Abstract
Immunotherapy that activates the host immune system to reverse immunosuppression has emerged as a new generation of cancer treatment in both preclinical studies and clinical trials. Although immunotherapy has shown significant achievements in the treatment of various cancers, it faces challenges that limit its further evolution such as poor permeation and modest responsiveness. The development of nanoparticle drug delivery system has provided an opportunity to overcome these drawbacks and to achieve optimized immunotherapy. Based on the research of our group, we here introduce the new strategies being employed using nanoscale intelligent drug delivery systems to enhance the effects of cancer immunotherapy. We also provide a perspective on the further possible application of nanoparticles in more effective antitumor immunotherapy.
Keywords: cancer immunotherapy, nanoparticles, tumor microenvironment, immune checkpoint inhibitors, tumor vaccine, natural killer cells, dendritic cells
Introduction
Effective treatment of cancer is an ongoing challenge for both basic researchers and clinicians worldwide. Traditional chemotherapy and radiotherapy have serious disadvantages, such as systemic toxicity and multidrug resistance [1–4]. In more recent years, cancer immunotherapy, which effectively kills cancer cells by enhancing immune system function in patients, has emerged as a new therapeutic approach [5, 6]. It is well known that the tumor microenvironment maintains an immunosuppressive state [7, 8]. There are many reasons for this, including: (1) tumor cells hyperactivate immune checkpoints, escaping immune surveillance [9]; (2) tumor antigens that are heterogeneous and have high mutation rates make surrounding immune cells unable to recognize and kill tumor cells [10]; and (3) tumor cells have low immunogenicity and are not easily recognized by antigen-presenting cells (APCs) [11]. Therefore, the intrinsic immune system cannot clear tumor cells in the same manner that it eliminates ordinary foreign substances. Many drawbacks of traditional therapy have been overcome by this new emerging therapy, which exhibits a rapid response, powerful effects, and long-lasting efficacy [12, 13]. However, one drawback of immunotherapy is its unsatisfactory effects on deep solid tumors [14]. Consequently, to improve cancer immunotherapy, there is an urgent demand for novel strategies to reverse and remodel immunosuppressive microenvironments [15]. The development of nanomedicine has provided a novel approach to enhance immunotherapeutic efficacy and minimize adverse toxicities [16, 17]. In contrast to free drugs, nanodrugs (10–200 nm) have a prolonged plasma half-life and high accumulation in tumor sites due to passive or/and active targeting effects [18]. Moreover, the easy functionalization of nanodrug surfaces makes it possible to release drugs solely in response to the tumor microenvironment, ensuring high biosafety and improved therapeutic efficiency [19]. Considering these outstanding advantages, nanomedicine presents a promising strategy for advancing tumor immunotherapy. In recent years, our group has been committed to the investigation of intelligent nanomedicine to regulate the tumor immune microenvironment [20, 21]. This review introduces three aspects of cancer immunotherapy based on the work of our group and provides a perspective on the combination of nanomedicine and immunotherapy.
Nanoparticle delivery system improves the therapeutic efficiency of immune checkpoint inhibitors in the tumor microenvironment
Under physiological conditions, immune checkpoint pathways maintain immune balance and protect against autoimmune diseases [22, 23]. However, tumor cells exploit this function, causing immune cells to recognize them as normal cells. Immune checkpoint inhibitors interfere with the camouflage of the surface of tumor cells and disrupt the receptor-ligand interaction so that T cells can be activated to eliminate tumor cells. CTLA-4 was the first immune checkpoint protein discovered to antagonize a T cell costimulatory signal and turn off T cells as a negative regulator [24]. The continuous success of PD-1/PD-L1 inhibitors has attracted widespread attention in recent years [25, 26]. Many commercially available PD-1/PD-L1 inhibitors have been used clinically with inspiring curative effects [27]. However, due to the dynamic and complicated nature of the tumor environment, only a small number of tumor-infiltrating lymphocytes are recruited to tumor sites [28]. In addition, the rapid proliferation of tumor cells and abnormality of tumor blood vessels lead to hypoxia in tumor tissues and overexpression of hypoxia-inducible factor (HIF)-1α, consequently resulting in chemoresistance and poor immunotherapeutic outcomes [29, 30]. Accordingly, remodeling the tumor microenvironment will be important to enhance the therapeutic efficacy of immune checkpoint inhibitors [31, 32]. Many nanodrug delivery systems have achieved encouraging therapeutic efficacy in tumor inhibition through the codelivery of immune checkpoint inhibitors with other drugs or compounds expected to regulate the tumor microenvironment [33, 34].
To enhance the efficacy of anti-PD-1 antibodies, many strategies involving hypoxia alleviation and combination with other immunomodulatory drugs have been proposed. Zou et al. prepared a multifunctional biomimetic nanoplatform containing zeolitic imidazolate framework (ZIF)-8 coated with a tumor cell membrane as the guidance part and antigen stimulation to codeliver catalase and doxorubicin [35]. Oxygen generation by catalysis of catalase could alleviate hypoxic conditions and enhance the antitumor effects of doxorubicin. Consistent with previous research, this nanoplatform was shown to decrease the expression of PD-L1 on the tumor cell membrane by inhibiting HIF-1α [36]. Encouraged by this outcome, Zou et al. immediately combined this nanoplatform with an anti-PD-1 antibody for immunotherapy. The effects of the combination therapy were significant compared with those of other treatments, indicating that hypoxia alleviation using nanomedicine in the tumor microenvironment could enhance the effects of immunotherapy. In addition, Zhang et al. generated engineered magnetosomes to achieve synergistic antitumor effects by integrating a checkpoint antibody and a TGF-β inhibitor into Fe3O4 magnetic nanoclusters. In this system, the anti-PD-1 antibody for activating exhausted T cells and the TGF-β inhibitor for modulating macrophage polarization from the M2 phenotype to the M1 phenotype had a synergistic effect that created an immunogenic microenvironment. This treatment could increase the amount of H2O2 in polarized M1 macrophages, while Fe3O4 magnetic nanoclusters induced the Fenton reaction to produce reactive oxygen species (ROS). The generated ROS subsequently resulted in lethal ferroptosis in tumor cells. The anticancer effects were evaluated in seven different tumor models, and all of the results were extraordinarily satisfactory [37].
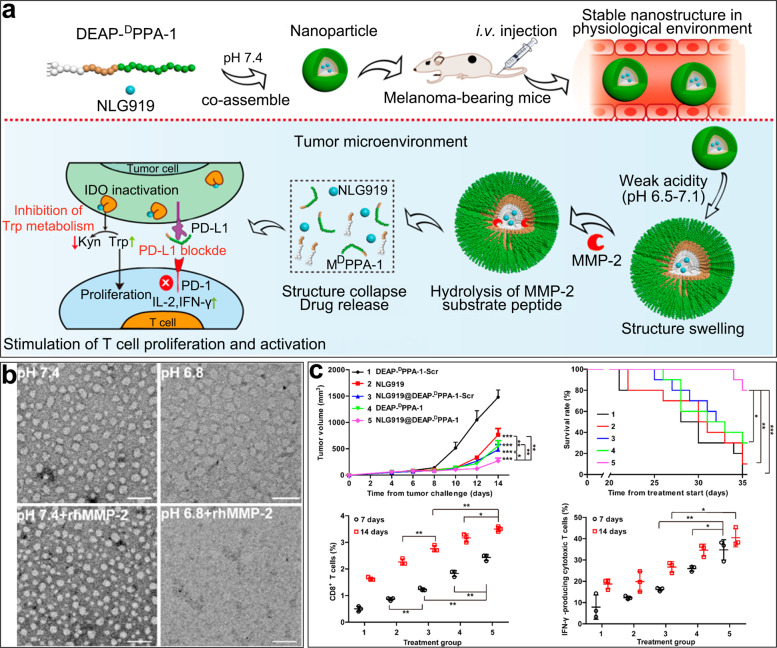
In addition to overexpression of PD-1/PD-L1 and CTLA4, overexpression of indoleamine 2,3-dioxygenase (IDO) in tumors has also been shown to be associated with immune tolerance [38]. IDO is a unique rate-limiting enzyme that regulates metabolism of tryptophan into kynurenine and is widely expressed in most organs except the liver [39]. Tryptophan is an essential amino acid that influences the proliferation and activation of T cells through two distinct molecular stress-response pathways (the GCN2/eIF-2α and GLK1/mTORC1 pathways) [40, 41]. Therefore, a reduction in the tryptophan level in the tumor microenvironment is not conducive to effector T cell activity [22, 42]. In addition, kynurenine products produced by IDO-1 are able to bind to aryl hydrocarbon receptors (AhR) as an endogenous ligand. Activation of AhR induces the differentiation of regulatory T Cells (Tregs), which suppress antitumor immunity and help tumors escape immune surveillance [43]. Therefore, several IDO inhibitors have been developed to prevent both the consumption of tryptophan and the accumulation of kynurenine, including the first-line IDO inhibitor NLG919 [44]. In our prior studies, we designed an amphiphilic peptide consisting of DEAP, PLGLAG (a short peptide substrate of MMP-2), and DPPA-1 (a bioactive D-peptide antagonist of PD-L1) [45]. This peptide self-assembled into nanoparticles to encapsulate NLG919 in the hydrophobic domain under physiological pH conditions. Intravenously administered nanoparticles swelled and exposed specific peptide substrates to abundant MMP-2 after protonation of DEAP in the acidic tumor microenvironment. Following structural disintegration of the nanoparticles, NLG919 and DPPA-1 were consequently released to inhibit the metabolism of tryptophan and block PD-L1, respectively (Fig. 1). Transmission electron microscopy showed that the peptide-assembling nanoparticles could be hydrolyzed rapidly in the tumor microenvironment. In vivo treatment indicated that the combination immunotherapeutic group showed the greatest antitumor efficacy and promoted T cell replication and activation to the greatest extent.
Fig. 1. NLG919@DEAP-DPPA-1 nanoparticles for cancer immunotherapy.
a The antitumor mechanism of the multifunctional peptide in the tumor microenvironment. b Transmission electron microscopy images of nanoparticles under different pH conditions with or without recombinant human MMP-2 (rhMMP-2). Scale bars: 100 nm. c Treatment efficacy of peptide nanoparticles and the quantity of CD8+ T cells measured in melanoma-bearing mice. Copyright (2018), American Chemical Society.
Nanoparticle facilitates the killing ability of natural killer cells in the tumor microenvironment
Natural killer (NK) cells, which account for 10%–15% of the circulating lymphocytes in the innate immune system, can spontaneously lyse tumor cells without requiring prior sensitization [46]; therefore, NK cells have unique roles in cancer immune surveillance and clearance [47, 48]. Following activation counterbalanced by various receptors, NK cells express activating receptors, including natural cytotoxicity receptors (e.g., NKp30 and NKp44) and Fc-gamma receptors (e.g., CD16), that recognize tumor cells or virus-infected cells [49]. In addition, NK cells are also equipped with some inhibitory receptors, such as killer immunoglobulin-like receptors, to protect normal cells [50]. Finally, NK cells also express some cytokine (e.g., IL-2 and TGF-β) receptors, which regulate their function [51, 52].
NK cells recognize foreign antigens through cell stress-induced recognition and missing-self recognition, which blocks the transmission of inhibitory signals or upregulates interactions between activating receptors and stress ligands on tumor cells [48, 53]. After recognition, perforin and granzyme B are released by activated NK cells, resulting in rapid tumor cell lysis [54]. However, many clinical and preclinical studies have reported dysfunction of NK cells, especially in various solid tumors [55], which is primarily due to the immunosuppressive microenvironment [56].
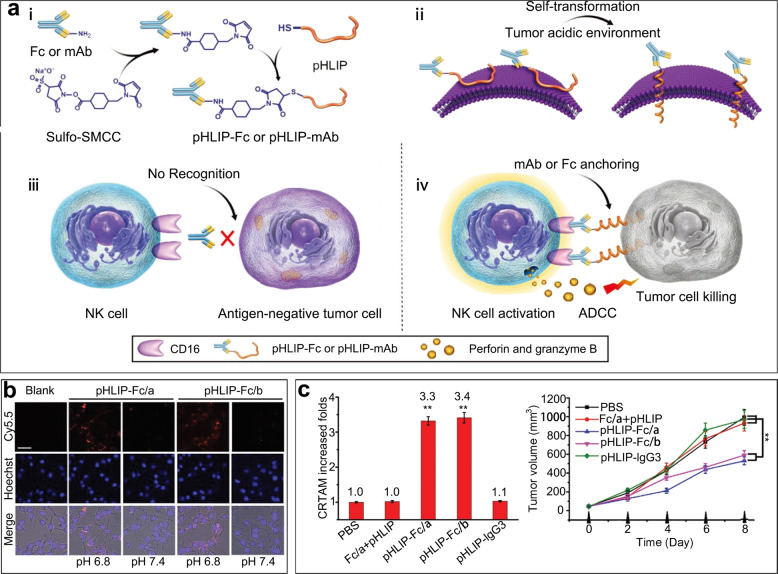
NK cells directly lyse tumor cells via antibody-dependent cell-mediated cytotoxicity (ADCC), wherein antibodies play key roles in the activation and ADCC of NK cells [48, 57]. FcγRIIIA (CD16), which is abundantly expressed on the NK cell surface, has been reported to trigger ADCC by specifically binding to an antibody-coated antigen [58, 59]. However, the heterogeneity of tumor antigens severely limits this recognition process, resulting in a reduction in the ADCC effect [60]. Therefore, our group combined Fc fragments or therapeutic antibodies with pH (low) insertion peptides (pHLIPs) [61]. A weakly acidic pH has been validated as the most significant feature of many solid tumors and has been widely applied in nanoparticle construction [62]. Under low-pH conditions, pHLIPs can transform coil conformations into α-helices and anchor themselves into tumor cell membranes so that the Fc fragments or therapeutic antibodies are exposed [63]. The subsequent recognition and binding of recruited NK cells can then trigger ADCC more efficiently. In vitro, pHLIP-Fc-mediated ADCC at pH 6.8 is superior to that at pH 7.4. In vivo, both primary tumor and metastatic disease models showed enhanced therapeutic effects, and the number of activated NK cells was increased more than three times with pHLIP-Fc therapy than with control treatment and led to distinctly outstanding therapeutic effects (Fig. 2). Given the excellent insertion capacity of pHLIPs, our group is also investigating whether pHLIPs can be applied to CAR-T cell therapy, increasing the possible applications of this therapy in solid tumors.
Fig. 2. pH (low) insertion peptide (pHLIP)-Fc enhanced immunotherapy with natural killer cells.
a Mechanism by which pHLIP-Fc or pHLIP-mAb activates ADCC. b Comparison of pHLIPs at physiological pH and a weakly acidic pH. Scale bar: 50 µm. c Measurement of CRTAM-positive cells as activated NK cells and the inhibition of tumor growth. Fc/a: Fc fragments from mouse IgG2a; Fc/b: Fc fragments from a mouse. Copyright (2018) John Wiley & Sons, Inc.
Zheng et al. also designed immunomodulatory core-shell assembled nanoparticles to activate NK cells in situ. The pH-responsive shell PMPC-b-PApm/Glu can disassemble following exposure to the acidic tumor microenvironment. The naked bifunctional core nBSA-PBA-IgG immediately binds sialic acid expressed on the tumor cell membrane to allow IgG to activate NK cells. These safe and effective immunomodulatory nanoparticles can avoid immune-related adverse events in nontarget tissues and exert immunotherapeutic effects exclusively on tumors [64].
Nanoparticle-engineered NK cells have also been extensively investigated, including NK cells with expression of an EGFR-targeted CAR on the cell membrane realized through gene delivery and recruitment and infiltration of NK cells into tumors elevated by magnetic traction [65, 66]. All of these approaches indicate that nanoparticles have broad applications in NK cell-mediated cancer immunotherapy.
Nanoparticle enhances the function of dendritic cells in the tumor microenvironment
Dendritic cells (DCs) are the primary APCs in the human body [67]. They have two responsibilities in the immune system; first, they catch and process tumor antigens, and second, they present processed antigens and stimulate T cells or other lymphocytes [68, 69]. DCs are classified as immature and mature [70]. Only mature DCs possess the ability to induce antitumor immunity [71]. As a consequence, activated DCs may be the key to reversing the immunosuppressive tumor microenvironment [72–74].
It is generally accepted that tumor vaccines enable targeted delivery of tumor-associated antigens (TAAs) or adjuvants to DCs and induce long-lasting antitumor immune responses [75–78]. Nevertheless, it is difficult for DCs to absorb soluble antigens; phagocytosis of antigen-loaded nanoparticles is preferred [79–81]. Nanoparticles with sizes similar to those of pathogens can simulate endocytosis of TAAs [82–84]. On account of this advantage, the application of nanoparticles will be important in the development of tumor vaccines delivering TAAs to DCs [85–87].
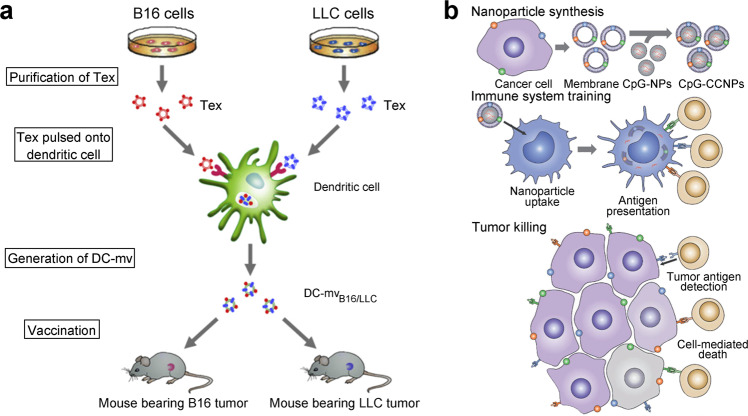
In recent years, biologically derived nanomaterials, such as exosomes, have received increasing attention [88]. Exosomes, which are vesicles secreted by parent cells, are widely used in disease diagnosis and treatment [89–92]. In our previous study, we prepared two types of DC-derived membrane vesicles (DC-mvs) that bore antigens from B16 or LLC tumor cells [93]. A DC-mv vaccine could lead to the activation of specific cytotoxic T lymphocytes and exhibited powerful antitumor effects when administered to tumor-bearing mice. Consequently, DC-mvs as antitumor vaccines exhibit an excellent antigen presentation ability. In tumor rejection studies, DC-mvs containing dual tumor antigens have been shown to be superior to those containing a single antigen (Fig. 3a).
Fig. 3. Biologically derived nanoparticles for activation of dendritic cells (DCs).
a Exosome-based dual vaccine. Tumor-derived exosomes were incubated with DCs to generate DC-derived membrane vesicles (DC-mvs), which were injected into mice. Copyright (2012) Elsevier Ltd. b Membrane-based tumor vaccine. The tumor cell membrane (antigen) and CpG (adjuvant) were codelivered to antigen-presenting cells (blue), and activated T cells (tan) killed tumor cells. Copyright (2017) John Wiley & Sons, Inc.
Recently, biomimetic nanoparticles coated with cancer cell membranes have emerged as a rising star in nanomedicine [94, 95]. Because of the abundance of tumor antigens on membranes, these nanoparticles are more suitable for application as tumor vaccines [96, 97]. Kroll et al. constructed a tumor vaccine that used the B16-F10 mouse melanoma cell membrane as the shell exposing an external tumor antigen to encapsulate the core, which was a PLGA nanoparticle that encapsulated CpG, a common adjuvant [98]. The mature APCs and activated T cells triggered by this vaccine eliminated tumor cells efficiently in female C57BL/6NHsd mice. While exploring whether the immune activation caused by this vaccine enhances immunotherapeutic efficiency, researchers found that combination of this vaccine with both anti-CTLA4 and anti-PD1 antibodies significantly reinforced the curative effects compared with checkpoint blockade cocktail alone (Fig. 3b). Therefore, tumor vaccines can be considered an effective tool to relieve immunosuppression and increase immunotherapeutic efficiency, especially with the development of personalized tumor vaccines in the future [99–101].
In addition to use of the tumor membrane, the application of bacteria in cancer immunotherapy has also received widespread attention [102]. We currently have unpublished data showing that a hybrid membrane-based tumor vaccine that consists of both a tumor membrane and a bacterial cytoplasmic membrane exhibits great potential as an adjuvant.
Conclusions and perspectives
Nanoparticle drug delivery systems have been widely used as well-known drug carriers in tumor immunotherapy. In addition to the three areas described above, our group continues to conceive nanomedicine strategies to achieve the following: promote the polarization of macrophages from the M2 phenotype to the M1 phenotype, eliminate the physical barrier around tumors prior to immune activation, and inhibit the differentiation of myeloid-derived suppressor cells.
The combination of immunotherapy with other strategies is believed to produce optimal results. Nanodelivery systems can subtly integrate different strategies to target the tumor microenvironment and release drugs or effectors. However, some nanoparticles are too complex to be applied for clinical diagnosis and therapy. To solve this problem, in our view, nanoparticles should be designed with a multifunctional component serving as both the bioactive and building elements. For instance, it is better to construct peptide-based self-assembled nanoparticles with an amphipathic peptide against PD-L1 than to conjugate an anti-PD-L1 antibody to presynthesized nanoparticles through additional building elements for blocking PD-L1 [37, 45]. Therefore, the construction of nanomedicine materials should never be regarded as only the integration of many components. As the components and steps increase, the probability of us achieving large-scale stable production and fast translation into clinical practice decreases. As mentioned by researchers in the medical field, we should always remember that our original intention is to invent a real drug to save lives, not to create sophisticated artwork. Achieving the most powerful effects with the simplest system should be the most important consideration in future nanomedicine development.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFE0205300 and 2018YFA0208900) and the National Natural Science Foundation of China (81871489 and 91859118).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Fei-long Qi, Mei-fang Wang
Contributor Information
Guang-jun Nie, Email: niegj@nanoctr.cn.
Su-ping Li, Email: lisuping@nanoctr.cn.
References
- 1.Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg Med Clin North Am. 2014;32:167–203. doi: 10.1016/j.emc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Cree IA, Charlton P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer. 2017;17:10. doi: 10.1186/s12885-016-2999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall S, Rudrawar S, Zunk M, Bernaitis N, Arora D, McDermott CM, et al. Protection against radiotherapy-induced toxicity. Antioxidants. 2016;5:22. doi: 10.3390/antiox5030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui C, Yang J, Li X, Liu D, Fu L, Wang X. Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance. Mol Cancer. 2020;19:58. doi: 10.1186/s12943-020-01180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–36. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020. 10.1038/s41577-019-0271-z [DOI] [PubMed]
- 9.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 11.Bai Y, Wang Y, Zhang X, Fu J, Xing X, Wang C, et al. Potential applications of nanoparticles for tumor microenvironment remodeling to ameliorate cancer immunotherapy. Int J Pharm. 2019;570:118636. doi: 10.1016/j.ijpharm.2019.118636. [DOI] [PubMed] [Google Scholar]
- 12.Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–29. doi: 10.1016/j.ejca.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell. 2015;27:439–49. doi: 10.1016/j.ccell.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 14.D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 2018;9:282. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Shi Z, Xu X, Yu Z, Mi J. The influence of microenvironment on tumor immunotherapy. FEBS J. 2019;286:4160–75. doi: 10.1111/febs.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52:1543–54. doi: 10.1021/acs.accounts.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 18.Ali ES, Sharker SM, Islam MT, Khan IN, Shaw S, Rahman MA, et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: current status and future perspectives. Semin Cancer Biol. 2020. 10.1016/j.semcancer.2020.01.011 [DOI] [PubMed]
- 19.Yang Z, Ma Y, Zhao H, Yuan Y, Kim BYS. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1590. doi: 10.1002/wnan.1590. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Di C, Li S, Yang X, Nie G. Smart nanotherapeutic targeting of tumor vasculature. Acc Chem Res. 2019;52:2703–12. doi: 10.1021/acs.accounts.9b00283. [DOI] [PubMed] [Google Scholar]
- 21.Qin H, Ding Y, Mujeeb A, Zhao Y, Nie G. Tumor microenvironment targeting and responsive peptide-based nanoformulations for improved tumor therapy. Mol Pharmacol. 2017;92:219–31. doi: 10.1124/mol.116.108084. [DOI] [PubMed] [Google Scholar]
- 22.Deng H, Zhang Z. The application of nanotechnology in immune checkpoint blockade for cancer treatment. J Control Release. 2018;290:28–45. doi: 10.1016/j.jconrel.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 25.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers. 2019;11:1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol. 2019;7:4. doi: 10.3389/fcell.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2017;36:439–45. doi: 10.1038/onc.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol. 2015;42:663–71. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 33.Ou W, Thapa RK, Jiang L, Soe ZC, Gautam M, Chang JH, et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J Control Release. 2018;281:84–96. doi: 10.1016/j.jconrel.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Kosmides AK, Sidhom JW, Fraser A, Bessell CA, Schneck JP. Dual Targeting nanoparticle stimulates the immune system to inhibit tumor growth. ACS Nano. 2017;11:5417–29. doi: 10.1021/acsnano.6b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou MZ, Liu WL, Li CX, Zheng DW, Zeng JY, Gao F, et al. A multifunctional biomimetic nanoplatform for relieving hypoxia to enhance chemotherapy and inhibit the PD-1/PD-L1 axis. Small. 2018;14:e1801120. doi: 10.1002/smll.201801120. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Wang Y, Bai R, Yang K, Tian Z. MiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1alpha regulation. Oncogenesis. 2016;5:e224. doi: 10.1038/oncsis.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Li F, Lu GH, Nie W, Zhang L, Lv Y, et al. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano. 2019;13:5662–73. doi: 10.1021/acsnano.9b00892. [DOI] [PubMed] [Google Scholar]
- 38.Ricciuti B, Leonardi GC, Puccetti P, Fallarino F, Bianconi V, Sahebkar A, et al. Targeting indoleamine-2,3-dioxygenase in cancer: scientific rationale and clinical evidence. Pharmacol Ther. 2019;196:105–16. doi: 10.1016/j.pharmthera.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines. 2015;3:703–29. doi: 10.3390/vaccines3030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–8. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian S, Zhang M, Chen Q, He Y, Wang W, Wang Z. IDO as a drug target for cancer immunotherapy: recent developments in IDO inhibitors discovery. RSC Adv. 2016;6:7575–81. [Google Scholar]
- 43.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors:from bench to bedside. Cancer Res. 2017;77:6795–811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng K, Ding Y, Zhao Y, Ye S, Zhao X, Zhang Y, et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 2018;18:3250–8. doi: 10.1021/acs.nanolett.8b01071. [DOI] [PubMed] [Google Scholar]
- 46.Lee HH, Kang H, Cho H. Natural killer cells and tumor metastasis. Arch Pharm Res. 2017;40:1037–49. doi: 10.1007/s12272-017-0951-9. [DOI] [PubMed] [Google Scholar]
- 47.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–36. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 48.Valipour B, Velaei K, Abedelahi A, Karimipour M, Darabi M, Charoudeh HN. NK cells: an attractive candidate for cancer therapy. J Cell Physiol. 2019;234:19352–65. doi: 10.1002/jcp.28657. [DOI] [PubMed] [Google Scholar]
- 49.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–24. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 51.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–88. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 53.Ewen EM, Pahl JHW, Miller M, Watzl C, Cerwenka A. KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur J Immunol. 2018;48:355–65. doi: 10.1002/eji.201747128. [DOI] [PubMed] [Google Scholar]
- 54.Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol. 2019;105:1319–29. doi: 10.1002/JLB.MR0718-269R. [DOI] [PubMed] [Google Scholar]
- 55.Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36:1191–9. doi: 10.1038/aps.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Y, Tian Z, Zhang C. Natural killer cell-based immunotherapy for cancer: advances and prospects. Engineering. 2019;5:106–14. [Google Scholar]
- 57.Sanseviero E. NK cell-Fc receptors advance tumor immunotherapy. J Clin Med. 2019;8:1667. doi: 10.3390/jcm8101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7:105. doi: 10.21037/atm.2019.01.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, Lopez-Diaz de Cerio A, Cabo M, et al. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 61.Ji T, Lang J, Ning B, Qi F, Wang H, Zhang Y, et al. Enhanced natural killer cell immunotherapy by rationally assembling Fc fragments of antibodies onto tumor membranes. Adv Mater. 2019;31:e1804395. doi: 10.1002/adma.201804395. [DOI] [PubMed] [Google Scholar]
- 62.Zhou F, Feng B, Yu H, Wang D, Wang T, Ma Y, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31:e1805888. doi: 10.1002/adma.201805888. [DOI] [PubMed] [Google Scholar]
- 63.Wyatt LC, Lewis JS, Andreev OA, Reshetnyak YK, Engelman DM. Applications of pHLIP technology for cancer imaging and therapy. Trends Biotechnol. 2017;35:653–64. doi: 10.1016/j.tibtech.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng C, Wang Q, Wang Y, Zhao X, Gao K, Liu Q, et al. In situ modification of the tumor cell surface with immunomodulating nanoparticles for effective suppression of tumor growth in mice. Adv Mater. 2019;31:e1902542. doi: 10.1002/adma.201902542. [DOI] [PubMed] [Google Scholar]
- 65.Kim KS, Han JH, Park JH, Kim HK, Choi SH, Kim GR, et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials. 2019;221:119418. doi: 10.1016/j.biomaterials.2019.119418. [DOI] [PubMed] [Google Scholar]
- 66.Wu L, Zhang F, Wei Z, Li X, Zhao H, Lv H, et al. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater Sci. 2018;6:2714–25. doi: 10.1039/c8bm00588e. [DOI] [PubMed] [Google Scholar]
- 67.Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol. 2019;113:31–7. doi: 10.1016/j.molimm.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadeghzadeh M, Bornehdeli S, Mohahammadrezakhani H, Abolghasemi M, Poursaei E, Asadi M, et al. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020: 117580. 10.1016/j.lfs.2020.117580 [DOI] [PubMed]
- 70.Kim MK, Kim J. Properties of immature and mature dendritic cells: phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019;9:11230–8. doi: 10.1039/c9ra00818g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 72.Radford KJ, Tullett KM, Lahoud MH. Dendritic cells and cancer immunotherapy. Curr Opin Immunol. 2014;27:26–32. doi: 10.1016/j.coi.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Galati D, Zanotta S. Empowering dendritic cell cancer vaccination: the role of combinatorial strategies. Cytotherapy. 2018;20:1309–23. doi: 10.1016/j.jcyt.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Menares E, Galvez-Cancino F, Caceres-Morgado P, Ghorani E, Lopez E, Diaz X, et al. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun. 2019;10:4401. doi: 10.1038/s41467-019-12319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–8. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 76.Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic cell-based immunotherapy: state of the art and beyond. Clin Cancer Res. 2016;22:1897–906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 77.Hanks BA, Jiang J, Singh RA, Song W, Barry M, Huls MH, et al. Re-engineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo. Nat Med. 2005;11:130–7. doi: 10.1038/nm1183. [DOI] [PubMed] [Google Scholar]
- 78.Sabado RL, Bhardwaj N. Cancer immunotherapy: dendritic-cell vaccines on the move. Nature. 2015;519:300–1. doi: 10.1038/nature14211. [DOI] [PubMed] [Google Scholar]
- 79.Gao S, Yang D, Fang Y, Lin X, Jin X, Wang Q, et al. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics. 2019;9:126–51. doi: 10.7150/thno.29431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:532–47. doi: 10.1002/wnan.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 82.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 83.Park YM, Lee SJ, Kim YS, Lee MH, Cha GS, Jung ID, et al. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 2013;13:177–83. doi: 10.4110/in.2013.13.5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheerlinck JP, Greenwood DL. Virus-sized vaccine delivery systems. Drug Discov Today. 2008;13:882–7. doi: 10.1016/j.drudis.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 85.Salem AK. Nanoparticles in vaccine delivery. AAPS J. 2015;17:289–91. doi: 10.1208/s12248-015-9720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett. 2014;162:59–67. doi: 10.1016/j.imlet.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Cao X. Intratumoral dendritic cells in the anti-tumor immune response. Cell Mol Immunol. 2015;12:387–90. doi: 10.1038/cmi.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019;31:e1802896. doi: 10.1002/adma.201802896. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–96. doi: 10.1039/c5lc01117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou J, Meng L, Ye W, Wang Q, Geng S, Sun C. A sensitive detection assay based on signal amplification technology for Alzheimer’s disease’s early biomarker in exosome. Anal Chim Acta. 2018;1022:124–30. doi: 10.1016/j.aca.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 91.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu H, Chen L, Peng Y, Yu S, Liu J, Wu L, et al. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget. 2018;9:2887–94. doi: 10.18632/oncotarget.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian X, Zhu M, Tian Y, Ramm GA, Zhao Y, Nie G. A membrane vesicle-based dual vaccine against melanoma and Lewis lung carcinoma. Biomaterials. 2012;33:6147–54. doi: 10.1016/j.biomaterials.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 94.Min H, Wang J, Qi Y, Zhang Y, Han X, Xu Y, et al. Biomimetic metal-organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv Mater. 2019;31:e1808200. doi: 10.1002/adma.201808200. [DOI] [PubMed] [Google Scholar]
- 95.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci USA. 2007;104:932–6. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kroll AV, Jiang Y, Zhou J, Holay M, Fang RH, Zhang L. Biomimetic nanoparticle vaccines for cancer therapy. Adv Biosyst. 2019;3:1800219. doi: 10.1002/adbi.201800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fontana F, Fusciello M, Groeneveldt C, Capasso C, Chiaro J, Feola S, et al. Biohybrid vaccines for improved treatment of aggressive melanoma with checkpoint inhibitor. ACS Nano. 2019;13:6477–90. doi: 10.1021/acsnano.8b09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29:1703969. doi: 10.1002/adma.201703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 2015;75:5–10. doi: 10.1158/0008-5472.CAN-14-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaiser J. Personalized tumor vaccines keep cancer in check. Science. 2017;356:122. doi: 10.1126/science.356.6334.122. [DOI] [PubMed] [Google Scholar]
- 101.Ali OA, Lewin SA, Dranoff G, Mooney DJ. Vaccines combined with immune checkpoint antibodies promote cytotoxic T-cell activity and tumor eradication. Cancer Immunol Res. 2016;4:95–100. doi: 10.1158/2326-6066.CIR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee DH, Kim SH, Kang W, Choi YS, Lee SH, Lee SR, et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011;29:8293–301. doi: 10.1016/j.vaccine.2011.08.102. [DOI] [PubMed] [Google Scholar]