Abstract
TMEM16A Ca2+-activated chloride channel (CaCC) plays an essential role in vascular homeostasis. In this study we investigated the molecular mechanisms underlying downregulation of TMEM16A CaCC activity during hypertension. In cultured basilar artery smooth muscle cells (BASMCs) isolated from 2k2c renohypertesive rats, treatment with angiotensin II (0.125−1 μM) dose-dependently increased endophilin A2 levels and decreased TMEM16A expression. Similar phenomenon was observed in basilar artery isolated from 2k2c rats. We then used whole-cell recording to examine whether endophilin A2 could regulate TMEM16A CaCC activity in BASMCs and found that knockdown of endophilin A2 significantly enhanced CaCC activity, whereas overexpression of endophilin A2 produced the opposite effect. Overexpression of endophilin A2 did not affect the TMEM16A mRNA level, but markedly decreased TMEM16A protein level in BASMCs by inducing ubiquitination and autophagy of TMEM16A. Ubiquitin-binding receptor p62 (SQSTM1) could bind to ubiquitinated TMEM16A and resulted in a process of TMEM16A proteolysis in autophagosome/lysosome. These data provide new insights into the regulation of TMEM16A CaCC activity by endophilin A2 in BASMCs, which partly explains the mechanism of angiotensin-II-induced TMEM16A inhibition during hypertension-induced vascular remodeling.
Keywords: TMEM16A, Ca2+-activated chloride channel, basilar smooth muscle cells, angiotensin II, endophilin A2, ubiquitination, autophagy, hypertension, vascular remodeling
Introduction
TMEM16A/Ano1 is the molecular identity of the Ca2+-activated chloride channel (CaCC) in eukaryotic cells [1–4]. The TMEM16A-mediated CaCC has been reported to regulate a variety of physiological functions, including neuronal excitability, nociception, transepithelial ion transport, and gastrointestinal peristalsis [5–9]. Our recent study found that CaCC activity in the cardiovascular system, as well as TMEM16A expression level, was decreased in basilar artery smooth muscle cells (BASMCs) isolated from two-kidney two-clip (2k2c) renohypertensive rats, possibly caused by a high concentration of angiotensin II during hypertension [10]. Although diverse physiological and pathological functions have been described, regulatory mechanisms of the TMEM16A channel have rarely been reported. Calmodulin, one of the many modulators, has been identified as a factor contributing to the Ca2+ dependence of TMEM16A channel activity [11–13]. Recently, another intriguing study reported that secreted calcium-activated chloride channel regulator 1 could modulate TMEM16A-dependent calcium-activated chloride currents by stabilizing TMEM16A on the cell surface in HEK293T cells [14], which gives rise to new insight into seeking proper adaptor proteins required for TMEM16A CaCC modulation. A recent proteomics study identified several endogenous proteins implicated in protein trafficking, folding, and stability that interact with TMEM16A, including SNARE complexes such as syntaxins and syntaxin-binding proteins, as well as ezrin–radixin–moesin scaffolding proteins [15]. These studies reveal the importance of the regulation of TMEM16A distribution and stability on CaCC activity. Identification of appropriate modulators in different tissues is crucial to precisely understand TMEM16A functions.
Endophilin A2 is a member of the endophilin protein family that is widely expressed in eukaryotic cells and profoundly exists in smooth muscle cells compared with the other family members [16]. Endophilins are composed of two conserved functional domains, including a sizeable N-terminal BAR (Bin–amphiphysin–Rvs) domain, which can induce and stabilize membrane curvature upon dimerization, and a C-terminal SH3 (Src homology 3) domain, which can recognize and bind to proline-rich domains (PRDs). There are other undiscovered domains that mediate essential functions as well [17–19]. Evidence has shown that endophilin A2 plays a vital role in regulating clathrin-independent endocytosis and clathrin-dependent endocytosis due to its unique protein structure [20]. In addition, amino acid residues 200–219 mediate the direct interaction of the endophilin-Ca2+ channel complex, whereas E264, which is flanked with a PRD, is required to form a binding site for Ca2+ and can affect the conformation of the endophilin A2 protein in a Ca2+-dependent manner, eventually modulating the formation of the endophilin A2–Ca2+ channel complex [21]. Based on multiple related functions of endophilin A2, we tested the hypothesis that endophilin A2 could regulate TMEM16A CaCC activity. In this study, we identified endophilin A2 as a vital regulator involved in TMEM16A CaCC activity in smooth muscle cells.
Materials and methods
Animals
For primary BASMC isolation, 4-week-old Sprague–Dawley rats were used. Endophilin A2 transgenic mice were generated as described previously [22]. All experiments were approved by and conducted in accordance with the ethical guidelines of the Sun Yat-Sen University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize any pain or discomfort, and the minimum number of animals was used.
Cell culture
Rat BASMCs were isolated and cultured from rat basilar arteries using the explant method as previously described [23, 24]. Briefly, 4-week-old male Sprague–Dawley rats (150~180 g) were anesthetized with pentobarbital sodium (60 mg/kg, intraperitoneally). The basilar arteries were collected immediately and immersed in Krebs solution containing 105 U/L penicillin and 100 mg/L streptomycin. After the connective tissue was cleaned, the basilar arteries were cut into small pieces ~0.5 mm long and the vessel segments were placed on the surface of the culture dish. The dish was then incubated in Dulbecco’s modified Essential medium/F-12 supplemented with 20% fetal calf serum at 37 °C and 5% CO2. Passages 8–12 of the cultured BASMCs were used for experiments.
Adenoviral infection
Ad-Endophilin A2 was designed and produced by Sunbio Medical Biotechnology (Shanghai, China). Briefly, cultured BASMCs at 50% confluence were infected with adenovirus encoding endophilin A2 for 4 h and then the cells were washed with phosphate-buffered saline (PBS) and incubated in fresh culture medium before experimentation.
Plasmid transfection
Plasmids were transfected with Lipofectamine 2000 reagent (InvitrogenTM, Life Technologies, Grand Island, NY, USA). Briefly, plasmids were mixed with Lipofectamine 2000 in OPTI-MEMRI medium (InvitrogenTM, Life Technologies) and incubated with cells according to the manufacturer’s instructions. Six hours later, the cells were rinsed with PBS and incubated in fresh culture medium for 42 h before cell lysate collection.
siRNA transfection
The sequences of small interfering RNA (siRNA) (Supplementary Table S1) against rat endophilin A2 and p62 were synthesized (Invitrogen, Life Technologies). Briefly, siRNA was diluted in 100 μL culture medium and mixed with 12 μL HiPerFect reagent (Qiagen, Valencia, CA, USA) for 20 min at room temperature. The mixture was then added to the cells and incubated for 3 h in serum-free medium (the final siRNA concentration was 40 nM). Finally, the cells were washed once with PBS and incubated in standard culture medium for another 45 h. The siRNA efficiency was detected by Western blotting (Supplementary Fig. S1).
Reverse-transcription PCR
Briefly, total RNA was isolated using Trizol reagent (InvitrogenTM, Life Technologies) according to the manufacturer’s instructions. One picogram of total RNA was reverse-transcribed in a total volume of 30 μL on a Thermal Cycler (Thermo Scientific, Waltham, MA, USA) according to the instructions of the One-step RT-PCR Kit (Qiagen, Valencia, CA, USA). Reactants underwent reverse transcription (50 °C, 30 min), an initial denaturation (95 °C, 15 min), 35 amplification cycles (94 °C, 45 s; 55 °C, 45 s; 72 °C, 60 s), and a final extension (72 °C, 10 min). The specific primers are listed in Supplementary Table S2.
Western blotting analysis
Cells or tissues were lysed with RIPA lysis buffer containing a protease inhibitor cocktail (Merck, Germany). Protein was separated with 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transformed to a polyvinylidene difluoride (PVDF) membrane. After blocking with 3%–5% milk at room temperature for 1 h, the membrane was incubated with primary antibody (Supplementary Table S3) at 4 °C overnight and then incubated for 1 h with secondary antibody at room temperature. Final detection was carried out with a chemiluminescent horseradish peroxidase substrate (Millipore) by using a BIO-RAD molecular imager, ChemiDoc XRS+. The densities of the target bands were determined by Image Lab 4.0.
Co-immunoprecipitation
Cell lysates were incubated with precoupled antibodies bound to protein G beads overnight at 4 °C. The beads were centrifuged and washed, and then boiled in protein SDS sample buffer. Samples were resolved on 10% SDS-PAGE gels and transferred to PVDF membranes. The bound proteins were determined by immunoblotting with the indicated antibodies.
Electrophysiology
The Ca2+-activated Cl− channel currents were recorded with an Axopatch 200B Amplifier (Axon Instruments, Foster City, CA, USA) using the whole-cell recording technique at room temperature. Patch pipettes were made from borosilicate capillary tubes with a Sutter P-97 horizontal puller (Sutter Instrument, Novato, CA, USA). The resistance of the pipettes was 3–6 MΩ after filling with pipette solution. The currents were elicited with voltage steps from −100 mV to +100 mV in a +20 mV increment for 250 ms with an interval of 5 s from a holding potential of −50 mV. Currents were filtered at 2 kHz and sampled at 5 kHz using pCLAMP8.0 software (Axon Instruments). The extracellular solution contained (mM): NMDG-Cl 125, KCl 5, CaCl2 1.5, MgSO4 1, HEPES 10, glucose 10 and pH was adjusted to 7.4 with NMDG. The pipette solution contained (mM): CsCl 130, Mg·ATP 1, MgCl2 1.2, HEPES 10, EGTA 2, CaCl2 1.639 and pH was adjusted to 7.4 with CsOH. The intracellular Ca2+ concentration was 260 nM.
Immunofluorescence
BASMCs were seeded onto poly-lysine-treated dishes and transfected with pEGFP-LC3 and red fluorescent protein (RFP) endophilin A2. After 24 h of incubation, the cells were fixed with 4% paraformaldehyde. After permeabilization with blocking buffer (PBS containing 5% normal donkey serum, 1% bovine serum albumin), the cells were incubated overnight in blocking buffer at 4 °C with the indicated antibodies (Supplementary Table S3). Subsequently, the dishes were rinsed in washing buffer (PBS with 0.2% TX-100) and incubated for 2 h at room temperature with fluorescently conjugated secondary antibodies and Hoechst diluted in blocking buffer. Pictures were acquired on a confocal microscope (Fluoview FV1000, Olympus).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). All data are expressed as the mean ± SD. Statistical differences were assessed with the unpaired two-tailed Student’s t-test for two experimental groups and one-way and two-way analysis of variance for more than two groups. P < 0.05 was considered statistically significant.
Results
Endophilin A2 is upregulated during hypertension
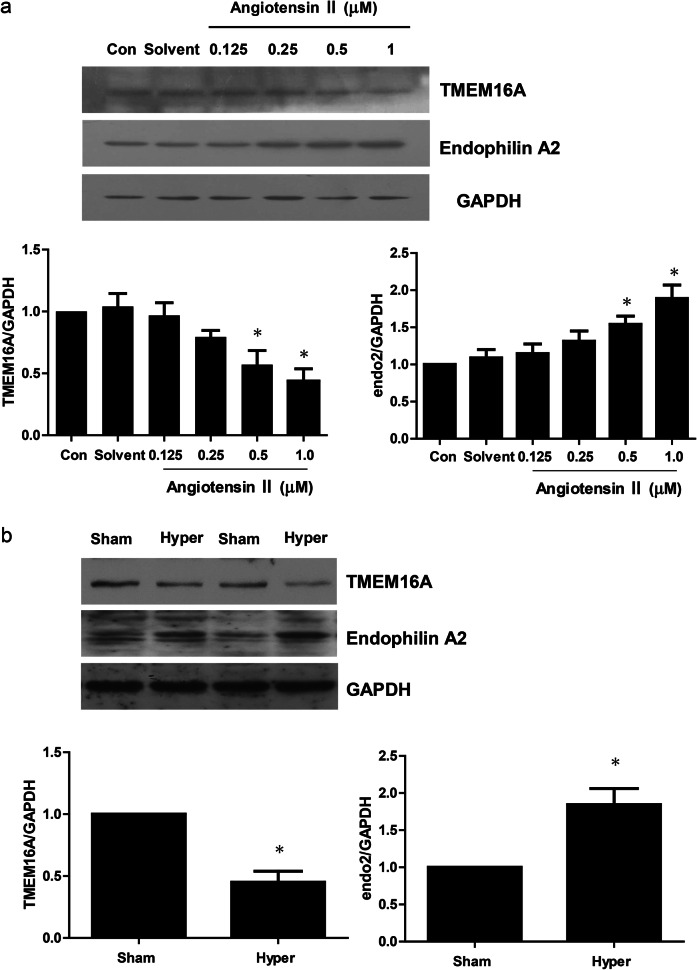
We previously identified TMEM16A as the molecular identity of the CaCC in smooth muscle cells and found that CaCC activity was associated with a high concentration of angiotensin II in the basilar artery of the 2k2c renohypertensive rat model. In this study, by employing BASMCs isolated from a 2k2c renohypertensive rat model, we found that angiotensin II could increase endophilin A2 levels and decrease TMEM16A expression in a dose-dependent manner (Fig. 1a). Similar results were also found in basilar arteries isolated from the 2k2c model. As shown in Fig. 1b, a significant increase in endophilin A2 was observed in 4-week-old 2k2c renohypertensive rat basilar arteries compared with Sham control arteries. There have been reports on the association between endophilin A2 and calcium regulation, as well as the association between endophilin A2 and TMEM16A expression during hypertension, and we therefore wanted to test the effects of endophilin A2 on TMEM16A CaCC activity.
Fig. 1.
Expression of Endophilin A2 and TMEM16A in hypertensive rat basilar artery and BASMCs in response to Angiotensin II stimuli. a BASMCs were incubated with Angiotensin II at concentrations indicated above for 48 h; TMEM16A and Endophilin A2 expression were detected by Western blotting. n = 6; *P < 0.05 vs. Control (Con). b Two-kidney two-clip (2k2c) renovascular stroke-prone hypertensive rats were operated 4 weeks later; TMEM16A and Endophilin A2 expression in sham operation (Sham and hypertension (Hyper) basilar artery were detected by Western blotting). n = 6; *P < 0.05 vs. Sham
Endophilin A2 modulates the activity of CaCCs
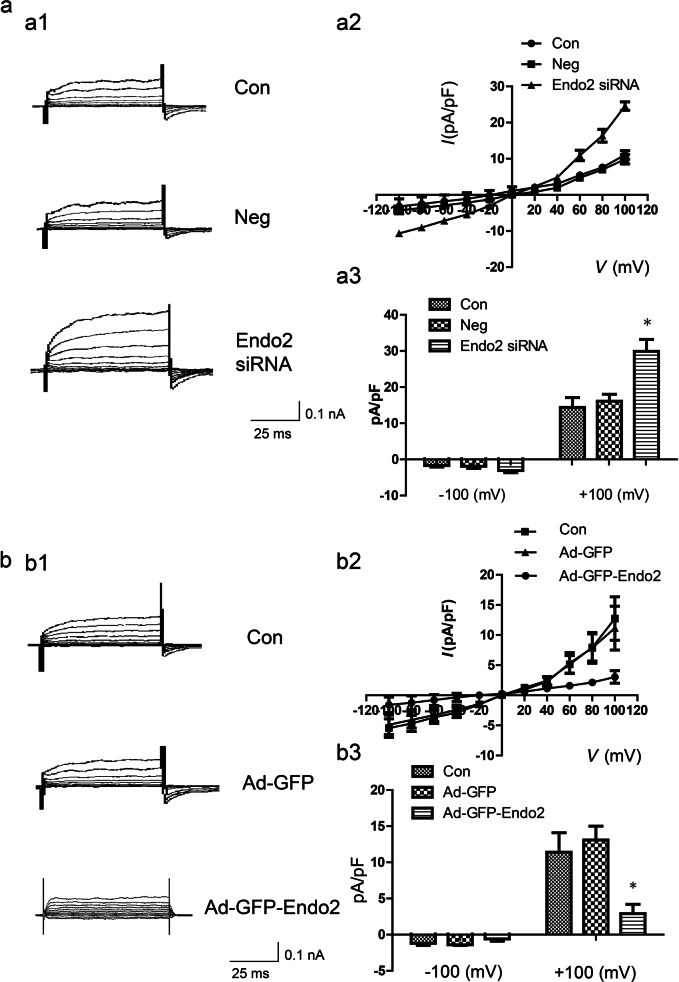
To determine whether endophilin A2 could regulate TMEM16A CaCC activity, we carried out experiments to record ICaCC. BASMCs were transfected with endophilin A2 fluorescent-labeled siRNA or nonspecific, scrambled RNA. ICaCC of the fluorescent-labeled cells was recorded by whole-cell patch clamp electrophysiology. In the presence of 260 nM intracellular Ca2+ and a physiological concentration of extracellular Cl−, a robust outward rectifying current was recorded in the endophilin A2 siRNA group compared with the nonspecific, scrambled RNA or untransfected group (Fig. 2a). By contrast, overexpression of endophilin A2 significantly decreased CaCC activity according to the recording of ICaCC of green fluorescent protein (GFP)-expressing cells infected with endophilin A2-GFP adenovirus or GFP control (Fig. 2b). Similar results were found in BASMCs freshly isolated from endophilin A2 transgenic mice [22] and their littermate controls (Fig. 3c). These data suggest that endophilin A2 can modulate CaCC activity in smooth muscle cells.
Fig. 2.
Endophilin A2 modulates the activity of calcium-activated Cl− channel. a Representative traces of calcium-activated Cl− current (a1), I–V curves (a2), and mean current densities measured at ±100 mV in Control (Con), nonspecific, scrambled RNA (Neg), Endophilin A2 siRNA knockdown (Endo2 siRNA), which were evoked by 260 nM [Ca2+]i. n = 8, *P < 0.05 vs. Con and Neg group. b Representative traces of calcium-activated Cl− current (b1), I–V curves (b2), and mean current densities measured at ±100 mV (b3) in control, GFP adenovirus (Ad-GFP), and GFP-Endophilin A2 adenovirus (Ad-GFP-Endo2) groups, which were evoked by 260 nM [Ca2+]i. n = 8, *P < 0.05 vs. Con and Ad-GFP group
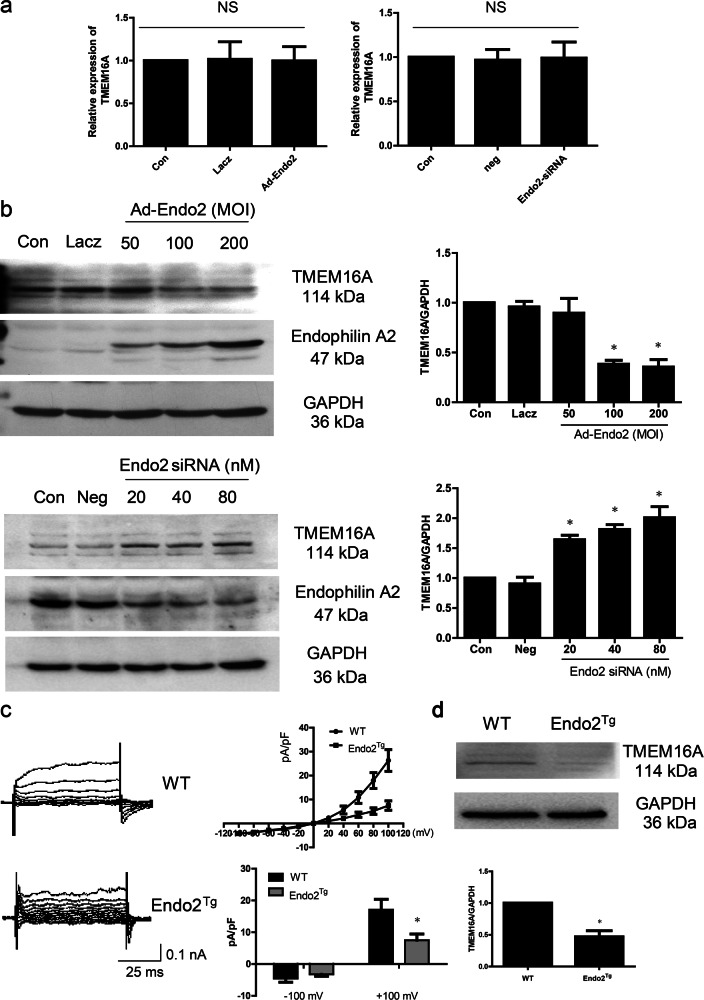
Fig. 3.
Endophilin A2 regulates TMEM16A protein expression. a Endophilin A2 had no effects on TMEM16A mRNA level under overexpression (left) or knockdown (right) of Endophilin A2 in BASMCs. b Western blotting showed overexpression (up) of Endophilin A2 decreased TMEM16A expression, whereas knockdown (down) of Endophilin A2 increased TMEM16A expression in BASMCs. Multiplicity of infection (MOI): the number of infected viral particles per cell. n = 6, *P < 0.05 vs. Con. c Representative traces of calcium-activated Cl− current, I–V curves, and mean current densities measured at ±100 mV in BASMCs isolated from wild-type (WT) and Endophilin A2 transgenic mouse (endo2Tg). n = 8, *P < 0.05 vs. WT. d Expression of TMEM16A in basilar artery from WT and Endophilin A2 transgenic mouse (endo2Tg). n = 6, *P < 0.05 vs. WT
Endophilin A2 regulates the expression of TMEM16A protein
Next, to evaluate how endophilin A2 modulates TMEM16A CaCC activity in smooth muscle cells, we determined the mRNA and protein levels of TMEM16A with overexpression or knockdown of endophilin A2. The quantitative reverse-transcription PCR (qRT-PCR) results showed that there was no significant effect on TMEM16A mRNA level by adenovirus-mediated endophilin A2 overexpression or endophilin A2 knockdown (Fig. 3a). However, the Western blotting results revealed that the TMEM16A protein level was significantly decreased following adenovirus-mediated overexpression of endophilin A2, whereas knockdown of endophilin A2 increased the protein level of TMEM16A (Fig. 3b). The expression of TMEM16A was also reduced in the basilar arteries isolated from endophilin A2 transgenic mice (Fig. 3d). Furthermore, the calcium-activated Cl− current was decreased in BASMCs freshly isolated from endophilin A2 transgenic mice compared with WT mice (Fig. 3c). Together, these data implicate that endophilin A2 plays a causal role in modulating TMEM16A CaCC activity and modulates TMEM16A protein expression by regulating TMEM16A posttranslational modification rather than transcriptional regulation.
Upregulation of TMEM16A ubiquitination by endophilin A2 as a prerequisite for selective autophagy-mediated degradation
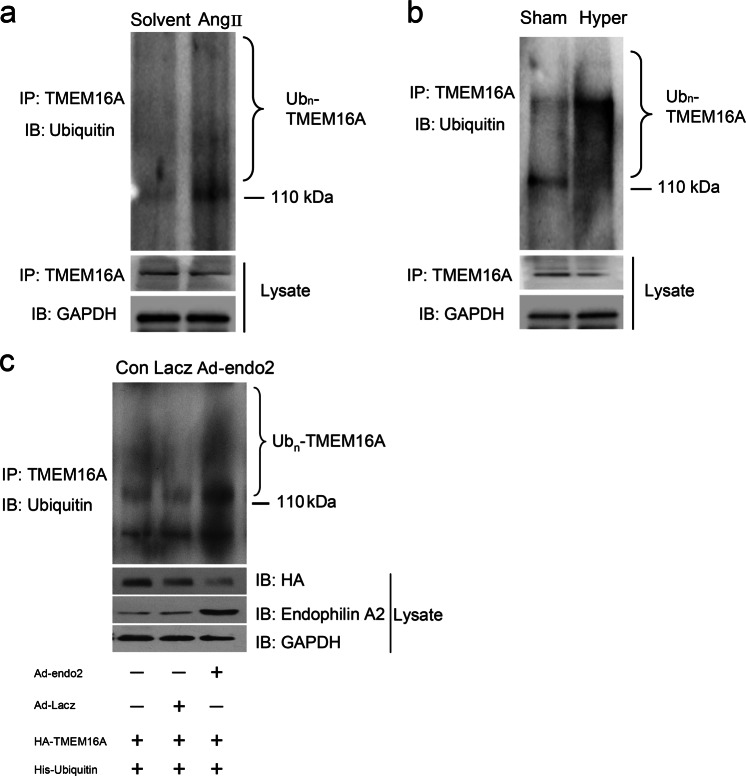
In our study, we showed that angiotensin II induced a decrease in TMEM16A protein in BASMCs. To elucidate how angiotensin II regulates TMEM16A protein expression, we detected the effects of TMEM16A ubiquitination following angiotensin II stimulation. The results showed that TMEM16A ubiquitination was significantly increased following stimulation of angiotensin II, whereas the expression level of TMEM16A was decreased (Fig. 4a). Similar results were found in the basilar arteries isolated from the hypertension model in vivo (Fig. 4b).
Fig. 4.
Involvement of TMEM16A ubiquitination in TMEM16A protein quality control. a BASMCs were treated with Angiotensin II (500 nM) for 48 h, then ubiquitination of TMEM16A was detected by Western blotting. b TMEM16A ubiquitination in basilar artery isolated from sham operation (Sham) and hypertensive (Hyper) rats. c Overexpression Endophilin A2 increased TMEM16A ubiquitination in BASMCs. Cells were transfected with HA-TMEM16A and His-ubiquitin plasmids. Then, the cells were infected with Lacz or Endophilin A2 adenovirus as indicated, ubiquitination of TMEM16A was detected by Western blotting
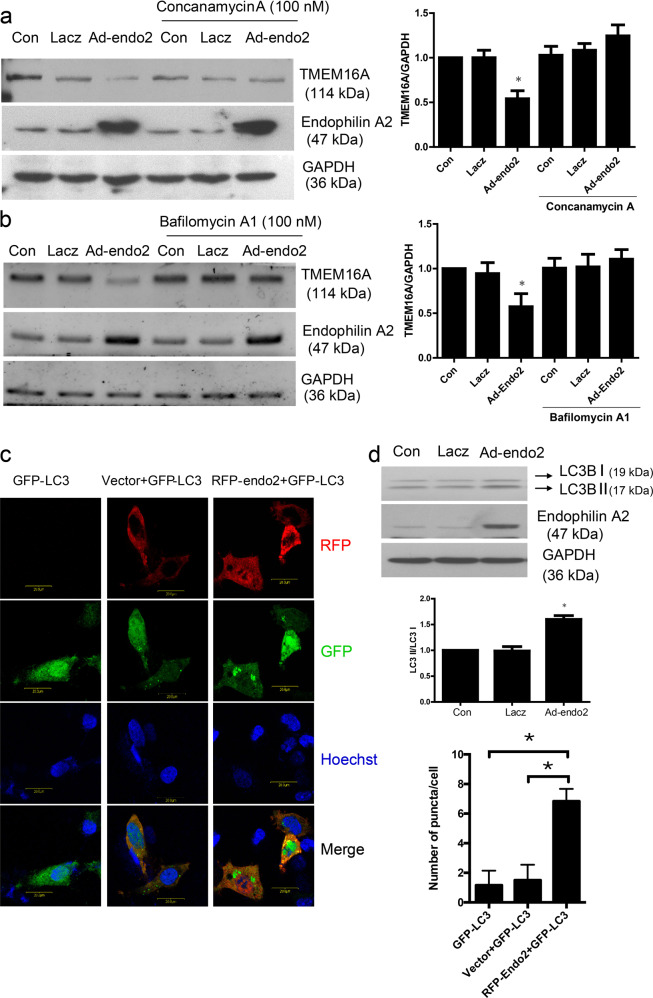
Considering the significant upregulation of endophilin A2 in the basilar arteries isolated from the hypertension model, we hypothesized that overexpression of endophilin A2 could reduce TMEM16A protein expression by modulating TMEM16A ubiquitination, which would further lead to downregulation of CaCC activation. By immunoprecipitating TMEM16A with ubiquitin, we found that overexpression of endophilin A2 resulted in robust polyubiquitination of TMEM16A (Fig. 4c). To identify the exact degradation mechanism by which endophilin A2 regulated TMEM16A protein homeostasis, we used concanamycin A, a specific inhibitor of V-type ATPases that can inhibit lysosome activity, to evaluate its effect on TMEM16A protein levels. As shown in Fig. 5a, the suppression of TMEM16A by endophilin A2 was significantly reversed by concanamycin A. Similar results were found by recruiting bafilomycin A1, another lysosome inhibitor that is involved in the maturation of autophagic vacuoles, as demonstrated by inhibiting fusion between autophagosomes and lysosomes (Fig. 5b). However, no significant difference could be detected when the cells were pretreated with or without the proteasome inhibitor MG132 (Supplementary Fig. S2). These data suggest that the modification of TMEM16A ubiquitination tends to be delivered to the lysosome and eventually degrades in the autophagosome/lysosome.
Fig. 5.
Autophagosome/lysosome inhibitors attenuate degradation of TMEM16A regulated by Endophilin A2. a Effects of concanamycin A (100 nM) on TMEM16A expression modulated by Endophilin A2. n = 6, *P < 0.05 vs. Con and Ad-Endo2 plus concanamycin A. b Effects of Bafilomycin A1 (100 nM) on TMEM16A expression modulated by Endophilin A2. n = 6, *P < 0.05 vs. Con and Ad-Endo2 plus Bafilomycin A1. c Cells cotransfected with RFP-Endophilin A2 and GFP-LC3 plasmids revealed more significant LC3 puncta in comparison with RFP vector and GFP-LC3 group. Statistically significant differences with P-value < 0.05 (*). d Overexpression of Endophilin A2 increased the expression of LC3 and enhanced conversion of LC3 I to LC3 II. n = 6, *P < 0.05 vs. Con
Endophilin A2 regulates autophagy in BASMCs
To confirm whether endophilin A2 was a regulator of autophagy in BASMCs and whether autophagosomes/lysosomes represent a major method by which TMEM16A is degraded, we detected LC3 I/II conversion and GFP-LC3 puncta, which are both hallmarks of autophagosome activation. Significantly increased expression levels of LC3 and evoked conversion of LC3 I to LC3 II were observed with adenovirus-mediated endophilin A2 overexpression (Fig. 5c). Obvious GFP-LC3 puncta were found in SMC cells that were cotransfected with an RFP-endophilin A2 plasmid and a GFP-LC3 plasmid (Fig. 5d). We also detected autophagy flux levels to confirm this observation by using bafilomycin A1 and the results revealed that overexpressing endophilin A2 dramatically increased autophagy flux compared with the LacZ control (Supplementary Fig. S3). These data suggest the critical role of endophilin A2 in regulating autophagosome/lysosome activity, which is associated with TMEM16A degradation.
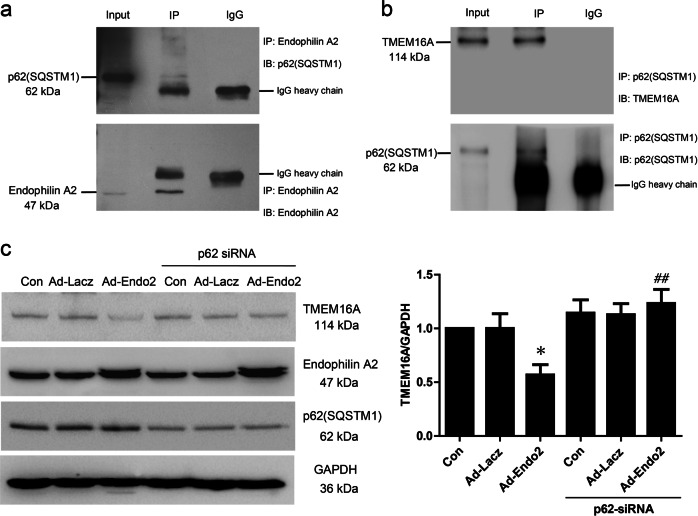
P62(SQSTM1) is required as a bridge coupling ubiquitinated TMEM16A and autophagosomes/lysosomes
To further understand how ubiquitinated TMEM16A is delivered to autophagosomes/lysosomes, we tested the interaction between TMEM16A and p62 (SQSTM1), a ubiquitin-binding receptor that can bind Ub and autophagosome-associated Ub-like (UBL) proteins such as LC3, which mediates wharfing of ubiquitinated protein to the autophagosome. Co-immunoprecipitation experiments showed that p62 (SQSTM1) could bind to both endophilin A2 and TMEM16A, implying the potential essential roles of p62 (SQSTM1) in crosslinking ubiquitinated TMEM16A and autophagosome-associated UBL proteins (Fig. 6a, b). To shed light on the significance of p62(SQSTM1) in the degradation of ubiquitinated TMEM16A, we took advantage of p62 (SQSTM1) siRNA to knock down endogenous p62. Similar to concanamycin A and bafilomycin A1, the disruption of p62 (SQSTM1) caused a significant reversal of the degradation of TMEM16A mediated by endophilin A2 (Fig. 6c). In addition, the immunofluorescence results showed that puncta positive for LC3, as well as p62 with TMEM16A, were increased in adenovirus-mediated endophilin A2-overexpressing cells (Supplementary Fig. S4). Altogether, these data suggest that ubiquitinated TMEM16A modulated by endophilin A2 is recognized by p62(SQSTM1) and is finally delivered to autophagosomes/lysosomes for degradation, which results in a decrease in TMEM16a protein expression and CaCC activity in SMCs.
Fig. 6.
p62 (SQSTM1) could interact with Endophilin A2 and TMEM16A. a Co-immunoprecipitation analysis of Endophilin A2 and p62 (SQSTM1) protein interaction in BASMCs. b Co-immunoprecipitation analysis of TMEM6A and p62 (SQSTM1) protein interaction in BASMCs. The normal IgG was used as a control. c Effects of p62 knockdown on TMEM16A expression modulated by Endophilin A2. n = 6, *P < 0.05 vs. Con. ##P < 0.01 vs. Ad-Endo2
Discussion
Our previous study demonstrated that TMEM16A played a significant role in vascular remodeling by inhibiting angiotensin II-induced proliferation in rat basilar smooth muscle cells. The activation of CaCC and expression of TMEM16A protein were negatively correlated with a medial cross-sectional area in the basilar artery during hypertension [10]. However, how TMEM16A channel activity is negatively regulated during hypertension-induced remodeling is still unclear. In this study, we found that endophilin A2 was significantly increased in basilar arteries isolated from a 2k2c renohypertensive rat model and in BASMCs following angiotensin II stimulus, and this result was accompanied by a downregulation of TMEM16A protein expression. Furthermore, by using the whole-cell patch clamp method, we verified that endophilin A2 could modulate CaCC activity, which implies that endophilin A2 is an important regulator of CaCC during hypertension. We also found that overexpression of endophilin A2 largely decreased the expression of TMEM16A protein, whereas its mRNA level did not change. These data suggest that endophilin A2 regulates the posttranslational modification of TMEM16A protein. Endophilin A2 is a protein with a proline-rich peptide ligand for SH3 domains [21]. Proteins with this functional domain are commonly involved in protein ubiquitination as previously reported [25–27]. Therefore, we speculated that endophilin A2 might play a vital role in TMEM16A ubiquitination during hypertension. The data showed that endophilin A2 undeniably enhanced TMEM16A ubiquitination following angiotensin II stimulus. Although ubiquitination, the process of transferring the target to the proteasome where most cytosolic and nuclear proteins are degraded, is the hallmark of protein degradation, the ability of the proteasome is restrictive due to its inability to degrade most membrane and extracellular proteins, especially oligomeric and aggregated proteins taken up by endocytosis [28, 29]. TMEM16A is also known to be an oligomeric membrane protein complex similar to many other ion channels, which raises the view that the formation of high-weight TMEM16A oligomeric complexes in the membrane might not result in sufficient degradation in the proteasome [30–32]. Therefore, we hypothesized that the modification of TMEM16A ubiquitination could be degraded through ubiquitin-mediated selective autophagy. By using concanamycin A and bafilomycin A1, our results revealed that autophagosome inhibitors reversed the downregulation effect of TMEM16A mediated by endophilin A2. Moreover, endophilin A2 increased the formation of autophagosomes and autophagy in BASMCs. Interestingly, it has been reported that endophilin B1 (Bif-1), another member of the endophilin family, is involved in the regulation of autophagy and is required for induced autophagy in Parkinson’s disease [33–36]. In our study, we found that endophilin A2 participated in the modulation of autophagy in Vascular Smooth Muscle Cells (VSMCs). However, how endophilin A2 regulates cell autophagy and the following functions in VSMCs requires further study. Furthermore, we demonstrated ubiquitin-binding receptor p62 (SQSTM1) as a critical link that bridged ubiquitinated TMEM16A to autophagosomes [37–40] and that knockdown of p62 (SQSTM1) could reverse the downregulation of TMEM16A modulated by endophilin A2.
Taken together, our studies disclose the associations between endophilin A2 and TMEM16A during hypertension. We found that angiotensin II upregulated endophilin A2 expression and facilitated the inhibition of TMEM16A by ubiquitin-mediated selective degradation, ultimately modulating CaCC activity. Our data provide new insights into the mechanisms by which endophilin A2 regulates CaCC activity and angiotensin-II-mediated inhibition of TMEM16A during hypertension-induced vascular remodeling. Future studies are warranted to determine the process of autophagy regulated by endophilin A2 in hypertension by using genetic models.
Supplementary information
Acknowledgements
YYG was supported by the National Natural Science Foundation of China (Key grant numbers 81230082, 81302771, 81525025, 81473206, 81573422, 81773721, and 81500226). XFL is supported by the Natural Science Foundation of Guangdong Province (2018A030310233), The Fundamental Research Funds for the Central Universities (number 18zxxt74).
Author contributions
CZL and FYL conceived and designed the experiments. CZL, FYL, XFL, MMM, XYL, and CXL performed the experiments. CZL and FYL analyzed the data. CZL, FYL, GLW, and YYG wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Can-zhao Liu, Fei-ya Li
Contributor Information
Can-zhao Liu, Email: liucanzhao0521@163.com.
Yong-yuan Guan, Email: guanyy@mail.sysu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41401-019-0298-5) contains supplementary material, which is available to authorized users.
References
- 1.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–4. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–29. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 4.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins) Physiol Rev. 2014;94:419–59. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest. 2010;120:1240–52. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15:1015–21. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, et al. Activation of the Cl- channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal. 2013;6:ra73. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA. 2012;109:16354–9. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, et al. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]
- 11.Tien J, Peters CJ, Wong XM, Cheng T, Jan YN, Jan LY, et al. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. Elife. 2014;3:e02772. doi: 10.7554/eLife.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T, Hendrickson WA, Colecraft HM. Preassociated apocalmodulin mediates Ca2+-dependent sensitization of activation and inactivation of TMEM16A/16B Ca2+-gated Cl- channels. Proc Natl Acad Sci USA. 2014;111:18213–8. doi: 10.1073/pnas.1420984111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG. Dynamic modulation of ANO1/TMEM16A HCO3- permeability by Ca2+/calmodulin. Proc Natl Acad Sci USA. 2013;110:360–5. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sala-Rabanal M, Yurtsever Z, Nichols CG, Brett TJ. Secreted CLCA1 modulates TMEM16A to activate Ca2+-dependent chloride currents in human cells. Elife. 2015;4:e05875. doi: 10.7554/eLife.05875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Cornejo P, Gokhale A, Duran C, Cui Y, Xiao Q, Hartzell HC, et al. Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network. Proc Natl Acad Sci USA. 2012;109:10376–81. doi: 10.1073/pnas.1200174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loll PJ, Swain E, Chen Y, Turner BT, Zhang JF. Structure of the SH3 domain of rat endophilin A2. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:243–6. doi: 10.1107/S1744309108007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gortat A, San-Roman MJ, Vannier C, Schmidt AA. Single point mutation in Bin/Amphiphysin/Rvs (BAR) sequence of endophilin impairs dimerization, membrane shaping, and Src homology 3 domain-mediated partnership. J Biol Chem. 2012;287:4232–47. doi: 10.1074/jbc.M111.325837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renard HF, Simunovic M, Lemiere J, Boucrot E, Garcia-Castillo MD, Arumugam S, et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517:493–6. doi: 10.1038/nature14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai J, Hu Z, Dittman JS, Pym EC, Kaplan JM. Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis. Cell. 2010;143:430–41. doi: 10.1016/j.cell.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Deng L, Maeno-Hikichi Y, Lai M, Chang S, Chen G, et al. Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. doi: 10.1016/S0092-8674(03)00726-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu CZ, Li XY, Du RH, Gao M, Ma MM, Li FY, et al. Endophilin A2 influences volume-regulated chloride current by mediating ClC-3 trafficking in vascular smooth muscle cells. Circ J. 2016;80:2397–406. doi: 10.1253/circj.CJ-16-0793. [DOI] [PubMed] [Google Scholar]
- 23.Liu YJ, Wang XG, Tang YB, Chen JH, Lv XF, Zhou JG, et al. Simvastatin ameliorates rat cerebrovascular remodeling during hypertension via inhibition of volume-regulated chloride channel. Hypertension. 2010;56:445–52. doi: 10.1161/HYPERTENSIONAHA.110.150102. [DOI] [PubMed] [Google Scholar]
- 24.Ma MM, Lin CX, Liu CZ, Gao M, Sun L, Tang YB, et al. Threonine 532 phosphorylation in ClC-3 is required for angiotensin II-induced Cl current and migration in cultured vascular smooth muscle cells. Br J Pharmacol. 2015;173:529–44. doi: 10.1111/bph.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trempe JF, Chen CX, Grenier K, Camacho EM, Kozlov G, McPherson PS, et al. SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol Cell. 2009;36:1034–47. doi: 10.1016/j.molcel.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Stamenova SD, Dunn R, Adler AS, Hicke L. The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J Biol Chem. 2004;279:16017–25. doi: 10.1074/jbc.M313479200. [DOI] [PubMed] [Google Scholar]
- 27.Khan MM, Strack S, Wild F, Hanashima A, Gasch A, Brohm K, et al. Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy. 2014;10:123–36. doi: 10.4161/auto.26841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell. 2009;33:505–16. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Kraft C, Peter M, Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol. 2010;12:836–41. doi: 10.1038/ncb0910-836. [DOI] [PubMed] [Google Scholar]
- 30.Tien J, Lee HY, Minor DL, Jr., Jan YN, Jan LY. Identification of a dimerization domain in the TMEM16A calcium-activated chloride channel (CaCC) Proc Natl Acad Sci USA. 2013;110:6352–7. doi: 10.1073/pnas.1303672110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan JT, Worthington EN, Yu K, Gabriel SE, Hartzell HC, Tarran R. Characterization of the oligomeric structure of the Ca2+-activated Cl- channel Ano1/TMEM16A. J Biol Chem. 2011;286:1381–8. doi: 10.1074/jbc.M110.174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohshiro J, Yamamura H, Saeki T, Suzuki Y, Imaizumi Y. The multiple expression of Ca2+-activated Cl- channels via homo- and hetero-dimer formation of TMEM16A splicing variants in murine portal vein. Biochem Biophys Res Commun. 2014;443:518–23. doi: 10.1016/j.bbrc.2013.11.117. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–51. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi Y, Meyerkord CL, Wang HG. BARgaining membranes for autophagosome formation: regulation of autophagy and tumorigenesis by Bif-1/Endophilin B1. Autophagy. 2008;4:121–4. doi: 10.4161/auto.5265. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Meyerkord CL, Wang HG. Bif-1/endophilin B1: a candidate for crescent driving force in autophagy. Cell Death Differ. 2009;16:947–55. doi: 10.1038/cdd.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong AS, Lee RH, Cheung AY, Yeung PK, Chung SK, Cheung ZH, et al. Cdk5-mediated phosphorylation of endophilin B1 is required for induced autophagy in models of Parkinson’s disease. Nat Cell Biol. 2011;13:568–79. doi: 10.1038/ncb2217. [DOI] [PubMed] [Google Scholar]
- 37.Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Kirkin V, Dikic I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr Opin Cell Biol. 2007;19:199–205. doi: 10.1016/j.ceb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.