Abstract
Purpose
To describe the spectrum of COVID-19 neurology in Singapore.
Method
We prospectively studied all microbiologically-confirmed COVID-19 patients in Singapore, who were referred for any neurological complaint within three months of COVID-19 onset. Neurological diagnoses and relationship to COVID-19 was made by consensus guided by contemporaneous literature, refined using recent case definitions.
Results
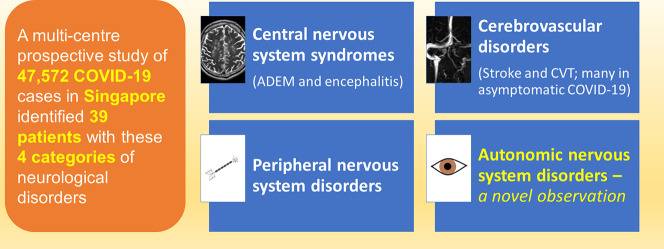
47,572 patients (median age 34 years, 98% males) were diagnosed with COVID-19 in Singapore between 19 March to 19 July 2020. We identified 90 patients (median age 38, 98.9% males) with neurological disorders; 39 with varying certainty of relationship to COVID-19 categorised as: i) Central nervous system syndromes-4 acute disseminated encephalomyelitis (ADEM) and encephalitis, ii) Cerebrovascular disorders-19 acute ischaemic stroke and transient ischaemic attack (AIS/TIA), 4 cerebral venous thrombosis (CVT), 2 intracerebral haemorrhage, iii) Peripheral nervous system-7 mono/polyneuropathies, and a novel group, iv) Autonomic nervous system-4 limited dysautonomic syndromes. Fifty-one other patients had pre/co-existent neurological conditions unrelated to COVID-19. Encephalitis/ADEM is delayed, occurring in critical COVID-19, while CVT and dysautonomia occurred relatively early, and largely in mild infections. AIS/TIA was variable in onset, occurring in patients with differing COVID-19 severity; remarkably 63.2% were asymptomatic. CVT was more frequent than expected and occurred in mild/asymptomatic patients. There were no neurological complications in all 81 paediatric COVID-19 cases.
Conclusion
COVID-19 neurology has a wide spectrum of dysimmune-thrombotic disorders. We encountered relatively few neurological complications, probably because our outbreak involved largely young men with mild/asymptomatic COVID-19. It is also widely perceived that the pandemic did not unduly affect the Singapore healthcare system.
Keywords: COVID-19, Neurological manifestations, Coronavirus, SARS-CoV-2, Pandemic
Graphical abstract
Highlights
-
•
Out of 47,572 COVID-19 patients, we identified 39 with neurological disorders.
-
•
‘CNS syndrome’ is delayed, occurring in critically ill COVID-19 patients.
-
•
Dysautonomia occurred relatively early and largely in mild infections.
-
•
63.2% of AIS/TIA patients had asymptomatic COVID-19.
-
•
We recorded 4 cerebral venous thromboses, in mild/asymptomatic COVID-19.
1. Introduction
The 2003 Severe Acute Respiratory syndrome (SARS) outbreak, caused by SARS coronavirus-1 (SARS-CoV-1) resulted in 206 patients with 32 deaths in Singapore. Five patients developed large vessel stroke, largely attributed to the predilection for thromboembolism in critically ill SARS patients [1]. A study covering all adult and paediatric patients admitted to public hospitals during the 2009 H1N1 Influenza pandemic in Singapore [2], identified 98 neurological complications developing at median of two days after onset. Majority were in patients less than 19 years old and the commonest disorder was seizures. Influenza associated encephalopathy occurred in 20%. A wider range of neurological conditions were seen among adults, half presenting with an exacerbation of their pre-existent neurological disease.
The current pandemic of a novel coronavirus SARS-CoV-2, causing the coronavirus disease 2019 (COVID-19) has caused more than 15 million cases to date [3]. Mao et al. documented neurological manifestations in 36.4% (78/214) of COVID-19 patients [4]. This was followed by reports of central and peripheral nervous systems (PNS) complications [[5], [6], [7]], including anosmia, ageusia, headache, seizures, cranial neuropathies [8], myelitis [9], stroke [[4], [5], [6],10,11], cerebral venous thrombosis [12], encephalopathy-encephalitis [4,7,10,13,14] and various neuropsychiatric manifestations [6]. Direct viral neurotropism has been reported rarely. Conversely, there have been increasing descriptions of secondary prothrombotic, dysimmune pathology [9,13,[15], [16], [17], [18]] and para-infectious phenomena such as Guillain-Barré [19,20] syndrome (GBS). The exact incidence of neurological complications in COVID-19 patients is uncertain. The confounding effect of the surge in COVID-19 cases on healthcare systems has to be accounted for as published studies of COVID-19 neurology are largely from countries that have been overwhelmed by the pandemic [4,7,10].
To comprehensively study the impact of COVID-19 on the nervous system, we set-up a multi-centre prospective database of all neurological disorders detected in microbiologically-confirmed COVID-19 patients. We aim to describe the spectrum of COVID-19 neurology.
2. Materials and method
2.1. Study design, source of data and eligibility criteria
In this prospective multi-centre cohort study, we included patients with microbiologically-confirmed COVID-19 under the care of public healthcare institutions in Singapore (listed fully in author designations), who were referred for any neurological complaint within three months of COVID-19 diagnosis. COVID-19 was confirmed by a positive naso- or oropharyngeal swab for SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) and or raised SARS-CoV-2 IgG [21]. Severity of COVID-19 was defined [22] as: Mild: Nil or mild pneumonia; Severe: dyspnoea, tachypnoea and hypoxia requiring supplemental oxygen; Critical: Respiratory failure requiring assisted ventilation, septic shock, multi-organ dysfunction. COVID-19 onset was defined as day 1 of acute respiratory illness (ARI). For asymptomatic individuals, the onset was taken as the date of confirmatory SARS-CoV-2 RT-PCR result or 14 days prior to date of positive SARS-CoV-2 serology. Date of discovery of neurological symptom was taken as the onset of neurological disorder. The study team comprised neurologists and neurosurgeons involved in clinical care of COVID-19 patients at the listed institutions. Pertinent clinical, laboratory and radiological data as well as final diagnosis were collated. Data was anonymised at entry and collected over a period needed to delineate fully the neurological diagnosis and its sequelae. We did not record anosmia and dysgeusia, common presenting complaints of COVID-19, as specific neurological disorders. The study group tele-conferenced fortnightly to discuss the diagnoses and adjudicate their relationship to SARS-CoV-2 by consensus guided by contemporaneous literature. Subsequently, the certainty of association with COVID-19 was graded ‘probable and ‘possible’ using the case definitions by Ellul et al. [16] (see Appendix A Table 1).The secondary aim of these sessions was to detect any trends that might have immediate impact on the management of COVID-19 patients. Several of the cases we describe (encephalitis, cerebral venous thrombosis, facial neuropathy, dysautonomia cases) have been previously submitted for publication as case reports/series by their managing physicians. As our aim is to comprehensively survey COVID-19 neurology in Singapore, we have chosen to include them and referenced them where applicable. The study was approved by the local institutional review board (CIRB 2020/2410).
2.2. Statistical analysis
Descriptive analysis is expressed as frequencies and percentages for categorical variables and median and range for continuous variables.
3. Results
From 19 March 2020 to 19 July 2020 there were 47,572 COVID-19 patients, including 81 paediatric cases (age < 16 years), in Singapore. Median age was 34 (1−102) years and 98% were males. Ninety-three patients needed critical care and 27 died. Ninety patients had neurological disorders; 98.9% of whom were males with a median age of 38 (22–75) years. 84.4% were mild or asymptomatic, 2.2% severe and 13.3% critical COVID-19. We recorded the following neurological diagnostic case-mix.
3.1. Central nervous system syndromes (n = 4)
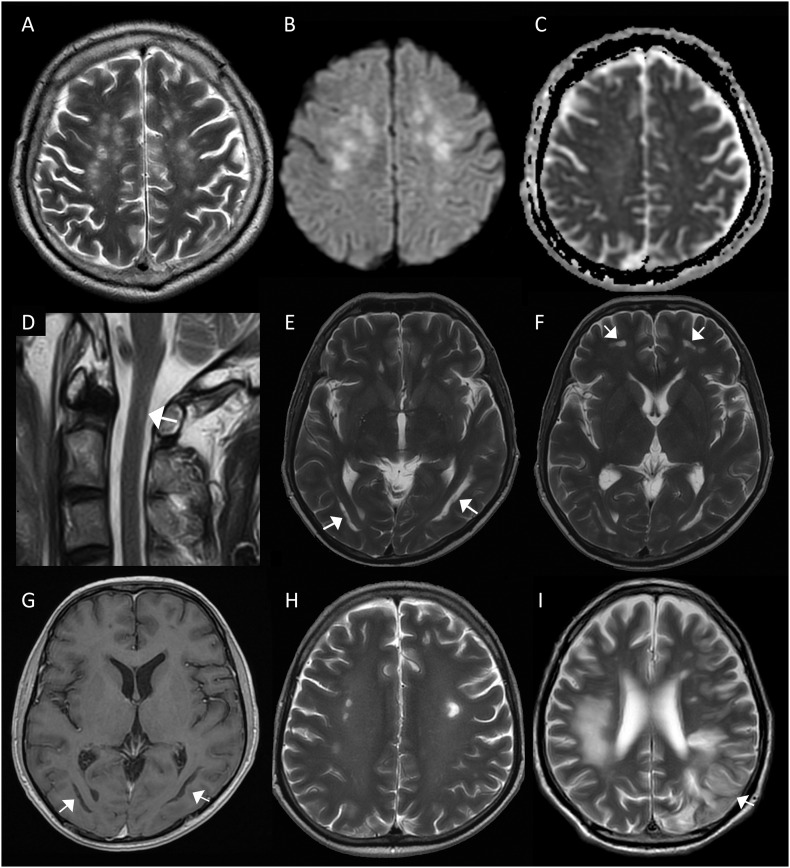
Four critically-ill patients (age 40–73 years, all males) developed severe encephalitis. They were noted to be persistently encephalopathic when weaned-off sedation, at a median interval of 24 (15–65) days into illness, while recovering from critical COVID-19 that was complicated by sepsis, acute respiratory distress syndrome (ARDS) and renal failure. Case 1 was treated for COVID-19 with convalescent plasma, Case 2 and 3 with interferon beta 1b and lopinavir/ritonavir and Case 4 with remdesivir. Case 1's neurology was remarkable for spastic quadriparesis and transient ocular flutter, Case 3 had two episodes of right and left hemiplegia while Cases 2 and 4 had no focal neurological deficit. Case 1's cerebrospinal fluid (CSF) was remarkable for mild pleocytosis (red blood cells 22/μL and white blood cells 6/μl) and raised protein 0.56 g/L, while Case 2's was normal. CSF SARS-CoV-2 RT-PCR was negative in both; viral culture and CSF SARS-CoV-2 serology were negative for Case 1. Cases 3 and 4 did not have CSF examined. MRI brain scans for Cases 1-3 showed multifocal abnormalities predominantly in the cerebral white matter, with varying involvement of the grey matter, brainstem and spinal cord (Fig. 1 in press). Radiological findings were consistent with post-infectious acute disseminated encephalomyelitis (ADEM) for Case 1; likewise Case 4's MRI brain, with multifocal haemorrhagic lesions predominantly in the white matter, suggested acute haemorrhagic leukoencephalitis (AHLE), a severe variant of haemorrhagic ADEM. Cases 2 and 3 were diagnosed as possible encephalitis (see Appendix A Table 1). The four patients also displayed blood markers, e.g. raised D-dimer, fibrinogen and lupus anticoagulant (LAC), of autoimmunity and hypercoagulability. Cases 1, 3 and 4 did not respond significantly to empirical, albeit delayed, therapy with intravenous immunoglobulin (IVIG), corticosteroids and corticosteroid-IVIG combination respectively. Case 2 eventually recovered, after three months of intensive physical therapy, with mild neuropsychiatric deficits in working memory, visuospatial-perceptual and planning abilities. He did not receive any specific immunomodulatory treatment. Case 3 died three months into illness.
Fig. 1.
Neuroimaging in 3 patients with COVID-19 associated central nervous system (CNS) syndromes.
Case 1 – Probable acute disseminated encephalomyelitis (A-D): Axial T2-weighted brain MRI (A) showing discrete hyperintense foci in the deep and subcortical white matter. Axial diffusion weighted imaging (DWI) (B) and apparent diffusion coefficient (ADC) (C) images demonstrating DWI hyperintensity of the lesions without restricted diffusion on ADC maps. Sagittal T2-weighted image of the cervical spine (D) showing a small linear lesion on the right side of the spinal cord at C1 (white arrow).
Case 2 – Possible encephalitis (E-H): Axial T2-weighted images showing (E) bilateral curvilinear high signal paralleling the ventricles and (F) in the inferior frontal white matter (white arrows). Corresponding axial T1-weighted image (G) demonstrating low signal in the white matter lesions (white arrows). Axial T2-weighted image (H) revealing discrete hyperintense lesions in the deep white matter on background of diffuse leukoencephalopathy.
Case 3 – Possible encephalitis (I): Axial T2-weighted MRI (I) showing T2W hyperintensity in the right frontal and parietal deep and subcortical white matter; and an evolving infarct in the left frontal and parietal lobes.
3.2. Cerebral venous thrombosis (n = 4)
Four cases of cerebral venous thrombosis (CVT) occurred in young men (age 27–38 years). Two patients had mild COVID-19 while two were asymptomatic. Only two of the four patients had raised inflammatory and procoagulant markers (Table 1 ). Median interval between onset of COVID-19 and CVT was 2 (0−21) days. All patients had unilateral thrombosis of transverse and sigmoid sinuses. Thrombosis extended into the internal jugular vein in three, one of whom had near complete thrombosis of superior sagittal sinus and another suffered a temporal lobe haematoma. None had risk factors except for one with a concomitant undisplaced occipital bone fracture from an accidental fall. Three received anticoagulation while one was not anticoagulated due to concomitant subdural hematoma. Three patients recovered and one died despite anticoagulation.
Table 1.
Distribution of cerebrovascular disorders, prothrombotic markers and severity of COVID-19 (^Abnormalities of any of the following: D-dimer, anticardiolipin immunoglobulin M/G (IgM/IgG), lupus anticoagulant, beta2-glycoprotein IgM/IgG, fibrinogen, Von-Willebrand factor, Factor VIII, anti-thrombin III, protein S, protein C and homocysteine; *2 missing data).
| Cerebrovascular disorders | Total number (n = 25) | Number of cases with abnormal prothrombotic markers^, n (%) | Number of asymptomatic COVID-19 patients diagnosed by SARS-CoV-2 serology at neurological presentation, n (%) | Number of cases with severe/critical COVID-19, n (%) |
|---|---|---|---|---|
| Cerebral venous thrombosis | 4 | 2 (50) | 1 (25) | 0 (0) |
| Acute ischaemic stroke (AIS) and transient ischaemic attack (TIA) | 19 | 9* (47.4) | 4 (21.1) | 3 (15.8) |
| Radiologically confirmed (MRI brain) acute ischaemic stroke | 14 | 8 (57.1) | 4 (28.6) | 3 (21.4) |
| Cardioembolic | 2 | 1 | ||
| Large vessel | 2 | 2 | ||
| Small vessel | 5 | 2 | ||
| Undetermined | 3 | 3 | ||
| Cryptogenic | 2 | 0 | ||
| Intracerebral haemorrhage | 2 | 1 (50) | 0 (0) | 2 (100) |
3.3. Acute ischaemic stroke and transient ischaemic attack (n = 19)
Out of a total of 19 patients,16 had acute ischaemic stroke (AIS), including two patients with clinical features of stroke and no intracranial bleed on brain CT. MRI brain could not be done for these two patients because of infection control restrictions. Three patients had transient ischaemic attack (TIA). The median age in this group was 52.5 (35–73) years and 18 were males. Twelve of the nineteen patients (63.2%) were asymptomatic for SARS-CoV-2 infection and presented with AIS/TIA as the initial manifestation, eight of whom were diagnosed by swab RT-RCR and four by SARS-CoV-2 serology. Three patients had critical COVID-19 and developed AIS in the intensive care unit. Fifteen of the nineteen patients had pre-existent or newly-diagnosed traditional vascular risk factors. The stroke subtypes of the 14 patients [median age 44.5 (35–73) years, all males] with radiologically-confirmed AIS (with MRI brain) are presented in Table 1. Remarkably, five were small-vessel stroke, two of whom had abnormal prothrombotic markers. Median interval between onset of COVID-19 and all AIS/TIA cases was 34 (0–68) days; and for radiologically-confirmed AIS cases it was 14 (0–55) days. Intravenous thrombolysis was administered to one patient and mechanical thrombectomy was performed for two patients. One patient died.
3.4. Intracerebral haemorrhage (n = 2)
Two men (age 64 and 60 years) had intracerebral haemorrhage (ICH). Both had critical COVID-19 with multi-organ failure requiring extracorporeal membrane oxygenation (ECMO) and abnormal coagulation from therapeutic heparin. The second case also had prothrombotic tendency (Table 1) with raised D-dimer, fibrinogen, Von-Willebrand factor (VWF), factor VIII, LAC and anticardiolipin IgM. Both suffered massive ICH and died.
3.5. Peripheral nervous system disorders (n = 7)
Seven cases developed neuropathy [median age 36 (27–73) years, seven males]. They included unilateral facial neuropathy (n = 5), axonal variant of GBS (n = 1) and small-fibre neuropathy (n = 1). Three had asymptomatic COVID-19. Median interval between onset of COVID-19 and neurological symptoms was 15 (0–37) days. Three of the seven cases (42.9%) improved approximately two weeks after immunotherapy (corticosteroids or plasma exchange), two (28.6%) improved spontaneously while two (28.6%) remained status-quo at last review. GBS was diagnosed in a patient with severe flaccid quadriparesis, approximately one month into COVID-19 illness that required prolonged ventilation. The initial and interval electrodiagnostic features, with characteristic sural-sparing, were indicative of acute motor and sensory axonal neuropathy (AMSAN). Spinal fluid showed cytoalbuminological dissociation. This patient also had thromboembolic ischaemic stroke with positive LAC and anti-cardiolipin IgM. Hence, he was treated with plasma exchange rather than IVIG. He made a good recovery. Another patient developed bilateral feet and asymmetric arm paraesthesia 24 days after onset of COVID-19. Nerve conduction study did not reveal large-fibre polyneuropathy but sympathetic skin responses (SSR) were absent in the lower limbs and delayed in the upper limbs. CSF analysis and SARS-CoV-2 PCR were unremarkable. Other causes (e.g. Sjogren's, autoimmune disorders, multiple myeloma, retroviral disease) were excluded. He was diagnosed with small-fibre neuropathy. His symptoms improved and SSR normalized after a course of symptomatic treatment.
3.6. Dysautonomia (n = 4)
We identified four patients (age 28–47, all males) with COVID-19-associated dysautonomia. Median interval between onset of COVID-19 and dysautonomia was 8 (1-18) days. A middle-aged man, with COVID-19 requiring supplemental oxygen therapy, developed acute ischaemic limb from aortic thrombosis secondary to COVID-19-associated immuno-thrombotic state. He had raised factor VIII, VWF, fibrinogen and LAC. He required prone-positioning and was started on remdesivir. On day 18, he developed acute hyperhidrosis and persistent symptomatic orthostatic tachycardia suggestive of acute dysautonomia (in press). Autonomic function test (AFT) showed marked tachycardia on standing (70 to 105/min after 3 mins) and during passive 60° tilt (67 to 98/min after 5 mins) without a corresponding drop in blood pressure. He improved with fludrocortisone and sodium chloride tablets. On day 74, hyperhidrosis and orthostatic tachycardia had resolved, albeit on medications. Three other patients with mild COVID-19 developed isolated, acute, near vision difficulty with asymmetric accommodation defects within two weeks of illness onset (in press). They had a variety of pupillary abnormalities, namely Argyll Robertson, inverse Argyll Robertson and Adie's pupils. The patient with Adie's pupil had absent ankle and H reflexes; and his AFT revealed postural tachycardia; heart rate rose from 91 to 122 beats/min on standing and from 83 to 114/min during tilt-table test without postural hypotension. Investigations for other causes of acute dysautonomia were unremarkable in all four patients.
3.7. Pre-existent or co-existent conditions likely unrelated to COVID-19 (n = 51)
Fifty-one cases [median age 36 (22–75) years, 46 males] were referred for neurological symptoms, eventually deemed due to either pre-existent or co-existent neurological disorders unrelated to COVID-19 (Appendix B Table 2). All except one had mild or asymptomatic COVID-19.
4. Discussion
We set out to prospectively describe the spectrum of COVID-19 neurology. We recorded 39 patients with neurological complications, out of 47,572 (0.08%) COVID-19 patients. There were no neurological complications reported in 81 paediatric patients. The total number of neurological cases associated with COVID-19 was small compared to other studies [4,6,10]. This might be related to the peculiar demographics of the local outbreak that involved young healthy migrant workers in large crowded dormitories, many of whom were asymptomatic or had mild infection. It is also largely perceived that the pandemic did not overwhelm Singapore's healthcare system.
The neurological disorders of COVID-19 patients fell into four categories with variable characteristics with regards to severity of COVID-19, patient demographic, latency, and differing certainty of association with COVID-19. The underlying pathophysiology appears to be dysimmunity and/or prothrombotic tendency. A recently-published study [7] proposed five categories of COVID-19-related neurological manifestations: 1. Encephalopathy, 2. Inflammatory central nervous system (CNS) syndromes, 3. Ischaemic stroke, 4. PNS syndromes and 5. Miscellaneous disorders. We expanded it to reflect the spectrum of manifestations seen in our cohort (Table 2 ) and ascribed certainty of association with COVID-19 for these conditions based on standardized case definitions (see Appendix A Table 1) [16]. We have combined their categories 1 and 2 [7] into one CNS category, as the distinction between the two is predicated upon the availability of detailed imaging and spinal fluid examination, both of which may be restricted by infection control rules and limited resources in under-resourced settings where the pandemic is taking its toll. As illustrated by our cases, it would be prudent to consider ‘CNS syndromes’ or ‘Cerebrovascular disorders’ (expanded to include CVT and ICH), in patients who appear to be disproportionately drowsy for their critical illness and dose of sedation. We have also introduced a novel category - ‘Autonomic nervous system’ for a group of patients with limited dysautonomia that we believe represents a distinct neurological manifestation of COVID-19.
Table 2.
Classification of neurological manifestations in COVID-19 (^One patient had Guillain-Barré syndrome and acute ischaemic stroke; ECMO: Extracorporeal membrane oxygenation).
| Category | Definition [16] | Number of cases (n = 39; 1 patient with 2 diagnoses^) | Interval between onset of COVID-19 and neurological disease, median (range), days |
|---|---|---|---|
| Central nervous system syndromes | Acute disseminated encephalomyelitis, encephalitis | 4 | 24 (15–65) |
| Cerebrovascular disorders | Acute ischaemic stroke and transient ischaemic attack; cerebral venous thrombosis and intracerebral haemorrhage | 25 | Acute ischaemic stroke and transient ischaemic attack: 34 (0–68) Cerebral venous thrombosis: 2 (0–21) Intracerebral haemorrhage related to ECMO:17 (13-21) |
| Peripheral nervous system | Mono- and polyneuropathies | 7^ | 15 (0–37) |
| Autonomic nervous system | Limited dysautonomia | 4 | 8 (1–18) |
To date there have only been a few convincing reports of SARS-CoV-2 encephalitis, including isolation of viral fragments in the CNS [13,15,23]. The main pathological findings in an autopsy series of 18 patients were hypoxic changes with no specific features of encephalitis or viral inclusions. On the other hand, the emerging reports of ADEM [17,18] and acute necrotizing encephalopathy (ANE) [13], the presence of LAC, and good response to immunomodulatory treatment [13,14] support a dysimmune-inflammatory pathophysiology. Cases 1 and 4 [24] were suggestive of ADEM and AHLE (a severe variant in ADEM spectrum) respectively. However, we could not completely discount the possibility of a direct viral effect, especially in Cases 2 and 3. Association with COVID-19, based on case definitions by Ellul et al. [16],was ‘probable’ in all cases. The median interval of 24 (15–65) days between onset of COVID-19 and neurological symptom suggests that encephalitis is a delayed phenomenon occurring at least two weeks after onset of ARI and while patients were critically-ill. The lack of efficacious antiviral therapy against SARS-CoV-2 and the presence of severe concurrent nosocomial infections made the decision for timely empirical immunomodulatory therapy challenging. Co-morbidities associated with critical illness and delay in investigations such as MRI brain because of infection control restrictions confounded diagnosis. Overall, we are not able to give specific recommendations with regards to the type and timing of immunomodulatory treatments.
We observed two key findings in patients with cerebrovascular disorders. Firstly, 12/19 (63.2%) of all AIS/TIA occurred in patients with asymptomatic COVID-19, compared to approximately 14% from published data [16]. Secondly, we recorded a higher ratio of CVT to AIS/TIA (approximately 1:5). CVT incidence in our cohort is approximately four times higher than that seen in previous studies (6.7 per 100,000 vs 1.6 per 100,000) [25,26]. Of the four CVT cases, two presented within a few days of mild COVID-19 while two were asymptomatic. Association with COVID-19 [16] was ‘probable’ in three and ‘possible’ in one with skull fracture [27]. A systematic review (manuscript under review) revealed 15 cases of CVT occurring unexpectedly in previously well, middle-aged men with COVID-19. Unlike our patients, about 1/3 occurred in patients with critical COVID-19, whilst 5.4% were asymptomatic. Furthermore, in our study, one of the four CVT and 4/19 AIS/TIA patients' COVID-19 was diagnosed by serology. In addition to increased vigilance for CVT and AIS/TIA in COVID-19 patients, we support testing for COVID-19 with swab RT-PCR and serology in patients who develop unprovoked CVT and AIS/TIA, including those who are asymptomatic for ARI.
The median age of AIS/TIA in COVID-19 patients was lower than that reported (52.5 vs 69.1 years) in the Singapore Stroke Registry [28], likely contributed by the young profile of COVID-19 patients in Singapore. Of the 19 AIS/TIA, four patients were classified as ‘probable’ COVID-19 associated, while fifteen were graded as ‘possible’, because of the presence of pre-existent or newly-diagnosed traditional vascular risk factors [16]. Remarkably, we also encountered five small-vessel stroke (two of whom had prothombotic factors, Table 1), in contrast to the predominantly large vessel stroke reported in other studies [5,11,29,30]. Thrombotic tendency of COVID-19 arising from SARS-CoV-2-induced inflammation and endothelial dysfunction [31] is likely contributory, although we detected commonly screened prothrombotic markers in only 2/4 ‘probable’ and 7/15 ‘possible’ cases. Likewise, only 2/4 CVT cases had demonstrable prothrombotic markers (Table 1).
The two ICH cases were likely ECMO-related, hence the association with COVID-19 was graded as ‘possible’ [16]. In a pooled review of COVID-19 ARDS patients, the mortality rate was higher in those who underwent ECMO (94.1% versus 70.9%). As in one of our cases, the excess mortality may be related to COVID-19 dysimmune-coagulopathy [32].
Association of the seven PNS disorders with COVID-19 was ranked ‘probable’ [16]. The median interval between onset of COVID-19 and neurological symptom of 15 (0–37) days, and improvement following immunomodulatory treatment suggested para-infectious dysimmunity. Isolated facial neuropathy (5/7, 71.4%) was the most common COVID-associated mononeuropathy in our cohort. To date, there have only been few reports of facial neuropathy in COVID-19 patients [8]. Spinal fluid SARS-CoV-2 viral RT-PCR was negative in one of our patients with facial neuropathy [8]. Recovery ranged from one week to few months, similar to classic Bell's palsy. In contrast to reports of increased incidence of GBS during the pandemic [20], we encountered only one case in a COVID-19 patient. Nonetheless, we emphasize the therapeutic importance of considering GBS as a differential diagnosis of critical illness neuromyopathy in COVID-19 patients who develop flaccid weakness. The case of acute small-fibre neuropathy might be part of the spectrum of dysautonomia elaborated below.
COVID-19 associated dysautonomia was a novel finding in our study. One patient with hyperhidrosis and orthostatic tachycardia, and three others with ocular dysautonomia; occurring at median 8 days from COVID-19 onset. We posit COVID-19 as a cause of dysimmune, limited autonomic dysfunction, similar to post-infectious autonomic neuropathy seen in diphtheria. Accommodation difficulty, inverse Argyll-Robertson pupil and delayed, mainly cardiovagal autonomic dysfunction, as well as hyperhidrosis have been reported in diphtheria patients [33,34], where the pathology is post-infectious segmental demyelination [35]. As expected in dysimmune aetiology, this complication did not correlate with the severity of COVID-19. Dysautonomia was reported in 21 out of 841 COVID-19 patients in the ALBACOVID registry [10]. No details of their autonomic dysfunction were given but the authors also suggested dysimmune pathology.
We recorded 51 other cases with pre- or co-existent neurological disorders unrelated to SARS-CoV-2 (Appendix B Table 2). Cognizant that COVID-19 is a novel disorder, we were wary of misattributing causality [16,36]. These cases were adjudicated unrelated to SARS-CoV-2 after scrutiny of the temporal relationship of their symptoms to COVID-19 and likelihood of alternative aetiology. Seizure, headache, PNS disorders and functional neurological disorders (FND) were the main conditions. Similar to other cohorts [4,6,10], headache was not uncommon in our study. In addition, we saw eight patients with FND. While direct effects of the infection cannot be separated from psychosocial impact of the pandemic on underlying or reactive psychopathology, we believe prolonged isolation played an important role. Furthermore, we anticipated pandemic-induced disruption of medical services to increase unrelated neurological disorders and exaggerate the neurological morbidity of COVID-19. However, this accounted for only two patients - one with relapsed manic depressive disorder and another with epilepsy.
A recent editorial called for a better understanding of COVID-19 neurology [36]. A major strength of our study is the inclusion of only microbiologically-confirmed COVID-19 cases. It is also a comprehensive nationwide survey that included all COVID-19 patients in Singapore and prospectively analysed all their neurological complaints. Furthermore, extensive surveillance and case-finding of COVID-19 in Singapore allows us to be confident of the denominator against which the 39 neurological complications are contextualised. We acknowledge the following limitations. Our attribution of neurological complications to COVID-19, while based on standardized case definitions [16], was not guided by specific pathological evidence. Restrictions from infection and movement control limited the evaluation of some patients. There was also referral bias. Although all COVID-19 patients in Singapore were isolated in hospitals or community care facilities that came under the care of the authors' institutions, some cases were likely missed. A number of patients with neurological disorders, presenting with symptoms such as encephalopathy and behavioural problems, may not have been referred by their primary physicians because they were deemed to have apparent non-neurological causes. There is also a tendency for clinicians to flag out more serious conditions and disorders already perceived to be related to COVID-19. Nevertheless, we believe we have captured the majority of neurological disorders occurring in this cohort of COVID-19 patients during the study period. Finally, the generalizability of our study is limited by the disproportionate number of young healthy males in our cohort.
5. Conclusion
COVID-19 neurology has a wide spectrum of distinct dysimmune-thrombotic disorders that can be divided into four groups. ADEM and encephalitis are delayed phenomena in critical COVID-19. Dysautonomia occurred relatively early and largely in mild infections. CVT was more frequent than expected and seen in mild and asymptomatic COVID-19. AIS/TIA was variable in onset and occurred in patients with differing COVID-19 severity, including 63.2% who were asymptomatic. It is widely perceived that the pandemic did not unduly affect the Singapore healthcare system. This, and the disproportionate number of young healthy males with mild infection, explain the small number of neurological disorders in our cohort.
Authors' contribution
JSK, HJC, KT, TU: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content, study supervision.
DADS, AMLQ, TMT, CYHS, RHMH, MS, ACH, JA, JRK, MHY, YH, WYY, TCCL, BYQT, KWPN, LLLY, YZP, KMP, AA, TT: Acquisition of data, analysis and interpretation of data, critical revision of manuscript for important intellectual content.
DCBL: Study supervision.
All authors approved the final version of the manuscript.
Funding
None. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Singapore Health Services institutional review board (CIRB 2020/2410). Waiver of consent was granted by the Singapore Health Services institutional review board.
Data availability statement
Data is available upon reasonable request. All data relevant to the study are included in the article and appendices. Patient-related data will be shared upon request from any qualified investigator, maintaining anonymisation of the individual patients.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thank Dr Matthias Paul Toh Han Sim (National Centre for Infectious Diseases) and Ms Ang Li Wei (National Centre for Infectious Diseases) for their help with the Singapore COVID-19 statistics.
Appendix A. Table 1
Diagnostic criteria and case definitions for the association of neurological disorders with COVID-19 (MRI: Magnetic resonance imaging; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; PCR: Polymerase chain reaction; CSF: Cerebrospinal fluid; CNS: Central nervous system; WHO: World Health Organization) (adapted from Ellul's et. al) [9].
| Diagnosis | Diagnostic Criteria (1–8) | Case definitions for association of neurological disorders to COVID-19, Ellul's et al. (9) |
|---|---|---|
| Acute disseminated encephalomyelitis (ADEM) | Probable: a) Unifocal or multifocal clinical central nervous system event OR b) Alteration in consciousness or behavioural change unexplained by fever/systemic illness/postictal symptoms AND c) Abnormal brain MRI with typical diffuse, poorly demarcated lesions >1 cm Definite: Any one of the following in addition to fulfilling a-c: absence of new clinical or MRI findings 3 months or more after symptom onset, signs/symptoms/MRI findings consistent with multiphasic ADEM |
Probable: (1) Neurological disease onset within 6 weeks of COVID-19 and (2) either SARS-CoV-2 PCR or positive serology detected in any sample suggestive of acute infection; (3) no evidence of other commonly associated causes Possible: (1) Neurological disease onset within 6 weeks of COVID-19 and (2) either SARS-CoV-2 PCR or positive serology detected in any sample suggestive of acute infection; (3) evidence of other commonly associated causes |
| Encephalitis | Possible: a) Acute or sub-acute (<4 weeks) alteration in consciousness, cognition (including delirium/coma), personality or behaviour persisting for more than 24 h AND b) Absence of an alternative diagnosis for symptoms AND c) Any one of the following: New focal neurological signs, new onset seizure, new movement disorders, fever, electroencephalogram with focal abnormalities Definite: Any one of the following in addition to fulfilling a-c: CSF total white cell count >5 cells/mm3, neuroimaging compatible with encephalitis, confirmation of brain inflammation on brain biopsy |
Definite: (1) SARS-CoV-2 detected in CSF or brain tissue or evidence of SARS-CoV-2-specific intrathecal antibody; and (2) no other explanatory pathogen or cause found Probable: (1) SARS-CoV-2 detected in respiratory or other non-CNS sample, or evidence of SARS-CoV-2-specific antibody in serum indicating acute infection; and (2) no other explanatory pathogen or cause found Possible: (1) Suspected case definition of COVID-19 based on WHO criteria; and (2) no other explanatory pathogen or cause found |
| Cerebral venous thrombosis | Infarction or haemorrhage in the brain, spinal cord, or retina because of thrombosis of a cerebral venous structure. Symptoms or signs caused by reversible edema without infarction or haemorrhage do not qualify as stroke. |
Probable: (1) Either SARS-CoV-2 detected in CSF or other sample, or evidence of SARS-CoV-2-specific antibody in serum indicating acute infection; and (2) no other known traditional risk factors Possible: (1) Either SARS-CoV-2 detected in CSF or other sample, or evidence of SARS-CoV-2-specific antibody indicating acute infection; and (2) other traditional risk factors |
| Ischaemic stroke | An episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction. | |
| Transient ischemic attack | A transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction | |
| Intracerebral haemorrhage | Rapidly developing clinical signs of neurological dysfunction attributable to a focal collection of blood within the brain parenchyma or ventricular system that is not caused by trauma. |
|
| Peripheral nerve disorders | Guillain-Barre syndrome and other other mono- and poly-neuropathies based on clinical and/or supportive electrodiagnostic features. | Probable: (1) Neurological disease onset within 6 weeks of COVID-19 and (2) either SARS-CoV-2 PCR or positive serology detected in any sample suggestive of acute infection; (3) no evidence of other commonly associated causes Possible: (1) Neurological disease onset within 6 weeks of COVID-19 and (2) either SARS-CoV-2 PCR or positive serology detected in any sample suggestive of acute infection; (3) evidence of other commonly associated causes |
| Dysautonomic disorders | Dysautonomia based on clinical and/or supportive electrodiagnostic features. |
Appendix A Table 1 references
1. Venkatesan A, Tunkel AR, Bloch KC, et al. Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin Infect Dis 2013; 57:1114–28.
2. Granerod J, Ambrose HE, Davies NWS, et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect Dis 2010. 10 [12]:835–44.
3. Sejvar JJ, Kohl KS, Bilynsky R, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007. 25 [31]:5771–92.
4. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barr. syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011. 29 [3]:599–612.
5. Yuki N, Hartung HP. Guillain-Barré syndrome N Engl J Med. 2012;366 [24]:2294–2304. doi:https://doi.org/10.1056/NEJMra1114525.
6. Sacco RL, Kasner SE, Broderick JP, et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013;44:2064–89.
7. Easton JD, Saver JL, Albers GW, et al. Definition and Evaluation of Transient Ischemic Attack. Stroke 2009;40:2276–93.
8. Dineen J, Freeman R. Autonomic Neuropathy. Semin Neurol. 2015;35 [4]:458–468.doi:https://doi.org/10.1055/s-0035-1,558,983.
9. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19 Lancet Neurol. 2020;S1474–4422(20)30221–0.doi:https://doi.org/10.1016/S1474-4422(20)30221-0.
Appendix B. Table 2
Pre- or co-existent neurological conditions unrelated to COVID-19.
(*Diagnosis was based on clinical, laboratory, electrodiagnostic and radiological data where applicable)
| Category (n = number of cases) | Diagnosis* |
|---|---|
| Headache (n = 11) | Primary headache (migraine, tension-type headache, cluster headache, thunderclap headache) |
| Others (non-specific, post-traumatic headache) | |
| Dizziness (n = 3) | Benign paroxysmal positional vertigo |
| Poor oral intake | |
| Vasovagal syncope | |
| Seizures (n = 9) | Focal, symptomatic (cystic brain lesions due to neurocysticercosis versus tuberculoma) |
| Traumatic head injury | |
| Unknown cause | |
| Epilepsy | |
| Stroke (n = 3) | Subacute ischaemic stroke |
| Intracranial haemorrhage due to hypertension | |
| Peripheral nervous system disorders (n = 10) | Entrapment neuropathies (ulnar neuropathy at elbow, peroneal neuropathy at fibular neck, bilateral meralgia paraesthetica from prolonged prone positioning for respiratory failure, sciatic neuropathy) |
| Polyneuropathy (Vitamin B12 deficiency, diabetes neuropathy) | |
| Mononeuritis multiplex from tuberculoid leprosy reactivation | |
| Lumbosacral or cervical radiculopathy | |
| Hirayama disease | |
| Functional neurological disorder (weakness, speech and comprehension difficulty, non-specific dizziness and numbness) (n = 8) | Anxiety, adjustment disorder secondary to prolonged isolation during COVID-19 pandemic |
| Others (n = 7) | Drug-induced dystonia, referred facial pain from tooth abscess, manic-depression relapse secondary to cessation of psychiatric medications, toxic-metabolic encephalopathy, right eye coloboma, non-specific musculoskeletal complaints |
References
- 1.Umapathi T., Kor A.C., Venketasubramanian N. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J. Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prerna A., Lim J.Y.X., Tan N.W.H. Neurology of the H1N1 pandemic in Singapore: a nationwide case series of children and adults. J. Neuro-Oncol. 2015;21(5):491–499. doi: 10.1007/s13365-015-0341-3. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; 2020. The Coronavirus Disease 2019 (COVID-19):Situation Report-36. [Google Scholar]
- 4.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the Young. N. Engl. J. Med. 2020;82:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro E., Priori A., Beghi E. The international EAN survey on neurological symptoms in patients with COVID-19 infection. Eur. J. Neurol. 2020:0–3. doi: 10.1111/ene.14407. [Internet]. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson R.W., Brown R.L., Benjamin L. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020:2020):2–37. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goh Y., Beh D.L.L., Makmur A. Pearls and Oy-sters: facial nerve palsy as a neurological manifestation of Covid-19 infection. Neurology. 2020 doi: 10.1212/WNL.0000000000009863. [DOI] [PubMed] [Google Scholar]
- 9.Sotoca J., Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5):1–4. doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garaci F., Di Giuliano F., Picchi E. Venous cerebral thrombosis in COVID-19 patient. J. Neurol. Sci. 2020;414 doi: 10.1016/j.jns.2020.116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virhammar J., Kumlien E., Fällmar D. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020 doi: 10.1212/WNL.0000000000010250. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poyiadji N., Shahin G., Noujaim D. COVID-19–associated acute Hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriguchi T., Harii N., Goto J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [Internet]. (March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellul M., Benjamin L., Singh B. Neurological Associations of COVID-19. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30221-0. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichard R.R., Kashani K.B., Boire N.A. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons T., Banks S., Bae C. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020;0123456789:3–6. doi: 10.1007/s00415-020-09951-9. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H., Shen D., Zhou H. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gigli G.L., Bax F., Marini A. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J. Neurol. 2020;0123456789:1–3. doi: 10.1007/s00415-020-09911-3. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 23.Solomon I.H., Normandin E., Bhattacharyya S. Neuropathological features of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong M.H., Chan F.Z.Y., Liu J. A rare case of acute hemorrhagic leukoencephalitis in a COVID-19 patient. J. Neurol. Sci. Neurol. 2020 doi: 10.1016/j.jns.2020.117035. (in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devasagayam S., Wyatt B., Leyden J. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke. 2016;47(9):2180–2182. doi: 10.1161/STROKEAHA.116.013617. [DOI] [PubMed] [Google Scholar]
- 26.Venketasubramanian N., Yoon B.W., Pandian J. Stroke epidemiology in south, east, and south-east asia: a review. J. Stroke. 2017;19(3):286–294. doi: 10.5853/jos.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabavizadeh S., Mowla A., Bress A. Thrombosis of posterior condylar vein with extension to internal jugular vein; a rare radiological finding in traumatic brain injury. Surg. Neurol. Int. 2015 doi: 10.4103/2152-7806.156157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NRDO NR of DO Singapore stroke registry annual report 2017. Singapore Stroke Regist. 2018:1–66. [Internet] [Google Scholar]
- 29.Merkler A.E., Parikh N.S., Mir S. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;2019:1–7. doi: 10.1001/jamaneurol.2020.2730. [Internet. (doi:https://jamanetwork.com/journals/jamaneurology/fullarticle/276809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaghi S., Ishida K., Torres J. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. (July):2002–11) PMID: 32432996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry B.M., Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J. Crit. Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idiaquez J. Autonomic dysfunction in diphtheritic neuropathy. J. Neurol. Neurosurg. Psychiatry. 1992;55(2):159–161. doi: 10.1136/jnnp.55.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piradov M.A., Pirogov V.N., Popova L.M. Diphtheritic polyneuropathy: clinical analysis of severe forms. Arch. Neurol. 2001;58(9):1438–1442. doi: 10.1001/archneur.58.9.1438. [DOI] [PubMed] [Google Scholar]
- 35.Fisher M., Adams R.D. Diphtheritic polyneuritis-a pathological study. J. Neuropathol. Exp. Neurol. 1956;15(3):243–268. doi: 10.1097/00005072-195607000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ellul M., Varatharaj A., Nicholson T.R. Defining causality in COVID-19 and neurological disorders. J. Neurol. Neurosurg. Psychiatry. 2020;91(8):811–812. doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon reasonable request. All data relevant to the study are included in the article and appendices. Patient-related data will be shared upon request from any qualified investigator, maintaining anonymisation of the individual patients.