Abstract
The role of the epidermal growth factor receptor (EGFR) mutation status testing in lung squamous cell carcinoma (SqCC) remains controversial. Evidence of the effectiveness of osimertinib in SqCC with EGFR T790M mutation is limited. Here, we describe a hitherto unreported case of a stage III SqCC patient with compound mutation of EGFR exon 19 deletion (19Del) and T790M mutation. Pathological complete tumor response was achieved after treatment with osimertinib. We suggest that EGFR mutation testing should be performed in Asian patients who have not been definitively diagnosed with SqCC due to small lung biopsy samples. Osimertinib has shown good efficacy in SqCC harboring a “primary” resistance mechanism (EGFR T790M).
Key points
An unreported case of stage III squamous cell carcinoma with synchronous occurrence of EGFR exon 19 deletion (19Del) and T790M mutation. Complete tumor response was achieved after treatment with osimertinib.
EGFR mutation testing should be performed in Asian patients who are not definitively diagnosed with SqCC due to small lung biopsy samples. Osimertinib has shown good efficacy in SqCC harboring a “primary” resistance mechanism (EGFR T790M).
Keywords: Epidermal growth factor receptor, lung squamous cell carcinoma, osimertinib, tyrosine kinase inhibitor
Introduction
Lung cancer remains the leading cause of cancer‐related death worldwide.1 Non‐small cell lung cancers (NSCLC) account for about 85% of lung cancers, and of these, approximately 30% are lung squamous cell carcinomas (SqCC).2 Epidermal growth factor receptor (EGFR) is a cell‐surface tyrosine kinase receptor that can activate pathways associated with cell growth and proliferation when activated. EGFR mutations have become an important therapeutic target for the treatment of nonsquamous NSCLC. EGFR exon 19 deletion (19Del) and exon 21 Leu858Arg point mutation (L858R), which are associated with favorable outcomes in patients treated with EGFR‐tyrosine kinase inhibitors (TKIs), account for 90% of all EGFR mutations. EGFR exon 20 Thr790Met point mutation (T790M) was present in approximately 50% to 60% of acquired resistance to EGFR‐TKI.3 EGFR T790M mutation can also be detected in a small proportion of tumors before treatment with EGFR‐TKIs.4 Third generation TKIs, such as osimertinib, have demonstrated efficacy in patients who develop resistance to first or second generation EGFR‐TKIs due to T790M mutation.5
EGFR mutation rate is 40% to 50% in lung adenocarcinoma (ADC) cases in east Asian populations.6 However, in Asian SqCC patients, incidence of EGFR mutation is relatively low, varying from 2% to 13%.7, 8, 9, 10 The role of EGFR mutation status testing and EGFR‐TKIs in SqCC remains controversial. Some oncology groups recommend EGFR mutation testing in all SqCC patients when clinical features indicate a higher probability of an oncogenic driver (ASCO, ACP/IASLC/AMP),11 while others recommend it only for patients with SqCC who have never smoked or who have mixed subtypes (ESMO and NCCN).12
Here, we report a case of locally advanced SqCC harboring EGFR exon 19Del/T790M mutation with a pathological complete tumor response after osimertinib treatment. We also discuss the literature regarding the efficacy of EGFR TKIs in SqCC, as well as their use in the neoadjuvant setting.
Case report
A 50‐year‐old Chinese Han male, who was a former light smoker with a smoking index of 200, presented with irritable cough and left chest pain in February 2019. Chest computed tomography (CT) scan revealed a pulmonary left upper lobe mass of 5.3 cm with enlarged mediastinal lymph nodes (station 4L, 5 and 6), suggesting lung cancer and the possibility of lymph node metastasis (Fig 1a). Fine needle biopsy of the primary tumor confirmed that it was SqCC (Fig 2a–e). Molecular status was tested with a panel including common mutated genes in lung cancer. Amplification refractory mutation system‐polymerase chain reaction (ARMS‐PCR) revealed compound occurrence of EGFR exon 19Del and T790M mutation (Fig 2g). ALK, ROS‐1 rearrangement and c‐MET amplification were lacking. The patient had no history of malignant tumors and family history. He had normal lung function, no comorbidities, and his clinical stage was cT3N2M0 IIIB (AJCC eighth version). According to NCCN guidelines, the third‐generation EGFR inhibitor osimertinib is the preferred first‐line therapy option for patients with metastatic NSCLC with sensitizing EGFR mutations. The patient started osimertinib (80 mg per os q.d.) immediately. He had no remarkable adverse effects (AEs). CT scans after one and three months showed a remarkable decrease of the lung lesion and lymph nodes (Fig 1b,c). Response evaluation criteria in solid tumors (RECIST) partial response was obtained.
Figure 1.
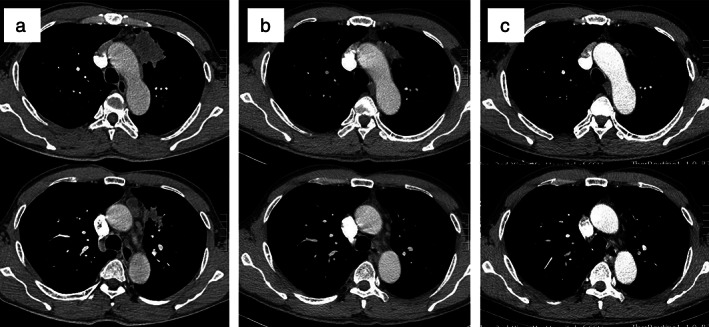
Evaluation by computed tomography (CT) scan. (a) At diagnosis. The upper left lobe mass was considered as the primary tumor while the mediastinal mass was considered as station 5 and 6 lymph‐node metastasis. (b) One month after osimertinib, both the primary tumor and lymph‐node had decreased in size. (c) Three months after osimertinib. The primary tumor had reduced in size remarkably, and the mediastinal mass had disappeared.
Figure 2.
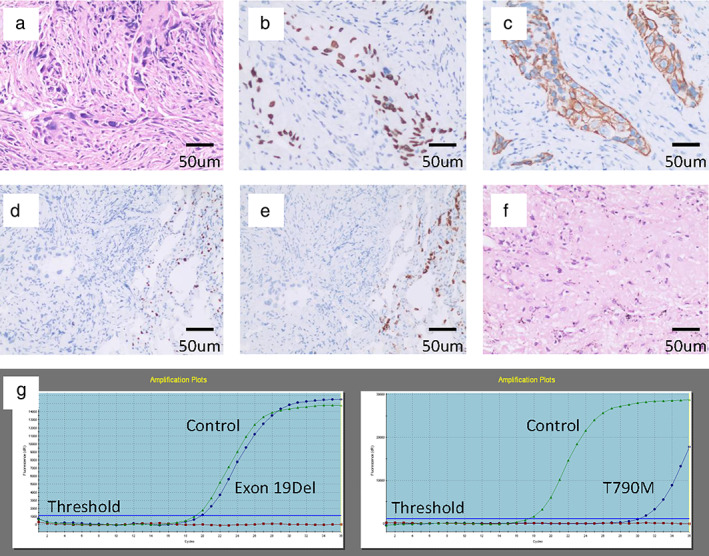
Pathology and amplification refractory mutation system‐polymerase chain reaction (ARMS‐PCR). (a) At the time of diagnosis. Hematoxylin and eosin (HE) staining showed neoplastic cells with morphological characteristics of non‐small cell lung cancer (NSCLC). (b–e) At the time of diagnosis. (b) Immunohistochemistry revealed diffuse expression of P40; (c) CK (5/6); (d) negative expression of TTF‐1; and (e) Napsin A, which led to the diagnosis of lung squamous cell carcinoma. (f) At the time of surgery. HE staining showed a necrotic area of former cancer tissue, with no residual viable cancer cells. (g) At the time of diagnosis. ARMS‐PCR showed coexistence of epidermal growth factor receptor (EGFR) exon 19Del and T790M (magnification: 200x; scale bar: 50 μm).
After multiple disciplinary team (MDT) discussion, a VATS left upper lobectomy following systemic lymph nodes resection was conducted. During surgery, regional lymph nodes including lower paratracheal, subaotic, para‐aortic, subcarinal, hilar and interlobar nodes were dissected. The involved mediastinal pleura and left phrenic nerve were resected en bloc, and the left phrenic diaphragm was suspended to the left chest wall to avoid paradoxical movement of the diaphragm. The patient recovered uneventfully and was discharged three days after surgery. Postoperative pathological examination showed coagulative necrosis of the lesion and proliferation of fibrous tissue. No cancer cells were found in the primary lung cancer lesion and lymph nodes (Fig 2f). The patient is in follow‐up with adjuvant osimertinib, and recent CT examination eight months after surgery found no evidence of recurrence or metastasis.
Discussion
Here we describe a hitherto unreported case of a stage III SqCC in a patient with synchronous occurrence of EGFR exon 19Del and T790M mutation treated with osimertinib. To date, only a few cases of squamous cell transformation from LADC treated with EGFR‐TKIs with concomitant T790M have been reported.13, 14, 15 There are no reports on the use of osimertinib in SqCC with primary EGFR exon 19Del and T790M compound mutation.
In Asian SqCC patients, incidence of EGFR mutation varies from 2% to 13%.8, 9 According to the updated CAP/IASLC/AMP Molecular Testing guideline, EGFR testing is recommended for adenocarcinomas and mixed lung cancers with an adenocarcinoma component in the setting of lung cancer resection specimens. In the setting of fully excised lung cancer specimens, EGFR testing is not recommended in lung cancers that lack any adenocarcinoma component, such as pure SqCC and pure small cell carcinomas.16 However, in the setting of small lung biopsies, adenosquamous carcinoma could be misdiagnosed as SqCC or NSCLC favor SqCC due to undersampling. Ho and colleagues studied 148 small lung biopsy cases with pathological diagnosis of SqCC or NSCLC favor SqCC, and found an EGFR mutation rate of 5.2% (7/135) in SqCC and 46.2% (6/13) in NSCLC favor SqCC. They concluded that EGFR mutation testing should be performed in Asian patients with SqCC diagnosed from small lung biopsies, especially in never‐smokers and patients with diagnosis of NSCC favor SqCC, which have a high probability of being adenosquamous carcinoma.17 Tests of multiple biopsies are helpful for accurate pathological and molecular diagnosis. In our case, the patient was a young, light smoker, and diagnosis came from small biopsy.
Response rates (RR) and median progression‐free survival (PFS) associated with EGFR TKI therapies among SqCC appear to be lower than among patients with adenocarcinoma, with 25% to 43.2% versus 54.4% to 80% for RR and 1.4–5.1 months versus 9–13 months for PFS, respectively.8, 18, 19, 20, 21 Some reports have argued that EGFR‐mutated SqCC have mixed ADC histology due to the diagnostic limitations of small biopsies and intratumoral heterogeneity. Meanwhile, the majority of the population are SqCC with wild‐type EGFR, resulting in inferior RR and PFS. Thus, the sensitivity of EGFR‐TKIs in patients with non‐ADC harboring EGFR mutations may depend on the proportion of EGFR‐mutated ADC components in the whole tumor.8 However, this does not explain why some EGFR‐mutated non‐ADC patients respond completely to EGFR‐TIKs, as in our case. The distinction between adenocarcinoma and squamous cell carcinoma can be extremely challenging in some cases. Thus, patients should not be deprived of potentially beneficial nontoxic therapies such as TKIs just on the basis of histology.19
Osimertinib is a third generation, irreversible EGFR‐TKI that selectively inhibits both EGFR‐TKI‐sensitizing and EGFR T790M resistance mutations. According to the FLAURA trial, osimertinib showed efficacy superior to that of standard EGFR‐TKIs in the first‐line treatment of EGFR mutation‐positive advanced NSCLC, with a similar safety profile and lower rates of serious adverse events.22 However, evidence of the effectiveness of osimertinib in SqCC with EGFR T790M mutation is limited. Zhang et al. reported a case of SqCC with secondary T790M mutation receiving osimertinib, and PFS was less than 10 months. In our case, a pathological complete response was achieved after three months of osimertinib. More cases are needed to clarify the efficacy of osimertinib in SqCC with EGFR T790M mutation.
The continuation maintenance therapy with osimertinib is still controversial. Several studies on adjuvant TKI treatment have concluded its safety and feasibility.23 However, there are relatively few clinical trials evaluating the efficacy of adjuvant EGFR‐TKIs in SqCC patients. More clinical trials are needed to provide convincing evidence for customized therapy for SqCC patients with EGFR mutations.
As far as we are aware, this is the first reported EGFR Exon 19Del/T790M mutation SqCC case with pathologic complete response to osimertinib, which serves as direct evidence of the effectiveness of osimertinib in SqCC. Earlier detection and surgical intervention have been shown to be beneficial for patient outcomes. Further clinical data in patients with SqCC harboring a “primary” resistance mechanism (T790M) to TKIs may be helpful in order to optimize the best treatment for these patients.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
This research is supported by the Hunan Provincial Natural Science Foundation (2018JJ3764), Key Research and Development Program of Hunan Province (2019SK2253).
Contributor Information
MuYun Peng, Email: pengmuyun@csu.edu.cn.
WenLiang Liu, Email: liuwenliang@csu.edu.cn.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Zappa C, Mousa SA. Non‐small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res 2016; 5: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu HA, Arcila ME, Rekhtman N et al Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol 2014; 11: 473–81. [DOI] [PubMed] [Google Scholar]
- 5. Janne PA, Yang JC, Kim DW et al AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015; 372: 1689–99. [DOI] [PubMed] [Google Scholar]
- 6. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 2006; 118: 257–62. [DOI] [PubMed] [Google Scholar]
- 7. Fang W, Zhang J, Liang W et al Efficacy of epidermal growth factor receptor‐tyrosine kinase inhibitors for Chinese patients with squamous cell carcinoma of lung harboring EGFR mutation. 2013; 5: 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hata A, Katakami N, Yoshioka H et al How sensitive are epidermal growth factor receptor‐tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene‐sensitive mutations? J Thorac Oncol 2013; 8: 89–95. [DOI] [PubMed] [Google Scholar]
- 9. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small‐cell lung cancer: Meta‐analyses by ethnicity and histology (mutMap). Ann Oncol 2013; 24: 2371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Y, Yin X, Wen MM et al EGFR mutations subset in Chinese lung squamous cell carcinoma patients. Mol Med Rep 2018; 17: 7575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalemkerian GP, Narula N, Kennedy EB et al Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 2018; 36: 911–9. [DOI] [PubMed] [Google Scholar]
- 12. Planchard D, Popat S, Kerr K et al Metastatic non‐small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2019; 30: 863–70. [DOI] [PubMed] [Google Scholar]
- 13. Bruno R, Proietti A, Ali G et al Squamous cell transformation and EGFR T790M mutation as acquired resistance mechanisms in a patient with lung adenocarcinoma treated with a tyrosine kinase inhibitor: A case report. Oncol Lett 2017; 14: 5947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scher KS, Saldivar JS, Fishbein M, Marchevsky A, Reckamp KL. EGFR‐mutated lung cancer with T790M‐acquired resistance in the brain and histologic transformation in the lung. J Natl Compr Canc Netw 2013; 11: 1040–4. [DOI] [PubMed] [Google Scholar]
- 15. Jukna A, Montanari G, Mengoli MC et al Squamous cell carcinoma "transformation" concurrent with secondary T790M mutation in resistant EGFR‐mutated adenocarcinomas. J Thorac Oncol 2016; 11: e49–51. [DOI] [PubMed] [Google Scholar]
- 16. Lindeman NI, Cagle PT, Aisner DL et al Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018; 13: 323–58. [DOI] [PubMed] [Google Scholar]
- 17. Ho HL, Kao HL, Yeh YC, Chou TY. The importance of EGFR mutation testing in squamous cell carcinoma or non‐small cell carcinoma favor squamous cell carcinoma diagnosed from small lung biopsies. Diagn Pathol 2019; 14: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morita S, Okamoto I, Kobayashi K et al Combined survival analysis of prospective clinical trials of gefitinib for non‐small cell lung cancer with EGFR mutations. Clin Cancer Res 2009; 15: 4493–8. [DOI] [PubMed] [Google Scholar]
- 19. Xu J, Chu T, Jin B et al Epidermal growth factor receptor tyrosine kinase inhibitors in advanced squamous cell lung cancer. Clin Lung Cancer 2016; 17: 309–14. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Zhang Y, Zhang L et al Efficacy of epidermal growth factor receptor‐tyrosine kinase inhibitors for lung squamous carcinomas harboring EGFR mutation: A multicenter study and pooled analysis of published reports. Oncotarget 2017; 8: 49680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joshi A, Zanwar S, Noronha V et al EGFR mutation in squamous cell carcinoma of the lung: Does it carry the same connotation as in adenocarcinomas? Onco Targets Ther 2017; 10: 1859–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378: 113–25. [DOI] [PubMed] [Google Scholar]
- 23. Wu JX, He Q, Ye F et al EGFR‐TKI‐based vs non‐EGFR‐TKI‐based adjuvant therapy in resected non‐small‐cell lung cancer with EGFR mutations: A meta‐analysis of randomized controlled trials. Onco Targets Ther 2018; 11: 6803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


