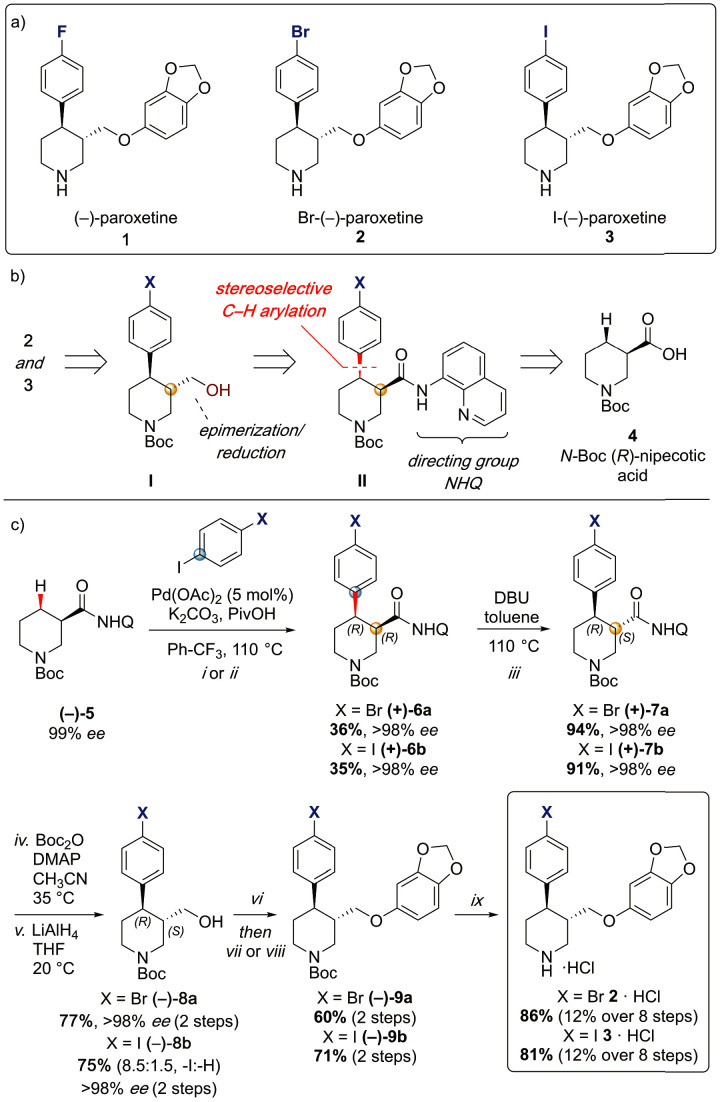
Figure 2. Synthesis of paroxetine analogues.
(a) Structures of (–)-paroxetine (1) and the targeted Br- (2) and I-analogues (3). (b) Retrosynthetic analysis of Br- and I-(–)-paroxetine. (c) Synthesis of Br- and I-(–)-paroxetine 2 and 3. Q = 8 quinolinyl-. Reaction conditions: i) X = Br: (–)−5 (4.0 mmol), 4-bromo iodobenzene (three equiv), Pd(OAc)2 (5 mol %), K2CO3 (one equiv), PivOH (one equiv), Ph-CF3 (2 mL, 2 M), 110°C, 18 hr; ii) X = I: (–)−5 (4.0 mmol), 1,4-diiodobenzene (four equiv), Pd(OAc)2 (5 mol %), K2CO3 (one equiv), PivOH (one equiv), Ph-CF3 (2 mL, 2 M), 110°C, 18 hr; iii) DBU (three equiv), toluene (1 M), 110°C, 24 hr; iv) Boc2O (four equiv), DMAP (20 mol %), CH3CN (0.5 M), 35°C, 22 hr; v) LiAlH4 (two equiv), THF, 20°C, 0.5 hr; vi) MsCl (1.3 equiv), Et3N (1.4 equiv), CH2Cl2, 0 to 25°C, 2 hr; vii) X = Br: sesamol (1.6 equiv), NaH (1.7 equiv), THF, 0°C to 70°C, 18 hr; viii) X = I: sesamol (2.0 equiv), NaH (2.2 equiv), DMF, 0°C to 90°C, 20 hr; ix) 4 N HCl in dioxane (10 equiv), 0°C to 25°C, 18 hr.