Abstract
Background
Cytokine release storm (CRS) is a potentially fatal, hyperinflammatory condition common to both coronavirus disease 2019 (COVID-19) and reactive hemophagocytic lymphohistiocytosis (rHLH). We present our experience with the use of a diagnostic score, developed for rHLH, in a kidney transplant recipient hospitalized with COVID-19.
Methods
We applied the H-Score to risk-stratify our patient to help predict his hospital course. This study was exempt from requiring specific Institutional Review Board approval, but met all the criteria required by our institution for this type of study and report including consent from the patient.
Results
The calculated H-Score for our patient fell below the diagnostic cut-off value for rHLH. Because rHLH is characterized by CRS, we expected him to have a milder hospital course with COVID-19. Correlating with his below cut-off H-score, the patient had a more benign than expected hospital course.
Conclusions
Because this is only a single case, we plan to retrospectively review a series of patients to validate our initial experience—that a low H-Score may correlate with a milder hospital course in kidney transplant patients with COVID-19.
Although a growing number of case reports have been published describing coronavirus disease 2019 (COVID-19) infections in solid organ transplant recipients, few have explored formulas to predict the hospital course of this high-risk patient group. Because outcomes have ranged from mild infection [1] to death due to acute respiratory distress syndrome (ARDS) and cytokine release storm (CRS), in the severe COVID-19 cases [2] we explored the utility of a risk stratification score that could help predict outcomes in these patients. We decided to implement the H-Score, the first validated score developed for the diagnosis of reactive hemophagocytic lymphohistiocytosis (rHLH) [3]. The H-Score is calculated by adding the values assigned to factors such as immunosuppression, fever, interleukin 6 (IL-6) levels, and anemia on admission to the hospital (Table 1 ). Standard admission laboratory tests and clinical features of patients suspected of COVID-19 provide the majority of variables needed to calculate the H-Score. Any data points listed on the table not available when the formula is used are assigned a score of 0. A value of 169 on the H-Score is 93% sensitive and 86% specific for the diagnosis of rHLH, accurately classifying 90% of patients studied. Additionally, the score calculator is available for free online [4]. We describe the use of this score and how it may help risk-stratify kidney transplant patients by predicting who is at higher risk of death from COVID-19.
Table 1.
H-Score for Reactive Hemophagocytic Lymphohistiocytosis
| Points Assigned |
|
|---|---|
| HIV + or known immunosuppression | 18 |
| Hemophagocytosis (bone marrow aspirate) | 35 |
| Body temperature | |
| 38.4ºC-39.4°C | 33 |
| >39.4°C | 49 |
| Hepatomegaly AND splenomegaly (both) | 38 |
| Hepatomegaly OR splenomegaly (either) | 23 |
| Cytopenias (Hgb <9.2, WBC <5, PLT <100) | |
| 3 lineages | 34 |
| 2 lineages | 24 |
| Ferritin | |
| 2000-6000 ng/mL | 35 |
| >6000 ng/mL | 50 |
| Serum aspartate aminotransferase ≥30 IU/L | 19 |
| Fibrinogen ≤2.5 g/L | 25 |
| Triglycerides | |
| Triglycerides 1.5-4.0 mmol/L | 44 |
| Triglycerides ≥4.0 mmol/L | 65 |
Abbreviations: HgB, hemoglobin; PLT, platelets; WBC, white blood cells.
Case
We present a 53-year-old white man whose original kidney disease was biopsy-proven focal segmental glomerulosclerosis and who had undergone a pre-emptive kidney transplant from his 60-year-old father 13 years earlier. He was induced with thymoglobulin and maintained on mycophenolate mofetil (750 mg, twice a day) and tacrolimus (3 mg, every 12 hours). He received no steroids beyond his transplantation admission. His tacrolimus level was kept between 4 to 5 μg/L. He never experienced a rejection episode or a recurrence of his original disease. His medical history included coronary artery disease, diabetes mellitus, and hypertension. He was last seen in clinic in October 2019 and was doing well with a serum creatinine of 1.8 mg/dL, a tacrolimus level of 4 ng/mL, and a normal urine protein to creatinine ratio of 0.2.
On February 4, 2020, he developed an upper respiratory infection and sinusitis. This was treated with azithromycin and he improved. On March 8, he was diagnosed with influenza type B and was treated with a 5-day course of oseltamivir. On March 18th, he was diagnosed with another bout of sinusitis and was given amoxicillin/clavulanic acid. According to his family, on March 21, the patient was exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during a car ride with a coworker, who became acutely ill on March 22 and tested positive for SARS-CoV-2. On March 23, our patient became acutely ill with fever (100.8°F), headache, dry cough, myalgias, chills, and labile blood pressure (range: 82/54-108/70 mm Hg). Prompted by his family, he attended a virtual video visit with his transplant nephrologist, during which he was noted to be very diaphoretic and pale. He was immediately sent to the Houston Methodist Hospital emergency room. The emergency room was given advance notice of the possibility of a COVID-19 case and the patient was instructed to wear a surgical mask. The family later notified the transplant nephrologist that his wife and 6 of his coworkers had tested positive for SARS-CoV-2.
Upon admission, he was put into immediate respiratory isolation in the emergency room. His initial oxygen saturation was 98% on 2 L by nasal cannula. He was found positive for SARS-CoV-2 by qualitative polymerase chain reaction. His chest x-ray and computed tomography scan were consistent with patterns previously described as COVID-19-associated pneumonia [5]. He was admitted to an intermediate care unit designated for suspected or confirmed COVID-19 patients. His maintenance immunosuppression was reduced to 250 mg of mycophenolate mofetil every 12 hours while continuing his tacrolimus to a goal trough level of 5 to 8 mcg/L. On hospital day 2, he was started on 500 mg of azithromycin followed by 250 mg daily and 400 mg daily of hydroxychloroquine, which he was administered for 5 days. On the night of hospital day 5 (symptom day 8), he developed worsening hypoxia (oxygen saturation 82%) on 2 L nasal cannula. The hypoxia improved with increase of oxygen support to 3.5 L nasal cannula. With his worsening clinical condition and increasing inflammatory markers (Table 2 ), his mycophenolate mofetil was held and his tacrolimus continued.
Table 2.
Laboratory Data During Hospital Stay
| Measures | Ref. Range | Baseline 10/04/19 |
Day 1 03/26/20 |
Day 3 03/28/20 |
Day 5 03/30/20 |
Day 7 04/01/20 |
Day 9 04/03/20 |
Day 11 04/05/20 |
Day 12 04/06/20 |
|---|---|---|---|---|---|---|---|---|---|
| AST; U/L | 10-50 | 13 | 49 | 51 | 65 | 68 | 39 | ||
| ALT; U/L | 5-50 | 17 | 44 | 36 | 46 | 47 | 34 | ||
| Alb; g/dL | 3.5-5 | 4.7 | 4.1 | 3.3 | 3.1 | 2.9 | 2.7 | ||
| Sodium; mEq/L | 135-148 | 134 | 137 | 136 | 131 | 132 | 129 | 134 | 137 |
| Potassium; mEq/L | 3.5-5 | 4.3 | 4.4 | 4.2 | 4.6 | 5.2 | 4.6 | 4.9 | 4.8 |
| Chloride; mEq/L | 98-112 | 102 | 100 | 100 | 96 | 96 | 92 | 96 | 99 |
| Bicarbonate; mEq/L | 24-31 | 22 | 22 | 19 | 18 | 22 | 19 | 23 | 23 |
| BUN; mg/dL | 6-20 | 29 | 31 | 25 | 24 | 32 | 34 | 36 | 35 |
| Creatinine; mg/dL | 0.7-1.2 | 1.8 | 3.2 | 2.3 | 2.1 | 2.55 | 2.52 | 2.52 | 2.3 |
| Glucose; mg/dL | 65-99 | 117 | 116 | 101 | 101 | 95 | 111 | 110 | 129 |
| eGFR; mL/min/1.73 m2 | >90 | 42 | 21 | 31 | 36 | 28 | 28 | 28 | 31 |
| Tacrolimus; ng/mL | Variable | 4.5 | 5 | 4.5 | 4 | 4.3 | 4.8 | 6.6 | 6.7 |
| CRP; mg/dL | 0-0.5 | NA | 2.1 | 7.75 | 7.92 | 25 | 33.7 | 30.2 | 18.67 |
| IL-6; pg/mL | <5 | NA | <5 | NA | NA | ||||
| PLTS; k/uL | 150-400 | 285 | 188 | 164 | 175 | 231 | 527 | ||
| HGB; g/dL | 13.2-17.1 | 14.3 | 14.5 | 13.3 | 13.2 | 12.3 | 11.4 | ||
| WBC; 1000 u/L | 3.8-10.8 | 6.9 | 5.1 | 3.2 | 3.3 | 5.8 | 7.3 | ||
| PMN; % | 39-69 | 69.5 | 77.5 | 72.5 | 70.6 | 68 | |||
| Lymph; % | 25-45 | 20.5 | 15.6 | 17.6 | 21.6 | 24 | |||
| Urine; Pr/CR mg/g | 22-128 | 234 | 239 | NA | NA | ||||
| Urine; mOsm/kg | 50-1400 | NA | NA | NA | 526 | 168 | |||
| BP; mm hg | Variable | 130/80 | 100/69 | 120/85 | 118/84 | 120/80 | 130/80 | 132-82 | 130-80 |
| Pulse; bpm | 60-100 | 60 | 90 | 86 | 92 | 100 | 80 | 70 | 70 |
| RR | 12-16 | 12 | 6-16 | 8-20 | 8-20 | 8-38 | 10-20 | 10-20 | 10-12 |
| Tmax; F | 98.6 | 98.6 | 101.2 | 102 | 103.8 | 102.6 | 101 | 101 | 99 |
| Oxygen saturation; % | 99∗ | 99∗ | 96∗ | 92 | 93 on 3.5∗,† | 95 on 3.5∗,† | 9 on 2L∗,† | 9 on 2L∗,† | 95∗ |
| Ferritin; ng/mL | 30-400 | NA | 1707 | 2812 | 4459 | 4347 | 4439 | 4466 |
Abbreviations: Alb, albumin; ALT, alanine aminotransferace; AST, aspartate aminotransferase; BUN, blood urea nitrogen; BP, blood pressure; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HGB, hemoglobin; IL-6, interleukin 6; PLTS, platelets; PMN, polymorphonuclear leukocytes; Pr/CR, protein /creatinine; RR, respiratory rate; WBC, white blood cells.
On room air.
Nasal cannula.
During our patient's worst clinical symptoms, his calculated H-Score was 165. This was below the 169 cut-off value used to diagnose rHLH characterized by CRS. As a result, we decided against the use of IL-6 inhibitor tocilizumab in favor of continuing supportive care. His IL-6 level later returned within the normal range consistent with minimal systemic inflammation and a better prognosis [6]. With continued supportive care including oxygen and nebulized albuterol he was successfully discharged on hospital day 12 on room air. The chronology of his vital signs and labs during his hospitalization are shown in Table 2.
The patient continued to have close follow-up with a SARS-CoV-2 molecular test obtained after discharge. His test returned negative. Although his fever and cough improved, his renal function did not. His baseline creatinine was 1.8 mg/dL and his creatinine post discharge rose to 2.3 mg/dL, which prompted a kidney allograft biopsy on June 12, 2020, 2 months after hospitalization. There was delay in the biopsy due to the need for 2 negative SARS-CoV-2 tests, spaced at least 24 hours apart, and the difficulty of performing an allograft biopsy in the setting of the pandemic.
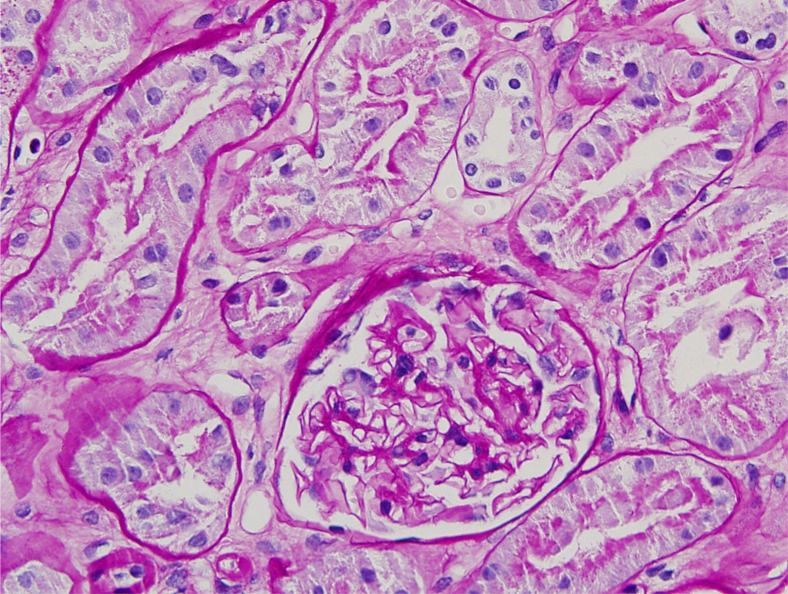
Percutaneous needle biopsy was obtained by 18-gauge biopsy needle. Three cores of renal cortex were divided for light microscopy, electron microscopy, and immunofluorescence studies. They had adequate cortical tissue. Microvascular inflammation (ie, acute glomerulitis and peritubular capillaritis) was not evident. Double contours of the glomerular basement membranes were not detected in periodic acid-Schiff-stained sections. Glomerular cellularity was within normal limits. Features of cell mediated rejection, namely interstitial inflammation, tubulitis, and intimal arteritis, were absent (Fig 1 ).
Fig 1.
Unremarkable glomerulus without acute glomerulitis, reduplication of the glomerular basement membrane. No peritubular capillaritis. Proximal renal tubules are intact. (periodic acid-Schiff stain; original magnification 20X).
Renal tubules were lined by intact, well-preserved epithelium that did not display reactive changes, necrosis, or viral cytopathic changes. A relatively large subcapsular scar composed of several sclerotic glomeruli and inflamed fibrotic interstitium was noted in one core.
Minor changes were noted in the deeper cortex including a few scattered obsolescent glomeruli and mild arterial hyaline sclerosis. Immunostaining for polyomavirus was negative. Fibrosis in the deep cortex was patchy, comprising less than 10% of the cortical surface. Immunofluorescence studies were essentially unremarkable without evidence of immune deposits in the glomeruli or the glomerular basement membranes.
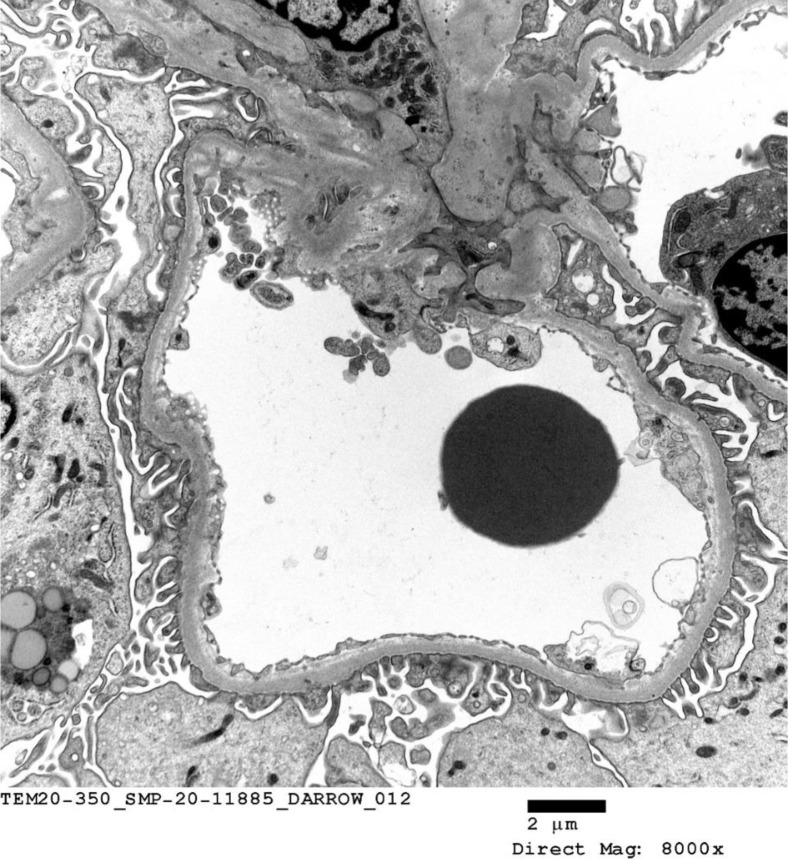
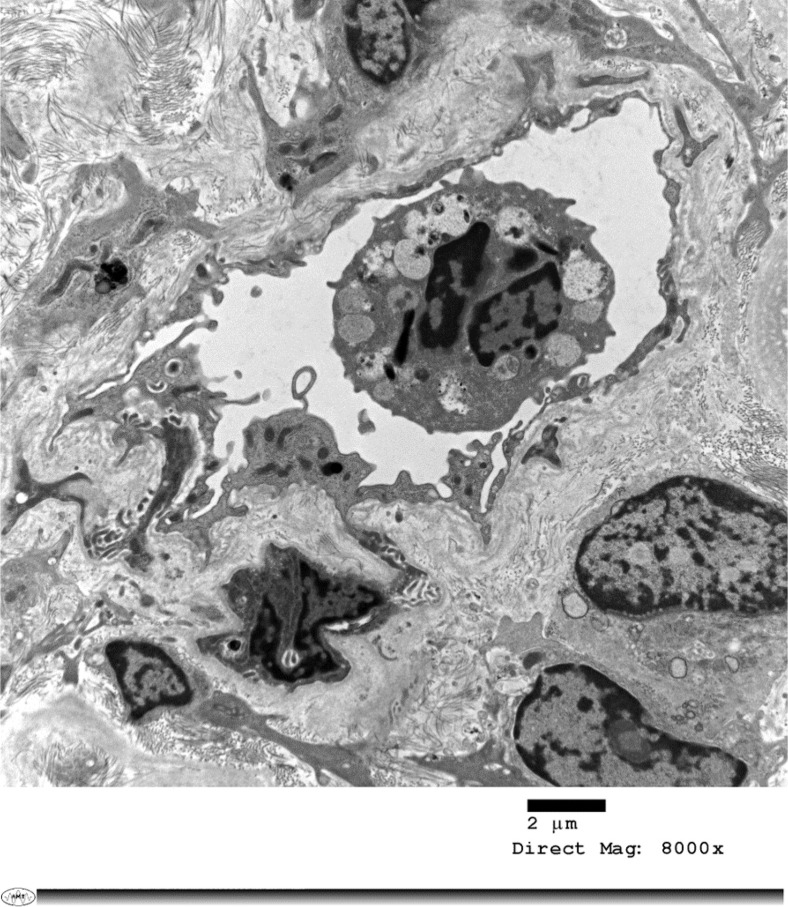
C4d staining was negative in the peritubular capillaries. Electron microscopy confirmed the absence of acute or chronic glomerulitis and peritubular capillaritis (Fig 2 ). Viral inclusions were not detected after careful examination of the samples submitted. A peculiar degenerative change in an intracapillary neutrophil manifested by numerous irregularly shaped and intracytoplasmic vacuoles that appear to contain some nonorganized debris (Fig 3 ).
Fig 2.
Electron micrograph depicting a glomerular capillary loop and a small segment of mesangium. Endothelial cells are not swollen. No margination of inflammatory cells is noted. The lamina densa shows no abnormality.
Fig 3.
Electron micrograph of a peritubular capillary. The endothelium lining the capillary is prominent. Intracapillary, multinucleated, granular leukocyte with intracellular debris and intracytoplasmic clear vesicles. Increased fibrosis in the surrounding interstitium.
Discussion
Based on published data for COVID-19, our patient had 3 of the major risk factors for increased mortality from COVID-19 infection: diabetes mellitus, coronary artery disease, and hypertension [5,7,8]. In an effort to predict his hospital course, we used the H-Score to risk-stratify him for treatment options. This score was previously validated in a multicenter retrospective cohort of 312 patients [3] and is the first score devoted to the diagnosis of rHLH. rHLH is a hyperinflammatory condition caused by the immune system’s exaggerated response to conditions such as infection, autoimmunity, or malignancy, most commonly in the setting of chronic immunosuppression. rHLH presents with fever, pancytopenia, hepatosplenomegaly, and widespread histiocytic infiltration. The H-Score includes immunosuppression as a risk factor, prompting us to consider its application in the transplant population. The score is based on biologic, cytologic, and clinical variables, each with different weights (Table 2). The H-Score generates the probability of rHLH. Our patient’s H-Score was generated by his use of immunosuppression (18 points), fever >103 (49 points), ferritin 4400 (35 points), aspartate aminotransferase >30 IU/L (19 points), and triglycerides (44 points). It is important to note that while a bone marrow aspirate is part of the score, it is not needed to make the diagnosis of rHLH. Our patient did not undergo a bone marrow biopsy, so this variable was assigned a value of zero on the online calculator. The use of this score helped guide our treatment and dissuaded us from using the anti-IL-6 receptor antibody mAB tocilizumab, thus conserving doses of medication for more critically ill patients.
CRS is becoming more clearly described in patients with severe cases of COVID-19 [9]. CRS is an abnormal proinflammatory state where cytokines, such as IL-6, are secreted by infected alveolar macrophages, leading to CD-4 and CD-8 positive T-cellular dysfunction. Both CRS and rHLH can manifest as ARDS and have a similarly hyperactive immune response. Although APACHE II scores are useful in predicting mortality in patients in the intensive care unit [2], we sought a risk assessment for less critically ill patients.
To balance worsening infection and risk of rejection, we withheld mycophenolate mofetil and increased tacrolimus. We did not use corticosteroids as these have not proven useful in treating COVID-19 lung injury [10]. Tacrolimus has been demonstrated to strongly inhibit the growth of SARS-CoV-2 in cell culture [11], so we continued it in case it could be of benefit against SARS-CoV-2 targeting slightly higher trough levels. What is not clear is if immunosuppression increases mortality in COVID-19 patients. Standard immunosuppression drugs are effective against naive T-cells, but do not block memory T and B cells. Tacrolimus may inhibit cytokine release initiated by the virus [12], contributing to reduced mortality.
This kidney allograft biopsy is the only biopsy done to date in our institution. We had several discussions with our pathology group and asked that all efforts be made to see if SARS-CoV-2 viral particles were identified in the biopsy. Electron microscopy did not identify viral particles. We are in the process of validating immunohistochemistry assays to try to identify viral antigens in formalin-fixed paraffin-embedded tissue.
The need to have control tissue from infected patients poses a serious challenge in setting up these assays. Most institutions do not have the negative pressure autopsy suites, which the CDC and other organizations have indicated are necessary to safely perform autopsies on patients who die with COVID-19, thus limiting access to tissue from these patients. Respiratory-related procedures on COVID-19 infected patients are also limited from safety concerns. As we continue to learn from each patient we care for and from each other, we will all be safer from COVID-19.
In summary, the H-Score may potentially have risk-stratification value in transplant patients diagnosed with COVID-19. However, because of the extremely small sample size of this case report (n = 1), it must be validated in a large series of transplant patients diagnosed with COVID-19. We plan to develop a retrospective case series to further investigate the H-Score’s utility in transplant patients diagnosed with COVID-19. We report one of the first kidney biopsies in a kidney transplant recipient who had a milder case of COVID-19 but remained with acute kidney injury over 2 months after returning home. Although his biopsy did not show SARS-CoV-2 viral particles by electron microscopic examination, it also did not show another explanation for his persistent elevation in creatinine. We acknowledge that the measurement of viral particles within the paraffin block would have been the best and most sensitive way to detect the virus. Unfortunately, at this time, this technique is not commercially available. Additionally, we could have overlooked the virus using both light microscopy and electron microscopy due to current limitations in available technology. Our plan is to assess for viral antigens in the biopsy after validation of immunohistochemical assays with appropriate controls.
References
- 1.Zhu L., Xu X., Ma K. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kądziołka I., Świstek R., Borowska K., Tyszecki P., Serednicki W. Validation of APACHE II and SAPS II scales at the intensive care unit along with assessment of SOFA scale at the admission as an isolated risk of death predictor. Anaesthesiol Intensive Ther. 2019;51:107–111. doi: 10.5114/ait.2019.86275. [DOI] [PubMed] [Google Scholar]
- 3.Fardet L., Galicier L., Lambotte O. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 4.HScore Calculator. https://www.mdcalc.com/hscore-reactive-hemophagocytic-syndrome [accessed 21.09.20].
- 5.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [e-pub ahead of print] JAMA. 2020 doi: 10.1001/jama.2020.4683. accessed March 25, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abadja F., Atemkeng S., Alamartine E., Berthoux F., Mariat C. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation. 2011;92:396–403. doi: 10.1097/TP.0b013e3182247b5f. [DOI] [PubMed] [Google Scholar]