. MMWR Morb. Mortal Wkly Rep. 2020;69:496–498. doi: 10.15585/mmwr.mm6916e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. National Collaborating Centre for Environmental Health; Vancouver, BC: 2020. Reducing COVID-19 Transmission Through Cleaning and Disinfecting Household Surfaces. [Google Scholar]
- China's National Health Commission, 2020. Technical Guidance on Disinfectant Use (Feb 2020 Update). China's National Health Commission, Beijing.
- Choi S.M., Roh T.H., Lim D.S., Kacew S., Kim H.S., Lee B.-M. Risk assessment of benzalkonium chloride in cosmetic products. J. Toxicol. Environ. Health, Part B. 2018;21:8–23. doi: 10.1080/10937404.2017.1408552. [DOI] [PubMed] [Google Scholar]
- Delmaar, J.E., Park, M.V.D.Z., van Engelen, J.G.M., 2005. ConsExpo – Consumer Exposure and Uptake Models (RIVM report no. 320104004).
- ECETOC . European Centre for Ecotoxicology and Toxicology of Chemicals; Brussels: 2010. Guidance on assessment factors to derive a DNEL. [Google Scholar]
- European Chemical Agency, 2020. Registered Substances Database (https://echa.europa.eu/information-on-chemicals/registered-substances). European Chemical Agency (ECHA), Helsinki (accessed May 25, 2020).
- Gramatica P., Cassani S., Chirico N. QSARINS-chem: Insubria datasets and new QSAR/QSPR models for environmental pollutants in QSARINS. J. Comput. Chem. 2014;35:1036–1044. doi: 10.1002/jcc.23576. [DOI] [PubMed] [Google Scholar]
- Gramatica P., Chirico N., Papa E., Cassani S., Kovarich S. QSARINS: a new software for the development, analysis, and validation of QSAR MLR models. J. Comput. Chem. 2013;34:2121–2132. [Google Scholar]
- Health Canada, 2020a. Hard-surface disinfectants and hand sanitizers (COVID-19): List of disinfectants for use against COVID-19 (available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/list.html). Health Canada, Ottawa.
- Health Canada, 2020b. Hard-surface disinfectants and hand sanitizers (COVID-19): List of hand sanitizers authorized by Health Canada (available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/hand-sanitizer.html). Health Canada, Ottawa.
- Hodges G., Eadsforth C., Bossuyt B., Bouvy A., Enrici M.H., Geurts M., Kotthoff M., Michie E., Miller D., Muller J., Oetter G., Roberts J., Schowanek D., Sun P., Venzmer J. A comparison of log K-ow (n-octanol-water partition coefficient) values for non-ionic, anionic, cationic and amphoteric surfactants determined using predictions and experimental methods. Enviro. Sci. Eur. 2019;31 [Google Scholar]
- Isaacs K.K., Phillips K.A., Biryol D., Dionisio K.L., Price P.S. Consumer product chemical weight fractions from ingredient lists. J. Expo Sci. Environ. Epidemiol. 2018;28:216–222. doi: 10.1038/jes.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R., Houck K., Martin M., Knudsen T., Thomas R.S., Sipes N., Shah I., Wambaugh J., Crofton K. In vitro and modelling approaches to risk assessment from the U.S. Environmental Protection Agency ToxCast programme. Basic Clin. Pharmacol. Toxicol. 2014;115:69–76. doi: 10.1111/bcpt.12239. [DOI] [PubMed] [Google Scholar]
- Judson R., Houck K., Martin M., Richard A.M., Knudsen T.B., Shah I., Little S., Wambaugh J., Woodrow Setzer R., Kothya P., Phuong J., Filer D., Smith D., Reif D., Rotroff D., Kleinstreuer N., Sipes N., Xia M., Huang R., Crofton K., Thomas R.S. Editor's highlight: analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space. Toxicol. Sci. 2016;152:323–339. doi: 10.1093/toxsci/kfw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmaus A.L., Filer D.L., Martin M.T., Houck K.A. Evaluation of food-relevant chemicals in the ToxCast high-throughput screening program. Food Chem. Toxicol. 2016;92:188–196. doi: 10.1016/j.fct.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Li L., Arnot J.A., Wania F. Revisiting the contributions of far- and near-field routes to aggregate human exposure to Polychlorinated Biphenyls (PCBs) Environ. Sci. Technol. 2018;52:6974–6984. doi: 10.1021/acs.est.8b00151. [DOI] [PubMed] [Google Scholar]
- Li L., Arnot J.A., Wania F. Towards a systematic understanding of the dynamic fate of polychlorinated biphenyls in indoor, urban and rural environments. Environ. Int. 2018;117:57–68. doi: 10.1016/j.envint.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Li L., Arnot J.A., Wania F. How are humans exposed to organic chemicals released to indoor air? Environ. Sci. Technol. 2019;53:11276–11284. doi: 10.1021/acs.est.9b02036. [DOI] [PubMed] [Google Scholar]
- Li X., Brownawell B.J. Quaternary ammonium compounds in urban estuarine sediment environments – a class of contaminants in need of increased attention? Environ. Sci. Technol. 2010;44:7561–7568. doi: 10.1021/es1011669. [DOI] [PubMed] [Google Scholar]
- Li X., Luo X., Mai B., Liu J., Chen L., Lin S. Occurrence of quaternary ammonium compounds (QACs) and their application as a tracer for sewage derived pollution in urban estuarine sediments. Environ. Pollut. 2014;185:127–133. doi: 10.1016/j.envpol.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Lu C., Fenske R.A. Air and surface chlorpyrifos residues following residential broadcast and aerosol pesticide applications. Environ. Sci. Technol. 1998;32:1386–1390. [Google Scholar]
- Mackay D., Celsie A.K., Parnis J.M. The evolution and future of environmental partition coefficients. Environ. Rev. 2016;24:101–113. [Google Scholar]
- Mansouri K., Grulke C.M., Judson R.S., Williams A.J. OPERA models for predicting physicochemical properties and environmental fate endpoints. J. Cheminf. 2018;10:10. doi: 10.1186/s13321-018-0263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready D., Pitt J., Fontaine D.D., Busby M.I. Potential sequential exposures to 2-butoxyethanol after use of a hard-surface cleaner. Hum. Ecol. Risk Assess. 2013;19:189–214. [Google Scholar]
- National Environment Agency, 2020. Interim List of Household Products and Active Ingredients for Disinfection of the COVID-19 Virus (available at: https://www.nea.gov.sg/our-services/public-cleanliness/environmental-cleaning-guidelines/guidelines/interim-list-of-household-products-and-active-ingredients-for-disinfection-of-covid-19).
- National Research Council . National Academies Press; Washington, DC: 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. [Google Scholar]
- Papa E., Sangion A., Arnot J.A., Gramatica P. Development of human biotransformation QSARs and application for PBT assessment refinement. Food Chem. Toxicol. 2018;112:535–543. doi: 10.1016/j.fct.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Pham N., Iyer S., Hackett E., Lock B.H., Sandy M., Zeise L., Solomon G., Marty M. Using ToxCast to explore chemical activities and hazard traits: a case study with ortho-phthalates. Toxicol. Sci. 2016;151:286–301. doi: 10.1093/toxsci/kfw049. [DOI] [PubMed] [Google Scholar]
- Popendorf W., Selim M. Exposures while applying commercial disinfectants. Am. Ind. Hyg. Assoc. J. 1995;56:1111–1120. doi: 10.1080/15428119591016331. [DOI] [PubMed] [Google Scholar]
- Punt A., Firman J., Boobis A., Cronin M., Gosling J.P., Wilks M.F., Hepburn P.A., Thiel A., Fussell K.C. Potential of ToxCast data in the safety assessment of food chemicals. Toxicol. Sci. 2020;174:326–340. doi: 10.1093/toxsci/kfaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H., Kampf G., Cinatl J., Doerr H. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson H., Counts J.L., Stanton K.L., Sedlak R.I. Exposure and prioritization - human screening data and methods for high production volume chemicals in consumer products: amine oxides a case study. Risk Anal. 2006;26:1637–1657. doi: 10.1111/j.1539-6924.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach R.P., Gschwend P.M., Imboden D.M. second ed. John Wiley & Sons; Hoboken, NJ: 2003. Environmental Organic Chemistry. [Google Scholar]
- Shin H.-M., Ernstoff A., Arnot J.A., Wetmore B.A., Csiszar S.A., Fantke P., Zhang X., McKone T.E., Jolliet O., Bennett D.H. Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ. Sci. Technol. 2015;49:6760–6771. doi: 10.1021/acs.est.5b00498. [DOI] [PubMed] [Google Scholar]
- Silva M., Pham N., Lewis C., Iyer S., Kwok E., Solomon G., Zeise L. A comparison of ToxCast test results with in vivo and other in vitro endpoints for neuro, endocrine, and developmental toxicities: a case study using endosulfan and methidathion. Birth Defects Res. B. 2015;104:71–89. doi: 10.1002/bdrb.21140. [DOI] [PubMed] [Google Scholar]
- Tilley S.K., Reif D.M., Fry R.C. Incorporating ToxCast and Tox21 datasets to rank biological activity of chemicals at Superfund sites in North Carolina. Environ. Int. 2017;101:19–26. doi: 10.1016/j.envint.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley A.E., Isaacs K.K., Wetmore B.A., Karmaus A.L., Embry M.R., Krishan M. Incorporating new approach methodologies in toxicity testing and exposure assessment for tiered risk assessment using the RISK21 approach: Case studies on food contact chemicals. Food Chem. Toxicol. 2019;134 doi: 10.1016/j.fct.2019.110819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention, 2020a. Cleaning and Disinfection for Households. U.S. Department of Health & Human Services.
- U.S. Centers for Disease Control and Prevention, 2020b. Interim Recommendations for U.S. Households with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19). U.S. Centers for Disease Control and Prevention, Washington, D.C.
- U.S. Environmental Protection Agency, 2014. Reference Dose (RfD): Description and Use in Health Risk Assessments. U.S. Environmental Protection Agency, Washington, D.C.
- U.S. Environmental Protection Agency, 2019. Exposure Factors Handbook (2011 Edition; 2019 Update). National Center for Environmental Assessment, Office of Research and Development, Washington, D.C.
- U.S. Environmental Protection Agency, 2020a. CompTox Chemistry Dashboard. U.S. Environmental Protection Agency, Research Triangle Park, NC.
- U.S. Environmental Protection Agency, 2020b. List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19) (available at: https://cfpub.epa.gov/giwiz/disinfectants/index.cfm). U.S. Environmental Protection Agency, Washington, D.C.
- U.S. Food and Drug Administration, 2016. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. 21 CFR Part 310 [Docket No. FDA–1975–N–0012; Formerly Part of Docket No. 1975N–0183H]. U.S. Food and Drug Administration, Washington, D.C.
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh J.F., Hughes M.F., Ring C.L., MacMillan D.K., Ford J., Fennell T.R., Black S.R., Snyder R.W., Sipes N.S., Wetmore B.A., Westerhout J., Setzer R.W., Pearce R.G., Simmons J.E., Thomas R.S. Evaluating in vitro-in vivo extrapolation of toxicokinetics. Toxicol. Sci. 2018;163:152–169. doi: 10.1093/toxsci/kfy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh J.F., Wang A., Dionisio K.L., Frame A., Egeghy P., Judson R., Setzer R.W. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ. Sci. Technol. 2014;48:12760–12767. doi: 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- Wambaugh J.F., Wetmore B.A., Pearce R., Strope C., Goldsmith R., Sluka J.P., Sedykh A., Tropsha A., Bosgra S., Shah I., Judson R., Thomas R.S., Setzer R.W. Toxicokinetic triage for environmental chemicals. Toxicol. Sci. 2015;147:55–67. doi: 10.1093/toxsci/kfv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Infer the in vivo point of departure with ToxCast in vitro assay data using a robust learning approach. Arch. Toxicol. 2018;92:2913–2922. doi: 10.1007/s00204-018-2260-6. [DOI] [PubMed] [Google Scholar]
- Weerdesteijn, M.C.H., Bremmer, H.J., Zeilmaker, M.J., van Veen, M.P., 1999. Hygienic Cleaning Products Used in the Kitchen: Exposure and Risks (RIVM report 612810008). Bilthoven, The Netherlands.
- Wegner S.H., Pinto C.L., Ring C.L., Wambaugh J.F. High-throughput screening tools facilitate calculation of a combined exposure-bioactivity index for chemicals with endocrine activity. Environ. Int. 2020;137 doi: 10.1016/j.envint.2020.105470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann T., Gerlich J., Heinrich S., Nowak D., Mutius E.V., Vogelberg C., Genuneit J., Lanzinger S., Al-Khadra S., Lohse T., Motoc I., Walter V., Radon K. Association of household cleaning agents and disinfectants with asthma in young German adults. Occup. Environ. Med. 2017;74:684–690. doi: 10.1136/oemed-2016-104086. [DOI] [PubMed] [Google Scholar]
- Weschler C.J., Nazaroff W.W. SVOC exposure indoors: fresh look at dermal pathways. Indoor Air. 2012;22:356–377. doi: 10.1111/j.1600-0668.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- Weschler C.J., Nazaroff W.W. Growth of organic films on indoor surfaces. Indoor Air. 2017;27:1101–1112. doi: 10.1111/ina.12396. [DOI] [PubMed] [Google Scholar]
- Wetmore B.A., Wambaugh J.F., Allen B., Ferguson S.S., Sochaski M.A., Setzer R.W., Houck K.A., Strope C.L., Cantwell K., Judson R.S., LeCluyse E., Clewell H.J., Thomas R.S., Andersen M.E. Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci. 2015;148:121–136. doi: 10.1093/toxsci/kfv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Hieda Y., Saito Y., Nomura T., Fujihara J., Takayama K., Kimura K., Takeshita H. Distribution and disposition of benzalkonium chloride following various routes of administration in rats. Toxicol. Lett. 2004;148:113–123. doi: 10.1016/j.toxlet.2003.12.068. [DOI] [PubMed] [Google Scholar]
- Zang Q., Mansouri K., Williams A.J., Judson R.S., Allen D.G., Casey W.M., Kleinstreuer N.C. In silico prediction of physicochemical properties of environmental chemicals using molecular fingerprints and machine learning. J. Chem. Inf. Model. 2017;57:36–49. doi: 10.1021/acs.jcim.6b00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zartarian V., Bahadori T., McKone T. Adoption of an official ISEA glossary. J. Expo Sci. Environ. Epidemiol. 2005;15:1–5. doi: 10.1038/sj.jea.7500411. [DOI] [PubMed] [Google Scholar]
- Zhang X., Arnot J.A., Wania F. Model for screening-level assessment of near-field human exposure to neutral organic chemicals released indoors. Environ. Sci. Technol. 2014;48:12312–12319. doi: 10.1021/es502718k. [DOI] [PubMed] [Google Scholar]
- Zhou S., Hwang B.C., Lakey P.S., Zuend A., Abbatt J.P., Shiraiwa M. Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations. Proc. Natl. Acad. Sci. 2019;116:11658–11663. doi: 10.1073/pnas.1902517116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Lee A.K.Y., McWhinney R.D., Abbatt J.P.D. Burial effects of organic coatings on the heterogeneous reactivity of particle-borne benzo[a]pyrene (BaP) toward ozone. J. Phys. Chem. A. 2012;116:7050–7056. doi: 10.1021/jp3030705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.