Abstract
Current cancer immunotherapy has limited response rates in a large variety of solid tumors partly due to the low immunogenicity of the tumor cells and the immunosuppressive tumor microenvironment (ITM). A number of clinical cancer treatment modalities, including radiotherapy, chemotherapy, photothermal and photodynamic therapy, have been shown to elicit immunogenicity by inducing immunogenic cell death (ICD). However, ICD-based immunotherapy is restricted by the ITM limiting its efficacy in eliciting a long-term antitumor immune response, and by severe systemic toxicity. To address these challenges, nanomedicine-based drug delivery strategies have been exploited for improving cancer immunotherapy by boosting ICD of the tumor cells. Nanosized drug delivery systems are promising for increasing drug accumulation at the tumor site and codelivering ICD inducers and immune inhibitors to simultaneously elicit the immune response and relieve the ITM. This review highlights the recent advances in nanomedicine-based immunotherapy utilizing ICD-based approaches. A perspective on the clinical translation of nanomedicine-based cancer immunotherapy is also provided.
Keywords: cancer immunotherapy, immunogenic cell death, immunosuppressive tumor microenvironment, nanomedicine, drug delivery systems
Introduction
Cancer immunotherapy has changed the paradigm of clinical cancer therapy by demonstrating that boosting the systemic immune response can lead to tumor regression [1, 2]. Despite the potential of immunotherapy to cure malignant tumors, the clinical application of current cancer immunotherapy has been hampered by several challenges, including insufficient intratumoral infiltration of cytotoxic T lymphocytes (CTLs) and the immunosuppressive tumor microenvironment (ITM) [3, 4]. Solid tumors with low immunogenicity are defined as “cold tumors,” and they differ from solid tumors with high immunogenicity, which are termed “hot tumors” [5]. Immune checkpoint blockade (ICB) therapy has been extensively investigated to overcome ITM [6, 7], while many other therapeutic modalities, including cancer vaccines, cytokine therapy and adoptive T-cell transfer therapy, have also been exploited for cancer immunotherapy [8–10].
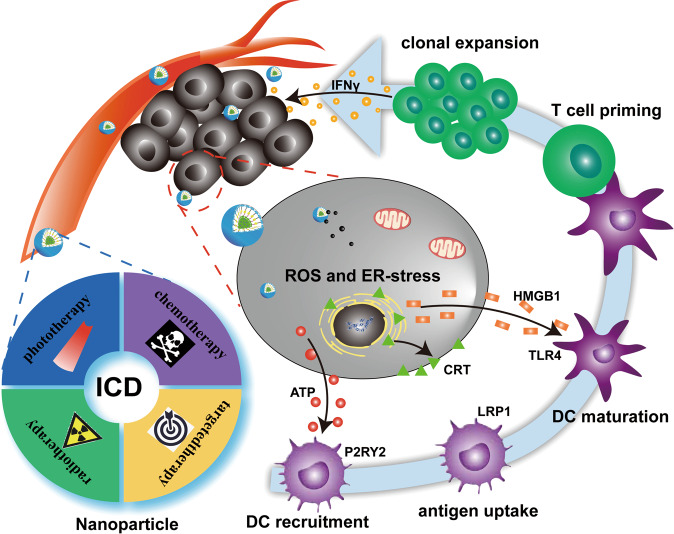
Immunogenic cell death (ICD) is an alternative approach that activates antitumor immunity to eradicate tumor cells [11]. Tumor cells undergoing ICD secrete distress signals through molecules called damage-associated molecular patterns (DAMPs), including calreticulin (CRT), adenosine triphosphate (ATP) and high-mobility group box 1 (HMGB1), to activate the immune response [12–14]. These DAMPs facilitate the cancer immunity cycle. For instance, the presentation of CRT, an endoplasmic reticulum (ER) chaperone, on the tumor cell surface, along with ATP release, induce antigen presenting cells (APCs), including dendritic cells (DCs), to phagocytose tumor cells [15]. In addition, the release of HMGB1 from dying tumor cells promotes interactions of toll-like receptor 4 (TLR-4), a pattern recognition receptor that signals through the TLR-4-MyD88 axis on DCs, that elicit potent immunostimulatory effects [16]. Exogenous HMGB1 also facilitates the maturation of DCs, enabling their trafficking to the lymphnodes for antigen presentation and T cells priming [17]. Thereafter, activated CTLs recognize tumor cells and induce tumor regression by secreting cytokines, including interferon-γ (IFN-γ), tumor necrosis factor-α and interleukin-6 [18–21] (Fig. 1).
Fig. 1. Schematic of nanoparticle-mediated ICD for synergistic cancer immunotherapy.
After overcoming pathophysiological barriers, multifunctional nanoparticles composed of ICD inducers and immune inhibitors induce tumor cell death via immunogenic pathways. The DAMPs released by dying cells play a crucial role in modulating the immunogenicity of the tumor microenvironment, including promoting the recruitment of APCs through tumor cell-derived secreted ATP and HMGB1 and facilitating DC maturation. Subsequently, activated DCs prime T cells, enabling CTLs to kill tumor cells via IFN-γ-dependent mechanisms.
Recently, it was demonstrated that chemotherapeutics (e.g., oxaliplatin (OXA), doxorubicin (DOX), and mitoxantrone) and tyrosine kinase inhibitors, such as crizotinib elicit antitumor immunity by inducing the immunogenic death of tumor cells [22–26]. Furthermore, several physical stimulis also induce ICD. For example, photodynamic therapy (PDT), irradiation and photothermal therapy (PTT) trigger reactive oxygen species (ROS) generation to induce the intracellular ER stress that initiates the ICD cascade and DAMPs release [27–30].
Despite the promising potential of ICD-based approaches for cancer therapy, inadequate induction of ICD by conventional approaches has hampered clinical translation. Shortcomings of conventional approaches include insufficient circulation time, limited intratumoral accumulation and retention, restricted tumor cellular uptake and slow release in tumor cells [31, 32]. Moreover, on-target but off-tumor toxicity of the ICD inducers has also limited the clinical translation of ICD-based cancer therapy approaches [33, 34].
To overcome these challenges with conventional ICD approaches, nanoparticle-based drug delivery systems with tunable size and surface properties offer unique advantages for ICD-based cancer therapy (Fig. 1). Nanoparticles enable targeted drug accumulation at the tumor site, enhance tissue penetration and increase cellular uptake, as well as trigger the tailored release of ICD inducers [35–40]. Furthermore, nanoparticle-based drug delivery systems may also deliver other immunomodulatory agents to diminish the ITM [41]. Thus, nanoparticle-based ICD approaches offer the potential of enhancing ICD and reversing the ITM in a controllable manner.
This review discusses recent advances in nanoparticle-mediated ICD and focuses on the effects induced by certain drugs and physical stimuli. Novel insights into engineering nanoparticles to enhance their antitumor efficacy utilizing a combination of ICD and ITM reprogramming are also provided.
Therapeutic modalities that induce ICD
Chemotherapy
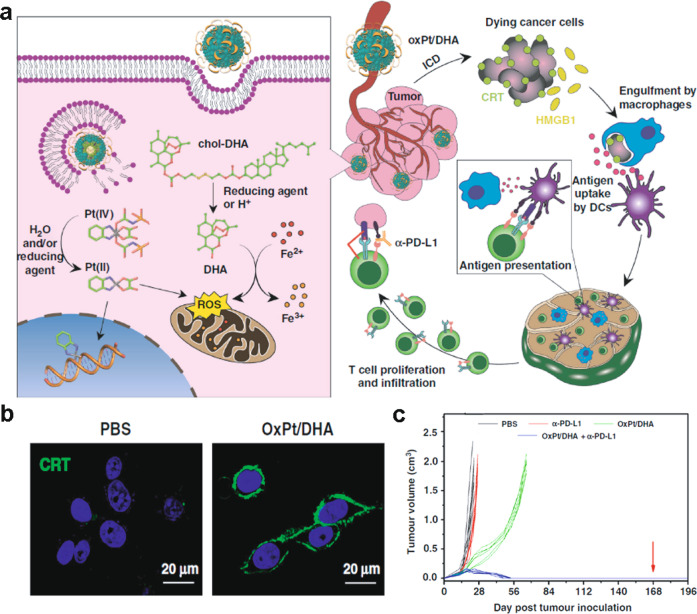
Chemotherapy is a main stream clinical cancer treatment, and recent evidence suggests that several chemotherapeutics have the capacity to induce ICD. Nevertheless, the potency of the ICD effect is reduced because of insufficient tumor accumulation and/or adverse nonspecific cytotoxic effects [42]. Systemic delivery of chemotherapies with nanosized drug delivery systems is a promising strategy to address these challenges because they have advantages in terms of sufficient circulation time, increased intratumoral accumulation and retention, highly efficient uptake by tumor cells and precise release at tumor tissues, compared with these features of chemotherapeutic agents administered alone. For instance, OXA encapsulated in a self-assembling methoxy poly(ethylene glycol)-block-poly(D, L-lactide-co-glycolide) (mPEG-b-PLGA) nanocarrier enhanced the induction of ICD to a greater extent than did OXA delivered alone via the promotion of DAMPs, enhanced maturation of DCs and elevated levels of intratumor infiltrating CTLs [43]. In addition, OXA encapsulated in mPEG-b-PLGA was more effective in inhibiting tumor growth in both immunocompetent and immunodeficient mice than was OXA. Similar results were also reported for OXA-loaded iron oxide nanoparticles that induced ICD by increasing ER stress. Self-assembled nanoscale coordination polymer (NCP) core-shell nanoparticles containing OXA in the core and dihydroartemesinin (DHA) in the shell conjugated to cholesterol via disulfide bonds were cleaved by glutathione (GSH) in tumor cells to release the chemotherapeutic and exert ICD effects (Fig. 2a) [44]. Both OXA and DHA induce oxidative stress (Fig. 2b) and were found to synergistically enhance these effects through delivered OXA-loaded NCP nanoparticles. In addition, these particles promoted intratumoral infiltration of CTLs and activated the immune system for tumor regression (Fig. 2c).
Fig. 2. Schematic of OXA-loaded DHA NCPs (OxPt/DHAs) with synergistic antitumor activity by inducing ICD.
a Chemical structure and synergistic mechanism of tumor inhibition; b Confocal images of cell surface localization of CRT upon exposure to OxPt/DHAs; c. Tumor growth over time with OxPt/DHAs or combination therapy with checkpoint blockade (anti-PD-L1) in a mouse model of CT26 colorectal tumor. Adapted with permission from [44]. Copyright (2019) Nature Publishing Group.
DOX has been investigated for the ICD induction of tumor cells [45]. For example, chimeric polypeptide-conjugated DOX nanoparticles promoted IFN-γ secretion, facilitated the intratumoral infiltration of CD8+ T cells by ICD induction and more efficiently reduced tumor size than did DOX administered alone [46]. In addition to OXA and DOX, mitoxantrone has also been reported to activate antigen release via the ICD cascade [47]. A promising strategy involves combining chemotherapeutic drugs with immune adjuvants to enhance host-specific antitumor immunity. Among the various adjuvants, CpG oligonucleotides, which function as TLR-9 agonists, have been widely investigated for immunotherapy applications [48]. For example, CpG-loaded lipid-polymer hybrid nanoparticles containing mitoxantrone induced an antigen-specific CD8+ T cell response with a higher level of ICD than did mitoxantrone administered alone and significantly inhibited tumor growth in a CT26 tumor model [49].
In addition to CpG oligonucleotides, interleukin-2 (IL-2) is an immune adjuvant used for activating CTLs and natural killer (NK) cells [50]. Thermosponge nanoparticles (TSNs) composed of mPEG-PLGA loaded with paclitaxel (PTX) in the core and IL-2 in the shell (PTX/IL-2-TSNs) reduced tumor growth and metastasis in a murine melanoma model to a greater extent than did either TSNs loaded with PTX or IL-2, demonstrating the benefit of combining chemotherapy with an immune adjuvant [51].
Radiotherapy
Radiotherapy is another main stream method to treat local solid tumors by destroying DNA double strands [52]. Moreover, several studies have demonstrated that radiotherapy induces the release of tumor-associated antigens (TAAs) that trigger antitumor immune responses [53, 54]. For example, PLGA-based core-shell nanoparticles have been shown to enhance the efficacy of radiotherapy [55]. A double emulsion strategy was used to encapsulate catalase (Cat) inside the aqueous core and to integrate imiquimod (also known as R837, a potent TLR-7 agonist) into the hydrophobic shell of PLGA (PLGA-R837@Cat). Cat was incorporated because it decomposes H2O2 into H2O and O2 to reduce hypoxia-associated radiation resistance. PLGA-R837@Cat induced ICD after X-ray irradiation in a CT26 murine tumor model, as demonstrated by the cell surface localization of CRT and increased DC maturation. These nanoparticles, in combination with X-rays, reduced the size of the primary tumors. In addition, the combination of PLGA-R837@Cat with clinically approved CTLA-4 checkpoint blockade therapy inhibited tumor metastasis and prevented cancer recurrence.
PTT
PTT utilizes a photoabsorber to convert photoenergy into thermal energy upon laser irradiation [56]. The hyperthermic effect induced by PTT destroys tumor cells by disrupting the cell membrane, denaturing proteins and inducing DNA damage [57]. In addition, PTT induces immune responses by releasing TAAs and other immunostimulatory molecules [58, 59]. To take advantage of PTT-induced ICD, Prussian blue nanoparticles combined with PTT administered at temperatures between 63.3 and 66.4 °C induced a robust ICD-mediated antitumor effect and long-term survival in a neuroblastoma tumor model [60]. In addition, magnetic iron oxide nanoparticles coated with myeloid-derived suppressor cell membrane (MNPs@MDSC) have been reported to accumulate in tumor tissues and act as PTT agents to induce local hyperthermia and trigger ICD, as demonstrated by increased tumor cell surface CRT expression and delayed tumor growth [61]. PTT-based cancer immunotherapy is limited by reduced laser penetration, insufficient activation of the ICD effect and an extant ITM. To address these challenges, extensive efforts have been made to boost PTT-based ICD. For instance, Ma et al. utilized near-infrared II (NIR-II) light with high tissue penetration ability to promote PPT-mediated cancer immunotherapy. An in vivo antitumor study demonstrated that NIR-II laser-based PTT-induced DAMP release in deep tumor tissue, eliciting DC maturation at high efficiency, and facilitated intratumoral infiltration of CTLs [62].
PDT
PDT destroys tumors by a combination of light, oxygen and photosensitizers (PS) due to the generation of highly toxic ROS [63]. Once activated by a laser emitting a specific wavelength, the generated cellular ROS inhibited tumor growth by destroying the vasculature within the tumor, specifically by damaging organelles and promoting the secretion of proinflammatory cytokines. PDT has been shown to enhance tumor immunogenicity by inducing ICD via the upregulation of tumor cell surface antigens and activation of CTLs [64]. Hence, PDT is a promising therapeutic modality for eliminating residual tumor cells [65]. However, PSs are generally hydrophobic molecules, and therefore, their photoactivity is compromised by their poor solubility in biological media and low accumulation at the tumor site [66].
Nanoparticle-based delivery systems offer enhanced delivery of PSs to tumors and reduced systemic toxicity [67]. A core-shell nanoparticle prepared on the basis of the coordination between Zn2+ and photoactive pyrophosphate was coated with the PS pyrolipid (ZnP-Pyro) and showed prolonged circulation in blood, high accumulation within tumors and high cell surface CRT expression [68]. ZnP-Pyro promoted ROS-induced ICD, suppressed primary tumors and inhibited lung metastasis. An alternative approach to achieve high tumor specificity is based on using the NK cell membrane to enhance tumor targeting and promote M1-type macrophage polarization [69]. A porphyrin-like PS was loaded onto the NK cell membrane (NK-NP) and achieved prolonged circulation in blood and induced ICD. Both the number of matured DCs and the ratio of CD8+ T cells in distal tumors were significantly increased after laser treatment compared with the number and ratio of the control group. NK-NPs not only eliminated the growth of the primary tumor but also prevented the progression of the distant tumor.
The antitumor efficacy of PDT is also limited by the hypoxic tumor environment [70, 71]. Thus, some approaches have explored oxygen nanocarriers, such as hemoglobin (Hb), hybridized with human serum albumin via disulfide bonds and loaded with the PS chlorine e6 (C@HPOC) [72]. The C@HPOC supplied oxygen to the tumor and upon laser irradiation increased ROS, as well as promoted the release of HMGB1 and ATP and the cell surface expression of CRT. Similarly, core-shell gold nanocages coated with manganese dioxide (MnO2) nanoparticles were found to degrade in acidic tumor environments and produce oxygen that promoted the antitumor immunity response, while nanoparticles without the ability to produce oxygen did not elicit a similar antitumor immune response [73]. Thus, approaches that supply oxygen to the tumor offer a new way to enhance antitumor activity and reverse the ITM.
ER targeting to trigger ER stress is another approach to enhance the ICD efficacy of PDT [74, 75]. Gold nanospheres containing ER-targeting pardaxin (FAL) peptides and indocyanine green (ICG) (FAL-ICG-HAuNSs) were explored for both PDT- and PTT-mediated tumor therapy [76]. These particles were found to accumulate in the ER. When delivered together with FAL-modified Hb liposomes (FAL-Hb lipos) and exposed to near-infrared (NIR) radiation, high levels of ROS were generated compared with those generated by the control. Delivery of this particle combination together with NIR irradiation showed effective tumor growth inhibition and increased survival rates in a CT26 tumor model.
Combinationtherapy
Cancer monotherapy is limited by tumor heterogenicity and the complex tumor immune microenvironment. Hence, combination therapies may offer some advantages [77, 78]. For example, a chemotherapeutic and PS-loaded self-assembling triblock copolymer composed of polyethylene glycol-poly(methyl methacrylate-co-2-amino ethyl methacrylate (thiol/amine))-poly(2-(dimethylamino)ethyl methacrylate) (PEG-P(MMA-co-AEMA (SH/NH2)-PDMA)) has been explored for its anticancer activity [79]. These particles were used to encapsulate a low dose of DOX and a photoactive agent, 2-(1-hexyloxyethyl)-2-devinylpyropheophorbide-a, that could be released via GSH-mediated cleavage for use in PDT [80]. These particles administered with laser irradiation increased the proportion of mature DCs in tumor draining lymph nodes and CD8+ T cells in MC38 colon adenocarcinoma cell tumors in mice [79]. In addition, the cell surface CRT expression was higher than that of the control, suggesting that the combination therapy exhibited enhanced efficacy compared with that of monotherapies.
Approaches targeting the ITM
The tumor microenvironment plays a dominant role in tumor growth, recurrence and metastasis; however, the efficacy of traditional therapeutics is limited by the tumor microenvironment. Recently, immunosuppressive factors, including indoleamine 2,3-dioxygenase 1 (IDO-1) and ICB, have been investigated to overcome the barriers placed by the ITM.
Inhibiting IDO-1 expression
The depletion of tryptophan (Trp) and accumulation of kynurenine (Kyn) by IDO-1 can hamper the antitumor immune response by suppressing the activity of CTLs and eliciting regulatory T cells [81]. Moreover, the IFN-γ secreted by CTLs has been demonstrated to promote the expression of IDO-1 [82]. Thus, the level of IDO-1 expression is key to modulating the ITM. Nanoparticle-based approaches have been explored, and a binary cooperative prodrug nanoparticle (BCPN) was designed to provide balance by codelivering an ICD inducer, OXA prodrug, and an IDO-1 inhibitor, NLG919, to augment the therapeutic efficacy of ICD [83]. BCPN released OXA and NLG919 in the acidic and reductant tumor microenvironment to promote ICD induction and IDO-1 inactivation [84, 85].
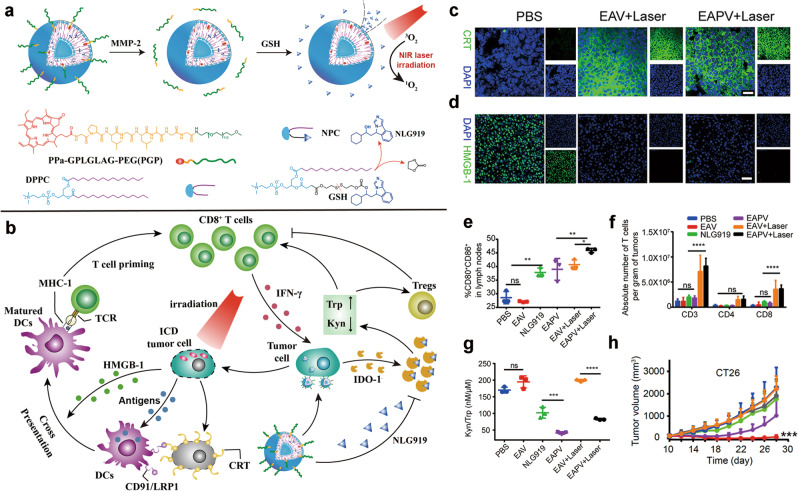
To achieve tumor-specific distribution and accumulation of the ICD inducer and IDO-1 inhibitor, enzyme-activatable prodrug vesicles (termed EAPVs), with the enzyme matrix metalloproteinase-2/9 (MMP-2/9), were codelivered with a PEGylated PS (e.g., PPa) and NLG919 (Fig. 3a, b) [86]. The prodrug vesicle was stable during blood circulation and accumulated in the tumor via the EPR effect. In the tumor, the PEG corona was cleaved via MMP-2-mediated degradation of the GPLGLAG peptide spacer, and NLG919 was released via the GSH-mediated reduction of the disulfide bonds. The EAPVs showed higher accumulation and retention in the tumors of a CT26 xenograft tumor model than they did in an MMP-2/9-insensitive control group, and the EAPVs showed increased ROS generation upon laser irradiation. The EAPVs also promoted CRT expression on the surface of the cell membrane and HMGB1 release, which subsequently supported DC maturation and recruited intratumorally infiltrating T lymphocytes to a greater extent than did the controls (Fig. 3c–f). Thus, EAPVs triggered PDT-mediated ICD as well as IDO-1 suppression, which supported tumor growth inhibition (Fig. 3g, h). Similarly, a synergistic nanoparticle containing a PS, protoporphyrin IX, and the IDO-1 inhibitor 1-methyltryptophan, conjugated via caspase-responsive peptides, also showed inhibition of the primary tumor and lung metastasis [87].
Fig. 3. Schematic illustration of shedding prodrug vesicles for cancer immunotherapy.
a The preparation procedure for enzyme-activatable prodrug vesicles (EAPV) designed to codeliver PEGylated PS and an IDO-1 inhibitor (NLG919); b The mechanisms by which EAPVs enhance antitumor efficacy via synergic triggering of ICD and combating IDO-1-mediated adaptive immune resistance; c Immunofluorescence image of CRT expressed on the surface of tumor cells in the CT26 tumor xenograft model (scale bar = 50 μm); d Immunofluorescence image of HMGB1 release from tumor cells in the CT26 tumor xenograft model (scale bar = 25 μm); e The ratio of mature DCs in tumor-draining lymph nodes; f The number of intratumor infiltrating T lymphocytes in tumor tissue; g Kyn to Trp molar ratio determined for the CT26 tumor tissue; and h Tumor growth curves of the CT26 xenograft tumors. Adapted with permission from [86]. Copyright (2019) American Chemical Society.
To precisely control drug release at the tumor site to promote antitumor immunogenicity and minimize systemic adverse effects, a light-inducible nanocargo (LINC) was designed for use in combination cancer immunotherapy [88]. A LINC was constructed with a GSH-responsive heterodimer of PPa and NLG919 and an ROS-labile PEGylated prodrug of OXA, which induced ICD and inhibited IDO-1 activity.
In a spatiotemporally controllable manner via dual laser irradiation steps (Fig. 4a). The first irradiation step promoted ROS generation and cleavage of the PEG corona following tumor accumulation to facilitate deep tumor penetration and intracellular uptake of the prodrug nanoparticles. Both OXA and NLG919 were restored inside the tumor cells via GSH-mediated reduction of the disulfide bond and OXA(IV) prodrug to trigger the immunogenic death of tumor cells. The second wave of laser irradiation enabled the PDT, while NLG919 reduced the ITM by suppressing the activity of IDO-1 (Fig. 4b). The combination of LINC treatment and two-wave laser irradiation (namely, LINCL) led to higher ROS generation efficacy than did LINC alone. In the 4T1 xenograft tumor model, LINCL significantly increased the cell surface expression of CRT, promoted HMGB1 release (Fig. 4c, d), and facilitated DC maturation in the tumor-draining lymph nodes to a greater extent than were realized in the laser-insensitive group. In addition, the ratio of intratumorally infiltrating CD8+ T cells was higher than that in the laser-insensitive group, thus resulting in a higher level of tumor eradication without lung metastasis (Fig. 4e, f).
Fig. 4. Schematic of LINC for cancer immunotherapy.
a The chemical structures and self-assembly process of the LINC; b The mechanisms of NIR light-inducible nanoparticles that induce ICD and reprogram the immunosuppressive tumor microenvironment; c CRT expression; d Intratumoral infiltration of CD8+ T cells; and hematoxylin and eosin (H&E) staining of the lung sections collected from the (e) saline and (f) LINCL-treated mouse groups, respectively. Adapted with permission from [88]. Copyright (2019) John Wiley & Sons, Inc.
Immune checkpoint blockade (ICB)
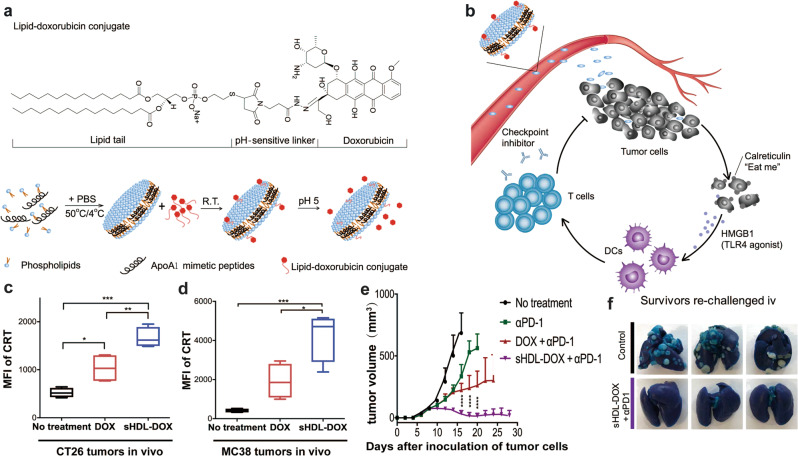
ICB therapy using either anti-programmed death 1 (αPD-1), anti-programmed death ligand 1 (αPD-L1) or anti-cytotoxic T lymphocyte-associated antigen 4 antibodies has shown potential in the clinic [89, 90]. Thus, combination ICB therapy using nanomedicine strategies is a promising approach. To promote immunogenicity and combat ITM simultaneously, high-density lipoprotein nanodiscs (sHDLs) composed of DOX, phospholipids and the apolipoprotein A1 mimetic peptide were designed (Fig. 5a, b) [91]. These sHDLs displayed prolonged blood circulation and tumor-specific accumulation of DOX that enhanced the release of HMGB1 and promoted cell surface expression of CRT in both CT26 and MC38 tumor models (Fig. 5c, d). Antitumor studies in vivo demonstrated that the combination therapy of sHDL and αPD-1 enhanced the frequency of intratumoral infiltrating CTLs compared with that shown in either αPD-1 monotherapy or combination therapy with DOX and αPD-1. sHDLs eliminated most of the established tumors and prevented lung metastasis (Fig. 5e, f).
Fig. 5. Schematic of sHDL-DOX to enhance cancer immunotherapy by combining ICD and αPD-1.
a The chemical structure and self-assembly process of a sHDL; b The mechanism of sHDL-triggered ICD-mediated antitumor immunity synergized with ICB. The level of CRT cell surface expression in c CT26 and d MC38 tumor models; e tumor volume over time and f Lung metastasis in CT26 tumor-bearing mice. Adapted with permission from [91]. Copyright (2019) Science Publishing Group.
In addition to the small molecule inhibitors and monoclonal antibodies for combating ITM, peptide-based immune checkpoint inhibitors have also been exploited for enhancing ICD by ICB. For example, a biomimetic nanoparticle has been designed to integrate PPa, a ROS-activated PTX prodrug, and αPD-L1 peptide (dPPA) into a single nanoplatform [92]. Combination immunotherapy with dPPA simultaneously enhanced tumor immunogenicity by inducing ICD and attenuated the ITM by blocking PD-L1, resulting in significant tumor regression.
CD47 is another crucial immune checkpoint protein that is abundantly expressed on the surface of various tumor cells. To prevent phagocytosis of tumor cells by APCs, CD47 acts as a “don’t eat me” signal by binding with signal regulatory protein-α (SIRP-α) on the surface of macrophages or DCs [93]. Therefore, CD47 suppresses the ICD effect by inhibiting antigen uptake by APCs. To address this challenge, a nanocage based on engineered human ferritin was designed to codeliver an SIRP-α variant and DOX (termed HFSIRP-α-DOX) [94]. The combination of ICD induction and CD47 blockade by HFSIRP-α-DOX provoked a long-term antitumor immune response. The in vivo antitumor studies showed that HFSIRP-α-DOX significantly increased the intratumoral infiltration of CD8+ T cells compared with that of the control group. HFSIRP-α-DOX efficiently suppressed tumor growth by activating the adaptive immune response and combating the ITM.
To further demonstrate the potential of ICD induction and CD47 blockade for combination immunotherapy, a tumor microenvironment-activatable nanoparticle was designed to destroy tumor cells and induce ICD. The nanoparticles were constructed from an MMP-2-activatable vesicle (MPV) and 2,3-dimethylmaleic anhydride-modified hexadecyl-oxaliplatindiethylene amine (HOAD), which was termed MPV-HOAD [95]. The MPV-HOAD vesicles specifically accumulated in tumors and were activated by tumor acidity and MMP-2/9. The combination of MPV-HOAD with NIR laser irradiation induced the immunogenic death of tumor cells in the CT26 tumor xenograft model, while the addition of an intratumoral injection of αCD47 was most effective at suppressing tumor growth.
Conclusion and future perspectives
In summary, several emerging therapeutic methods, including chemotherapy, phototherapy, and radiotherapy, have been shown to be effective for eliciting ICD. However, ICD-activated immune responses only moderately suppressed tumor recurrence and metastasis due to insufficient tumor distribution of these therapies. To improve the therapeutic performance of ICD-based cancer immunotherapy, nanoparticle-based drug delivery systems enable codelivery and multiple irradiation regimens to enhance the performance of ICD-based therapies. The combination of nanotechnology and ICD-based cancer immunotherapy shows potential for improved cancer immunotherapy by simultaneously eliciting antitumor immunity and suppressing the ITM.
Despite great success using these nanoparticle-based ICD therapies in preclinical studies, their clinical application is still hindered by several challenges. First, the nanoparticle-based ICD strategy is in its infancy, with many novel tactics remaining to be explored. Tumor-specific drug distribution and activation are crucial for eliciting antitumor immunity. Second, simple nanoparticle designs and quality control mechanisms are essential for the clinical translation of nanomedicine-based cancer immunotherapy. Third, further efforts to elucidate the mechanisms underlying ICD-based cancer immunotherapy are required to facilitate its clinical translation.
Acknowledgements
Financial support from the National Natural Science Foundation of China (51873228, 31671024, 31900990, and 81521005), the Science and Technology Research Foundation of Shenzhen Bureau of Science and Technology & Information (JCYJ20180227175420974), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2014218) and UNSW-CAS Collaborative Research Seed Program 2019 are gratefully acknowledged.
Competing interests
The authors declare no competing interests.
References
- 1.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669–82. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 4.Smyth MJ, Ngiow SF, Ribas A, Teng Michele WL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2015;13:143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 6.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2006;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 12.Matzinger P. Tolerance, danger, and the extended family. Annu Rev lmmunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 13.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P, et al. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. BBA-REV Cancer. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 15.Valle AS, Anel A, Naval J, Marzo I. Immunogenic cell death andimmunotherapy of multiple myeloma. Front Cell Dev Biol. 2019;7:1–22. doi: 10.3389/fcell.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saenz R, Futalan D, Leutenez L, Eekhout F, Fecteau JF, Sundelius S, et al. TLR4-depend activation dendritic cells byan HMGB1-derived peptide adjuvant. J Transl Med. 2014;12:211. doi: 10.1186/1479-5876-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 18.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 20.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 21.Lesterhuis W, De Vries I, Aarntzen E, De BA, Scharenborg NM, Van, et al. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. Br J Cancer. 2010;103:1415–21. doi: 10.1038/sj.bjc.6605935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menger L, Vacchelli E, Adjemian S, Martins I, Ma YT, Shen S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra99. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 23.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 24.Krysko DV, Kaczmarek A, Krysko O, Heyndrickx L, Woznicki J, Bogaert P, et al. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ. 2011;18:1316–25. doi: 10.1038/cdd.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarasiuk J, Mazerski J, Tkaczyk-Gobis K, Borowski E. Molecular basis of the low activity of antitumor anthracenediones, mitoxantrone and ametantrone, in oxygen radical generation catalyzed by NADH dehydrogenase. Enzymatic and molecular modelling studies. Eur J Med Chem. 2005;40:321–8. doi: 10.1016/j.ejmech.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10:1486. doi: 10.1038/s41467-019-09415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–79. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–4. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 29.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P, et al. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med. 2012;18:589–98. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sriraman SK, Aryasomayajula B, Torchilin VP. Barriers to drug delivery in solid tumors. Tissue Barriers. 2014;2:e29528. doi: 10.4161/tisb.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang HL, Sengupta S. Transcellular transfer of nanomedicine. Nat Nanotechnol. 2019;14:731–2. doi: 10.1038/s41565-019-0494-y. [DOI] [PubMed] [Google Scholar]
- 33.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–92. [PubMed] [Google Scholar]
- 34.Binnewies M, Roberts EW, Kersten K, Fearon DF, Merad M, Coussens LM, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Q, Zhou Z, Qiu N, Shen YQ. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 36.Duan X, Chan C, Lin WB. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019;58:670–80. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J, Wang WQ, Yu HJ. Acid-activatable polymeric drug delivery systems for cancer therapy. Acta Pharm Sin. 2019;50:1156–66. [Google Scholar]
- 38.Hou B, Zhou L, Wang H, Saeed M, Wang DG, Xu ZA, et al. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2019. 10.1002/adma.201907210. [DOI] [PubMed]
- 39.Wang WQ, Jin YL, Xu ZA, Liu X, Bajwa SZ, Khan WS, et al. Stimuli-activatable nanomedicine for chemodynamic therapy of cancer. Nanomed Nanobiotechnol. 2020. 10.1002/wnan.1614. [DOI] [PubMed]
- 40.Ruan SB, Xie R, Qin L, Yu MN, Xiao W, Hu C, et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019;19:8318–32. doi: 10.1021/acs.nanolett.9b03968. [DOI] [PubMed] [Google Scholar]
- 41.Saeed M, Gao J, Shi Y, Lammers T, Yu HJ. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics. 2019;9:7981–8000. doi: 10.7150/thno.37568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X, Yang K, Zhao R, Ji TJ, Wang XC, Yang X, et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187–97. doi: 10.1016/j.biomaterials.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 44.Duan XP, Chan C, Han WB, Guo NN, Weichselbaum RR, Lin WB. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat Commun. 2019;10:1899. doi: 10.1038/s41467-019-09221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen QJ, Liu LS, Lu YF, Chen XL, Zhang YJ, Zhou WX, et al. Tumor microenvironment-triggered aggregated magnetic nanoparticles for reinforced image-guided immunogenic chemotherapy. Adv Sci. 2019;6:1802134. doi: 10.1002/advs.201802134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastria EM, Cai LY, Kan MJ, Li XH, Schaal JL, Fiering S, et al. Nanoparticle formulation improves doxorubicin efficacy by enhancing host antitumor immunity. J Control Release. 2018;269:364–73. doi: 10.1016/j.jconrel.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alev M, Egenberger L, Mühleisen L, Weigel B, Frey B, Friedrich RP, et al. Targeting of drug-loaded nanoparticles to tumor sites increases cell death and release of danger signals. J Control Release. 2018;285:67–80. doi: 10.1016/j.jconrel.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 49.Fan YC, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ. Immunogenic cell death amplified by co-localized adjuvant delivery for cancer immunotherapy. Nano Lett. 2017;17:7387–93. doi: 10.1021/acs.nanolett.7b03218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bluestone JA, Crellin N, Trotta E. IL-2: change structure change function. Immunity. 2015;42:779–81. doi: 10.1016/j.immuni.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhao YD, Song QL, Yin YJ, Wu TT, Hu XM, Gao XQ, et al. Immunochemotherapy mediated by thermosponge nanoparticles for synergistic anti-tumor effects. J Control Release. 2018;269:322–36. doi: 10.1016/j.jconrel.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 52.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 53.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubner Y, Wunderlich R, Rühle PF, Kulzer L, Werthmöller N, Frey B, et al. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol. 2012;2:75. doi: 10.3389/fonc.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Q, Chen JW, Yang ZJ, Xu J, Xu LG, Liang C, et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv Mater. 2019;31:1802228. doi: 10.1002/adma.201802228. [DOI] [PubMed] [Google Scholar]
- 56.Hwang S, Nam J, Jung S, Song J, Doh H, Kim S, et al. Gold nanoparticle-mediated photothermal therapy: current status and future perspective. Nanomedicine. 2014;9:2003–22. doi: 10.2217/nnm.14.147. [DOI] [PubMed] [Google Scholar]
- 57.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Onco Hema. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 58.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–49. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian M, Chen LL, Du YL, Jiang HL, Huo TT, Yang YF, et al. Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett. 2019;19:8409–17. doi: 10.1021/acs.nanolett.9b02448. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14:1800678. doi: 10.1002/smll.201800678. [DOI] [PubMed] [Google Scholar]
- 61.Yu GT, Rao L, Wu H, Yang LL, Bu LL, Deng WW, et al. Myeloid-derived suppressor cell membrane-coated magnetic nanoparticles for cancer theranostics by inducing macrophage polarization and synergizing immunogenic cell death. Adv Funct Mater. 2018;28:1801389. [Google Scholar]
- 62.Ma YC, Zhang YX, Li XQ, Zhao YY, Li M, Jing W, et al. Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano. 2019;13:11967–80. doi: 10.1021/acsnano.9b06040. [DOI] [PubMed] [Google Scholar]
- 63.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–37. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–45. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473:347–64. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam J, Son S, Park KS, Zou WP, Shea LD, Moon JJ. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater. 2019;4:398–414. [Google Scholar]
- 68.Duan XP, Chan C, Guo NN, Han WB, Weichselbaum RR, Lin WB. Photodynamic therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc. 2016;138:16686–95. doi: 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng GJ, Sun ZH, Li SP, Peng XH, Li WJ, Zhou LH, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 70.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 71.Dang JJ, He H, Chen DL, Yin LC. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT) Biomater Sci. 2017;5:1500–11. doi: 10.1039/c7bm00392g. [DOI] [PubMed] [Google Scholar]
- 72.Chen Z, Liu L, Liang R, Luo Z, He H, Wu Z, et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12:8633–45. doi: 10.1021/acsnano.8b04371. [DOI] [PubMed] [Google Scholar]
- 73.Liang R, Liu L, He H, Chen Z, Han Z, Luo Z, et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@ manganese dioxide to inhibit tumor growth and metastases. J Biomater Sci Polym Ed. 2018;177:149–60. doi: 10.1016/j.biomaterials.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 74.Abels C, Fickweiler S, Weiderer P, Bäumler W, Hofstädter F, Landthaler M, et al. Indocyanine green (ICG) and laser irradiation induce photooxidation. Arch Dermatol Res. 2000;292:404–11. doi: 10.1007/s004030000147. [DOI] [PubMed] [Google Scholar]
- 75.Verfaillie T, Garg AD, Agostinis P, Targeting ER. Stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–64. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10:3349. doi: 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang M, Song J, Zhou F, Hoover AR, Murray C, Zhou B, et al. NIR-triggered phototherapy and immunotherapy via an antigen-capturing nanoplatform for metastatic cancer treatment. Adv Sci. 2019;6:1802157. doi: 10.1002/advs.201802157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang XT, Hu C, Tong X, Liu R, Zhou Y, Qin L, et al. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv Funct Mater. 2019;29:1901896. [Google Scholar]
- 79.Yang W, Zhu G, Wang S, Yu G, Yang Z, Lin L, et al. In situ dendritic cell vaccine for effective cancer immunotherapy. ACS Nano. 2019;13:3083–94. doi: 10.1021/acsnano.8b08346. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Xiao K, Zhu W, Deng W, Lam KS. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv Drug Deliv Rev. 2014;66:58–73. doi: 10.1016/j.addr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, et al. Tumor-expressed IDO-1 recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 2015;13:412–24. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14:603–22. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 83.Feng B, Zhou FY, Hou B, Wang DG, Wang TT, Fu Y, et al. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv Mater. 2018;30:1803001. doi: 10.1002/adma.201803001. [DOI] [PubMed] [Google Scholar]
- 84.Lu J, Liu X, Liao YP, Salazar F, Sun B, Jiang W, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811. doi: 10.1038/s41467-017-01651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang H, Jiang CT, Shen S, Liu A, Gan YJ, Tong QS, et al. Nanoenabled reversal of IDO-1-mediated immunosuppression synergizes with immunogenic chemotherapy for improved cancer therapy. Nano Lett. 2019;19:5356–65. doi: 10.1021/acs.nanolett.9b01807. [DOI] [PubMed] [Google Scholar]
- 86.Gao A, Chen B, Gao J, Zhou FQ, Saeed M, Hou B, et al. Sheddable prodrug vesicles combating adaptive immune resistance for improved photodynamic immunotherapy of cancer. Nano Lett. 2020;20:353–62. doi: 10.1021/acs.nanolett.9b04012. [DOI] [PubMed] [Google Scholar]
- 87.Song W, Kuang J, Li CX, Zhang M, Zheng D, Zeng X, et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12:1978–89. doi: 10.1021/acsnano.7b09112. [DOI] [PubMed] [Google Scholar]
- 88.Feng B, Hou B, Xu Z, Saeed M, Yu HJ, Li YP. Self-amplified drug delivery with light-inducible nanocargoes to enhance cancer immunotherapy. Adv Mater. 2019;31:1902960. doi: 10.1002/adma.201902960. [DOI] [PubMed] [Google Scholar]
- 89.Ribas A, Wolchok J. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mccracken MN, Cha AC, Weissman IL. Molecular pathways: activating T cells after cancer cell phagocytosis from blockade of CD47 “Don’t Eat Me” signals. Clin Cancer Res. 2015;21:3597–601. doi: 10.1158/1078-0432.CCR-14-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y, et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4:eaao1736. doi: 10.1126/sciadv.aao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu W, He X, Yang Z, Yang X, Xiao W, Liu R, et al. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. doi: 10.1016/j.biomaterials.2019.119309. [DOI] [PubMed] [Google Scholar]
- 93.Vonderheide RH. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21:1122–3. doi: 10.1038/nm.3965. [DOI] [PubMed] [Google Scholar]
- 94.Lee EJ, Nam GH, Lee NK, Kih M, Koh E, Kim YK, et al. Nanocage-therapeutics prevailing phagocytosis and immunogenic cell death awakens immunity against cancer. Adv Mater. 2018;30:1705581. doi: 10.1002/adma.201705581. [DOI] [PubMed] [Google Scholar]
- 95.Zhou FY, Feng B, Yu HJ, Wang DG, Wang TT, Ma Y, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31:1805888. doi: 10.1002/adma.201805888. [DOI] [PubMed] [Google Scholar]