Abstract
Assisted reproductive technologies (ARTs) are widely used in the animal industry, human clinics, and for basic research. In small laboratory animal species such as mice, ARTs are essential for the production of animals for experiments, the preservation of genetic resources, and for the generation of new strains of genetically modified animals. The RIKEN BioResource Research Center (BRC) is one of the largest repositories of such animal bioresources, and maintains approximately 9,500 strains of mice with a variety of genetic backgrounds. We have sought to devise ARTs specific to the reproductive and physiological characteristics of each strain. Such ARTs include superovulation, in vitro fertilization (IVF), the cryopreservation of embryos and spermatozoa, transportation of cryopreserved materials and embryo transfer (ET). Of these, superovulation likely has the most influence on animal production because it determines the quantity of starting material for other ARTs. Superovulation using anti-inhibin serum combined with estrous synchronization has resulted in approximately a three-fold increase in production efficiency with IVF–ET in the C57BL/6J strain. Wild-derived strains are important as genetically diverse resources for murine rodents (Genus Mus), and many are unique to the BRC. We have also successfully developed ARTs for more than 50 wild-derived strains, which have been cryopreserved for future use. Our work to improve and develop ARTs for mice and other small laboratory species will contribute to the cost-effectiveness of routine operations at repository centers, and to the provision of high quality animals for research use.
Keywords: Assisted reproductive technologies (ARTs), Cryopreservation, In vitro fertilization (IVF), Murine rodents, Superovulation
Introduction
Laboratory species, especially mammals, are essential for the advancement of biomedical research and human health and welfare because they provide irreplaceable in vivo model systems. To ensure that research using laboratory animals is reliable and reproducible, it is critical that animals of high quality are readily available. For this purpose, global repository centers of mice and rats serve as core facilities for the collection, maintenance, and distribution of laboratory rodents [1, 2]. The mouse bank (Experimental Animal Division) at the RIKEN BioResource Research Center (BRC) is the largest mouse strain repository center in Japan [3]. The BRC was founded in 2001, as the national center for bioresources for research involving mice, cell lines, experimental plants, and genes (later extended to include microbes). Since 2002, projects for the biobanking of these resources have been supported by the National BioResource Project (NBRP), Japan, in recognition of the BRC’s services to the research community. The BRC mouse bank is registered with the International Mouse Strain Resource database (IMSR; http://www.findmice.org/index), and as of January 2020 it maintains approximately 9,500 mouse strains. The BRC mouse bank is growing steadily, and collects more than 200 new strains every year, most of which are deposited by Japanese scientists. Annually, more than 2,500 strains are distributed to universities, institutes, and private companies around the world. Therefore, the development of techniques that allow for the maintenance of large numbers of high quality mouse strains, in a safe and cost-effective manner is critical. We have developed a number of assisted reproductive technologies (ARTs), specifically, superovulation, in vitro fertilization (IVF), the cryopreservation of embryos and spermatozoa, and embryo transfer (ET). We have also developed specialized technologies such as intracytoplasmic sperm injection (ICSI) and somatic cell nuclear transfer (SCNT) that allow for the rescue of strains with severe infertility that may have arisen accidentally or for genetic reasons.
In general, the primary type of ART used at mouse strain repositories is the cryopreservation of embryos and gametes because it has numerous merits in relation to the routine operations performed. These include the protection of valuable strains from infectious diseases and genetic drift as a result of mutations accumulating during breeding; prevention of the escape and death of mice during transportation; and the reduction of costs for maintaining live mice. However, the efficiency of producing offspring from cryopreserved embryos or gametes can vary greatly between strains. Therefore, the development of techniques should focus on those that are applicable to as many mouse strains as possible. The use of ethylene glycol-based solutions for embryo cryopreservation is one possible approach. As an alternative, we have also attempted to investigate strain-specific differences in the characteristics of embryos and gametes in relation to the efficiency of their cryopreservation, in order to devise appropriate techniques for each strain. In this review article, we introduce the ARTs we developed originally with special emphasis on the significant improvements in the final yield of offspring obtained from a single female. We also summarize the ARTs we have developed for small laboratory rodents other than mice, which have provided invaluable information regarding how to solve species-specific problems in the handling of embryos and gametes.
Cryopreservation of Embryos
Cryopreservation of embryos is a reliable technique for strain preservation in mice. As the number of genetically modified strains with defined genetic backgrounds (e.g., C57BL/6) has increased at a rapid rate, sperm freezing has become the primary method of strain preservation because of its high offspring productivity and the availability of standard (non-genetically modified) females for IVF. However, there are many important inbred strains with undefined genetic backgrounds, such as historical spontaneous mutant strains. As inbred mouse strains are maintained by brother-sister mating, inbred strains with undefined genetic backgrounds can be safely cryopreserved as embryos, but not as spermatozoa. Furthermore, embryo cryopreservation is more preferable than sperm cryopreservation in the case that homozygous mutants should be prepared as early as possible.
Since the first successful cryopreservation of mice embryos by Whittingham et al. in 1972, the slow freezing method has become a reliable technique for cryopreserving mammalian embryos including those of rabbits and rats in early years (Table 1) [4,5,6,7]. However, slow freezing methods require a programmable freezer and are not always applicable to large cells such as 1-cell or 2-cell embryos. To overcome this problem, in 1985 Rall and Fahy developed a vitrification method [8], which enabled the rapid cryopreservation of mammalian embryos even at the 1-cell or 2-cell stages. Vitrification is not a freezing method per se because there is no intracellular ice formation and the method’s success is based on the selection of appropriate vitrification solutions and containers. So far, the most reliable vitrification solutions are those based on ethylene glycol. Kasai et al. [9] named the vitrification solution “EFS” based on its ingredients: ethylene glycol, Ficoll and sucrose. These EFS solutions have been used successfully for the vitrification of embryos from mice, rats, rabbits, bovines, and horses [9,10,11,12,13]. They have also been used for the vitrification of embryos from mastomys (Praomys coucha), Syrian hamsters (Mesocricetus auratus), and Mongolian gerbils (Meriones unguiculatus) [14,15,16,17,18,19]. For example, we have produced healthy offspring from vitrified embryos of mastomys and Mongolian gerbils (Fig. 1). Mastomys is a genus of African small rodents that is still not well known in the scientific community, but provides unique animal models for lysosomal glycolipid storage disease [20], infectious diseases [21], and gastric cancers [22]. As hamster embryos are known to be very difficult to culture in vitro, the first hamster offspring were obtained from cryopreserved embryos using a vitrification method with a mixture of ethylene glycol and dimethyl sulfoxide (DMSO; acronym EDFS) 36 years after the first successful use of IVF in this species [23]. It was reported that vitrification of unfertilized oocytes could be possible by increasing the cooling and warming speeds using a special device for small volumes of EDFS. Although laboratory mice and rats represent standard animal models for many scientific studies, it is often desirable to use a broader range of nontraditional animal models and, as such, the development of basic reproductive technologies for these species is also required.
Table 1. Laboratory species yielding offspring following the transfer of cryopreserved embryos [4].
| Species | Cryopreservation method | Cryoprotective agents | Authors | Year | References |
|---|---|---|---|---|---|
| Mouse | Slow freezing | 1 M DMSO | Whittingham et al. | 1972 | [5] |
| Rabbit | Slow freezing | 1.6 M DMSO | Bank et al. | 1974 | [6] |
| Rat | Slow freezing | 1.5 M DMSO | Whittingham et al. | 1975 | [7] |
| Mastomys | Vitrification (2-step) | 20% EG+F+S and 40% EG+F+S | Mochida et al. | 1998 | [14, 15] |
| Hamster | Vitrification (2-step) | 10% DMSO+10% EG and 20% DMSO+20% EG+F+S | Lane et al. | 1999 | [16, 17] |
| Mongolian gerbil | Vitrification (2-step) | 20% EG+F+S and 40% EG+F+S | Mochida et al. | 1999 | [18, 19] |
DMSO, dimethyl sulfoxide; EG, ethylene glycol; F, Ficoll; S, sucrose.
Fig. 1.
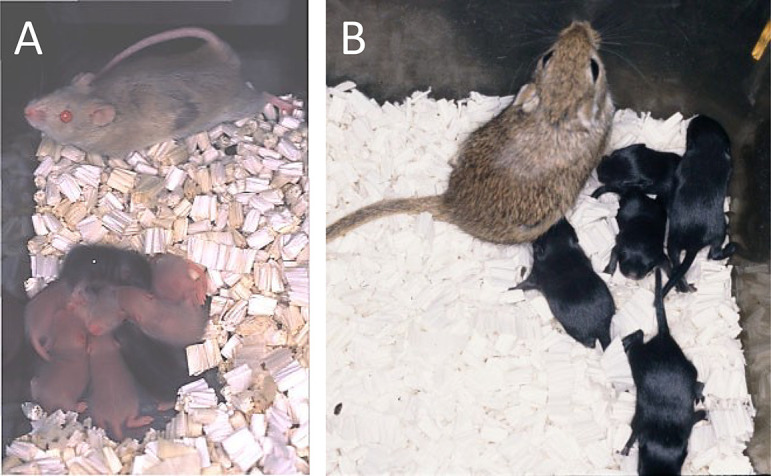
Mastomys (African rodent, Praomys coucha) and Mongolian gerbil (Meriones unguiculatus) pups born after the transfer of vitrified–warmed embryos. (A) Mastomys pups from an agouti coat color strain, born from a pregnant female of a chamois (light brown) coat color strain. The pups were nursed together with chamois coat color pups (the female’s own). (B) Mongolian gerbil pups from a black coat color strain born from a pseudopregnant female of an agouti coat color strain.
The BRC maintains a large number of mouse strains with a variety of genetic backgrounds and the vitrification protocol that has been optimized for standard strains such as C57BL/6 does not always work well for other strains. We found that the EFS solutions are more broadly applicable to a variety of mouse strains. Thus, we developed a technique using a new EFS solution, designed for routine use at the BRC. Importantly, we optimized the technique for vitrification by using cryotubes as containers instead of the plastic straws used in the original method, in order to avoid accidental breakages during handling or transportation. This method has now been used routinely for most strains maintained at the BRC for 18 years. The detailed protocol is available as a video journal for researchers and operators [24]. Generally, vitrified embryos should be kept supercooled below –130°C to avoid damage. This means that liquid nitrogen and special containers for maintaining supercooled temperatures (dry shippers) are necessary for the storage and transportation of embryos, respectively. To bypass this, we developed an equilibrium vitrification (or high osmolality vitrification, HOV) method (Table 2) [25] to preserve embryos in a vitrified state at dry ice (CO2) temperature (–80°C) in collaboration with Dr Kasai and others [26,27,28,29]. This maintains the viability of vitrified embryos in a conventional deep freezer or dry ice box. This method was deemed successful, as normal mice were recovered at the UK and USA after such embryos had been transported in a conventional dry ice package from our center [28].
Table 2. Survival rates and developmental ability of 2-cell embryos in several mouse strains after vitrification using the high osmolality vitrification (HOV) method [25].
| Strain | No. (%) of embryos |
No. (%) of recipients |
No. (%) of |
|||||
|---|---|---|---|---|---|---|---|---|
| Frozen | Recovered (%) | Survived (%) | Transferred | Delivered (%) | Transferred embryos | Implantation sites (%) | Offspring (%) | |
| C57BL/6J | 265 | 263 (99) | 256 (97) | 9 | 9 (100) | 118 | 104 (88) | 85 (72) |
| C57BL/6N | 175 | 173 (99) | 168 (97) | 3 | 3 (100) | 40 | 36 (90) | 21 (53) |
| BALB/cA | 210 | 210 (100) | 206 (98) | 3 | 3 (100) | 40 | 31 (78) | 18 (45) |
| 129/SvJ | 100 | 100 (100) | 93 (93) | 3 | 3 (100) | 41 | 33 (80) | 27 (66) |
| DBA/2N | 200 | 200 (100) | 193 (97) | 6 | 6 (100) | 77 | 44 (57) | 25 (32) |
| C3H/HeN | 100 | 99 (99) | 96 (97) | 3 | 3 (100) | 41 | 27 (66) | 19 (46) |
Sperm Cryopreservation and IVF
Cryopreservation of spermatozoa is an effective method for the preservation of gene-modified strains with a defined genetic background because haploid gametes are sufficient for the propagation of such mouse strains and a large number of spermatozoa can be obtained from a single male mouse. It is important to note that the first reliable protocol for mouse sperm cryopreservation was developed approximately 30 years ago [30] and the contents of the cryopreservation solution (raffinose and skim milk) have remained largely unchanged. However, recently several important modifications have been made to improve the IVF fertilization rates and handling efficiency. The original containers recommended for this technique were plastic straws, but we have since optimized the protocol for freezing in cryotubes, which are preferably used in many facilities in the USA and Europe [31, 32]. The original protocol developed by Takeshima et al. is not always applicable for spermatozoa from C57BL/6J mice, one of the standard strains. Choi et al. first reported that methyl-β-cyclodextrin (MBCD) significantly accelerated the capacitation of spermatozoa, most likely because cholesterol is removed from the sperm plasma membrane due to its strong binding affinity with MBCD [33]. Takeo et al. found that this MBCD-based IVF system significantly improved fertilization rates using frozen–thawed C57BL/6J spermatozoa [34]. Similarly, we reported that the combination of MBCD with d-penicillamine, sodium citrate, and hypotaurine improved the capacitation of frozen-thawed C57BL/6J spermatozoa [35]. Takeo and Nakagata further improved the fertilization rates using frozen-thawed C57BL/6J spermatozoa through the addition of glutamine to the MBCD medium [36]. Another important modification related to IVF using frozen–thawed spermatozoa has been the addition of reduced glutathione (GSH) to the IVF medium [37]. GSH relaxes the S–S bonds of the zona pellucida of oocytes and increases the probability of the frozen–thawed spermatozoa penetrating this barrier. Importantly, the combination of MBCD in the sperm preincubation medium and GSH in the IVF medium synergistically improved the fertilization ability of frozen–thawed spermatozoa [38,39,40]. Furthermore, we reported a microdroplet IVF method in which the volume was reduced to 1 μl, which meant that an optimal concentration of spermatozoa could be achieved even with small numbers [41]. With this method, the oocyte/sperm ratio could be reduced to 1/240, which enabled us to fertilize oocytes using spermatozoa rendered poorly motile due to inadequate freezing or for genetic reasons.
While the efficiency of IVF using frozen–thawed C57BL/6J spermatozoa has reached a practical level, as referenced above, it is still not understood why spermatozoa are highly sensitive to freeze-thawing. To determine the molecular mechanisms, we attempted to map the genetic regions responsible for this susceptibility. We performed IVF using spermatozoa from recombinant inbred strains of mice derived from the C57BL/6J and DBA/2J strains, whose spermatozoa showed distinct fertilization abilities after freezing and thawing. Genome-wide interval mapping identified two suggestive quantitative trait loci (QTL) associated with fertilization on chromosomes 1 and 11 [42]. We confirmed that at least four and three of the genes on these chromosomes, respectively, possessed a single nucleotide polymorphism between the B6J and D2J strains, according to the MGI database (http://www.informatics.jax.org/). Of them, Abl2 on chromosome 1 and Nlrp3 on chromosome 11 have amino acid substitutions. The Abl2 protein is known to coordinate actin remodeling and is a key regulator of subcellular structures [43]. The Nlrp3 protein is a member of the family of Nod-like receptor (NLR) proteins, which are important for immunity, helping to start and regulate the immune system’s response to injury, toxins, or invasion by microorganisms [44].
IVF can be used to generate large numbers of zygotes without requiring a significant number of single-caged males for mating. Therefore, the quality of spermatozoa from the donor male is critical. In order to achieve high fertilization rates (>70%) it is usually necessary to use male mice older than 12 weeks [45]. Consequently, at least 15 weeks (including the pregnancy period) are required to obtain a new generation of mice. If the technique of microinjecting mouse oocytes was available in laboratories, it would allow for the use of the first-wave of round spermatids from males of 17 days old [46]. Ogonuki et al. [47] established a high-speed congenic strategy using round spermatids from immature males aged 22–25 days, in which a new generation of mice can be obtained within 50 days. Subsequently, we sought to determine the youngest age that males could be used for effective IVF. We found that in approximately half of male mice aged 35 days, the spermatozoa had reached the caudal regions of the epididymides, but did not show any progressive motility. Furthermore, on average fertilization rates of 72% could be obtained using spermatozoa from males of 40 days old [48]. This indicates that our protocol will reduce the turnover time required for the generation of mice by approximately 1 month compared with that of the conventional IVF protocol. This is especially advantageous for the establishment of congenic strains by repeated backcrossing.
Superovulation
Collecting sufficient number of oocytes from females through the induction of superovulation is a critical step for successful ARTs. In mice, superovulation driven by consecutive injections of equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG) has been the gold standard [49]. However, exogenous eCG collected from mares is not always effective for mice and certain strains are known to be unresponsive to this hormone [50]. Alternatively, regulation of the endogenous endocrine system could be a more effective superovulation regimen. Taya and Watanabe established such a system using anti-inhibin serum (AIS). According to their protocol, endogenous inhibin secretion can be neutralized by injecting AIS, and as a result, endogenous follicle-stimulating hormone (FSH) secretion from the pituitary gland is maintained at high levels, thus inducing much larger numbers of developing follicles in a variety of species including laboratory and domestic animals [51,52,53,54,55,56]. Using AIS–hCG injections we succeeded in collecting approximately 25 oocytes per female mouse in MSM and JF1 mice [57] and in wild-derived strains of Mus musculus molossinus [58]. This corresponds to a fivefold increase in oocyte release compared with that of eCG–hCG injections. We also applied this protocol to other wild-derived strains and classified them into AIS–hCG-susceptive and eCG–hCG-susceptive strains. Accordingly, we optimized the superovulation protocols for each wild-derived strain and successfully cryopreserved embryos from 37 of them [25]. Among them, 20 strains were more effectively superovulated with AIS–hCG than with eCG–hCG injections.
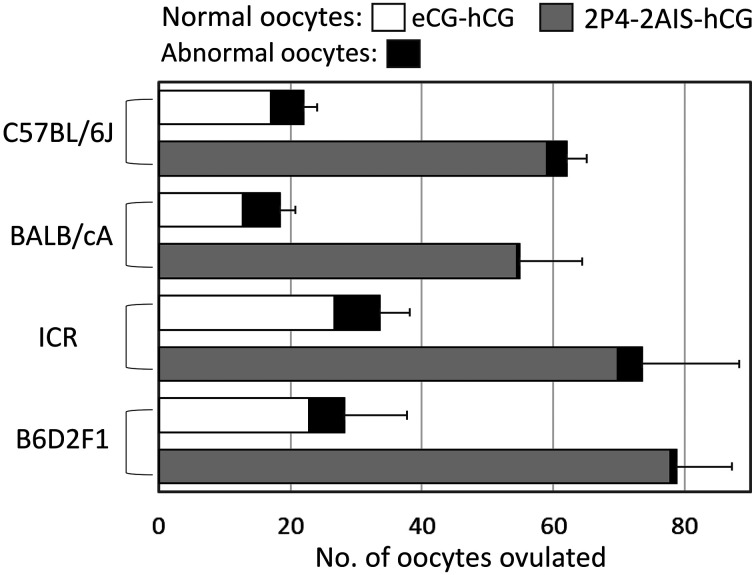
We then aimed to optimize the superovulation protocol for conventional laboratory strains using AIS. We found that the effectiveness of the AIS–hCG protocol largely depended on the stage of the female estrous cycle. When C57BL/6 mice of 10–14 weeks of age were injected with AIS–hCG at defined estrous stages, the highest number of oocytes was obtained from females treated at metestrus. We, therefore, examined whether the estrous cycle could be synchronized using two daily injections of progesterone (P4) (designated Days 1 and 2), based on the protocol established for guinea pigs [59,60,61]. As a result, 93% of mice were synchronized to metestrus at Day 4 irrespective of the estrous stage at the time of P4 treatment. Using this method of estrous synchronization followed by the AIS–hCG treatment, we collected 59 normal oocytes on average from each C57BL/6J female mouse (Fig. 2, Tables 3, 4) [40, 62]. This corresponds with an approximately 3.5-fold increase in oocyte recovery compared with that following eCG–hCG treatment. We confirmed that this protocol was also effective for other mouse strains, BALB/cA, ICR, and B6D2F1 (3.0-, 2.2-, and 2.8-fold increases, respectively; Fig. 2). In our laboratory, we routinely use this superovulation protocol when only limited numbers of females are available e.g., when using a small number of conditional knockout females for the analysis of maternal (oocyte) factors during development.
Fig. 2.
Results of superovulation with equine chorionic gonadotropin (eCG)- human chorionic gonadotropin (hCG) injections or two daily injections of both progesterone (P4) and anti-inhibin serum (AIS)-hCG in inbred (C57BL/6J and BALB/cA), outbred (ICR) and hybrid (B6D2F1) strains [62].
Table 3. Superovulation and in vitro fertilization (IVF) rates with various treatments in the C57BL/6J strain [40, 62].
Table 4. Total yield of offspring per female treated with Assisted reproductive technologies (ARTs) in the C57BL/6J strain at each facility: data added to those in reference [40].
| Abbreviation of facility [Reference no.] | Age of females (wks) | Superovulation method | Normal oocytes/female | IVF (%) | ET (%) | No. pups/females superovulated |
|---|---|---|---|---|---|---|
| NIRS [64] | 8–12 | eCG | 18.8 | 91.1 | 52.0 | 8.9 |
| JAX [65] | 3–4 | eCG | 25.0 | 66.3 | 53.1 | 8.8 |
| CIE [66] | 8–16 | eCG | 20.0 | 83.2 | 60.0 | 10.0 |
| CARD [67] | 4 | eCG | 27.7 | 96.4 | 43.6 | 11.6 |
| 4 | IASe * | 107.2 | 89.8 | NT ** | 42.0 *** | |
| BRC [25, 62] | 10–20 | eCG | 21.3 | 88.2 | 72.0 | 13.1 |
| 10–20 | AIS **** | 59.0 | 85.2 | 60.0 | 30.2 |
NIRS, National Institute of Radiological Sciences; JAX, The Jackson Laboratory; CIEA, Central Institute for Experimental Animals; CARD, Center for Animal Resources and Development; BRC, RIKEN BioResource Research Center. AIS, anti-inhibin serum; eCG, equine chorionic gonadotropin; IVF, in vitro fertilization; ET, embryo transfer. * Mixture of AIS–eCG injected to prepubertal females. ** Not tested. *** Estimated from the result of the eCG treatment. **** Twice daily injections of progesterone (P4) (estrous cycle synchronization) and AIS.
Effective Production of Pseudopregnant and Pregnant Females
The threefold increase in the number of oocytes that can be collected from one female, achieved through estrous cycle synchronization followed by AIS–hCG injections, significantly reduces the number of females required for experiments. Following this, we examined whether the same synchronization protocol could be used for reducing the size of female colonies required for ET experiments. After randomly selected ICR females were treated with two daily injections of P4 (Days 1 and 2), 85% of females were synchronized at metestrus on Day 3, which is consistent with the results from C57BL/6 females [63]. Subsequently, when P4-treated females were paired with vasectomized male mice for 4 days (Days 4–8), a vaginal plug was found in 63% of females on Day 7. The females were subsequently used as recipients on Day 7 for vitrified–warmed 2-cell embryos, 52% of which developed into offspring, a similar rate to that of the conventional ET procedure. Similarly, 77% of the P4-injected females became pregnant after mating with intact males for 3 days, which allowed the scheduled preparation of foster mothers [63]. Thus, our estrous cycle synchronization method can help bypass the conventional experience-based process of choosing females for sterile or fertile mating. Importantly, we estimate that the size of female stocks required as recipients in ET procedures can be reduced to less than 20% of those needed for conventional approaches, which would confer significant benefits for facilities undertaking mouse ARTs.
Towards the Goals of ARTs
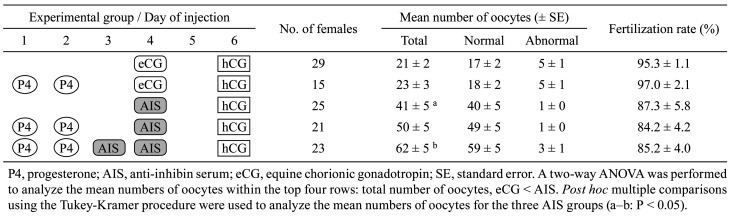
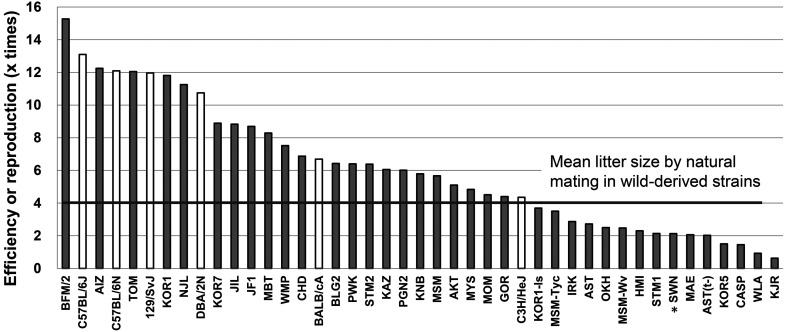
The most important parameter measured as part of our ART development program is the final yield of pups obtained per single female. Through our cumulative ART advancements, we have significantly improved the final yields of pups in standard inbred strains as well as in wild-derived strains (Fig. 3) [25]. Generally, the number of offspring produced from one female can be estimated by multiplying the number of oocytes obtained per female, fertilization rates, survival rates after vitrification at the 2-cell stage, and birthrates after ET into the oviducts of pseudopregnant females at Day 1. For example, in the standard C57BL/6J strain, the final yield in the past was: 21.3 oocytes × 88% IVF rate × 97% survival after vitrification × 72% birth rate = 13.1 pups/female. However, due to improvements in superovulation methods with AIS, this final yield has increased to 30 pups/female at the BRC and 42 pups/female at the Center for Animal Resources and Development (CARD; using immature females) in the C57BL/6J strain (Table 4) [40].
Fig. 3.
Overall reproduction efficiencies in wild-derived strains (gray bars) and standard inbred strains (white bars) [25]. The efficiencies were calculated as the number of offspring produced from one superovulated female i.e., the multiplication of the average of the number of collected oocytes per female based on equine chorionic gonadotropin (eCG) or anti-inhibin serum (AIS) treatment, the fertilization rate with fresh spermatozoa, the survival rate of embryos vitrifed using the high osmolality vitrification (HOV) method, and the birthrate after embryo transfer (ET) via the conventional or improved method. For the SWN strain, birthrate data were obtained based on embryos derived from natural mating (*).
It should be emphasized that the majority of researchers who use mice benefit from recent ART advancements because of the high pup production in the standard C57BL/6 strain. However, inefficiency at any of the ART steps will cause a significant decrease in pup production, and many mouse strains have a low conception rate following treatment with ARTs because of strain-specific technical difficulties. As genetically modified strains with different backgrounds can be generated rapidly using modern gene-editing technologies, it is important to ensure these strains are receptive to ART for easier maintenance and research use. Typical examples include the A, BALB/c and DBA/2 strains, which have been used for many years in specific biomedical fields such as immunology. They remain poor breeders even with the most advanced ARTs, primarily because their embryos easily loose viability after handling in vitro. We are now developing new methods to minimize the in vitro-induced damage to these embryos by improving the ET and embryo culture protocols.
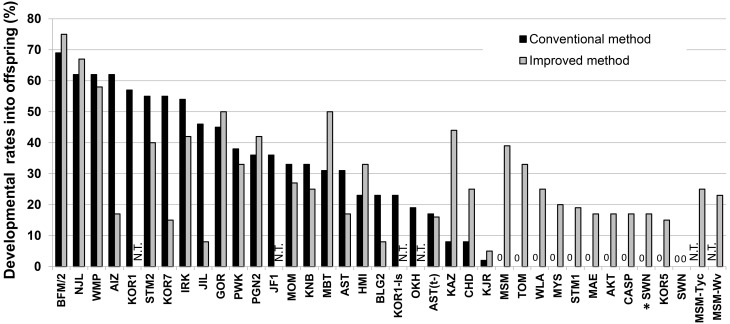
When we started developing ARTs for wild-derived strains approximately 10 years ago, embryos from most strains were unable to develop to term after being transferred into pseudopregnant ICR females. In the wild-derived strains belonging to M. m. molossinus, MSM/Ms embryos, but not JF1/Ms embryos, failed to develop to term after embryo transfer because of intrauterine death at mid to late gestation. Although the exact reason for this strain-specific difference within M. m. molossinus is unknown, it may be related to the fact that JF1/Ms is genetically more identical to laboratory strains than MSM/Ms [68]. We overcame this problem by developing an improved ET method, which combined the cotransfer of ICR strain embryos with an injection of the immunosuppressant cyclosporine A to recipient females at Day 4. Interestingly, fetal death at midgestation is found only in MSM/Ms embryos transferred into ICR recipients. Although some other wild-derived strains of M. m subspecies do not develop in ICR recipients, their death occurs at the peri-implantation stage or earlier, unlike in MSM/Ms embryos. Nonetheless, healthy pups in at least 14 wild-derived strains were obtained for the first time using our improved ET method (Fig. 4) [25]. It is likely that cotransfer with ICR embryos rescues a small number of surviving wild-derived mouse embryos by maintaining pregnancy to term. In support of this hypothesis, we noted that our new ET method increased the pup delivery rate by 1–3 pups/litter (from 7/37 ET to 16/35 ET) and decreased the number of cases where there was no delivery (from 9/37 ET to 1/35 ET).
Fig. 4.
Full-term development of cryopreserved embryos following conventional or improved embryo transfer (ET) methods in wild-derived strains of mice [25]. Living offspring were obtained from all 37 strains with either the conventional (black) or the improved (gray) ET method. Using the conventional method, 10 out of 35 strains failed to produce offspring, whereas offspring was produced successfully with the improved method. Thus, all the wild-derived strains tested produced offspring with ET using vitrified-warmed embryos (33 strains). Offspring from the SWN strain were born using a combination of embryos derived from natural mating [instead of in vitro fertilization (IVF)] and the improved ET method (*).
However, the production of offspring is still difficult in strains belonging to the subspecies M. m. castaneous and in the species M. spretus and M. spicilegus. While we are making efforts to develop ARTs specifically for these strains, we are also attempting to preserve their genetic resources by using embryonic stem cells (ESCs). We have established ESCs derived from natural fertilization, IVF, and SCNT using peripheral leukocytes, at least some of which were shown to have the potential to contribute to generating chimeric mice with ICR blastocysts (Fig. 5; unpublished data). The SCNT method is better than the former two methods as it does not require the sacrifice of valuable wild-derived mice, although the mitochondrial genome is largely derived from the recipient oocyte strain [69]. If preservation of mouse strains is possible with ESCs, it may be possible to prevent their extinction. Furthermore, they may also be useful for generating gene-modified strains and for mouse genomic research, especially with ESCs from wild-derived strains carrying abundant genetic polymorphisms.
Fig. 5.
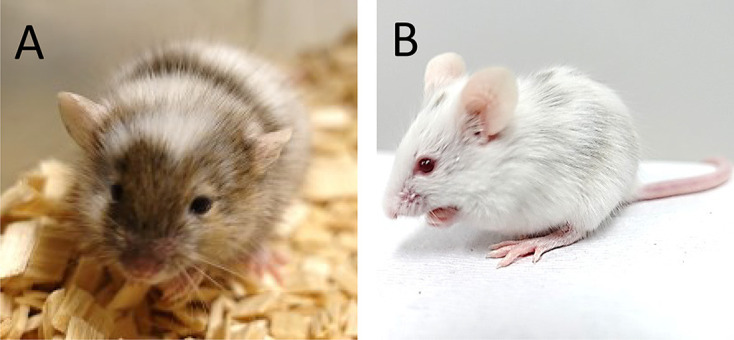
Interspecific chimeric mice generated from ESCs derived from M. spicilegus (A, ZBN/Ms strain; B, SPI/TUA strain) and M. m. domesticus (ICR strain) embryos.
Acknowledgments
I would like to thank Drs Gen Watanabe and Kazuyoshi Taya for providing AIS; and Drs Magosaburo Kasai and Keisuke Edashige for their guidance and collaboration during investigations. I would also like to thank Dr Kazuo Moriwaki, Dr Yuichi Obata, and the graduates and current members of the Experimental Animal Division and Bioresource Engineering Division of the RIKEN BioResource Research Center for their invaluable suggestions and for maintaining the mouse strains.
References
- 1.Holt WV, Pickard AR. Role of reproductive technologies and genetic resource banks in animal conservation. Rev Reprod 1999; 4: 143–150. [DOI] [PubMed] [Google Scholar]
- 2.Agca Y. Genome resource banking of biomedically important laboratory animals. Theriogenology 2012; 78: 1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshiki A, Ike F, Mekada K, Kitaura Y, Nakata H, Hiraiwa N, Mochida K, Ijuin M, Kadota M, Murakami A, Ogura A, Abe K, Moriwaki K, Obata Y. The mouse resources at the RIKEN BioResource center. Exp Anim 2009; 58: 85–96. [DOI] [PubMed] [Google Scholar]
- 4.Mochida K, Ogura A. Cryopreservation of embryos in laboratory species. J Mamm Ova Res 2010; 27: 87–92. [Google Scholar]
- 5.Whittingham DG, Leibo SP, Mazur P. Survival of mouse embryos frozen to -196 ° and -269 ° C. Science 1972; 178: 411–414. [PubMed] [Google Scholar]
- 6.Bank H, Maurer RR. Survival of frozen rabbit embryos. Exp Cell Res 1974; 89: 188–196. [DOI] [PubMed] [Google Scholar]
- 7.Whittingham DG. Survival of rat embryos after freezing and thawing. J Reprod Fertil 1975; 43: 575–578. [DOI] [PubMed] [Google Scholar]
- 8.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 1985; 313: 573–575. [DOI] [PubMed] [Google Scholar]
- 9.Kasai M, Komi JH, Takakamo A, Tsudera H, Sakurai T, Machida T. A simple method for mouse embryo cryopreservation in a low toxicity vitrification solution, without appreciable loss of viability. J Reprod Fertil 1990; 89: 91–97. [DOI] [PubMed] [Google Scholar]
- 10.Han MS, Niwa K, Kasai M. Vitrification of rat embryos at various developmental stages. Theriogenology 2003; 59: 1851–1863. [DOI] [PubMed] [Google Scholar]
- 11.Kasai M, Hamaguchi Y, Zhu SE, Miyake T, Sakurai T, Machida T. High survival of rabbit morulae after vitrification in an ethylene glycol-based solution by a simple method. Biol Reprod 1992; 46: 1042–1046. [DOI] [PubMed] [Google Scholar]
- 12.Tachikawa S, Otoi T, Kondo S, Machida T, Kasai M. Successful vitrification of bovine blastocysts, derived by in vitro maturation and fertilization. Mol Reprod Dev 1993; 34: 266–271. [DOI] [PubMed] [Google Scholar]
- 13.Hochi S, Fujimoto T, Braun J, Oguri N. Pregnancies following transfer of equine embryos cryopreserved by vitrification. Theriogenology 1994; 42: 483–488. [DOI] [PubMed] [Google Scholar]
- 14.Mochida K, Matsuda J, Noguchi Y, Yamamoto Y, Nakayama K, Takano K, Suzuki O, Ogura A. Birth of pups by transfer of mastomys embryos cryopreserved by vitrification. Biol Reprod 1998; 58(Suppl 1): 180–181. (abstract). [Google Scholar]
- 15.Mochida K, Matsuda J, Suzuki O, Nakahira M, Noguchi A, Nakayama K, Kurosawa S, Takano K, Noguchi Y, Yamamoto Y, Ogura A. Development of reproductive biotechniques in mastomys. In: Miyamoto H, Manabe N (eds.), Reproductive Biotechnology and Related Physiology. Kyoto: Hokuto Shobo; 2001: 279–284. [Google Scholar]
- 16.Lane M, Forest KT, Lyons EA, Bavister BD. Live births following vitrification of hamster embryos using a novel container-less technique. Theriogenology 1999; 51: 167 (abstract). [Google Scholar]
- 17.Lane M, Bavister BD, Lyons EA, Forest KT. Containerless vitrification of mammalian oocytes and embryos. Nat Biotechnol 1999; 17: 1234–1236. [DOI] [PubMed] [Google Scholar]
- 18.Mochida K, Wakayama T, Takano K, Noguchi Y, Yamamoto Y, Suzuki O, Ogura A, Matsuda J. Successful cryopreservation of Mongolian gerbil embryos by vitrification. Theriogenology 1999; 51: 171 (abstract). [Google Scholar]
- 19.Mochida K, Wakayama T, Takano K, Noguchi Y, Yamamoto Y, Suzuki O, Matsuda J, Ogura A. Birth of offspring after transfer of Mongolian gerbil (Meriones unguiculatus) embryos cryopreserved by vitrification. Mol Reprod Dev 2005; 70: 464–470. [DOI] [PubMed] [Google Scholar]
- 20.Fujimura H, Ogura A, Asano T, Noguchi Y, Mochida K, Takimoto K. Lysosomal glycolipid storage in the renal tubular epithelium in mastomys (Praomys coucha). Histol Histopathol 1996; 11: 171–174. [PubMed] [Google Scholar]
- 21.Hasche D, Rösl F. Mastomys species as model systems for infectious diseases. Viruses 2019; 11: E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oettlé AG. Spontaneous carcinoma of the glandular stomach in Rattus (mastomys) natalensis, an African rodent. Br J Cancer 1957; 11: 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature 1963; 200: 281–282. [DOI] [PubMed] [Google Scholar]
- 24.Mochida K, Hasegawa A, Taguma K, Yoshiki A, Ogura A. Cryopreservation of mouse embryos by ethylene glycol-based vitrification. J Vis Exp 2011; 57: e3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochida K, Hasegawa A, Otaka N, Hama D, Furuya T, Yamaguchi M, Ichikawa E, Ijuin M, Taguma K, Hashimoto M, Takashima R, Kadota M, Hiraiwa N, Mekada K, Yoshiki A, Ogura A. Devising assisted reproductive technologies for wild-derived strains of mice: 37 strains from five subspecies of Mus musculus. PLoS One 2014; 9: e114305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin B, Mochida K, Ogura A, Hotta E, Kobayashi Y, Ito K, Egawa G, Seki S, Honda H, Edashige K, Kasai M. Equilibrium vitrification of mouse embryos. Biol Reprod 2010; 82: 444–450. [DOI] [PubMed] [Google Scholar]
- 27.Jin B, Mochida K, Ogura A, Koshimoto C, Matsukawa K, Kasai M, Edashige K. Equilibrium vitrification of mouse embryos at various developmental stages. Mol Reprod Dev 2012; 79: 785–794. [DOI] [PubMed] [Google Scholar]
- 28.Mochida K, Hasegawa A, Li MW, Fray MD, Kito S, Vallelunga JM, Lloyd KCK, Yoshiki A, Obata Y, Ogura A. High osmolality vitrification: a new method for the simple and temperature-permissive cryopreservation of mouse embryos. PLoS One 2013; 8: e49316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mochida K, Kasai M. Equilibrium vitrification of mouse embryos. In: Nagy A, Gertsenstein M, Vintersten K, Behringer R. (eds.), Manipulating the Mouse Embryo: A Laboratory Manual. Fourth edition. New York: Cold Spring Harbor Laboratory Press; 2013. [Google Scholar]
- 30.Takeshima T, Nakagata N, Ogawa S. Cryopreservation of mouse spermatozoa. Jikken Dobutsu 1991; 40: 493–497 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 31.Thornton CE, Brown SD, Glenister PH. Large numbers of mice established by in vitro fertilization with cryopreserved spermatozoa: implications and applications for genetic resource banks, mutagenesis screens, and mouse backcrosses. Mamm Genome 1999; 10: 987–992. [DOI] [PubMed] [Google Scholar]
- 32.Sztein JM, Farley JS, Mobraaten LE. In vitro fertilization with cryopreserved inbred mouse sperm. Biol Reprod 2000; 63: 1774–1780. [DOI] [PubMed] [Google Scholar]
- 33.Choi YH, Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod 1998; 59: 1328–1333. [DOI] [PubMed] [Google Scholar]
- 34.Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod 2008; 78: 546–551. [DOI] [PubMed] [Google Scholar]
- 35.Taguma K, Nakamura C, Ozaki A, Suzuki C, Hachisu A, Kobayashi K, Mochida K, Ogura A, Kaneda H, Wakana S. A practical novel method for ensuring stable capacitation of spermatozoa from cryopreserved C57BL/6J sperm suspension. Exp Anim 2009; 58: 395–401. [DOI] [PubMed] [Google Scholar]
- 36.Takeo T, Nakagata N. Combination medium of cryoprotective agents containing L-glutamine and methyl-beta-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 2010; 44: 132–137. [DOI] [PubMed] [Google Scholar]
- 37.Bath ML. Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione. PLoS One 2010; 5: e9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa A, Yonezawa K, Ohta A, Mochida K, Ogura A. Optimization of a protocol for cryopreservation of mouse spermatozoa using cryotubes. J Reprod Dev 2012; 58: 156–161. [DOI] [PubMed] [Google Scholar]
- 39.Guan M, Bogani D, Marschall S, Raspa M, Takeo T, Nakagata N, Fray M. In vitro fertilization in mice using the MBCD-GSH protocol. Curr Protoc Mouse Biol 2014; 4: 67–83. [DOI] [PubMed] [Google Scholar]
- 40.Mochida K, Hasegawa A, Ogura A. Recent technical breakthroughs for ARTs in mice. J Mamm Ova Res 2017; 34: 13–21. [Google Scholar]
- 41.Hasegawa A, Mochida K, Tomishima T, Inoue K, Ogura A. Microdroplet in vitro fertilization can reduce the number of spermatozoa necessary for fertilizing oocytes. J Reprod Dev 2014; 60: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Mochida K, Hasegawa A, Inoue K, Ogura A. Identification of quantitative trait loci associated with the susceptibility of mouse spermatozoa to cryopreservation. J Reprod Dev 2018; 64: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courtemanche N, Gifford SM, Simpson MA, Pollard TD, Koleske AJ. Abl2/Abl-related gene stabilizes actin filaments, stimulates actin branching by actin-related protein 2/3 complex, and promotes actin filament severing by cofilin. J Biol Chem 2015; 290: 4038–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechtenberg BC, Mace PD, Riedl SJ. Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol 2014; 29: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cryopreservation, rederivation, and transport of mice. In: Nagy A, Gertsenstein M, Vintersten K, Behringer R. (eds.), Manipulating the Mouse Embryo: A Laboratory Manual. Third edition. New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 46.Miki H, Lee J, Inoue K, Ogonuki N, Noguchi Y, Mochida K, Kohda T, Nagashima H, Ishino F, Ogura A. Microinsemination with first-wave round spermatids from immature male mice. J Reprod Dev 2004; 50: 131–137. [DOI] [PubMed] [Google Scholar]
- 47.Ogonuki N, Inoue K, Hirose M, Miura I, Mochida K, Sato T, Mise N, Mekada K, Yoshiki A, Abe K, Kurihara H, Wakana S, Ogura A. A high-speed congenic strategy using first-wave male germ cells. PLoS One 2009; 4: e4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mochida K, Hasegawa A, Ogonuki N, Inoue K, Ogura A. Early production of offspring by in vitro fertilization using first-wave spermatozoa from prepubertal male mice. J Reprod Dev 2019; 65: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarrow MX, Wilson ED. The influence of age on superovulation in the immature rat and mouse. Endocrinology 1961; 69: 851–855. [DOI] [PubMed] [Google Scholar]
- 50.Ostermeier GC, Wiles MV, Farley JS, Taft RA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS One 2008; 3: e2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishigame H, Medan MS, Watanabe G, Shi Z, Kishi H, Arai KY, Taya K. A new alternative method for superovulation using passive immunization against inhibin in adult rats. Biol Reprod 2004; 71: 236–243. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Herath CB, Xia G, Watanabe G, Taya K. Superovulation, fertilization and in vitro embryo development in mice after administration of an inhibin-neutralizing antiserum. Reproduction 2001; 122: 809–816. [DOI] [PubMed] [Google Scholar]
- 53.Kishi H, Okada T, Otsuka M, Watanabe G, Taya K, Sasamoto S. Induction of superovulation by immunoneutralization of endogenous inhibin through the increase in the secretion of follicle-stimulating hormone in the cyclic golden hamster. J Endocrinol 1996; 151: 65–75. [DOI] [PubMed] [Google Scholar]
- 54.Takedomi T, Kaneko H, Aoyagi Y, Konishi M, Kishi H, Watanabe G, Taya K. Effects of passive immunization against inhibin on ovulation rate and embryo recovery in holstein heifers. Theriogenology 1997; 47: 1507–1518. [DOI] [PubMed] [Google Scholar]
- 55.Nambo Y, Kaneko H, Nagata S, Oikawa M, Yoshihara T, Nagamine N, Watanabe G, Taya K. Effect of passive immunization against inhibin on FSH secretion, folliculogenesis and ovulation rate during the follicular phase of the estrous cycle in mares. Theriogenology 1998; 50: 545–557. [DOI] [PubMed] [Google Scholar]
- 56.Shi F, Ozawa M, Komura H, Watanabe G, Tsonis CG, Suzuki AK, Taya K. Induction of superovulation by inhibin vaccine in cyclic guinea-pigs. J Reprod Fertil 2000; 118: 1–7. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa A, Mochida K, Matoba S, Yonezawa K, Ohta A, Watanabe G, Taya K, Ogura A. Efficient production of offspring from Japanese wild-derived strains of mice (Mus musculus molossinus) by improved assisted reproductive technologies. Biol Reprod 2012; 86: 167–: 1–7.. [DOI] [PubMed] [Google Scholar]
- 58.Moriwaki K, Miyashita N, Mita A, Gotoh H, Tsuchiya K, Kato H, Mekada K, Noro C, Oota S, Yoshiki A, Obata Y, Yonekawa H, Shiroishi T. Unique inbred strain MSM/Ms established from the Japanese wild mouse. Exp Anim 2009; 58: 123–134. [DOI] [PubMed] [Google Scholar]
- 59.Kosaka T, Hokao R, Takahashi KW, Saito TR. Copulatory behavior of sexually inexperienced male guinea pigs paired with synchronized estrus females. Jikken Dobutsu 1993; 42: 261–264. [DOI] [PubMed] [Google Scholar]
- 60.Ueda H, Kosaka T, Takahashi KW. Intraperitoneal insemination of the guinea pig with synchronized estrus induced by progesterone implant. Exp Anim 1998; 47: 271–275. [DOI] [PubMed] [Google Scholar]
- 61.Shi F, Mochida K, Suzuki O, Matsuda J, Ogura A, Tsonis CG, Watanabe G, Suzuki AK, Taya K. Development of embryos in superovulated guinea pigs following active immunization against the inhibin alpha-subunit. Endocr J 2000; 47: 451–459. [DOI] [PubMed] [Google Scholar]
- 62.Hasegawa A, Mochida K, Inoue H, Noda Y, Endo T, Watanabe G, Ogura A. High yield superovulation in adult mice by anti-inhibin serum treatment combined with estrous cycle synchronization. Biol Reprod 2016; 94: 21. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa A, Mochida K, Ogonuki N, Hirose M, Tomishima T, Inoue K, Ogura A. Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization. J Reprod Dev 2017; 63: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kito S, Hayao T, Noguchi-Kawasaki Y, Ohta Y, Hideki U, Tateno S. Improved in vitro fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comp Med 2004; 54: 564–570. [PubMed] [Google Scholar]
- 65.Eto T, Takahashi R, Kamisako T. Strain preservation of experimental animals: vitrification of two-cell stage embryos for multiple mouse strains. Cryobiology 2015; 70: 150–155. [DOI] [PubMed] [Google Scholar]
- 66.Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 2006; 65: 1716–1726. [DOI] [PubMed] [Google Scholar]
- 67.Takeo T, Nakagata N. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 2015; 10: e0128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takada T, Ebata T, Noguchi H, Keane TM, Adams DJ, Narita T, Shin-I T, Fujisawa H, Toyoda A, Abe K, Obata Y, Sakaki Y, Moriwaki K, Fujiyama A, Kohara Y, Shiroishi T. The ancestor of extant Japanese fancy mice contributed to the mosaic genomes of classical inbred strains. Genome Res 2013; 23: 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inoue K, Ogonuki N, Yamamoto Y, Takano K, Miki H, Mochida K, Ogura A. Tissue-specific distribution of donor mitochondrial DNA in cloned mice produced by somatic cell nuclear transfer. Genesis 2004; 39: 79–83. [DOI] [PubMed] [Google Scholar]