Abstract
Accumulating evidence suggests that KNDy neurons located in the hypothalamic arcuate nucleus (ARC), which are reported to express kisspeptin, neurokinin B, and dynorphin A, are indispensable for the gonadotropin-releasing hormone (GnRH) pulse generation that results in rhythmic GnRH secretion. The aims of the present study were to investigate the effects of peripheral administration of the neurokinin 3 receptor (NK3R/TACR3, a receptor for neurokinin B) antagonist, SB223412, on GnRH pulse-generating activity and pulsatile luteinizing hormone (LH) secretion in ovariectomized Shiba goats treated with luteal phase levels of estrogen. The NK3R antagonist was infused intravenously for 4 h {0.16 or 1.6 mg/(kg body weight [BW]·4 h)} during which multiple unit activity (MUA) in the ARC was recorded, an electrophysiological technique commonly employed to monitor GnRH pulse generator activity. In a separate experiment, the NK3R antagonist (40 or 200 mg/[kg BW·day]) was administered orally for 7 days to determine whether the NK3R antagonist could modulate pulsatile LH secretion when administered via the oral route. Intravenous infusion of the NK3R antagonist significantly increased the interval of episodic bursts of MUA compared with that of the controls. Oral administration of the antagonist for 7 days also significantly prolonged the interpulse interval of LH pulses. The results of this study demonstrate that peripheral administration of an NK3R antagonist suppresses pulsatile LH secretion by acting on the GnRH pulse generator, suggesting that NK3R antagonist administration could be used to modulate reproductive functions in ruminants.
Keywords: Gonadotropin-releasing hormone pulse generator, KNDy neuron, Multiple unit activity, Neurokinin B, Neurokinin 3 receptor
Mammalian reproductive function is regulated by the hypothalamus-pituitary-gonadal axis. Pulsatile gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus at a physiological frequency stimulates gonadotropin release from the pituitary to enhance gametogenesis and steroidogenesis in mammals of both sexes [1]. The hypothalamic GnRH pulse generator, a neuronal network located in the mediobasal hypothalamus, has been proposed to regulate pulsatile GnRH/gonadotropin secretion [2]. A group of kisspeptin neurons is considered to be a master regulator of mammalian reproduction via the direct stimulation of GnRH secretion, because mutation or knockout of the genes encoding kisspeptin (KISS1) or its receptor (KISS1R) leads to hypogonadotropic hypogonadism and subsequent loss of reproductive function in humans [3, 4] and mice [5, 6]. Accumulating evidence has suggested that the kisspeptin neurons located in the hypothalamic arcuate nucleus (ARC), which co-express neurokinin B (NKB) and dynorphin A in rodents [7, 8] and ruminants [9,10,11], and are, therefore, referred to as KNDy neurons [12], would be responsible for GnRH pulse generation [13]. Thus, the neurons are proposed to be a component of the GnRH pulse generator [14]. Indeed, rhythmic increases in multiple unit activity (MUA) were synchronized with luteinizing hormone (LH) pulses in goats when an electrode was placed near the KNDy neurons in the ARC [9, 15]. KNDy neuropeptides like NKB and dynorphin A, as well as their respective agonists and antagonists, have attracted considerable interest as pharmacological regulators for use in the artificial control of reproductive functions. Previous studies have demonstrated that agonists and antagonists of KNDy neuropeptide affect gonadotropin and gonadal steroid secretion in mammals, including domestic animals like cattle, goats, and sheep [16,17,18,19,20,21,22].
NKB, encoded by the tachykinin precursor 3 (TAC3) gene, is a decapeptide belonging to the tachykinin family of peptides that show a high affinity for the neurokinin 3 receptor (NK3R; encoded by TACR3) [23, 24]. NKB is suggested to be critical for the generation of pulsatile GnRH secretion, owing to the reported association between loss-of-function mutations in either TAC3 or TACR3 and hypogonadal hypogonadism stemming from impaired gonadotropin secretion in humans [25, 26]. In goats, NKB intracerebroventricular injection led to a rise in the frequency of episodic increases in MUA (MUA volley), an electrophysiological manifestation of GnRH pulse generator activity [27], indicating that NKB stimulates GnRH pulse generator activity [9]. Furthermore, bilateral NKB microimplants in the ARC were shown to stimulate pulsatile LH secretion in sheep [28], while intravenous injection of NKB or senktide, an NK3R agonist, stimulated pulsatile LH secretion in monkeys [29]. These observations indicate that NKB/NK3R signaling has a stimulatory effect on GnRH pulse generator activity, which in turn stimulates reproductive functions.
Considering the stimulatory role of NKB/NK3R signaling in GnRH pulse generation, antagonism of NK3R could be a useful strategy for contraception in wild animals such as wild deer or wild boar, and thereby reduce their population sizes. To achieve this, the effects of peripheral administration of NK3R antagonists on reproduction and mechanism mediating the effect should first be clarified. Previous studies have demonstrated that oral administration of SB223412, a selective NK3R antagonist, suppresses testosterone secretion in male dogs [21] and guinea pigs [20]. Peripherally administered ESN234, another NK3R antagonist, was also indicated to suppress pulsatile LH secretion in ovariectomized (OVX) ewes and decrease plasma estrogen levels, both in female monkeys [22] and in women [30]. These results imply that NK3R antagonist administered via the peripheral route may be capable of suppressing GnRH pulse generator activity. Interestingly, intracerebroventricular administration of SB223412 failed to suppress pulsatile LH secretion in female rats [31], thus, it is possible that the effect of NK3R antagonism on reproduction may differ among mammalian species.
In this study, using female goats as a ruminant model, we examined the effects of intravenous infusion of SB223412, an NK3R antagonist, on GnRH pulse generator activity. For this, we recorded MUA in OVX female Shiba goats treated with luteal phase levels of estradiol to test if peripheral administration of SB223412 would affect GnRH pulse generator activity. We also examined the effect of oral administration of the NK3R antagonist on pulsatile LH secretion in estrogen-treated OVX goats to test whether an NK3R antagonist administered via the oral route was capable of modulating the endocrine mechanisms that regulate reproductive functions in ruminants.
Materials and Methods
Animals
Adult (2–6 years old) female Shiba goats (Capra hircus) with body weight (BW) of 29.4–34.3 kg were used for the experiments (Experiment 1, n = 3; Experiment 2, n = 4). Goats were loosely tied to a stanchion in a room with a controlled environment (23°C and 50% relative humidity) and under a 12-h light/dark cycle. Goats were fed twice a day with a standard pelleted diet and hay and had free access to water and supplemental minerals. GnRH pulse generator activity is known to be considerably affected by ovarian steroid hormones such as estrogen and progesterone [32]. Therefore, goats were ovariectomized and treated with levels of estradiol sufficient to induce negative feedback to avoid the influence of endogenous ovarian steroids. Silicone tubing (inner diameter, 3 mm; outer diameter, 5 mm; length, 20 mm) filled with crystalline estradiol (E2, Sigma-Aldrich, St. Louis, MO, USA) was subcutaneously implanted in each OVX goat at least 2 weeks before the experiments to produce plasma E2 levels similar to those found in the luteal phase (4–8 pg/ml) [33]. All the animal experiments were approved by the Committee for the Care and Use of Experimental Animals of the Graduate School of Bioagricultural Sciences, Nagoya University.
Experiment 1: Intravenous infusion of SB223412
The NK3R antagonist, SB223412, was purchased from ChemShuttle (Hayward, CA, USA). A solution of SB223412 (0.16 or 1.6 mg SB223412/kg BW in 40 ml of 1% dimethyl sulfoxide [DMSO] in distilled water) or 1% DMSO in distilled water was infused intravenously for 4 h through a jugular catheter using a syringe pump with the infusion flow rate set at 10 ml/h. Doses of the antagonist were determined by referring to the following studies: a half-life of the NK3R antagonist in plasma after intravenous administration (1 mg/kg BW) was 404 ± 22 min in dogs [34]; a single intravenous injection (5 mg per goat) of SB222220, an NK3R antagonist with similar bioactivity and physicochemical properties to SB223412, blocked the occurrence of the pheromone-induced MUA volley in OVX goats [35]. Bilateral 18-gauge jugular catheters (Medicut; Nippon Sherwood Medical Industries, Tokyo, Japan) were inserted in the animals, one for NK3R antagonist infusion and the other for blood sampling, at least 1 day before the experiment. MUA was recorded throughout the experimental period. The methods employed for the bilateral implantation of the electrode array into the ARC and to record MUA have been described elsewhere [15]. Blood samples were collected every 6 min for 8 h before and after the start of the infusion of the drug or vehicle. Plasma was separated from the blood samples by centrifugation and stored at −20°C until measurement of LH.
Experiment 2: Oral administration of SB223412
The NK3R antagonist, SB223412, was purchased from AstaTech Biopharmaceutical Corp. (Chengdu, China), and a custom ordered pelleted diet containing 10 or 50 mg/g of SB223412 was provided by Oriental Yeast (Tokyo, Japan). Animals were fed with 4 g/[kg BW·day] of the pelleted diet containing the NK3R antagonist twice a day (0800 and 2000 h) for 7 days. Thus, two doses (40 and 200 mg/[kg BW·day]) of SB223412 were employed, and a diet without the NK3R antagonist was used as a control. Doses of the antagonist were determined according to the results of our preliminary study in which oral administration of the NK3R antagonist (20 mg/[kg BW·day]) failed to change pulsatile LH secretion in estrogen-treated OVX female goats (data not shown). The first day of oral administration was designated as Day 1. For blood sampling, an 18-gauge catheter was inserted unilaterally into the jugular vein of the animals at least 1 day before the experiment. Blood samples were collected every 6 min for 4 h (1200–1600 h) on Days 0 (the day before administration), 2, 4, 7, and 9 for the LH assay and daily at 1400 h from Day 0 to Day 9 to measure plasma SB223412 concentrations. Plasma was separated and then stored at −20°C until used for the assays.
Assays
Plasma LH concentrations were determined by a double-antibody radioimmunoassay, as previously described [18]. For 50-µl plasma samples, the lowest detectable LH concentration was 0.098 ng/ml, and the intra- and inter-assay coefficients of variation were 6.2% at 3.14 ng/ml and 7.3% at 3.11 ng/ml, respectively. Plasma SB223412 concentrations were determined by liquid chromatography–mass spectrometry using a previously described method [20].
Data analysis
In each experiment, peaks of LH pulses were identified using PULSAR computer program [36]. In Experiment 1, the MUA volley interval was used to evaluate GnRH pulse generator activity. When the difference between the values of two consecutive points exceeded twice the standard deviation calculated during the baseline period before starting the blood collection, the point was designated as the beginning of an MUA volley [18]. The mean MUA volley interval was calculated for each animal, and then each group in each treatment. Each parameter of pulsatile LH secretion during a 4-h period before and after infusion of the NK3R antagonist or vehicle was calculated for each animal, and then each group. In Experiment 2, for each day, each parameter of pulsatile LH secretion during a 4-h period was calculated for each animal, and then each group. For statistical analysis and data presentation, MUA volley interval values and those for each parameter of pulsatile LH secretion were expressed as percentages of pretreatment values (Experiment 1, vs. 4-h periods before infusion; Experiment 2, vs. Day 0). Significant differences between groups were analyzed using one-way ANOVA followed by the Bonferroni’s test for multiple comparisons, or two-way ANOVA for repeated measures (between = treatment, within = day) followed by a contrast test for multiple comparisons (JMP 7, SAS Institute Japan, Tokyo, Japan).
Results
Effects of intravenous administration of the NK3R antagonist on GnRH pulse generator activity and pulsatile LH secretion (Experiment 1)
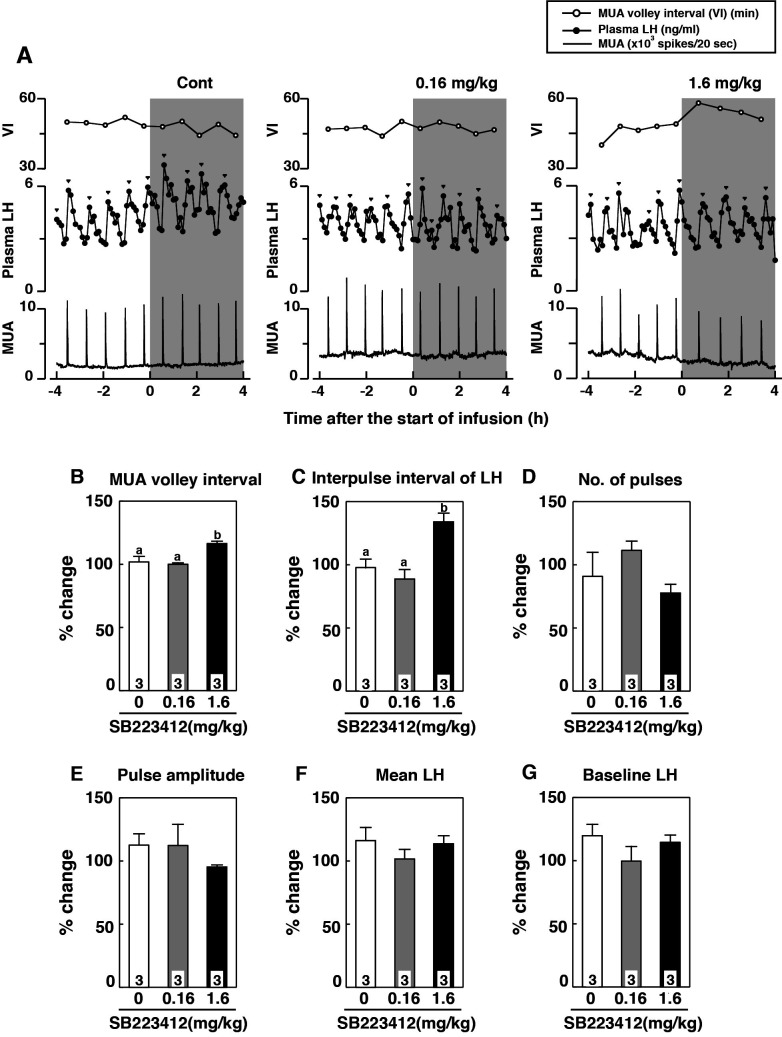
Plasma LH, MUA, and MUA volley interval profiles in a representative animal intravenously administered vehicle or SB223412 (0.16 and 1.6 mg/kg BW) are shown in Fig. 1A. MUA volley intervals were significantly increased in animals treated with 1.6 mg/kg BW of the NK3R antagonist compared with those of vehicle-treated controls (P < 0.05, Fig. 1B). The interpulse interval of LH pulses in the animals administered the higher dose of the antagonist was significantly increased compared with that of the vehicle-treated group or the group treated with the lower dose of the NK3R antagonist (P < 0.05, Fig. 1C). No significant difference was found between groups in the number and amplitude of LH pulses, or mean and baseline concentrations of plasma LH (Fig. 1D–G).
Fig. 1.
Effects of intravenous infusion of the neurokinin 3 receptor (NK3R) antagonists, SB223412, on gonadotropin-releasing hormone (GnRH) pulse generator activity and pulsatile luteinizing hormone (LH) secretion. (A) Profiles of multiple unit activity (MUA), MUA volley intervals, and plasma concentrations of LH in a representative goat receiving intravenous infusion of vehicle [1% dimethyl sulfoxide (DMSO)] or SB223412 (0.16 or 1.6 mg/kg BW). The shaded area represents the period of intravenous infusion. (B–G) Percent changes (means ± SEM) in MUA volley intervals (B), interpulse intervals (C), number of pulses (D), peak amplitude (E), mean LH concentration (F), and mean baseline LH concentration (G). Values are expressed as percent changes compared with before administration. Groups with different letters on the bars differ significantly (P < 0.05, one-way ANOVA followed by Bonferroni’s test).
Effects of oral administration of the NK3R antagonist on pulsatile LH secretion (Experiment 2)
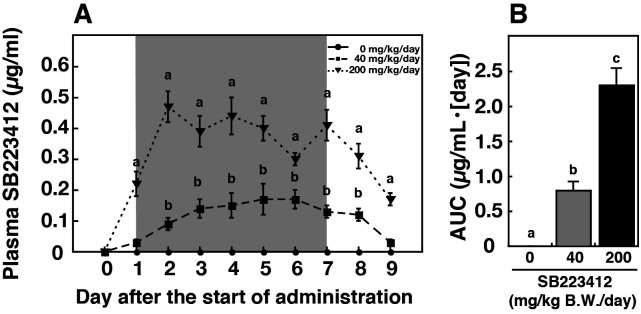
All goats consumed the pelleted diet (containing the NK3R antagonist or control) completely. The amount of food was comparable between the treatment and control groups. Plasma concentrations of SB223412 increased dose-dependently after the start of oral administration, and then decreased after the termination of administration (P < 0.05, Fig. 2A). The area under the curve (AUC) of plasma SB223412 concentrations increased dose-dependently during the oral administration period (P < 0.05, Fig. 2B).
Fig. 2.
Effect of daily oral administration of SB223412 on plasma SB223412 concentrations. (A) Changes in plasma SB223412 concentrations in estradiol-treated, ovariectomized goats receiving daily oral administration of an SB223412-containing diet (40 or 200 mg/[kg BW·day]) or control diet. The shaded area represents the period of oral administration (Day 1 to Day 7). Values are means ± SEM. Values with different letters differ significantly (P < 0.05, two-way ANOVA for repeated measures followed by a contrast test for multiple comparisons). (B) Mean (± SEM) area under the curve (AUC) of plasma SB223412 concentrations with daily oral administration (Day 1 to Day 7). Values with different letters differ significantly (P < 0.05, one-way ANOVA followed by Bonferroni’s test).
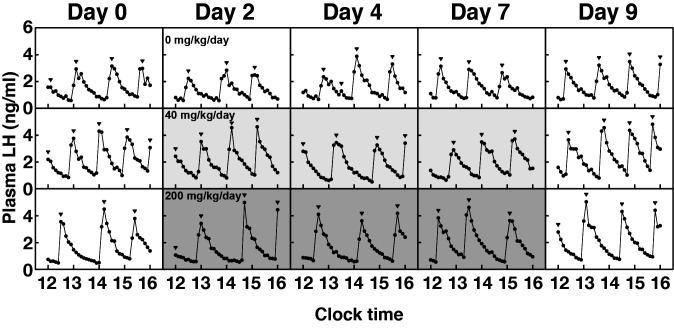
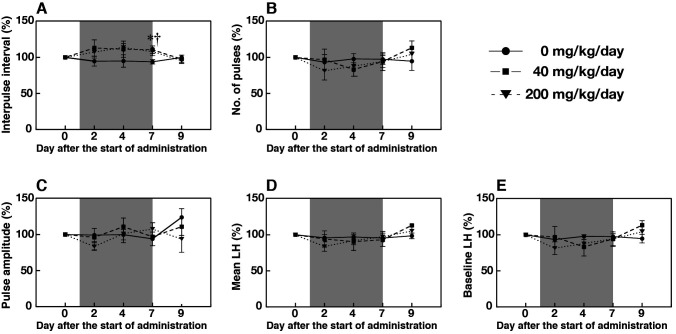
Profiles of plasma LH concentrations in a representative animal fed either a control diet or a pelleted diet containing the NK3R antagonist (40 and 200 mg/[kg BW·day]) are shown in Fig. 3. The interpulse intervals in animals administered the NK3R antagonist (40 and 200 mg/[kg BW·day]) tended to be prolonged on Days 2 and 4, and were significantly increased on Day 7 compared with those in animals fed the control diet (P < 0.05, Fig. 4A). No significant difference was found between groups in the number and amplitude of LH pulses, or mean and baseline concentrations of plasma LH (Fig. 4B–E).
Fig. 3.
Profiles of plasma concentrations of luteinizing hormone (LH) in a representative goat receiving daily oral administration of an SB223412-containing diet (40 or 200 mg/[kg BW·day]) or control diet. The shaded area represents the period of oral administration of an SB223412-containing diet (Day 1 to Day 7). Arrowheads indicate the peaks of LH pulses identified using PULSAR computer program.
Fig. 4.
Effect of daily oral administration of SB223412 on pulsatile luteinizing hormone (LH) secretion. Percent changes (means ± SEM) in interpulse intervals (A), number of pulses (B), peak amplitude (C), mean LH concentration (D), and mean baseline LH concentration (E). Values are expressed as percent changes compared with before administration (Day 0). Shaded areas represent periods of daily oral administration (Day 1 to Day 7, twice a day at 0800 and 2000 h). Blood samples were collected for 4 h (1200–1600 h) on Days 0, 2, 4, 7, and 9. An asterisk and a dagger indicate significant differences in the 40 and 200 mg/(kg BW·day) groups, respectively, compared with the control group (P < 0.05, two-way ANOVA for repeated measures followed by a contrast test for multiple comparisons).
Discussion
In the present study, we demonstrated that intravenous infusion or oral administration of SB223412, a selective NK3R antagonist, exerts a suppressive effect on GnRH pulse generator activity in E2-treated OVX goats. Intravenous infusion of the NK3R antagonist led to an increase in intervals of MUA volley, an electrophysiological manifestation of GnRH pulse generator activity, as well as interpulse intervals of LH pulses. The results suggest that peripheral administration of the NK3R antagonist suppressed pulsatile LH secretion by acting on the GnRH pulse generator. The results also suggest that endogenous NKB signaling has a facilitatory role in GnRH pulse generation, and is consistent with previous studies showing that NK3R activation by central administration of NKB [9] or intravenous injection of senktide, a selective NK3R agonist [37], increases MUA volley frequency, which reflects GnRH pulse generator activity.
The NK3R antagonist used in this study likely acted directly on KNDy neurons, the most likely candidates to be the GnRH pulse generator, because expression of NK3R mRNA and/or protein can be clearly detected in KNDy neurons in mice [7], rats [38], sheep [39], and monkeys [29]. Electrophysiological studies on brain slices obtained from male mice have indicated that exogenous NKB treatment stimulates KNDy neuron activity [40], while central NKB injection increases MUA volley frequency in the goat ARC [9]. In our previous in vitro study, we also demonstrated that primary cultured mice KNDy neurons, which express NK3R, were activated by incubation with senktide [41]. These studies indicate that NKB would act directly on KNDy neurons through NK3R and elicit pulsatile activation of KNDy neurons to stimulate GnRH neurons. Furthermore, SB223412 can be detected in rat brain tissue following intravenous administration, suggesting that SB223412 can cross the blood-brain barrier [34]. This suggests that SB223412 administered into peripheral circulation penetrates the blood-brain barrier, and acts directly on KNDy neurons to suppress GnRH pulse generation and, consequently, pulsatile GnRH secretion. Additionally, because NK3R expression was clearly detected in a mouse pituitary gonadotroph cell line [42], it is possible that peripherally administered NK3R antagonists may act not only on the hypothalamic GnRH pulse generator, but also on the pituitary gonadotroph to suppress LH secretion.
Because SB223412 has been reported to be active when administered orally [34], we examined the effect of oral administration of SB223412 on pulsatile LH secretion in goats. Plasma SB223412 concentrations increased during the periods of oral administration in a dose-dependent manner, suggesting that orally administered NK3R antagonist was successfully transferred into peripheral circulation. The result is consistent with a previous report, indicating that the SB223412 shows high bioavailability, therefore most of the orally administered SB223412 was transferred into peripheral circulation in dogs and rats [34]. In the present study, 7-day treatment with the NK3R antagonist slightly, but significantly, increased the interpulse intervals of LH pulses. Previous studies have demonstrated that oral administration of NK3R antagonist suppresses testosterone secretion in male dogs [21] and male guinea pigs [20], and decreases plasma E2 levels in cyclic female monkeys [22] and women [30]. Combined, the present results indicate that the endocrine mechanisms regulating reproductive functions in ruminants, as well as other mammalian species, could be modulated through oral administration of NK3R antagonists such as SB223412. However, the present study suggests that, as there was no significant difference in interpulse intervals of LH pulses between the two doses of orally administered NK3R antagonist, a much higher blood concentration of NK3R antagonist is needed to suppress GnRH pulse generator activity and pulsatile LH secretion in goats. Further studies are required to determine the optimal dosage and period for NK3R antagonist treatment for the development of practical regimens.
In the present study, both intravenous and oral administration of NK3R antagonist decreased MUA volley frequency but failed to completely abolish the GnRH pulse generator activity and pulsatile LH secretion. This result is consistent with a previous study showing that administration of another NK3R antagonist, SB222200, into the ARC prolonged the interpulse intervals of LH pulses but failed to abolish the pulsatility of LH secretion in OVX ewes [28]. We previously reported that intracerebral administration of SB223412 in rats failed to suppress pulsatile LH secretion, whereas CS-003, a triple antagonist for three neurokinin receptors, namely neurokinin 1 receptor (NK1R), neurokinin 2 receptor (NK2R) and NK3R, could suppress it [31]. Moreover, antagonism of the triple neurokinin receptors was required to abolish the stimulatory effect of NKB on the electrophysiological activity of KNDy neurons in mouse brain slices [40]. A previous study in goats demonstrated that pharmacological activation of NK1R and NK2R was able to induce MUA volley, albeit with a much lower efficacy than that of NK3R [37]. These studies suggest that NK1R and NK2R complementarily mediate the stimulatory effect of NKB on the GnRH pulse generator activity when NK3R is pharmacologically blocked, and thus, prolonged intervals of GnRH pulse generation by pharmacological blockade of NK3R could be due to delayed NKB stimulation to induce synchronized firing of KNDy neurons [23]. Improved knowledge of the localization of neurokinin receptors in the central nervous system and the effects of antagonists of the three neurokinin receptors on GnRH pulse generator activity is required to effectively suppress reproductive functions in goats.
In conclusion, we demonstrated that peripheral administration of SB223412, an NK3R antagonist, suppressed pulsatile LH secretion by acting on the GnRH pulse generator in female goats. The results of the present study suggest that peripheral and/or oral administration of NK3R antagonists could be used to modulate reproductive functions in ruminants.
Acknowledgments
We are grateful to Ms A Morishima and Ms R Suzumura for their technical support. We also thank Ms K Yamazaki, Mr Y Kono, and Mr F Yoshimura for their careful animal care and technical assistance. The radioimmunoassay was performed at the Nagoya University Radioisotope Center. This work was supported in part by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry from the Ministry of Agriculture, Forestry, and Fisheries of Japan (25022A); KAKENHI Grants from the Japan Society for the Promotion of Science (JSPS) Fellows to TaS (16J05264) and KY (17J10582); and JSPS KAKENHI Grant Numbers 24380154, 15K14842 and 16H05014 to SaO, 18H03973 and 18K19267 to HT, and 15H04654 to NF.
References
- 1.Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 1980; 207: 1371–1373. [DOI] [PubMed] [Google Scholar]
- 2.Maeda K, Tsukamura H, Ohkura S, Kawakami S, Nagabukuro H, Yokoyama A. The LHRH pulse generator: a mediobasal hypothalamic location. Neurosci Biobehav Rev 1995; 19: 427–437. [DOI] [PubMed] [Google Scholar]
- 3.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 6.Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M, Castellano JM, Dissen GA, Ojeda SR, Tena-Sempere M. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 2014; 155: 3088–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett 2016; 612: 161–166. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007; 148: 5752–5760. [DOI] [PubMed] [Google Scholar]
- 11.Hassaneen A, Naniwa Y, Suetomi Y, Matsuyama S, Kimura K, Ieda N, Inoue N, Uenoyama Y, Tsukamura H, Maeda K-I, Matsuda F, Ohkura S. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J Reprod Dev 2016; 62: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151: 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA 2017; 114: E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 2010; 1364: 103–115. [DOI] [PubMed] [Google Scholar]
- 15.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 16.Endo N, Rahayu LP, Ito Y, Tanaka T. Ovarian and hormonal responses to single or continuous peripheral administration of senktide, a neurokinin 3 receptor agonist, during the follicular phase in goats. Domest Anim Endocrinol 2015; 53: 136–143. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Wakabayashi Y, Yamamura T, Ohkura S, Matsuyama S. A neurokinin 3 receptor-selective agonist accelerates pulsatile luteinizing hormone secretion in lactating cattle. Biol Reprod 2017; 97: 81–90. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, Ito D, Sonoda T, Morita Y, Wakabayashi Y, Yamamura T, Okamura H, Oishi S, Noguchi T, Fujii N, Uenoyama Y, Tsukamura H, Maeda K-I, Matsuda F, Ohkura S. Peripheral administration of κ-opioid receptor antagonist stimulates gonadotropin-releasing hormone pulse generator activity in ovariectomized, estrogen-treated female goats. Domest Anim Endocrinol 2019; 68: 83–91. [DOI] [PubMed] [Google Scholar]
- 19.Nestor CC, Briscoe AMS, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology 2012; 153: 2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Ito Y, Yamamoto K, Takahashi C, Dai M, Tanahashi M, Uenoyama Y, Tsukamura H, Oishi S, Maeda K-I, Matsuda F. SB223412, a neurokinin-3 receptor-selective antagonist, suppresses testosterone secretion in male guinea pigs. Theriogenology 2017; 102: 183–189. [DOI] [PubMed] [Google Scholar]
- 21.Noritake K, Suzuki J, Matsuoka T, Makino T, Ohnishi H, Shimomura K, Uenoyama Y, Tsukamura H, Maeda K, Sanbuissho A. Testicular toxicity induced by a triple neurokinin receptor antagonist in male dogs. Reprod Toxicol 2011; 31: 440–446. [DOI] [PubMed] [Google Scholar]
- 22.Fraser GL, Hoveyda HR, Clarke IJ, Ramaswamy S, Plant TM, Rose C, Millar RP. The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology 2015; 156: 4214–4225. [DOI] [PubMed] [Google Scholar]
- 23.Mussap CJ, Geraghty DP, Burcher E. Tachykinin receptors: a radioligand binding perspective. J Neurochem 1993; 60: 1987–2009. [DOI] [PubMed] [Google Scholar]
- 24.Seabrook GR, Bowery BJ, Hill RG. Pharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slices. Eur J Pharmacol 1995; 273: 113–119. [DOI] [PubMed] [Google Scholar]
- 25.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK, Semple R. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 2009; 41: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, Meduri G, Brailly-Tabard S, Chanson P, Lecomte P, Guiochon-Mantel A, Young J. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS One 2011; 6: e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology 1991; 53: 392–395. [DOI] [PubMed] [Google Scholar]
- 28.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013; 154: 4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010; 151: 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser GL, Ramael S, Hoveyda HR, Gheyle L, Combalbert J. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J Clin Endocrinol Metab 2016; 101: 417–426. [DOI] [PubMed] [Google Scholar]
- 31.Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev 2011; 57: 409–415. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Ozawa T, Hoshino K, Mori Y. Changes in the gonadotropin-releasing hormone pulse generator activity during the estrous cycle in the goat. Neuroendocrinology 1995; 62: 553–561. [DOI] [PubMed] [Google Scholar]
- 33.Ichimaru T, Mori Y, Okamura H. A possible role of neuropeptide Y as a mediator of undernutrition to the hypothalamic gonadotropin-releasing hormone pulse generator in goats. Endocrinology 2001; 142: 2489–2498. [DOI] [PubMed] [Google Scholar]
- 34.Sarau HM, Griswold DE, Potts W, Foley JJ, Schmidt DB, Webb EF, Martin LD, Brawner ME, Elshourbagy NA, Medhurst AD, Giardina GAM, Hay DWP. Nonpeptide tachykinin receptor antagonists: I. Pharmacological and pharmacokinetic characterization of SB 223412, a novel, potent and selective neurokinin-3 receptor antagonist. J Pharmacol Exp Ther 1997; 281: 1303–1311. [PubMed] [Google Scholar]
- 35.Sakamoto K, Wakabayashi Y, Yamamura T, Tanaka T, Takeuchi Y, Mori Y, Okamura H. A population of kisspeptin/neurokinin B neurons in the arcuate nucleus may be the central target of the male effect phenomenon in goats. PLoS One 2013; 8: e81017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 37.Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev 2015; 61: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 2006; 498: 712–726. [DOI] [PubMed] [Google Scholar]
- 39.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 2010; 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013; 154: 2750–2760. [DOI] [PubMed] [Google Scholar]
- 41.Ikegami K, Minabe S, Ieda N, Goto T, Sugimoto A, Nakamura S, Inoue N, Oishi S, Maturana AD, Sanbo M, Hirabayashi M, Maeda K-I, Tsukamura H, Uenoyama Y. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J Neuroendocrinol 2017; 29: 1–14. [DOI] [PubMed] [Google Scholar]
- 42.Mijiddorj T, Kanasaki H, Purwana IN, Oride A, Sukhbaatar U, Miyazaki K. Role of Neurokinin B and Dynorphin A in pituitary gonadotroph and somatolactotroph cell lines. Endocr J 2012; 59: 631–640. [DOI] [PubMed] [Google Scholar]