Abstract
Background
Coronavirus disease 2019 (COVID-19) playing havoc across the globe caused 585,727 deaths and 13,616,593 confirmed cases so far as per World Health Organization data released till 17th July 2020. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV- 2) is responsible for causing this pandemic across different continents. It is not only impacting the world economy but also quarantined millions of people in their homes or hospitals.
Purpose
At present, there is no Food and Drug Administration-approved drug or vaccine available to treat this disease. Still, people are trying various pre-existing medicines that are known to have anti-viral or anti-parasitic effects. In view of this, the present study aimed to study the binding potential of various phytochemicals present in multiple natural plant extract as a secondary metabolite to non-structural protein 15 (Nsp15) protein, a drug target known to play a crucial role in virulence of coronavirus.
Method
Nsp15 protein was selected because it shows 89% similarity to the other SARS-CoV, which caused the earlier outbreak. The assumption is that inhibition of Nsp15 slowdowns the viral replication. Phytochemicals are selected as these are present in various plant parts (seed, flower, roots, etc.), which are used in different food cuisines in different geographical regions across the globe. The molecular docking approach was performed using two different software, i.e., Autodock, and Swissdock, to study the interaction of various phytochemicals with Nsp15 protein. Hydroxychloroquine is used as a positive control as it is used by medical professionals showing some positive effects in dealing with coronavirus.
Results
The present study demonstrated the binding potential of approximately 50 phytochemicals with Nsp15 and capable of inhibiting the viral replication, although in vitro and in vivo tests are required to confirm these findings.
Conclusions
In conclusion, the present study successfully demonstrated the binding of phytochemicals such as sarsasapogenin, ursonic acid, curcumin, ajmalicine, novobiocin, silymarin and aranotin, piperine, gingerol, rosmarinic acid, and alpha terpinyl acetate to Nsp15 viral protein and they might play a key role in inhibiting SARS-CoV-2 replication.
Keywords: Phytochemicals, Non-structural protein 15 (Nsp15), Molecular docking, Sarsasapogenin, SARS-CoV-2, Coronavirus disease 2019 (COVID-19)
Abbreviations: Nsp15, Non-structural protein 15; SARS-CoV, Severe acute respiratory syndrome coronavirus; COVID-19, Coronavirus disease 2019; WHO, World health organization; RCSB, Research collaboratory for structural bioinformatics; PDB, Protein data bank; RTC, Replicase-transcriptase complex; Mpro, Main protease; MD, Molecular dynamics; RMSD, Root mean square deviation; RMSF, Root mean square function; ACE-2, Angiotensin converting enzyme
Graphical abstract
Introduction
Human coronavirus is an RNA virus that belongs to the family of coronaviruses that can infect both humans and animals, which causes severe acute respiratory syndrome coronavirus (SARS-CoV) (Weiss and Navas-Martin, 2005). It has created difficult pandemic situations across the globe by causing severe respiratory syndrome-associated with cough, sore throat, fever, headache, body ache, and 2314,621 confirmed cases were detected (WHO). Healthcare facilities are on the verge of breakdown in many countries. To date, no specific drug or vaccine is available to treat this infectious disease. Scientists across the world are looking for novel diagnostic, preventive, and therapeutic interventions.
The COVID-19 virus infects the host by binding to host cells, gain entry into the cells than multiply by replication and transcription of viral genome achieved by assembling a multi-subunit RNA-synthesis complex of viral non-structural proteins (Nsp). Based on genome sequence published in RCSB, several proteins were identified that includes spike (S) proteins, envelope (E) protein, membrane (M) protein, nucleocapsid (N) proteins, and a non-structural protein (Nsp) that can be used as suitable drug targets (Gordon et al., 2020). Nsps (such as Nsp12, Nsp13, Nsp14 and Nsp15 are the product of the cleavage of polyproteins by the main protease (Mpro), which gathers into a replicase-transcriptase complex (RTC) (Kong et al., 2020). Nsp 15, also called uridylate-specific endoribonuclease preferentially cleaves 3′ end of uridines. It is encoded by the coronaviruses as RNA-processing enzymes and hence, identified to play is essential for viral replication in the host cells. Studies have shown that inhibition of Nsp15 can slow viral replication (Deng and Baker, 2018). This emphasizes on Nsp15 as a drug target for the development of a potential inhibitor of COVID-19.
Various plants and herbs are necessary for maintaining good health, nutrition, and have medicinal values as they are part of many home remedies for many common illnesses. Phytochemicals present in these plants and herbs are generally nontoxic and have the potential for preventing chronic disorders. Therefore, the present study focusses on the role of these medicinally useful phytochemicals against Nsp15, the RNA-processing enzymes in COVID-19.
Methodology
Software used are autodock and swissdock
Autodock is a molecular docking software developed by Scripps institute based upon a genetic algorithm, available online for predicting the binding affinity of a small-molecule with specific drug targets. Autodock gives binding energy along with free energy hence predicting the stability of the ligand and receptor protein complex. This software is extensively used worldwide for its known accuracy and reliability (Morris et al., 2009).
SwissDock server was used to perform the in-silico docking, whereas UCSF chimera was used for visualization and analysis of docking results. In the SwissDock server, the target protein and ligands (phytochemicals) were submitted. The result obtained in .zip files were viewed in UCSF chimera analysis different docking poses.
Molecular docking analysis by autodock 4.2
Molecular interactions of the phytochemicals with anti-viral activity were studied using Autodock 4.2, to obtain their potential protease activity against Nsp15 of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) endonuclease replication. Nsp15 plays a vital role in viral replication and is responsible for interference with the innate immune response (Kindler et al., 2017). The dimer structure of Nsp15 of novel SARS-CoV-2 (6VWW) was processed using PyMol to prepare protein for docking. Three-dimensional structures of phytochemicals and suspected inhibitors (nelfinavir, hydroxychloroquine) were downloaded from the PubChem Database. Ligand processing was performed using PyMol, and hydrogen was added to the structures. Docking protocol was developed with a grid dimension of −90.6 × 22.12*−31.68 and validated by performing blind docking with inhibitors in the active site, and the docking maps were generated. Post validation of the docking protocol, bioactive phytochemicals were individually docked with target receptor protein in the study. Molecular interactions, ligand conformation,s and binding energies were obtained.
Molecular docking analysis using swissdock
The crystal structure of Nsp15 (PDB ID: 6VWW) was downloaded from the protein databank (http://www.rcsb.org) (Kim et al., 2020). Before docking, the protein structure was prepared using the Dock Prep tool of free software package UCSF Chimera 1.10.1 (Petterson et al., 2004). During preparation, all the non-pro atoms, including water, were removed, hydrogen atoms were added and, partial charges were assigned using AMBER99 force field. The 3D chemical structure of each phytochemical was downloaded from PubChem in PDB and smiles format. For molecular docking studies, we performed blind docking using Swissdock, an online server that uses EADock DSS software (Grosdidier et al., 2011), where we submitted both our protein and phytochemicals one by one. After each docking run, output clusters are obtained, which were ranked by a specific SwissDock algorithm. This algorithm ranks the cluster based on FullFitness (FF) scoring function, where cluster 0 has the best FullFitness score. The individual conformer of each cluster was further arranged according to the FF score. A more favorable binding mode has higher negative FF score and hence, a better fit. UCSF Chimera package was used to visualize the results obtained from SwissDock.
Molecular dynamics studies
Molecular dynamics (MD) studies were performed using GROMACS to understand the conformational dynamics of docked complexes with SARS-CoV-2 Nsp15. MD simulations of the two lead phytochemicals, sarsasapogenin and ursonic acid complexes were performed with the GROMACS 2018.1 package using CHARMM36 force field (Huang et al., 2017). All atoms simulation method was used to gain insight by solving newton's equation of motion. The topology of Nsp15 was generated using pdb2gmx modules of GROMACS. Besides, PRODRG 2.5, an automated server, was used to generate the topology of ligand separately (Schuettelkopf and van Aalten, 2004). The complex was placed in a dodecahedron periodic box using editconf module keeping complex at the center and with 10 Å distance from the edges. The system was then solvated with a simple point charges water model [SPC216] to maintain equilibrium. Solvated systems were then minimizing at maximum force of 1000.0 KJ/mol/nm using with 50,000 steps cut-off. Further, systems reached 300 K to perform equilibration in NVT (number of atoms, volume, and temperature of the system) ensemble. The systems were equilibrated for about 100 ps under both ensemble processes (NVT and NPT) with restrained position dynamics. The LINCS algorithm was used for constraining all the bonds. Finally, the systems were submitted to molecular dynamics simulation for 1 ns to observe the stability of sarsasapogenin and urosonic acid complexes. Structural analysis (RMSD, RMSF, and radius of gyration) was performed and their 3D graphs were generated with xm grace (Graphing, Advanced Computation, and Exploration program).
Results
Molecular docking of phytochemicals using autodock
The active site of SARS-CoV-2 is located in a shallow groove between two anti-parallel β-sheets with six primary catalytic residues- His235, His250, Lys290, Ser294, Thr341, and Tyr343. Where His235, His250, and Lys290, which are proposed to form a catalytic triad of the protein. As evident from the table, the phytochemicals interacted with the main catalytic site residues with strong binding energies indicating irreversible protein inhibition. Table 1 shows the binding energies obtained with phytochemicals along with interacting residues and bond length of molecular interaction.
Table 1.
Phytochemicals with binding energy score and interacting residue wit Nsp15 protein target.
| Phytochemicals | Binding energy | Hydrogen bond | Interacting residues | Bond length |
|---|---|---|---|---|
| Positive control | ||||
| Nelfinavir | −7.3 | 4 | Thr341, His250, Gly248, His235 | (2.6, 2.8), 1.9, 2.7, (2.1, 2.4) |
| Hydroxychloroquine | −5.8 | 4 | His235, His250, Pro344, Ser294 | 1.9, 1.9, 2.4, 2.0 |
| Phytochemicals | ||||
| Sarsasapogenin | −8.5 | 1 | Lys290 | 2.4 |
| Ursonic acid | −8.4 | 5 | Lys290, Gly248, Thr341, His250, His235 | 2.8, 2.0, 2.0, 2.3, 2.5 |
| Novobiocin | −8.2 | 5 | His250, Glu340, His235, Leu246 | 2.2, (2.1, 2.2), 3.1, 3.4 |
| Aranotin | −8.2 | 10 | Leu246, Val292, Lys290, Gly247, Gly248, His250, Thr341, His235 | 3.4, 3.2, (2.5, 2.5), 3.3, 2.2, (1.8, 2.6), 1.9, 2.0 |
| Ajmalicine | −8.1 | 5 | Lys290, Gly248, His235, Thr341, His250 | 2.4, 1.9, 2.1, 2.0, 2.1 |
| Beta sitosterol | −8.1 | 2 | Ser294 | 2.3, 2.4 |
| Alpha amyrin | −8.1 | 1 | Leu346 | 2.4 |
| Silymarin | −8.0 | 2 | Ser294 | 2.3, 2.5 |
| Pomolic acid | −7.9 | 2 | Gln245, His235 | 2.5, 2.8 |
| Carnosol | −7.8 | 2 | Lys290, Ser294 | 2.1, 2.2 |
| Rutin | −7.8 | 6 | Lys290, His250, Thr341, His235, ASP240 | 2.3, 1.9, 2.4, 2.4, (2.2, 3.0) |
| Naringin | −7.8 | 5 | His235, Gly248, His250, Lys290, Val292 | 2.2, 2.1, 2.2, 2.4, 2.7 |
| Arjunolic acid | −7.6 | 1 | Ser294 | 2.7 |
| Asiatic acid | −7.4 | 5 | Lys290, Gly248, Thr341, His235, His250 | 2.8, 2.0, 2.0, 2.5, 2.2 |
| Reserpine | −7.4 | 4 | Glu340, Lys290, His235, Thr341 | 3.4, 2.5, 2.1, 2.1 |
| Betulinic acid | −7.3 | Hydrophobic | ||
| Platanic acid | −7.3 | 5 | Gln245, Leu246, His250, Thr341, His235 | 2.8, 2.6, 2.2, 2.1, 1.9 |
| Berberine | −7.3 | Hydrophobic | ||
| Taspine | −7.3 | 4 | Lys290, Gly248, His250, His235 | 2.6, 2.0, 2.3, 2.1 |
| Alphitolic acid | −7.2 | Hydrophobic | ||
| Taxifolin | −7.2 | 6 | Ser294, Val292, Lys290, His250, His235, Thr341 | 2.4, 3.0, 2.4, 2.3, 1.8, 2.5 |
| Luteolin | −7.2 | 5 | Pro344, Leu346, Lys290, Gly248, His250 | 2.2, 2.6, 2.4, 2.4, 2.4 |
| Apigenin | −7.2 | 4 | His235, Gly248, Tyr343, His250 | 2.4, 2.3, 3.1, 2.2 |
| Myricetin | −7.0 | 5 | Ser294, His250, His235, Gly248 | 3.0, 2.1, (1.9, 2.8), 2.2 |
| Wogonin | −6.9 | 4 | Tyr343, Gly248, His235 | 2.9, 2.4, (2.2, 2.6) |
| Epigallocatechin | −6.8 | 5 | His235, His250, Thr341 | (2.1, 2.2), 1.9, (2.1, 2.3) |
| Chlorogenic acid | −6.8 | 6 | Gln245, Ser294, Val292, His235, Thr341, His250 | 2.2, 2.3, 2.1, 2.0, 2.2, 2.2 |
| Afromosin | −6.7 | 5 | Ser294, His250, Tyr343, Thr341 | (2.0, 2.7), 2.7, 3.2, 2.7 |
| Gliotoxin | −6.7 | 4 | His235, His250, Gly248 | 2.1, (2.1, 2.8), 2.5 |
| Psoralen | −6.7 | 6 | Val292, Lys290, His250, His 235, Gly248 | 3.3, 2.3, (2.4, 2.6), 2.3, 2.3 |
| Carinatine | −6.6 | 5 | Lys290, Ser294, His235, Cys293 | 2.7, (2.1, 2.2), 1.9, 2.3 |
| Rhinacanthin | −6.5 | 6 | Tyr343, Lys290, Gly248, His235, His250, Cys293 | 2.3, 2.4, 2.4, 2.1, 2.2, 3.5 |
| Caffeic acid | −6.3 | 6 | Ser294, His250, His235, Gly248 | (2.6, 2.2), 1.8, (1.9, 2.4), 2.7 |
| Coriandrin | −6.2 | 3 | His250, Lys290, Tyr343 | 2.4, 2.2, 3.2 |
| Scopoletin | −6.1 | 5 | Val292, Cys293, His250, His235, Thr341 | 2.1, 2.3, 2.5, 2.0, 3.1 |
| Cordycepin | −5.6 | 4 | Cys293, His235, Thr341, His250 | 2.3, 2.0, 2.6, 2.0 |
| Ricinoleic acid | −5.0 | 3 | Thr341, His250, His235 | 2.2, 1.9, 2.2 |
| Alpha asarone | −4.9 | 1 | Ser294 | 2.7 |
| Valproic acid | −4.6 | 4 | His235, Lys290, Gly248, His250 | 2.0, 2.6, 2.0, 2.0 |
| Allicin | −3.8 | 3 | Thr341, His235, His250 | 2.2, 2.9, 2.1 |
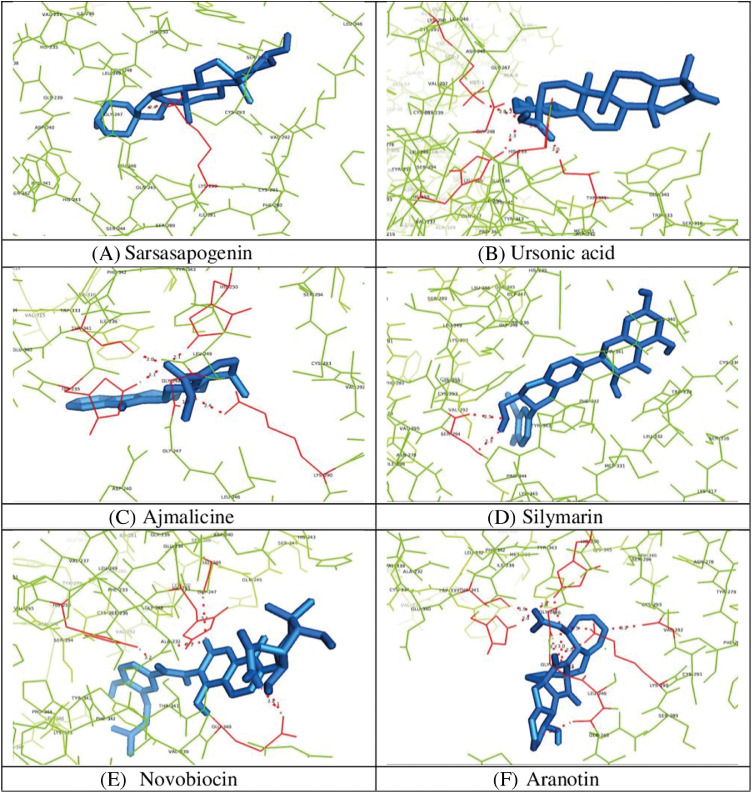
Amongst all studied phytochemicals, promising strong inhibitions could be observed with sarsasapogenin, ursonic acid, ajmalicine, novobiocin, silymarin, and aranotin (Fig. 1 ). Sarsasapogenin interacted with catalytic triad residue Lys290 with a strong single hydrogen bond of such low binding energy of −8.5, which made sarsasapogenin a potential inhibitor with the highest binding energy of −8.5.
Fig. 1.
Molecular interaction of SARS-CoV-2 Nsp15 with (A) Sarsasapogenin; (B) Ursonic acid; (C) Ajmalicine; (D) Silymarin; (E) Novobiocin and (F) Aranotin.
Predicted pharmacokinetics analysis
ADMET studies of phytoconstituents showed that sarsasapogenin, ursonic acid, ajmalicine, silymarin, and aranotin are the phytochemicals with strong interactions and decent ADMET profile as they obey the Lipinski's rule of five (Table 2 ). DruLito software was used to carry out ADMET analysis (www.niper website) for studying the ideal pharmacokinetics profile of the phytochemicals for drug designing. Lipinski's rule was used to screen the phytochemicals. According to the Lipinski rule, the weight of an ideal drug molecule should be below 500 g/mol, groups donating hydrogen bond should be less than or equal to 5 and groups accepting hydrogen bond should be less than or equal to 10 along with a partition coefficient of 5 or less. Bioavailability was analyzed based on TPSA (total polar surface area) of the drug molecule. Veber's rule states that the TPSA less than or equal to 140 Å indicates good oral bioavailability
Table 2.
Pharmacokinetics ADMET prediction by Drulito against Lipinski rule of five and blood-brain-barrier filter.
| Phytochemicals | MW | logP | AlogP | HBA | HBD | TPSA | nHB | nAcidic group | Filter l |
|---|---|---|---|---|---|---|---|---|---|
| Nelfinavir | 567.31 | 3.948 | 1.311 | 7 | 4 | 127.2 | 11 | 0 | |
| Hydroxychloroquine | 335.18 | 1.548 | −0.746 | 4 | 2 | 47.86 | 6 | 0 | l |
| Sarsasapogenin | 416.33 | 7.306 | 0.341 | 3 | 1 | 38.69 | 4 | 0 | |
| Ursonic acid | 454.34 | 8.347 | 2.053 | 3 | 1 | 54.37 | 4 | 1 | |
| Novobiocin | 612.23 | 2.575 | 1.49 | 13 | 5 | 196.1 | 18 | 0 | |
| Aranotin | 462.06 | −1.162 | −1.579 | 9 | 1 | 156.21 | 10 | 0 | l |
| Ajmalicine | 352.18 | 1.906 | −0.068 | 5 | 1 | 50.8 | 6 | 0 | l |
| Beta-sitosterol | 414.39 | 11.595 | 1.3 | 1 | 1 | 20.23 | 2 | 0 | |
| Alpha amyrin | 426.39 | 11.448 | 2.366 | 1 | 1 | 20.23 | 2 | 0 | |
| Silymarin | 482.12 | 0.855 | −1.848 | 10 | 5 | 155.14 | 15 | 0 | l |
| Pomolic acid | 472.36 | 6.778 | 1.045 | 4 | 3 | 77.76 | 7 | 1 | |
| Carnosol | 330.18 | 5.108 | 0.628 | 4 | 2 | 66.76 | 6 | 0 | |
| Rutin | 610.15 | −0.735 | −4.581 | 16 | 10 | 265.52 | 26 | 0 | |
| Naringin | 580.18 | −1.35 | −3.492 | 14 | 8 | 225.06 | 22 | 0 | |
| Arjunolic acid | 488.35 | 7.537 | 0.816 | 5 | 4 | 97.99 | 9 | 1 | |
| Asiatic acid | 488.35 | 7.439 | 0.17 | 5 | 4 | 97.99 | 9 | 1 | |
| Reserpine | 608.27 | 2.672 | −0.857 | 11 | 1 | 114.02 | 12 | 0 | |
| Betulinic acid | 456.36 | 9.407 | 2.35 | 3 | 2 | 57.53 | 5 | 1 | |
| Platanic acid | 458.34 | 7.533 | 0.831 | 4 | 2 | 74.6 | 6 | 1 | |
| Berberine | 336.12 | 2.473 | −2.525 | 4 | 0 | 39.93 | 4 | 0 | l |
| Taspine | 369.12 | 1.176 | 0.241 | 7 | 0 | 74.3 | 7 | 0 | l |
| Alphitolic acid | 472.36 | 8.595 | 2.127 | 4 | 3 | 77.76 | 7 | 1 | |
| Taxifolin | 304.06 | 0.803 | −1.369 | 7 | 5 | 127.45 | 12 | 0 | l |
| Luteolin | 286.05 | 1.486 | −0.787 | 6 | 4 | 107.22 | 10 | 0 | l |
| Apigenin | 270.05 | 1.138 | −0.224 | 5 | 3 | 86.99 | 8 | 0 | l |
| Myricetin | 318.04 | 2.182 | −1.807 | 8 | 6 | 147.68 | 14 | 0 | |
| Wogonin | 284.07 | 2.092 | −0.16 | 5 | 2 | 75.99 | 7 | 0 | l |
| Epigallocatechin | 306.07 | 1.2 | −1.499 | 7 | 6 | 130.61 | 13 | 0 | |
| Chlorogenic acid | 354.1 | −0.7 | −1.194 | 9 | 6 | 164.75 | 15 | 1 | |
| Afromosin | 298.08 | 1.685 | −0.258 | 5 | 1 | 64.99 | 6 | 0 | l |
| Gliotoxin | 326.04 | −2.142 | −0.153 | 6 | 2 | 131.68 | 8 | 0 | l |
| Psoralen | 186.03 | 1.097 | 0.181 | 3 | 0 | 35.53 | 3 | 0 | l |
| Carinatine | 303.15 | −0.608 | −1.107 | 5 | 3 | 73.16 | 8 | 0 | l |
| Rhinacanthin | 408.12 | 2.339 | 1.496 | 7 | 1 | 99.13 | 8 | 0 | l |
| Caffeic acid | 180.04 | 0.888 | 0.203 | 4 | 3 | 77.76 | 7 | 1 | l |
| Coriandrin | 230.06 | 1.478 | 0.033 | 4 | 0 | 44.76 | 4 | 0 | l |
| Scopoletin | 192.04 | 0.97 | −0.031 | 4 | 1 | 55.76 | 5 | 0 | l |
| Cordycepin | 251.1 | −2.195 | −3.23 | 8 | 3 | 116.03 | 11 | 0 | l |
| Ricinoleic acid | 298.25 | 6.107 | −2.565 | 3 | 2 | 57.53 | 5 | 1 | |
| Alpha asarone | 208.11 | 2.704 | 0.619 | 3 | 0 | 27.69 | 3 | 0 | l |
| Valproic acid | 144.12 | 2.742 | −0.347 | 2 | 1 | 37.3 | 3 | 1 | l |
| Allicin | 162.02 | 0.237 | 1.508 | 1 | 0 | 61.58 | 1 | 0 | l |
MW = molecular weight; logP= partition coefficient; AlogP = octanol-water partition coefficient; HBA= hydrogen bond acceptor; HBD= hydrogen bond donor; TPSA= total polar surface area; nHB= number of hydrogen bond; nAcidic group= number of acidic group; Filter l= Lipinski rule of five and B= blood-brain barrier.
Molecular docking of phytochemicals using swissdock
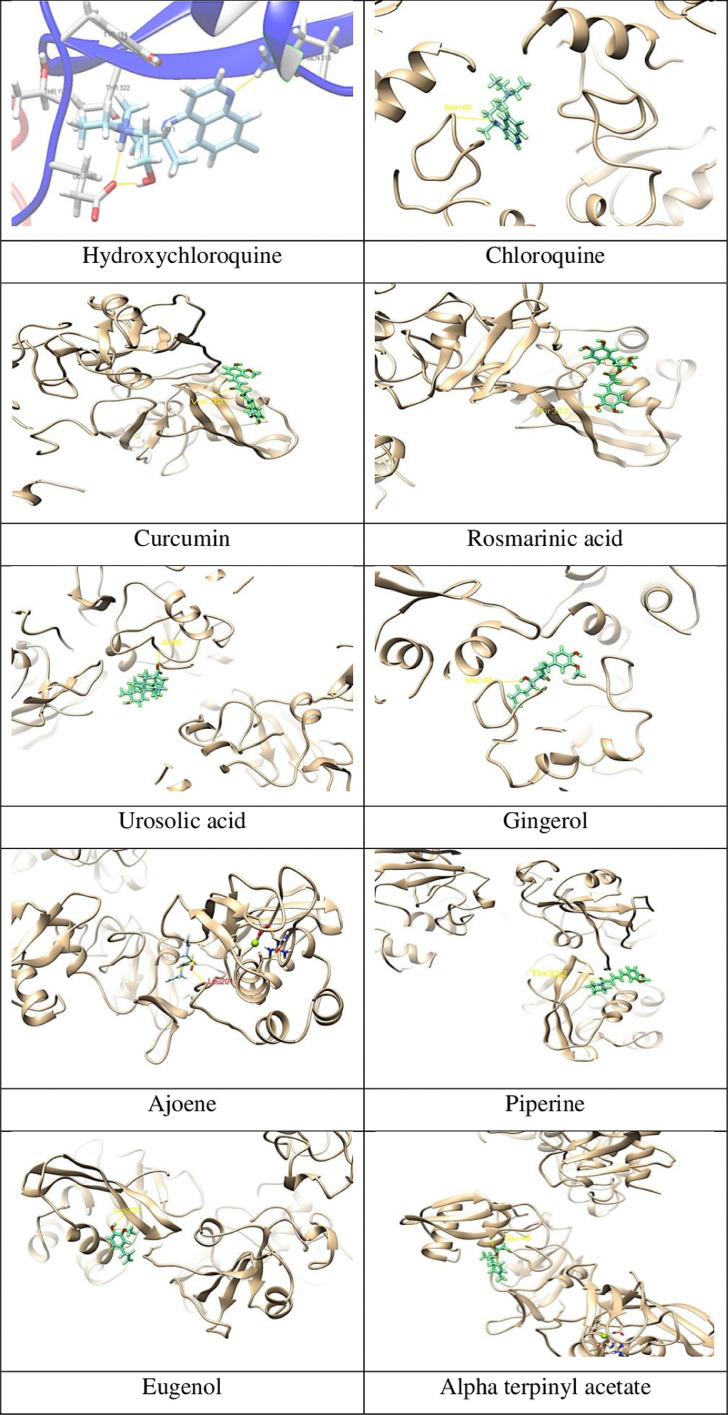
The structure used for COVID-19 endonuclease with nsp-15 had PDB ID 6VWW (Kim et al., 2020). The Nsp15 protein and phytochemicals were docked with Swissdock using default parameters. Docking results were observed using offline with UCSF-Chimera. The values were obtained in terms of energy full fitness and deltaG Kcal/mol. Different docking poses were screened based on deltaG Kcal/mol value; the appropriateness of the docked pose means lowest deltaG Kcal/mol energy. The best deltaG Kcal/mol was found with apigenin, curcumin, rosmarinic acid, and ursonic acid about −8.05, −7.74, −7.26, and −7.10 respectively. Whereas, docking result (Table 3 ) of phytochemicals best full fitness was observed for piperine, gingerol, and alpha terpinyl acetate (−3382.5708, −3381.75, and −3380.0696). Hydroxychloroquine and chloroquine were used as a standard drug against NSP-15 with deltaG value of −9.27 and 8.71 kcal/mol, respectively. Chloroquine, a known anti-malaria, and the anti-inflammatory drug showed deltaG −7.43 Kcal/mol and full fitness score of −3382.6494. In the case of hydroxychloroquine, a total of three hydrogen bonds are observed, two with Glu192 and one with Gln310, whereas, in the case of chloroquine, one hydrogen was observed with Met105 amino acid (Fig. 2 ). All the phytochemicals exhibited one hydrogen bond, whereas zingerone demonstrated two hydrogen bonds. Furthermore, piperine, gingerol, alpha terpinyl acetate, menthol, ajoene, and rosmarinic acid were found with a better FF score in comparison to hydroxychloroquine and chloroquine.
Table 3.
Docking result of phytochemicals in terms of full fitness, and estimated deltaG values predicted by SwissDock.
| Phytochemicals | deltaG | FullFitness | DeltaG vdw | DeltaG ligsolvnonpol | DeltaG ligsolvpol | DeltaG compsolvnonpol | DeltaG compsolvpol | surfFull | solvFull | IntraFull | InterFull | Interacting residues |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxychloroquine | −8.01 | −3366.96 | −49.81 | 8.25 | −30.29 | 552.85 | −3918.99 | 552.85 | −3918. 99 | 49.00 | −49.81 | Glu192, Gln310 |
| Chloroquine | −7.55 | −3391.97 | −42.76 | 8.33 | −29.50 | 552.99 | −3921.75 | 552.99 | −3921.75 | 19.54 | −42.76 | Met105 |
| Curcumin | −7.74 | −3358.21 | −47.66 | 8.00 | −15.26 | 555.45 | −3907.24 | 555.45 | −3907.24 | 41.25 | −47.66 | Val321 |
| Rosmarinic acid | −7.26 | −3367.92 | −40.41 | 8.30 | −20.04 | 555.27 | −3914.66 | 555.27 | −3914.66 | 31.88 | −40.41 | Thr322 |
| Ursonic acid | −7.10 | −3329.95 | −29.00 | 15.89 | −8.63 | 557.54 | −3910.05 | 557.54 | −3910.05 | 51.56 | −29.00 | Ala95 |
| Gingerol | −6.94 | −3381.75 | −29.51 | 7.47 | −8.16 | 552.874 | −3911.97 | 552.874 | −3911.97 | 6.85 | −29.51 | Met105 |
| Ajoene | −6.93 | −3377.19 | −35.48 | 4.77 | −6.54 | 554.52 | −3907.65 | 554.52 | −3907.65 | 11.42 | −35.48 | Leu201 |
| Piperine | −6.45 | −3382.57 | −29.66 | 6.93 | −6.89 | 556.22 | −3908.40 | 556.22 | −3908.40 | −0.74 | −29.66 | Thr322 |
| Eugenol | −6.27 | −3352.90 | −25.91 | 4.75 | −4.67 | 555.68 | −3911.73 | 555.68 | −3911.73 | 29.05 | −25.91 | Lys320 |
| Alpha terpinyl acetate | −6.15 | −3380.07 | −19.01 | 7.14 | −3.38 | 555.89 | −3915.74 | 555.89 | −3915.74 | −1.21 | −19.01 | Glu45 |
| Menthol | −6.06 | −3378.20 | −18.72 | 6.78 | −2.64 | 556.82 | −3915.86 | 556.82 | −3915.86 | −0.44 | −18.72 | Glu114 |
| Zingerone | −6.03 | −3365.89 | −21.35 | 5.33 | −7.86 | 555.95 | −3917.45 | 555.95 | −3917.45 | 16.95 | −21.35 | Leu201, Leu266 |
| Cuminaldehyde | −6.04 | −3356.90 | −23.38 | 4.58 | −4.77 | 556.14 | −3912.5 | 556.15 | −3912.5 | 22.83 | −23.38 | Thr322 |
| Cinnamaldehyde | −5.84 | −3360.45 | −23.89 | 3.53 | −5.28 | 555.14 | −3909.35 | 555.14 | −3909.35 | 17.64 | −23.88 | Lys159 |
Fig. 2.
Molecular interaction of different phytochemicals with Nsp 15 viral protein using Swissdock software.
Molecular dynamics analysis
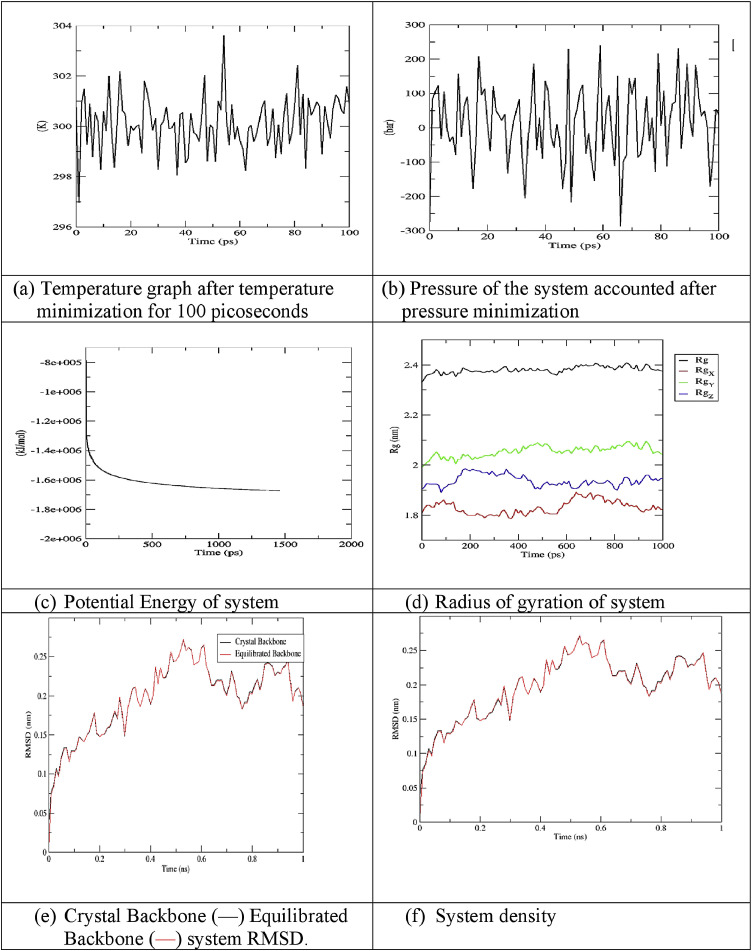
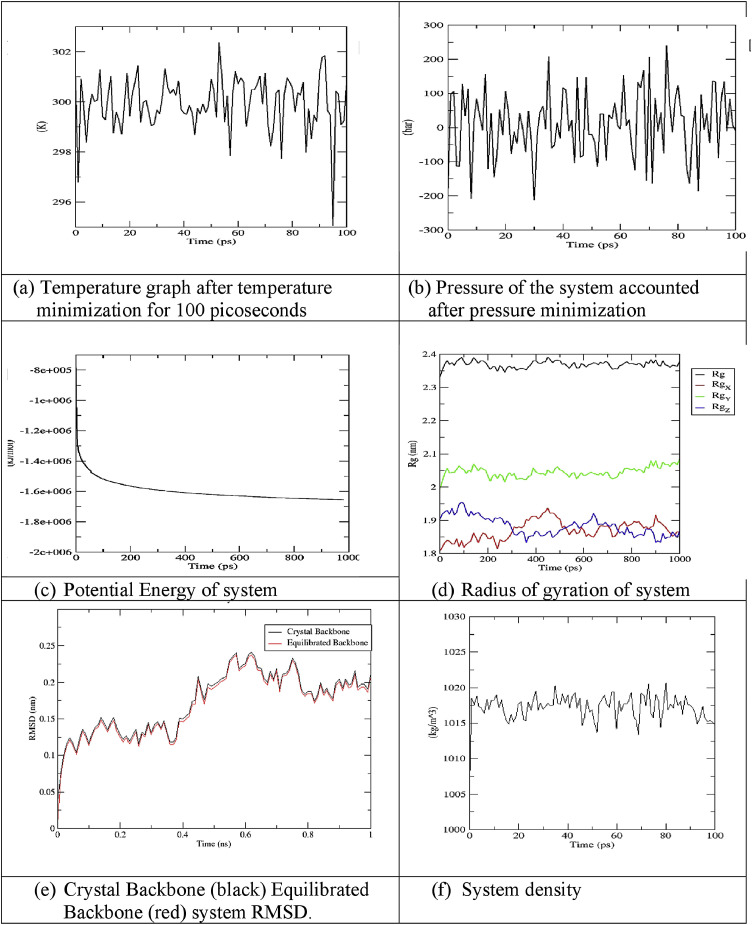
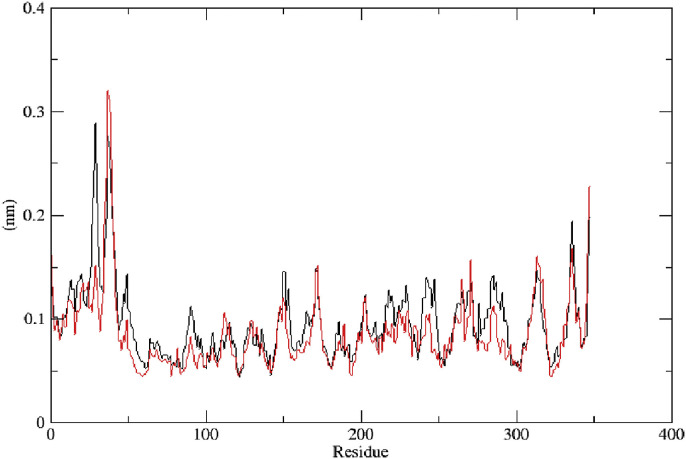
Sarsasapogenin and ursonic acid protein complexes were solvated and made electro neutral by adding 12 sodium ions in each system using genion. The volume and density of both systems were observed with values 954.784 nm3 and 1007.86 (g/l), respectively. After NVT and NPT ensemble, proteins were at a dynamic state at 300 K ((Figs. 3 a and 4 a) and constant pressure (Figs. 3b and 4b). The potential energy graph revealed a sudden drop in potential energy of the system in the first few ps but reached a constant value thereafter. Potential energy minimization of the ursonic acid system achieved at 980 EM steps as compared to 1460 EM steps of sarsasapogenin, indicating that the structure ursonic acid equilibrated more quickly than sarsasapogenin (Fig. 3c and 4c). To study the degree of compactness of protein during simulation, the radius of gyration was calculated. Over a span of 1000 ps, a variation of the only 0.05 nm was observed, stating that proteins have folded in its stable form. Lesser value of Radius of gyration for ursonic acid system shows its compactness over sarsasapogenin (Fig. 3d and 4d). Further, RMSD value was calculated to determine the fluctuations in 3D structure of proteins with time. Changes in the order of 1–3 Å are acceptable, and higher values indicate misbehavior of protein during the simulation. It was seen that protein under simulation undergoes minute changes resulting in a slight increase of RMSD. The blackline with red dots represents the protein backbone during simulation, which remained under acceptable value with reference to crystal structure. Ursonic acid equilibrated system shows little more deviation to its crystal structure as compared to the sarsasapogenin system (Fig. 3e and 4e). The density of systems during the course varied from 1012 to 1020 kg/m3, but the average was close to value 1017 kg/m3. Density of both the systems was found stable over time, indicating that systems were well-equilibrated with respect to pressure and density (Figs. 3f and 4f). MD followed this for a total of 1000 ps. RMSF per residue was calculated, which shows fluctuation over all residues of the protein. Peak shows the protein area undergoing maximum fluctuation over the simulation. Lesser RMSF of the ursonic acid system shows its stability over sarsasapogenin during simulations (Fig. 5 ).
-
(a)
Temperature graph after temperature minimization for 100 picoseconds
-
(b)
Pressure of the system accounted after pressure minimization
-
(c)
Potential Energy of system
-
(d)
Radius of gyration of system
-
(e)
Crystal Backbone (black) Equilibrated Backbone (red) system RMSD.
-
(f)
System density
Fig. 3.
Molecular dynamic analysis of sarsasapogenin system (a) Temperature graph after temperature minimization for 100 picoseconds, (b) Pressure of the system accounted after pressure minimization, (c) Potential Energy of system, (d) Radius of gyration of system, (e) Crystal Backbone ( ) (Black) and Equilibrated Backbone (
) (Black) and Equilibrated Backbone ( ) (Red) system RMSD, and (f) System density.
) (Red) system RMSD, and (f) System density.
Fig. 4.
Molecular dynamic analysis of ursonic acid system (a) Temperature graph after temperature minimization for 100 picoseconds, (b) Pressure of the system accounted after pressure minimization, (c) Potential Energy of system, (d) Radius of gyration of system, (e) Crystal Backbone ( ) and Equilibrated Backbone (
) and Equilibrated Backbone ( ) system RMSD, and (f) System density.
) system RMSD, and (f) System density.
Fig. 5.
Comparative RMSF of sarsasapogenin system ( ), and ursonic acid system (
), and ursonic acid system ( )
)
Discussion
COVID-19 caused by the deadly coronavirus SARS-CoV-2 is responsible for pandemic across the world and at present has caused thousands of deaths as per WHO report (WHO website). The identification of novel coronavirus led to the sequence of the viral genome, which revealed several genes that are encoding the protein and are responsible for the virulence of this virus strain (Wu et al., 2020). Major proteins responsible for virulence are spike (S) proteins, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) proteins, which play a crucial role in DNA replication. These proteins, when enters into a human cell, binds to human angiotensin-converting enzyme 2 (ACE2) receptor (Gordon et al., 2020). Therefore, these viral proteins can be used as a drug target to inhibit tiral replication. In the present study, Nsp15 was selected as a drug target which is a nidoviral RNA uridylate-specific endoribonuclease (NendoU) protein, conserved among coronaviruses and thought to directly participates in viral replication. The NendoU protein is conserved among the coronaviruses, arteriviruses and, toroviruses. Recently, NendoU activity of Nsp15 is found responsible for the protein interference with the innate immune response (Deng et al., 2017). Therefore, Nsp15 is essential in coronavirus biology. Because of this, the Nsp15 protein was chosen for the molecular docking study with phytochemicals that were reviewed from the literature. Natural phytochemicals are known for their non-toxicity and possess anti-viral activity (Naithani et al., 2008). The benefits of using phytochemicals are immense, as these are less toxic than synthetic drugs. They also provide health benefits by playing a synergetic rolewithinn the human body. They are eco-friendly molecules which can have therapeutic value. Several phytochemicals such as alkaloids, terpenoids, saponins,and polyphenols are reported to possess antiviral activity (Liu and Du., 2012). The previous study by Umesh et al., 2020 showed carnosol, rosmanol, and arjunglucoside-I, natural phytochemicals as potential inhibitors of SARS-CoV main protease using molecular docking approach. Similarly, Das et al., 2020 showed rutin with the highest inhibition efficiency for SARS-CoV main protease, among other phytochemicals. Phytochemical piperine and curcumin also showed anti-viral property against influenza, chikungunya, and zika viruses (Couceiro et al., 2005; Mounce et al., 2017).
The present study demonstrated sarsasapogenin, ursonic acid, apigenin, curcumin, ajmalicine, novobiocin, silymarin, and aranotin are strongly binding to Nsp15 target and could be used as promising strong inhibitors. Sarsasapogenin interacted with catalytic triad residue Lys290 with a strong single hydrogen bond of such low binding energy of −8.5, which made sarsasapogenin a lead potential inhibitor. Other phytochemical ursonic acid showed low binding energy of −8.4 with Nsp 15 protein. However, an MD study based on PEM, Equilibrated NVT-NPT, radius of gyration, RMSD and RMSF revealed that ursonic acid complex is more stable than sarsasapogenin complex, making ursonic acid a more potent inhibitor of Nsp15 when compared with sarsasapogenin. Previous studies have also shown anti-viral property of sarsasapogenin and ursonic acid against chikungunya viruses and rotavirus, respectively (Troost et al., 2020; Tohme et al., 2019). This study thus showed the significance of phytochemicals based drug discovery to find novel coronavirus drugs.
Conclusion
In conclusion, the present study successfully demonstrated the binding of phytochemicals such as sarsasapogenin, ursonic acid, curcumin, ajmalicine, novobiocin, silymarin and aranotin, piperine, gingerol, rosmarinic acid, and alpha terpinyl acetate to Nsp15 viral protein and they might play a key role in inhibiting SARS-CoV-2 replication. Among the phytochemicals, sarsasapogenin and ursonic acid can be considered as the lead phytochemicals. Further, in vivo and in vitro assay are required in the BSL 3 category lab to confirm the above finding.
CRediT authorship contribution statement
Suresh Kumar: Conceptualization, Supervision, Visualization, Writing - original draft, Writing - review & editing. Priya Kashyap: Methodology, Software, Data curation, Writing - original draft. Suman Chowdhury: Methodology, Software, Data curation, Writing - original draft. Shivani Kumar: Methodology, Software, Data curation, Writing - original draft. Anil Panwar: Methodology, Software, Data curation, Writing - original draft. Ashok Kumar: Methodology, Software, Data curation, Validation, Supervision, Writing - original draft.
Declaration of Competing Interest
None
Funding source
Nil
References
- Couceiro J.N., da Silva P.M., dos Santos M.G., Ribeiro T.S., de Lima M.E. Natural piperine as a new alternative against influenza viruses. Virus Rev. Res. 2005;10:27–32. [Google Scholar]
- Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dynam. 2020:1–18. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Baker S.C. An “Old” protein with a new story: coronavirus endoribonuclease is important for evading host antiviral defenses. Virology. 2018;517:157–163. doi: 10.1016/j.virol.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Hackbart M., Mettelman R.C., O'Brien A., Mielech A.M., Yi G., Kao C.C., Baker S.C. Proceedings of the National Academy of Sciences. Vol. 114. 2017. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages; pp. E4251–E4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang M.G., Bouhaddou M., Krogan N.J. A SARS-CoV-2-human protein-protein interaction map reveals drug. Targets Potential Drug-Repurpos. 2020;295:4773–4779. [Google Scholar]
- Grosdidier A., Zoete V., Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–W277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Rauscher S., Nawrocki G., Ran T., Feig M., de Groot B.L., Grubmüller H., MacKerell A.D. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods. 2017;14:71–73. doi: 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Jedrzejczak R., Maltseva N.I., Endres M., Godzik A., Michalska K., Joachimiak A. Protein Science; 2020. Crystal Structure of Nsp15 Endoribonuclease NendoU from SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler E., Gil-Cruz C., Spanier J., Li Y., Wilhelm J., Rabouw H.H., Züst R., Hwang M., V'kovski P., Stalder H., Marti S. Early endonuclease-mediated evasion of RNA sensing ensures efficient coronavirus replication. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R., Yang G., Xue R., Liu M., Wang F., Hu J., Guo X., Chang S. COVID-19 Docking Server: an interactive server for docking small molecules, peptides and antibodies against potential targets of COVID-19. arXiv Preprint. 2020 doi: 10.1093/bioinformatics/btaa645. arXiv:2003.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A.L., Du G.H. Springer; Dordrecht: 2012. Antiviral Properties of phytochemicals. In Dietary Phytochemicals and Microbes; pp. 93–126. [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce B.C., Cesaro T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Naithani R., Huma L.C., Holland L.E., Shukla D., McCormick D.L., Mehta R.G., Moriarty R.M. Antiviral activity of phytochemicals: a comprehensive review. Mini Rev. Med. Chem. 2008;8:1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Schüttelkopf A.W., Van Aalten D.M. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallograph. Sec. D: Biol. Crystallogr. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Tohmé M.J., Giménez M.C., Peralta A., Colombo M.I., Delgui L.R. Ursonic acid: a novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents. 2019;54:601–609. doi: 10.1016/j.ijantimicag.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Troost B., Mulder L.M., Diosa-Toro M., van de Pol D., Rodenhuis-Zybert I.A., Smit J.M. tomatidine, a natural steroidal alkaloid shows antiviral activity towards the chikungunya virus in vitro. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-63397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh, Kundu D., Selvaraj C., Singh S.K., Dubey V.K. Identification of new anti-nCoV drug chemical compounds from Indian spices exploiting SARS-CoV-2 main protease as target. J. Biomol. Struct. Dynam. 2020:1–9. doi: 10.1080/07391102.2020.1763202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]