Abstract
The present study aimed to evaluate whether novel conditional kisspeptin neuron-specific Kiss1 knockout (KO) mice utilizing the Cre-loxP system could recapitulate the infertility of global Kiss1 KO models, thereby providing further evidence for the fundamental role of hypothalamic kisspeptin neurons in regulating mammalian reproduction. We generated Kiss1-floxed mice and hypothalamic kisspeptin neuron-specific Cre-expressing transgenic mice and then crossed these two lines. The conditional Kiss1 KO mice showed pubertal failure along with a suppression of gonadotropin secretion and ovarian atrophy. These results indicate that newly-created hypothalamic Kiss1 KO mice obtained by the Cre-loxP system recapitulated the infertility of global Kiss1 KO models, suggesting that hypothalamic kisspeptin, but not peripheral kisspeptin, is critical for reproduction. Importantly, these Kiss1-floxed mice are now available and will be a valuable tool for detailed analyses of roles of each population of kisspeptin neurons in the brain and peripheral kisspeptin-producing cells by the spatiotemporal-specific manipulation of Cre expression.
Keywords: Cre/loxP system, Gonadotropin, Kisspeptin, Pubertal failure
It has been well established that kisspeptin (encoded by KISS1/Kiss1)-GPR54 (a kisspeptin receptor encoded by GPR54/Gpr54) signaling plays a critical role in the central mechanism controlling reproductive function in mammals including primates [1,2,3], and rodents [4,5,6,7]. Loss-of-function mutations in KISS1 or GPR54 in humans resulted in hypogonadotropic hypogonadism manifested by pubertal failure [1,2,3]. Similarly, global Kiss1 or Gpr54 knockout (KO) mice showed pubertal failure and gonadal atrophy [4,5,6]. In addition, Kiss1 KO rats showed a lack of both pulse and surge modes of gonadotropin secretion [7]. The most plausible interpretation is that kisspeptin-GPR54 signaling in the hypothalamus is fundamental for controlling reproductive function via direct activation of gonadotropin-releasing hormone (GnRH) neurons. This is because a GnRH neuron-targeted deletion of Gpr54 recapitulated the infertility of Kiss1 or Gpr54 KO animal models [8, 9]. Further, GnRH neuron-specific rescue of Gpr54 expression recovered reproductive function in Gpr54 KO mice [8].
Circumstantial evidence suggests that the hypothalamic kisspeptin neurons, located in two nuclei, such as the anteroventral periventricular nucleus-periventricular nucleus (AVPV-PeN) continuum (also known as the rostral periventricular region of the third ventricle, or RP3V) and the hypothalamic arcuate nucleus (ARC), are functionally distinct: AVPV-PeN kisspeptin neurons are indicated to be responsible for GnRH/luteinizing hormone (LH) surge generation in rodents [10,11,12,13,14,15], whereas the ARC ones are suggested to be involved in GnRH/LH pulse generation in rodents and ruminants [16,17,18,19,20,21,22]. Indeed, AVPV-PeN Kiss1 ablation by neonatal sex steroid exposure resulted in a deficiency of the LH surge in female rats [14, 15]. As for ARC kisspeptin neurons, rhythmic increases in the multiple unit activity recorded by the electrodes placed in close proximity to the ARC kisspeptin neurons corresponded to LH pulses in goats [16, 17]; the pulsatile kisspeptin secretion detected at the median eminence largely corresponded to GnRH pulses in monkeys [23]; chronic estrogen exposure in the neonatal period caused an irreversible suppression of ARC Kiss1 expression and LH pulses in male and female rats [24, 25]; optogenetic stimulation or inhibition of ARC kisspeptin neurons could stimulate or inhibit pulsatile LH secretion in Kiss1-Cre mice receiving adeno-associated virus (AAV) vectors carrying channelrhodopsin-2 or archaerhodopsin, respectively [26, 27]; ARC kisspeptin neurons exhibited a rhythmic increase in in vivo levels of intracellular Ca2+ that correspond to LH pulses in Kiss1-Cre mice receiving AAV vectors carrying GCaMP6, a Ca2+ biosensor [27]. In addition, previous studies showed that kisspeptin neurons are also located in the medial amygdala (MeA) of mice and rats and that kisspeptin administration into the MeA stimulated LH secretion, indicating that MeA kisspeptin neurons may integrate the limbic system and GnRH/LH secretion [28,29,30,31].
In addition to such an indispensable role of central kisspeptin in controlling pulsatile and surge-mode of GnRH/gonadotropin secretion, kisspeptin is now considered as a multi-functional molecule in the peripheral tissues [32,33,34]. Previous studies demonstrated that Kiss1 and Gpr54 expression were evident in the ovary and uterine of rodents and suggested local roles of kisspeptin signaling in follicular development, ovulation/corpus luteum formation, and implantation [35,36,37]. KISS1/Kiss1 and GPR54/Gpr54 expression were also found in the pancreas and adipose tissue of humans and rodents, wherein peripheral kisspeptin was suggested to be involved in metabolic function: Previous in vitro studies showed that kisspeptin increased glucose-induced insulin secretion from the pancreas and decreased glucose uptake and lipid accumulation via decreasing lipogenesis and increasing lipolysis in the adipose tissue [32, 34]. The Cre-loxP system for generating tissue- or cell type-specific Kiss1 KO is increasingly important to further elucidate local roles of kisspeptin in those peripheral organs as well as the central nervous system.
The present study aimed to evaluate whether our newly-created conditional kisspeptin neuron-specific Kiss1 KO mice obtained by the Cre-loxP system could recapitulate the infertility of global Kiss1 KO animal models, thereby providing further evidence for the fundamental role of central kisspeptin signaling in regulating reproduction in mammals. For this purpose, we here have generated Kiss1-floxed mice (Kiss1fl/fl mice), which could be useful for a better understanding of the brain region, tissue- or cell type-specific roles of kisspeptin. We also generated hypothalamic kisspeptin neuron-specific Cre-expressing transgenic mice (Kiss1-Cre mice) based on our previous findings on the brain region-specific Kiss1 enhancer [38, 39]. Further, we generated conditional Kiss1 KO mice by crossing the aforementioned two mouse lines and analyzed the reproductive function of the conditional Kiss1 KO mice to investigate if the mice replicate the phenotype, such as pubertal failure, suppression of gonadotropin secretion in global Kiss1 KO mice.
Materials and Methods
Animals
Gene-modified mice and wild-type (ICR, Charles River Laboratories Japan, Kanagawa, Japan; and BDF1, Japan SLC, Shizuoka, Japan) mice were housed under a controlled environment (14 h of light and 10 h of darkness; lights on at 0500 h; temperature, 22 ± 3ºC). Animals were weaned at postnatal day 21 and allowed free access to standard laboratory mouse chow (CE-2; CLEA Japan, Tokyo, Japan) and water. Genotypes of animals were determined by polymerase chain reaction (PCR) analyses of genomic DNA extracted from the ear tissue. The primer sequences for genotyping are listed in Table 1. The present study was approved by the Committees on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University and the National Institute for Physiological Sciences.
Table 1. Primer sequences for genotyping of animals and embryonic stem (ES) cell selection.
| Purpose | Primers | |
|---|---|---|
| Genotyping of animals | ||
| Kiss1-floxed | Forward, 5'-cacaggatggaagcagagca-3' | |
| Reverse, 5'-actgcccttcccctaaatgc-3' | ||
| Kiss1-Cre | Forward, 5'-gcagaacctgaagatgttcgcgat-3' | |
| Reverse, 5'- aggtatctctgaccagagtcatcc-3' | ||
| ES selection | ||
| 5'-region | Forward, 5'-gttgtttggggtggaatgagtc-3' | |
| Reverse, 5'-gcgataccgtaaagcacgag-3' | ||
| 3'-region | Forward, 5'- caggacgtgacaaatggaag-3' | |
| Reverse, 5'-accaaacattcctccagcag-3' | ||
| loxP site | Forward, 5'-gagccttgttgtctgtgaagtg-3' | |
| Reverse, 5'-ggagttccagttgtaggtggac-3' | ||
| Probe preparation | Forward, 5'-agaggctattcggctatgactg-3' | |
| for southern blotting | Reverse, 5'-actcgtcaagaaggcgatagaa-3' | |
Generation of Kiss1fl/fl mice
The targeting vector harbored a floxed exon 3 of the Kiss1 gene coding for the 52-amino-acid mouse kisspeptin and a floxed neomycin resistance cassette as shown in Fig. 1A. The targeting vector was electroporated into the TT2 (CBA × C57BL/6) line of mouse embryonic stem (ES) cells [40]. Successfully targeted ES cell clones were selected via a neomycin-supplemented medium. Genomic DNA was isolated to screen ES cell clones for homologous recombination of the Kiss1 locus. The presence of the loxP site in the Kiss1 locus was confirmed by PCR (Fig. 1B) and then confirmed by Southern blot analysis (Fig. 1C). The primer sequences for the probe preparation and PCR analyses for the ES selection are listed in Table 1. The targeted ES clones were injected into ICR 8-cell-stage embryos. The embryos containing the targeted ES clones were transplanted into the uterus of pseudopregnant foster mice. The resultant chimeric males were coupled with ICR females in order to test the germline transmission. Kiss1-floxed heterozygous mice (Kiss1fl/+ mice) without a floxed neomycin resistance cassette were produced by an injection of Cre recombinase-expressing plasmid (pCre-Pac; kindly provided by Dr Yagi, Osaka University) [41] into the fertilized oocytes obtained from the germline offspring. The resultant Kiss1fl/+ males and females were mated in order to generate Kiss1-floxed homozygous mice (Kiss1fl/fl mice). Kiss1fl/fl males and females were also fertile.
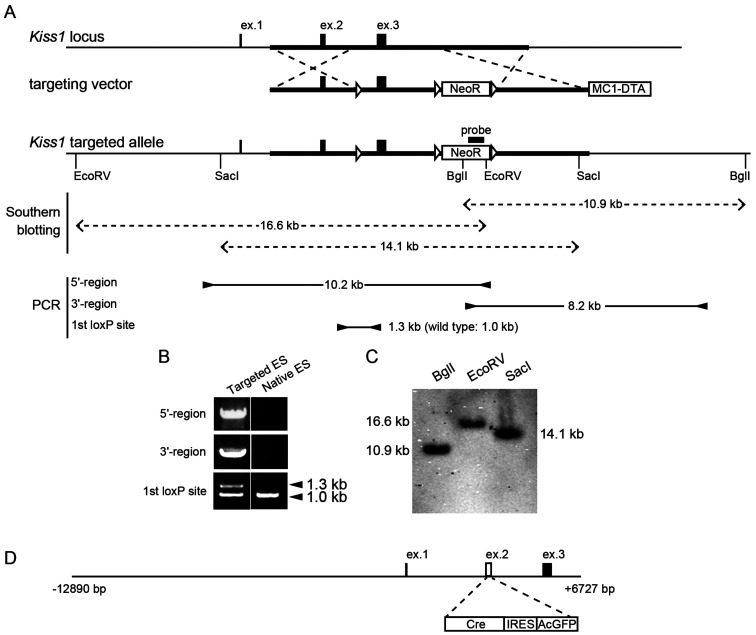
Fig. 1.
Generation of Kiss1-floxed mice and Kiss1-Cre mice. (A) Structure of the wild-type Kiss1 allele (top), targeting vector for the generation of Kiss1-floxed mice (middle), and Kiss1 targeted allele (bottom), resulting from replacement at dotted lines. The Kiss1 targeted allele was designed by insertion of three loxP sites (open triangles) and a neomycin resistance (NeoR) selection cassette. A diphtheria toxin A (DTA) expression cassette was used for negative selection in embryonic stem (ES) cells. Note that the NeoR selection cassette was removed by an injection of Cre recombinase-expressing plasmid into the fertilized oocytes obtained from the germline offspring. (B) Screening of ES cell clones by polymerase chain reaction (PCR) using three sets of primers (5'-region, 3'-region, and 1st loxP site). The locations of primers are shown by the arrowheads in panel A. The product sizes are also provided in the panel A. (C) Southern blot analysis of BglI-, EcoRV-, or SacI-digested DNA using the probe on the NeoR cassette detected 10.9-, 16.6-, and 14.1-kb fragments in the targeted allele. Predicted sizes of the DNA fragments are shown by dotted double arrows in panel A. (D) Structure of construct for Kiss1-Cre mice. The construct was designed by substitution of Cre, internal ribosome entry site (IRES), and Aequorea coerulescens green fluorescent protein (AcGFP) cassette (white boxes) for exon 2 of Kiss1 gene in which transcriptional start site is located.
Generation of Kiss1-Cre mice
Kiss1-Cre mice, in which Cre recombinase is expected to be driven by the Kiss1 promoter and the ARC-specific Kiss1 enhancer identified in our previous study [39], were generated as follows: Cre recombinase gene was inserted into a pIRES-AcGFP vector (Takara Bio, Kusatsu, Japan) and the resultant Cre-IRES-AcGFP transgene was substituted for the site between the translational start point and 3' end of exon 2 of the Kiss1 gene (accession no. AB666166) in a bacterial artificial chromosome (BAC) clone RP24-299J2 (BACPAC Resources, Oakland, CA, USA) by using a counterselection BAC modification kit (Gene Bridges, Heidelberg, Germany). The 3'-downstream-truncated DNA construct (Fig. 1D) was linearized according to our previous study [39]. The transgenic mice were generated by microinjection of the linearized construct to pronuclear-stage oocytes of BDF1 mice as previously described elsewhere [39].
Generation of conditional Kiss1 KO mice by crossing the Kiss1fl/fl mice and Kiss1-Cre mice
The Kiss1-Cre mice were crossed onto Kiss1fl/fl mice two times to generate offspring, in which Cre recombinase theoretically deletes the floxed Kiss1 exon 3, encoding a functional region of kisspeptin, in both alleles.
The vaginal opening was checked daily in the resultant conditional Kiss1 KO mice and their littermate Cre-negative Kiss1fl/fl controls until 40 days of age. Animals were then subjected to the collection of the ovary, blood, and brain samples.
Ovary collection and estradiol treatment
The conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl control mice were bilaterally ovariectomized (OVX) under aseptic conditions with isoflurane anesthesia (1–3% in air). Animals then immediately received subcutaneous Silastic implants (internal diameter: 1.57 mm; outer diameter: 3.18 mm; 3 mm in length; Dow Corning, Midland, MI, USA) that were filled with estradiol-17β (E2; Sigma-Aldrich, St. Louis, MO, USA) dissolved in peanut oil at 10 μg/ml. The E2 implant was chosen based on our previous studies [42,43,44] to visualize Kiss1 gene expression in both the AVPV and ARC: the size and dose were adjusted according to the animal body weight. Ovaries were weighed and stored at –80oC until analysis for Kiss1 and Cre mRNA expression.
Brain sampling and in situ hybridization for Kiss1 and Cre mRNA expression
One week after the OVX and E2 treatment, the animals were deeply anesthetized with pentobarbital (70 mg/kg, Kyoritsu Seiyaku, Tokyo, Japan), and then intracardially perfused with RNase-free 0.05 M phosphate-buffered saline (PBS; pH 7.5) followed by 4% paraformaldehyde (Sigma-Aldrich) in 0.05 M phosphate buffer (PB; pH 7.5). The brains were immediately removed, post-fixed with the same fixative for overnight at 4ºC, and then kept in 30% sucrose in 0.05 M PB until they sank at 4oC under the RNase-free conditions. Frozen frontal sections (50-μm thickness) of the brain containing the AVPV, ARC and medial amygdala (MeA), in which the previous study showed Kiss1 expression in mice [28, 29], were prepared using a cryostat (CM1800; Leica, Wetzlar, Germany) on the day or a day before the in situ hybridization and then stored in PBS at 4oC. Every two AVPV section and every four ARC and MeA section were used for in situ hybridization to visualize Kiss1 and Cre mRNA expression.
Digoxigenin (DIG)-labeled Kiss1 cRNA probe (position 38-372, GenBank accession no. NM_178260) and DIG-labeled Cre cRNA probe (position 485-1516, GenBank accession no. X03453) were synthesized by using a DIG-labeling kit (Boehringer Mannheim, Mannheim, Germany). Kiss1 and Cre expression was detected by free-floating in situ hybridization as described previously with slight modification [10]. Briefly, the sections were hybridized overnight at 60oC with 1 μg/ml Kiss1 or Cre cRNA probes. The DIG-labeled probes were detected by an alkaline phosphatase-conjugated anti-DIG antibody (1:1000; Roche Diagnostics, Indianapolis, IN, USA) and a chromogen (338 μg/ml 4-nitroblue tetrazolium chloride and 175 μg/ml 5-bromo-4-chloro-3-indolyl-phosphate, Roche Diagnostics). The sections were mounted on gelatin-coated slides and cover-slipped with 90% glycerol in 0.05 M PB. The signals of Kiss1 or Cre mRNA expression were observed under a light microscope (BX53; Olympus, Tokyo, Japan) and the numbers of Kiss1- or Cre-positive cells were counted throughout the AVPV and ARC. The specificity of signals was confirmed by in situ hybridization using corresponding sense probes, and no signals were detected with the sense probes.
Blood sampling and radioimmunoassay for LH and follicle-stimulating hormone (FSH)
Fifty-µl blood samples were collected from the descending aorta of both the conditional Kiss1 KO and Cre-negative Kiss1fl/fl control mice under the anesthetized condition just before the brain perfusion.
Plasma LH concentrations in 25-μl plasma samples were determined with a mouse LH-RIA kit provided by the National Hormone and Peptide Program (Bethesda, MD, USA) as previously described [45]. LH concentrations were expressed in terms of NIDDK mouse LH-RP. The least detectable concentration of LH in 25-μl plasma samples was 0.156 ng/ml. The intra- and inter-assay coefficients of variation were 4.7 and 14.5% at 1.6 ng/ml, respectively.
Plasma FSH concentrations in 25-μl plasma samples were determined with a mouse FSH RIA kit provided by the National Hormone and Peptide Program. FSH concentrations were expressed in terms of NIDDK mouse FSH-RP. The least detectable concentration of FSH in 25-μl plasma samples was 1.25 ng/ml. The intra- and inter-assay coefficient of variation was 0.32 and 13.8% at 9.6 ng/ml, respectively.
Ovarian Kiss1 and Cre expression
DNA-free total RNA was purified from the ovary by using ISOGEN (Nippon Gene, Tokyo, Japan) and the cDNA was synthesized with High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). The quantitative PCR analysis was performed by using an ABI 7500 real-time system (Thermo Fisher Scientific) with Thunderbird SYBR qPCR Mix (TOYOBO, Osaka, Japan) with specific primers for mouse Kiss1 (5'-gctgctgcttctcctctgtgt-3' and 5'-gcataccgcgattccttttc-3'), Cre (5'-cagcaacatttgggccagcta-3' and 5'-ccgccgcataaccagtgaaac-3') and mouse Actb (5'-ggtgggaatgggtcagaagg-3' and 5'-gtacatggctggggtgttga-3'). The cycling protocol was as follows: pre-denature for 1 min at 95ºC, 40 cycles amplification of 15 sec at 95ºC and 1 min at 60ºC. The specificity of the amplicons was confirmed by a dissociation curve analysis (60 to 95°C) after 40-cycle amplification. A distinct single peak was considered that only a single DNA sequence was amplified. The expression levels of Kiss1 and Cre were normalized to that of Actb.
Statistical analysis
Statistical differences in ovarian weights, plasma gonadotropin concentrations, the number of hypothalamic Kiss1-expressing cells, as well as ovarian Kiss1 and Cre expression levels between the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls were determined by Welch’s-t test (R version 3.6.0, http://www.R-project.org/).
Results
Pubertal failure and atrophy of ovaries in conditional Kiss1 KO mice
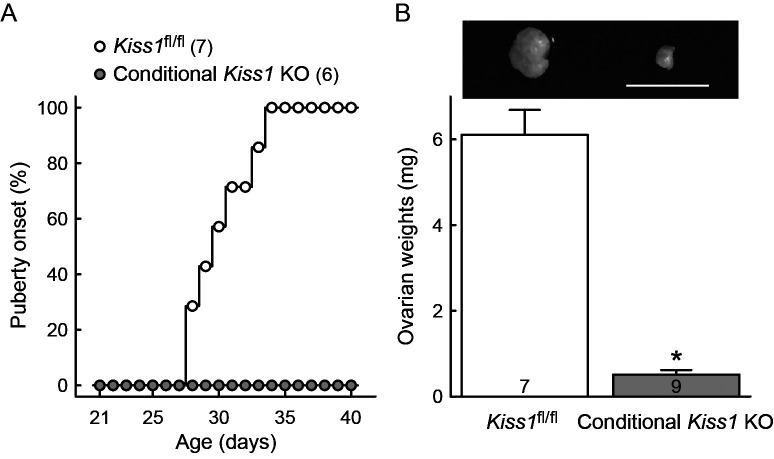
The conditional Kiss1 KO female mice by crossing Kiss1-Cre mice and Kiss1fl/fl mice showed no vaginal opening as an external sign of pubertal onset by 40 days of age, whereas the Cre-negative Kiss1fl/fl controls showed vaginal opening at 28–34 postnatal days (Fig. 2A). The ovarian weight was significantly lower in the conditional Kiss1 KO mice than the Cre-negative Kiss1fl/fl control mice (P < 0.05, Fig. 2B).
Fig. 2.
The conditional Kiss1 knockout (KO) mice failed to show puberty onset and ovarian atrophy. (A) Timing of vaginal opening as an external sign of pubertal onset is expressed as a percentage of the total number of animals for each genotype. Numbers in the parentheses indicate the number of animals used. (B) Representative photograph of ovary and ovarian weights in the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls. Values are indicated as mean ± SEM. Numbers in each column indicate the number of animals used. * P < 0.05 between the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls (Welch’s-t test). Scale bar, 5 mm.
Reduction of plasma gonadotropin levels in the conditional Kiss1 KO mice
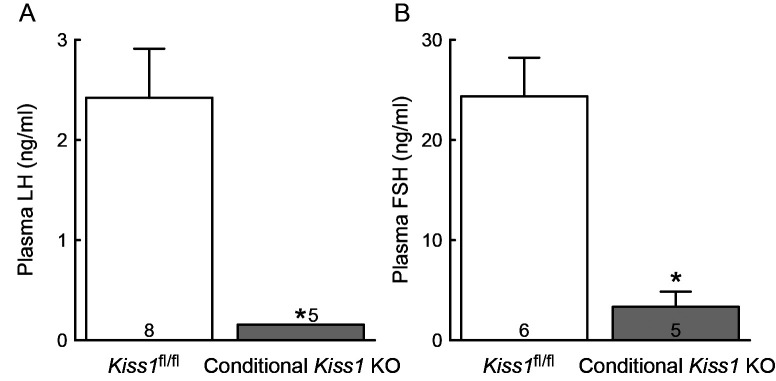
Plasma LH levels were undetectable in all conditional Kiss1 KO female mice and the levels were significantly lower in the conditional Kiss1 KO mice compared with those in the Cre-negative Kiss1fl/fl control mice (P < 0.05, Fig. 3A). Plasma FSH levels were undetectable in three out of five conditional Kiss1 KO mice, resulting in significant lower levels of FSH in the conditional Kiss1 KO mice compared with those in the Cre-negative Kiss1fl/fl controls (P < 0.05, Fig. 3B).
Fig. 3.
The conditional Kiss1 knockout (KO) mice showed suppression of gonadotropin secretion. Plasma luteinizing hormone (LH, A) and follicle-stimulating hormone (FSH, B) levels of the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls. Values are indicated as mean ± SEM. Note that plasma LH levels were undetectable in all conditional Kiss1 KO mice and expressed as the least detectable concentration of LH (0.156 ng/ml). Numbers in or on each column indicate the number of animals used. * P < 0.05 between the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls (Welch’s-t test).
Kiss1 expression in the brain of the conditional Kiss1 KO female mice and Cre expression in the brain of the Kiss1-Cre female mice
Figure 4 shows representative photomicrographs of Cre-expressing cells in the ARC (Fig. 4A) and AVPV (Fig. 4B) of Kiss1-Cre mice and Cre-negative controls. A number of Cre-expressing cells (432.9 ± 50.5, n = 4) were found in the ARC of Kiss1-Cre mice, but not in the Cre-negative control mice (Fig. 4A). Very few Cre-expressing cells (58.1 ± 8.3 cells, n = 4) with weak signals were found in the AVPV of Kiss1-Cre mice, but not in Cre-negative control mice (Fig. 4B).
Fig. 4.
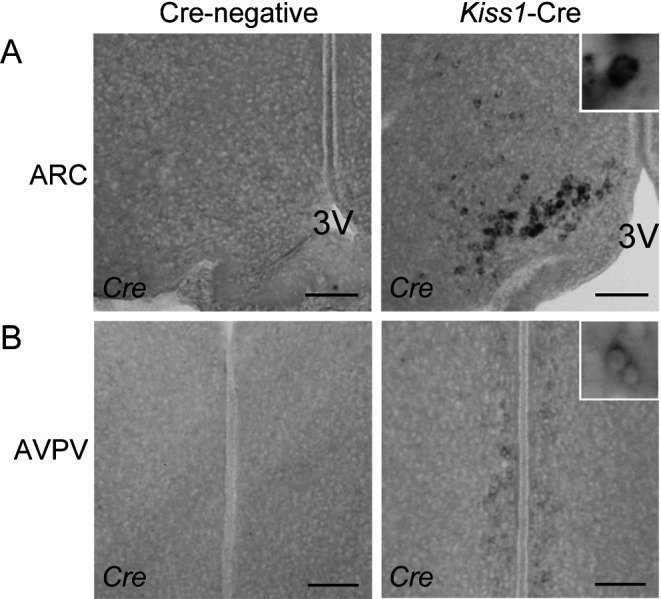
Determination of Cre expression in the hypothalamus of Kiss1-Cre mice. (A) Cre-expressing cells in the arcuate nucleus (ARC) of a representative Kiss1-Cre mouse (right panel). No Cre-expressing cells were found in the ARC of Cre-negative controls (left panel). 3V, third cerebroventricle. (B) Few Cre-expressing cells with weak signals in the anteroventral periventricular nucleus (AVPV) of a representative Kiss1-Cre mouse (right panel). No Cre-expressing cells were found in the AVPV of Cre-negative controls (left panel). Scale bars, 100 µm.
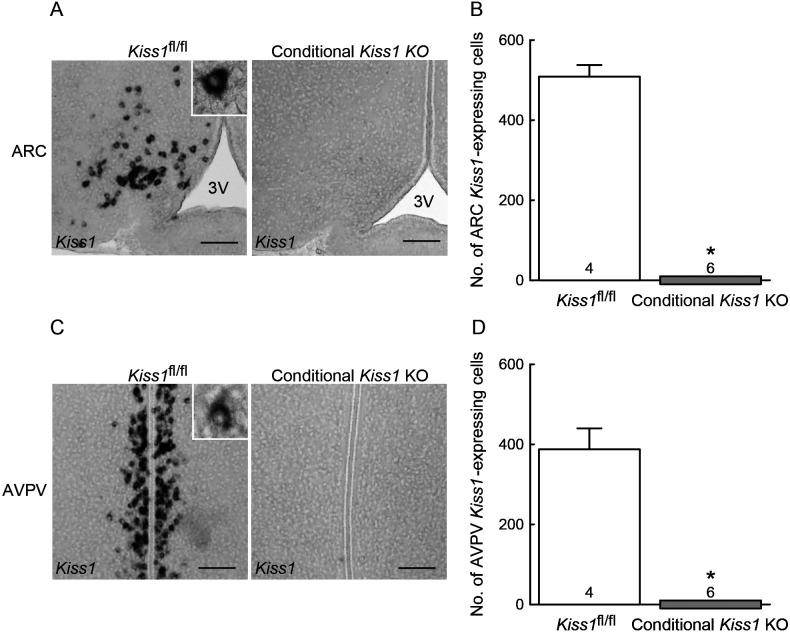
Figure 5 shows representative photomicrographs of Kiss1-expressing cells in the ARC (Fig. 5A) and AVPV (Fig. 5C) of the OVX+E2 conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls. No Kiss1-positive cells were found in the ARC of conditional Kiss1 KO female mice, whereas a number of Kiss1-positive cells were found in the ARC of Cre-negative Kiss1fl/fl controls (Fig. 5A). The number of ARC Kiss1-expressing cells were significantly lower in the conditional Kiss1 KO mice compared with Cre-negative Kiss1fl/fl controls (P < 0.05, Fig. 5B). Unexpectedly, no Kiss1-expressing cells were found in the AVPV of conditional Kiss1 KO female mice, whereas a number of Kiss1-positive cells were found in the AVPV of Cre-negative Kiss1fl/fl controls (Fig. 5C). The number of AVPV Kiss1-positive cells were also significantly lower in the conditional Kiss1 KO mice than the Cre-negative Kiss1fl/fl controls (P < 0.05, Fig. 5D).
Fig. 5.
The conditional Kiss1 knockout (KO) mice showed completely suppression of Kiss1 expression in the hypothalamus. (A) Kiss1-expressing cells in the arcuate nucleus (ARC) of representative conditional Kiss1 KO mouse and Cre-negative Kiss1fl/fl control. 3V, third cerebroventricle. (B) The number of Kiss1-expressing cells throughout the ARC. Note that no Kiss1-expressing cells were found in the ARC of conditional Kiss1 KO mice. (C) Kiss1-expressing cells in the anteroventral periventricular nucleus (AVPV) of representative conditional Kiss1 KO mouse and Cre-negative Kiss1fl/fl control. (D) The number of Kiss1-expressing cells throughout the AVPV. Note that no Kiss1-expressing cells were found in the AVPV of conditional Kiss1 KO mice. Values are indicated as mean ± SEM. Numbers in or on each column indicate the number of animals used. * P < 0.05 between the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl controls (Welch’s-t test). Scale bars, 100 µm.
No Kiss1-expressing cells were found in the MeA of both the conditional Kiss1 KO and Cre-negative Kiss1fl/fl OVX + E2 mice (Fig. 6A). In addition, no Cre-expressing cells were found in the MeA of Kiss1-Cre mice as well as Cre-negative controls (Fig. 6B).
Fig. 6.
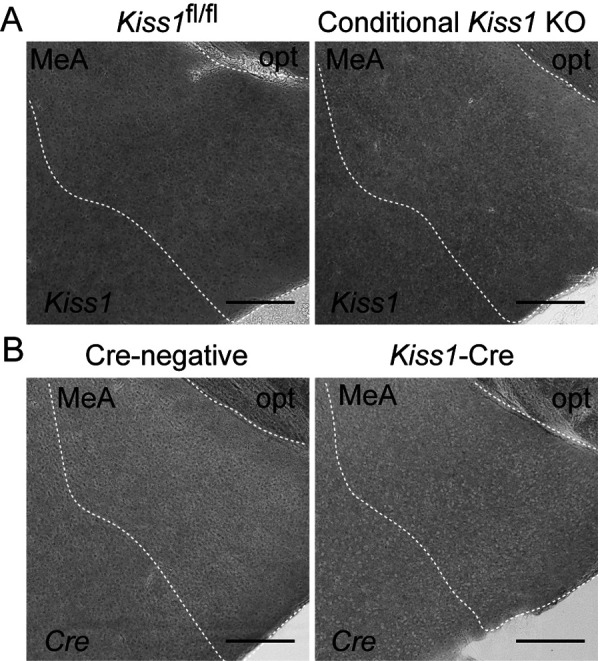
Neither Kiss1 nor Cre expression in the medial amygdala (MeA). (A) Representative photomicrographs showing no Kiss1-expressing cells in the MeA of both the conditional Kiss1 knockout (KO) mice and Cre-negative Kiss1fl/fl controls. (B) Representative photomicrographs showing no Cre-expressing cells in the MeA of Kiss1-Cre mice and Cre-negative controls. opt, optic tract. Scale bars, 200 µm.
Kiss1 and Cre expression in the ovary of the conditional Kiss1 KO female mice
Kiss1 mRNA was slightly detected in the ovary of both conditional Kiss1 KO mice (0.0031 ± 0.0012% of Actb) and Cre-negative Kiss1fl/fl control mice (0.0060 ± 0.0016% of Actb), and the levels were comparable between the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl control mice. Note that Kiss1 expression was highly detected in the wild-type mouse hypothalamus (1.02% of Actb, n = 2). Cre mRNA was slightly detected in the ovary of conditional Kiss1 KO mice (0.050 ± 0.022% of Actb), whereas it was undetectable in Cre-negative Kiss1fl/fl control mice.
Discussion
The present study demonstrates that the newly-created conditional kisspeptin neuron-specific Kiss1 KO mice generated by the Cre-loxP system replicated a hypogonadal phenotype of global Kiss1 KO mice [5, 6], because the animals generated by crossing Kiss1fl/fl mice and Kiss1-Cre mice showed no puberty onset along with an undetectable level of plasma gonadotropin and ovarian atrophy. It should be noted that high Cre expression was found in the ARC, but little in the AVPV, MeA, and ovary of the current Kiss1-Cre mice. Indeed, ovarian Kiss1 expression levels were comparable between the conditional Kiss1 KO mice and Cre-negative Kiss1fl/fl control mice. Collectively, the present results provide further evidence that hypothalamic kisspeptin neurons are fundamental to puberty onset and subsequent reproductive function in mammals and suggest that the Kiss1 expression outside of the hypothalamus may have a less important role for reproductive function in female mice.
The current result that Kiss1-Cre mice, which were generated by a microinjection of 3'-truncated Kiss1 locus replaced with Cre gene, mainly expressed Cre mRNA in the ARC at adulthood, was consistent with our previous finding showing that 5'-upstream sequence of Kiss1 locus serves as an ARC-specific Kiss1 enhancer in mice [39]. As expected, the conditional Kiss1 KO mice successfully lacked Kiss1 mRNA expression in the ARC. A previous study showed that Kiss1 is first expressed in the ARC during prenatal development: specifically, from embryonic day 12.5 in rats [46]. Taken together with this previous finding, the Kiss1 KO by Cre-loxP recombination is likely to occur in the ARC during the prenatal period in the current conditional Kiss1 KO female mice.
Interestingly, AVPV Kiss1 expression was also deprived in the current conditional Kiss1 KO female mice, even though only a few Cre-expressing cells were detected in the AVPV of the Kiss1-Cre mice. It is likely that such little Cre mRNA expression was enough to knock out Kiss1 in AVPV kisspeptin neurons. On the other hand, it is tempting to speculate that the AVPV Kiss1 mRNA expression would be somehow introduced depending on the ARC kisspeptin neurons. There are three possibilities to explain this result as follows: 1) AVPV kisspeptin neurons could be derived from ARC kisspeptin neurons. If this is the case, we envision that Kiss1 expression had been already suppressed before the migration of Kiss1-expressing cells from the ARC to AVPV because of the Kiss1 knocked out in the ARC by the Cre-loxP recombination; 2) Cre recombinase could be expressed in both the ARC and AVPV kisspeptin neurons during the fetal developmental period, although Cre mRNA expression was exclusively found only in the ARC at adulthood; 3) Kiss1 expression in the ARC kisspeptin neurons may be required for Kiss1 expression in the AVPV kisspeptin neurons at the adulthood, since a previous anterograde tracing study indicated the projection of ARC kisspeptin neurons toward AVPV kisspeptin neurons [47]. Further studies are needed to address this issue.
In summary, the current conditional kisspeptin neuron-specific Kiss1 KO mice newly utilizing the Cre-loxP system recapitulated the infertility of global Kiss1 KO animal models. The current Kiss1-floxed mice can be used as a valuable model for more elaborate analyses of the roles of distinct populations of kisspeptin neurons and kisspeptin-producing cells in the brain as well as the peripheral organs by the spatiotemporal manipulation of Cre expression.
Acknowledgments
The authors are grateful to the National Hormone and Peptide Program (HNPP), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Dr AF Parlow for providing the LH and FSH assay kit. The radioimmunoassays were performed at the Nagoya University Radioisotope Center. We wish to thank Dr Nicola Skoulding for editorial assistance and Takashi Hirashima, Tatsuya Fukanuma, Moe Yanagihara, Hitomi Abe, Yuhei Takayama, Ren Ishigaki, Saki Okamoto and Koki Yamada for their technical support. This work was supported in part by a Grant-in-Aid for the JSPS Fellows (No. 26-4247 to KI); the Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development Grant (REP2002 to HT); the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (to HT); a Grants-in Aid from the Japan Society for the Promotion of Science (18H03973 and 18K19267 to HT); and the Cooperative Study Program of National Institute for Physiological Sciences.
References
- 1.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 3.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 2012; 366: 629–635. [DOI] [PubMed] [Google Scholar]
- 4.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 2005; 102: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 2007; 104: 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 7.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 8.Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schütz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun 2013; 4: 2492. [DOI] [PubMed] [Google Scholar]
- 9.Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol 2014; 28: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 11.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007; 148: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 12.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 2007; 27: 8826–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides 2009; 30: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 15.Tsukamura H, Homma T, Tomikawa J, Uenoyama Y, Maeda K. Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann N Y Acad Sci 2010; 1200: 95–103. [DOI] [PubMed] [Google Scholar]
- 16.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013; 154: 4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol 2013; 784: 297–323. [DOI] [PubMed] [Google Scholar]
- 20.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology 2014; 99: 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman RL, Ohkura S, Okamura H, Coolen LM, Lehman MN. KNDy hypothesis for generation of GnRH pulses: evidence from sheep and goats. In: Herbison AE, Plant TM (eds.), The GnRH Neuron and its Control. Hoboken, NJ: Wiley; 2018: 289–324. [Google Scholar]
- 22.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 2010; 1364: 103–115. [DOI] [PubMed] [Google Scholar]
- 23.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 2008; 149: 4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minabe S, Ieda N, Watanabe Y, Inoue N, Uenoyama Y, Maeda KI, Tsukamura H. Long-term neonatal estrogen exposure causes irreversible inhibition of LH pulses by suppressing arcuate kisspeptin expression via estrogen receptors α and β in female rodents. Endocrinology 2017; 158: 2918–2929. [DOI] [PubMed] [Google Scholar]
- 25.Minabe S, Sato M, Inoue N, Watanabe Y, Magata F, Matsuda F, Uenoyama Y, Ozawa H, Tsukamura H. Neonatal estrogen causes irreversible male infertility via specific suppressive action on hypothalamic Kiss1 neurons. Endocrinology 2019; 160: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 26.Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA 2015; 112: 13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA 2017; 114: E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073–4077. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 2011; 152: 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, Jayasena CN, Ghatei MA, Bloom SR, Matthews PM, O’Byrne KT, Bell JD, Dhillo WS. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct 2016; 221: 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala kisspeptin neurons: putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology 2017; 104: 223–238. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya M, Babwah AV. Kisspeptin: beyond the brain. Endocrinology 2015; 156: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 33.Uenoyama Y, Pheng V, Tsukamura H, Maeda KI. The roles of kisspeptin revisited: inside and outside the hypothalamus. J Reprod Dev 2016; 62: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudek M, Ziarniak K, Sliwowska JH. Kisspeptin and metabolism: the brain and beyond. Front Endocrinol (Lausanne) 2018; 9: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod 2015; 93: 15. [DOI] [PubMed] [Google Scholar]
- 36.Ricu MA, Ramirez VD, Paredes AH, Lara HE. Evidence for a celiac ganglion-ovarian kisspeptin neural network in the rat: intraovarian anti-kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology 2012; 153: 4966–4977. [DOI] [PubMed] [Google Scholar]
- 37.Calder M, Chan YM, Raj R, Pampillo M, Elbert A, Noonan M, Gillio-Meina C, Caligioni C, Bérubé NG, Bhattacharya M, Watson AJ, Seminara SB, Babwah AV. Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology 2014; 155: 3065–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, Abe H, Ieda N, Minabe S, Deura C, Inoue N, Sanbo M, Tomita K, Hirabayashi M, Tanaka S, Imamura T, Okamura H, Maeda K, Tsukamura H. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci USA 2012; 109: E1294–E1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto T, Tomikawa J, Ikegami K, Minabe S, Abe H, Fukanuma T, Imamura T, Takase K, Sanbo M, Tomita K, Hirabayashi M, Maeda K, Tsukamura H, Uenoyama Y. Identification of hypothalamic arcuate nucleus-specific enhancer region of Kiss1 gene in mice. Mol Endocrinol 2015; 29: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T, Saga Y, Takeda N, Ikawa Y, Aizawa S. A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem 1993; 214: 70–76. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi M, Sanbo M, Watanabe S, Naruse I, Mishina M, Yagi T. Efficient production of Cre-mediated site-directed recombinants through the utilization of the puromycin resistance gene, pac: a transient gene-integration marker for ES cells. Nucleic Acids Res 1998; 26: 679–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 2009; 21: 527–537. [DOI] [PubMed] [Google Scholar]
- 43.Assadullah, Ieda N, Kawai N, Ishii H, Ihara K, Inoue N, Uenoyama Y, Tsukamura H. Co-expression of the calcitonin receptor gene in the hypothalamic kisspeptin neurons in female rats. Reprod Med Biol 2018; 17: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ieda N. Assadullah, Minabe S, Ikegami K, Watanabe Y, Sugimoto Y, Sugimoto A, Kawai N, Ishii H, Inoue N, Uenoyama Y, Tsukamura H. GnRH(1-5), a metabolite of gonadotropin-releasing hormone, enhances luteinizing hormone release via activation of kisspeptin neurons in female rats. Endocr J 2020; 67: 409–418. [DOI] [PubMed] [Google Scholar]
- 45.Minabe S, Uenoyama Y, Tsukamura H, Maeda K. Analysis of pulsatile and surge-like luteinizing hormone secretion with frequent blood sampling in female mice. J Reprod Dev 2011; 57: 660–664. [DOI] [PubMed] [Google Scholar]
- 46.Desroziers E, Droguerre M, Bentsen AH, Robert V, Mikkelsen JD, Caraty A, Tillet Y, Duittoz A, Franceschini I. Embryonic development of kisspeptin neurones in rat. J Neuroendocrinol 2012; 24: 1284–1295. [DOI] [PubMed] [Google Scholar]
- 47.Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 2011; 152: 2387–2399. [DOI] [PubMed] [Google Scholar]