Abstract
Background
The efficacy of anti‐programmed cell death‐1/ligand 1 antibody monotherapy (anti‐PD‐1/PD‐L1 monotherapy) in patients with active brain metastases (BMs) is not established. Here, we aimed to evaluate the efficacy of anti‐PD‐1/PD‐L1 monotherapy in non‐small cell lung cancer (NSCLC) patients with active BMs.
Methods
This retrospective study included NSCLC patients treated with second‐line or later‐line anti‐PD‐1/PD‐L1 monotherapy between December 2015 and August 2019. Patients were classified into those with or without active BMs, including symptomatic BMs requiring systemic steroids and untreated BMs. The progression‐free survival (PFS) and overall survival (OS) of the patients with and without active BMs were compared. Intracranial and extracranial tumor responses were evaluated in patients with active BMs.
Results
We analyzed 197 patients who had received anti‐PD‐1/PD‐L1 monotherapy. Among them, 24 had active BMs. Among those without active BMs, 145 had no BMs and 28 had treated asymptomatic BMs. The PFS and OS of patients with active BMs were significantly shorter than those of patients without active BMs (1.3 vs. 2.7 months; P < 0.001, and 4.5 vs. 16.3 months; P = 0.001 respectively). For patients with active BMs, the intracranial and extracranial response rates were 13.3% and 26.7%, respectively. On multivariate analysis, active BMs, poor performance status (PS), and EGFR/ALK positivity were significant factors associated with shorter PFS. Active BMs and poor PS were significant factors associated with shorter OS.
Conclusions
This study suggested that anti‐PD‐1/PD‐L1 monotherapy was not effective for NSCLC patients with active BMs. Further studies on immunotherapy are needed for patients with active BMs.
Key points
Significant findings of the study: The present study showed that anti‐PD‐1/PD‐L1 antibody monotherapy was not effective for non‐small cell lung cancer patients with active brain metastases. Intracranial and extracranial response rates were 13.3% and 26.7%, respectively.
What this study adds: Further studies on immunotherapy are needed for patients with active BMs.
Keywords: Anti PD‐1/PD‐L1 antibody, brain metastases, central nervous system metastases, immune checkpoint inhibitor
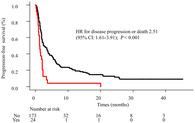
The present study showed that anti‐PD‐1/PD‐L1antibody monotherapy was not effective for non‐small cell lung cancer patients with active brain metastases. Intracranial and extracranial response rates were 13.3% and 26.7% respectively. Further studies on immunotherapy are needed for patients with active BMs.
Introduction
Lung cancer is the leading cause of cancer death worldwide. 1 However, the treatment of patients with non‐small cell lung cancer (NSCLC) has made marked progress in the past two decades. Several anticancer agents have been approved for the treatment of NSCLC, such as cytotoxic agents, angiogenesis inhibitors, and molecular targeted drugs. In particular, immune checkpoint inhibitors have improved survival times. 2 Despite recent advancements in the treatment of patients with NSCLC, brain metastases (BMs) are frequent and serious complications; approximately 10% of patients have BMs at diagnosis, and 20%–40% develop BMs during their disease course. 3 , 4 , 5 , 6 The treatment approach for patients with BMs is of particular significance, as the development of BMs often leads to deterioration of the patient's quality of life, and confers a poor prognosis. 7 , 8
In general, chemotherapeutic agents have limited effects on BMs, as they can hardly penetrate the blood‐brain barrier (BBB). 9 , 10 A previous study reported an intracranial response rate of approximately 30%, and a median overall survival of 7.7 months 11 with chemotherapeutic agents.
Recently, anti‐programmed cell death‐1/ligand 1 antibodies (anti‐PD‐1/PD‐L1 Ab), including nivolumab, pembrolizumab, and atezolizumab, have become instrumental in the treatment of NSCLC. Several phase III studies have reported that anti‐PD‐1/PD‐L1 Ab significantly improved overall survival compared with cytotoxic chemotherapy. 12 , 13 , 14 , 15 , 16 , 17 However, the efficacy of anti‐PD‐1/PD‐L1 Ab in the treatment of active BMs, including untreated, symptomatic, and unstable BMs, has not been established, as most clinical trials have excluded patients with active BMs. 12 , 13 , 14 , 15 , 16 , 17 In clinical practice, the patient population with active BMs is significant, yet advances in treatment have been limited. Therefore, the purpose of this study was to evaluate the efficacy of anti‐PD‐1/PD‐L1 Ab in NSCLC patients with active BMs.
Methods
Data collection
This retrospective study included 242 patients with histologically‐confirmed advanced NSCLC, who received second‐line or later‐line anti‐PD‐1/PD‐L1 Ab monotherapy with nivolumab, pembrolizumab, or atezolizumab at the Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan, between December 2015 and August 2019. The patients were treated with nivolumab (3 mg/kg bodyweight or 240 mg per patient) every two weeks, pembrolizumab (200 mg per patient) every three weeks, or atezolizumab (1200 mg per patient) every three weeks. We excluded patients who were not evaluated for BMs by computed tomography (CT) or magnetic resonance imaging (MRI) within 56 days before the initiation of anti‐PD‐1/PD‐L1 Ab monotherapy. Patients with carcinomatous meningitis were also excluded from this study. Clinical data including age, sex, Eastern Cooperative Oncology Group performance status (PS), smoking status, tumor histology, epidermal growth factor receptor mutation or anaplastic lymphoma kinase rearrangement status (EGFR/ALK status), PD‐L1 expression status, and number of previous chemotherapy regimens, were collated.
We defined active BMs as untreated or symptomatic BMs requiring systemic steroids equivalent to 10 mg of prednisolone at initiation of anti‐PD‐1/PD‐L1 Ab treatment. Patients were classified into “active” and “nonactive” BM groups. We compared the efficacy of anti‐PD‐1/PD‐L1 Ab between the two groups in terms of progression‐free survival (PFS) and overall survival (OS). All patients had CT or MRI scans at least every three months as per standard clinical practice, and intracranial and extracranial tumor responses in patients with measurable BMs within the active BM group were evaluated. The tumor response was further categorized into complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) in accordance with RECIST 1.1. PFS and OS were defined as the time interval from the initiation of anti‐PD‐1/PD‐L1 Ab monotherapy to disease progression/death from a related cause and to death, respectively. Moreover, in patients with evident BMs within the active BMs group, intracranial and extracranial PFS were evaluated. The protocol was reviewed and approved by the Ethics Committee of the Cancer Institute Hospital, Japanese Foundation for Cancer Research (approval number 2019–1164). Informed consent to using their clinical data was obtained from all patients using the opt‐out method on the website, per the instructions of the Ethics Committee of the Cancer Institute Hospital, Japanese Foundation for Cancer Research.
Statistical analysis
Patient age was compared between the two groups using the Mann‐Whitney U test, and the other patient characteristics were compared using Fisher's exact test. Kaplan‐Meier curves of PFS and OS for each group were generated and compared using the log‐rank test. Univariate and multivariate Cox regression analyses were used to determine the association between patient characteristics and PFS or OS. All significant factors identified on univariate analysis were entered into multivariate analysis. We performed all statistical analyses using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). 18 We considered P < 0.05 statistically significant.
Results
Patient categorization
Fig 1 shows a flow chart of the patient selection process; 242 NSCLC patients treated with anti‐PD‐1/PD‐L1 Ab were investigated, and 197 were analyzed based on our inclusion criteria. The median length of follow‐up for censored cases was 14.3 months (range, 1.1–45.6 months). A total of 24 patients formed the active BM group: 21 patients were not treated while the remaining three patients received systemic steroids equivalent to 10 mg of prednisolone. The remaining 173 patients, 28 of whom had treated BMs and 145 with no BMs, comprised the nonactive BM group.
Figure 1.
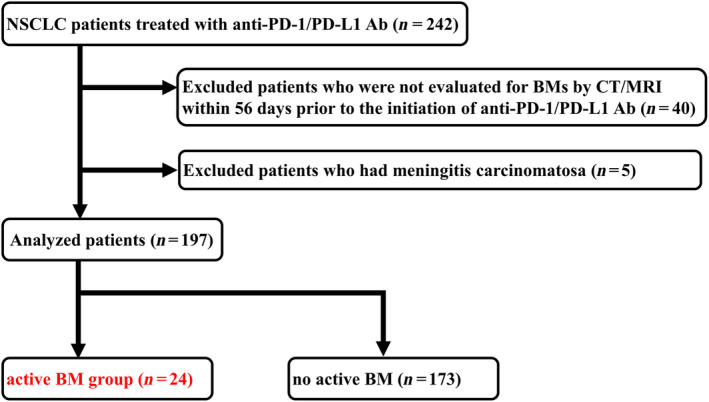
Patient selection flow chart. Anti‐PD‐1/PD‐L1 Ab; anti‐programmed cell death‐1/ligand 1 antibody, BMs, brain metastases; NSCLC, non‐small cell lung cancer.
For the active BM group, the PFS and OS of the 24 patients were analyzed; 15 patients had measurable intracranial and extracranial lesions. In terms of tumor response, two patients died before the initial CT/MRI evaluation, and one patient underwent radiological evaluation of the brain, without extracranial radiological evaluation at the time of disease progression.
The patient characteristics are shown in Table 1. The percentage of patients with a PS of 2–4 was higher in the active BM group than in the nonactive BM group (38% vs. 17%, P = 0.029). Compared with the nonactive BM group, more patients in the active BM group received anti‐PD‐1/PD‐L1 Ab as fourth or later line treatment (42% vs. 20%, P = 0.034). The active BM group had more patients with nonsquamous cell carcinoma than the nonactive BM group (92% vs. 73%, P = 0.073). EGFR/ALK status and PD‐L1 expression status were not significantly different between the two groups. All patients had not received previous anti‐PD‐1/PD‐L1 Ab treatment.
Table 1.
Patient characteristics
| Active BM (n = 24) | No active BM (n = 173) | P‐value | |
|---|---|---|---|
| Age, median (range) | 66.5 (41–83) | 67 (27–82) | 0.495 |
| Sex | |||
| Male | 13 (54%) | 122 (71%) | 0.157 |
| Female | 11 (46%) | 51 (29%) | |
| Performance status | |||
| 0, 1 | 15 (62%) | 143 (83%) | 0.029 |
| 2–4 | 9 (38%) | 30 (17%) | |
| Smoking status | |||
| Current/former | 15 (62%) | 135 (78%) | 0.123 |
| Never | 9 (38%) | 38 (22%) | |
| Histology | |||
| Squamous | 2 (8%) | 46 (27%) | 0.073 |
| Nonsquamous | 22 (92%) | 127 (73%) | |
| EGFR or ALK | |||
| Positive | 6 (25%) | 28 (16%) | 0.264 |
| Negative | 18 (75%) | 145 (84%) | |
| PD‐L1 | |||
| Positive | 8 (33%) | 77 (45%) | 0.485 |
| Negative | 4 (17%) | 31 (18%) | |
| Unknown | 12 (50%) | 65 (37%) | |
| Number of previous chemotherapeutic regimens | |||
| ≤2 | 14 (58%) | 138 (80%) | 0.034 |
| ≥3 | 10 (42%) | 35 (20%) | |
The details of patients with brain metastases are presented in Table 2. The number and largest size of BMs did not differ between the active and nonactive BM groups. In the active BM group, three patients received local treatment for BMs, but they received steroid treatment during initiation of anti‐PD‐1/PD‐L1 treatment. In the nonactive BM group, all patients with BMs received local treatment. Four patients received whole brain radiotherapy (WBRT), 21 received stereotactic radiosurgery (SRS), and two received WBRT and SRS. Only one patient underwent surgery.
Table 2.
Details of patients with brain metastases
| Active BM (n = 24) | No active BM (n = 28) | |
|---|---|---|
| Number of BMs; n (%) | ||
| 1–3 | 11 (46%) | 9 (32%) |
| 4–9 | 9 (37%) | 11 (39%) |
| 10< | 4 (17%) | 8 (29%) |
| Largest size of BMs | ||
| Median (range) | 10 mm (2–42 mm) | 13 mm (6–33 mm) |
| Local treatment; n (%) | ||
| WBRT | 2 (8%) | 4 (14%) |
| SRS | 1 (4%) | 21 (75%) |
| WBRT and SRS | 0 (0%) | 2 (7%) |
| Surgery and SRS | 0 (0%) | 1 (4%) |
| None | 21 (88%) | 0 (0%) |
| Steroid treatment for symptoms of BMs (≥10 mg of PSL); n (%) | ||
| Yes | 4 (17%) | 0 (0%) |
| No | 20 (83%) | 28 (100%) |
Efficacy
PFS curves for each group are shown in Fig 2a. The median PFS was 1.3 months (95% confidence interval [CI], 0.9 to 2.1) in the active BM group, and 2.7 months (95% CI: 2.0–3.8) in the nonactive BM group. The PFS of the active BM group was significantly shorter than that of the nonactive BM group (hazard ratio for disease progression or death [HR], 2.51; 95% CI: 1.61–3.91; P < 0.001). OS curves for each group are shown in Fig 2b. The median OS was 4.5 months (95% CI: 3.1–9.3) in the active BM group, and 16.3 months (95% CI: 13.9–19.3) in the nonactive BM group. The OS of the active BM group was significantly shorter than that of the nonactive BM group (HR for death, 2.25; 95% CI: 1.3–3.68; P = 0.001).
Figure 2.
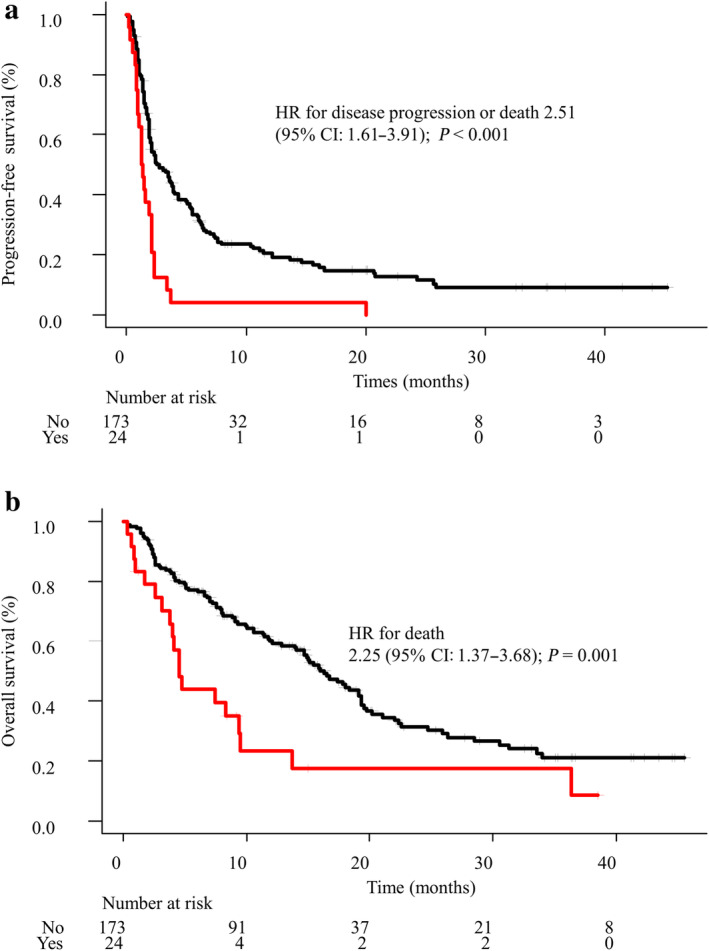
(a) Progression‐free survival ( ) active BM (
) active BM ( ) nonactive BM (b) Overall survival (
) nonactive BM (b) Overall survival ( ) active BM (
) active BM ( ) nonactive BM. HR, hazard ratio; CI, confidence intervals.
) nonactive BM. HR, hazard ratio; CI, confidence intervals.
On univariate analysis, male sex, PS0‐1, current or former smoking status, EGFR/ALK negativity, receipt of second or third line of treatment, no active BM, and no steroid treatment were significant factors associated with longer PFS. On multivariate Cox regression analysis, gender, PS, smoking status, EGFR/ALK status, line of treatment, presence of active BMs and steroid treatment were included as significant factors. Active BM (HR, 1.77; 95% CI: 1.09–2.86; P = 0.022), PS 0–1 (HR, 0.45; 95% CI: 0.30–0.67; P < 0.001), and steroid treatment (HR, 4.06; 95% CI: 1.61–10.23; P = 0.003) were significant factors associated with PFS (Table 3). On univariate analysis, PS 2–4, active BMs, and steroid treatment were significant factors associated with shorter OS. On multivariate Cox regression analysis, PS, presence of active BMs, and steroid treatment were included as significant factors. Active BM (HR, 1.87; 95% CI: 1.13–3.11; P < 0.001), PS 0–1 (HR, 0.26; 95% CI: 0.18–0.40; P < 0.001), and steroid treatment (HR, 2.96; 95% CI: 1.06–8.25; P < 0.001) were significant factors associated with OS (Table 3).
Table 3.
Cox regression analysis of progression‐free survival and overall survival
| Progression‐free survival | Overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI | P‐value | HR | 95%CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age ≥75 vs. <75 | 0.91 | 0.59, 1.39 | 0.647 | 1.29 | 0.81, 2.05 | 0.284 | ||||||
| Sex male vs. female | 0.62 | 0.45, 0.85 | 0.004 | 0.89 | 0.57, 1.39 | 0.608 | 0.76 | 0.52, 1.10 | 0.146 | |||
| PS 0,1 vs. 2–4 | 0.41 | 0.28, 0.60 | <0.001 | 0.45 | 0.30, 0.67 | <0.001 | 0.26 | 0.17, 0.38 | <0.001 | 0.26 | 0.18, 0.40 | <0.001 |
| Smoking status never vs. current/former | 1.77 | 1.24, 2.51 | 0.002 | 1.48 | 0.92, 2.38 | 0.109 | 1.22 | 0.81, 1.83 | 0.336 | |||
| Histology sq vs. nonsq | 1.06 | 0.75, 1.51 | 0.735 | 1.22 | 0.81, 1.84 | 0.351 | ||||||
| EGFR/ALK positive vs. negative | 2.10 | 1.40, 3.14 | <0.001 | 1.61 | 0.99, 2.61 | 0.055 | 1.41 | 0.90, 2.22 | 0.138 | |||
| PD‐L1 positive vs. negative | 0.76 | 0.49, 1.17 | 0.212 | 1.22 | 0.67, 2.20 | 0.517 | ||||||
| Line of therapy second/third vs. fourth | 0.52 | 0.37, 0.74 | <0.001 | 0.84 | 0.53, 1.31 | 0.437 | 0.76 | 0.51, 1,14 | 0.185 | |||
| Active BM vs. nonactive BM | 2.51 | 1.61, 3.91 | <0.001 | 1.77 | 1.09, 2.86 | 0.022 | 2.25 | 1.34, 3.68 | 0.001 | 1.87 | 1.13, 3.11 | <0.001 |
| Steroid treatment (≥10 mg of PSL) Yes vs. No | 3.63 | 1.48, 8.91 | 0.005 | 4.06 | 1.61, 10.23 | 0.003 | 2.97 | 1.09, 8.13 | 0.034 | 2.96 | 1.06, 8.25 | <0.001 |
ALK, anaplastic lymphoma kinase rearrangement; CI, confidence intervals; EGFR, epidermal growth factor receptor; HR, hazard ratio; PD‐L1, programmed cell death‐ligand 1; PSL, prednisolone; squamous, squamous cell carcinoma; nonsq, nonsquamous.
The intracranial and extracranial response rates were 13.3% (2/15) and 26.7% (4/15) respectively. Although extracranial lesions were controlled (PR or SD), the progression of intracranial lesions was observed in three patients (Table S1). For the 15 patients who had both, measurable intracranial and extracranial lesions in the active BM group, the intracranial and extracranial PFS are shown in Fig 3. The median intracranial and extracranial PFS were 1.4 months (95% CI: 0.8–2.2) and 2.2 months (95% CI: 0.9–6.3) respectively.
Figure 3.
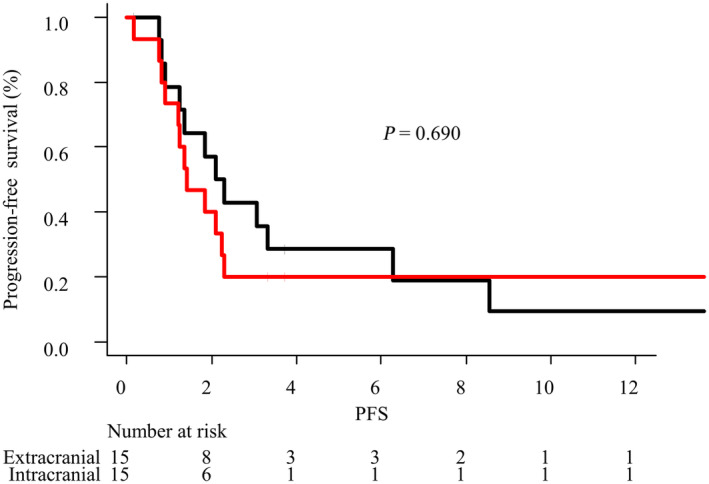
Intracranial progression‐free survival (PFS) and extracranial PFS in the active BM group. ( ) Intracranial (
) Intracranial ( ) Extracranial.
) Extracranial.
Discussion
Several recent clinical trials evaluated the efficacy of anti‐PD‐1/PD‐L1 Ab for patients with previously treated BMs, but the results have been controversial. 8 , 9 , 10 In a subgroup analysis of the Checkmate 057 trial, OS was not found to be significantly longer for nivolumab than for docetaxel in NSCLC patients with treated stable BMs (HR for death, 1.06; 95% CI: 0.62–1.76). 13 In a subgroup analysis of the OAK trial, the OS for atezolizumab was longer than that for docetaxel in patients with treated asymptomatic BMs (median OS, 16.0 vs. 11.9 months; HR for death, 0.74; 95% CI: 0.49–1.13). 19 Cumulative analysis of KEYNOTE‐001, 010, 024, and 042, particularly for patients with treated stable BMs, showed that the PFS and OS for pembrolizumab were longer than those for DTX (HR for disease progression or death, 0.96; 95% CI: 0.73–1.2 vs. HR for death, 0.83; 95% CI: 0.62–1.10). 20 In addition, these clinical trials excluded patients with active BMs; therefore, the efficacy of anti‐PD‐1/PD‐L1 Ab was not evaluated.
Another study retrospectively investigated the prognostic impact of the presence of BMs in routine clinical care as well as clinical trials. 21 The cohort included 1025 NSCLC patients who had received anti PD‐1/PD‐L1 Ab with or without anti‐cytotoxic T‐lymphocyte antigen 4 (anti‐CTLA‐4), and multivariate analysis included the following factors: age, smoking status, histology, number of organs with metastases, line of treatment, PS, use of corticosteroids, and the presence of BMs. The study showed that the presence of BMs was not a significant factor for PFS and OS. As anticipated, the PFS and OS of patients with unstable BMs (untreated, and new or growing brain lesions) were significant shorter than those of patients with stable BMs (irradiated and no growing brain metastases): (HR for disease progression or death, 0.62; 95% CI: 0.44–0.88; P = 0.007; HR for death, 0.62; 95% CI: 0.41–0.93; P = 0.019). However, the study did not consider whether the BMs were active or not. Therefore, the different outcomes of active BMs and nonactive BMs, though potentially significant, were not assessed.
The intracranial tumor response of anti‐PD‐1/PD‐L1 Ab was not well examined because in general, the intracranial lesion was not the target lesion. In our study, 15 patients had measurable intracranial and extracranial lesions in the active BM group. The intracranial response rate was 13.3%, and the extracranial tumor response rate was 26.7%. In three of the 15 patients, the tumor response varied (intracranial PD, but extracranial PR or SD). The median intracranial and extracranial PFS were 1.4 months and 2.2 months, respectively. The results therefore suggested that the efficacy of anti‐PD‐1/PD‐L1 Ab may be lower for intracranial than for extracranial tumors.
Recently, a phase II study evaluated the efficacy and safety of pembrolizumab for melanoma and NSCLC patients, who had at least one untreated or progressive BM. 22 Patients with neurological symptoms, or in need of corticosteroids were excluded. NSCLC patients with a PD‐L1 tumor proportion score > 1% were considered; the use of pembrolizumab resulted in an intracranial response rate of 33% (6/18) and a median OS of 7.7 months. The overall response rate was 33% (6/18); the intracranial response rate and OS were more favorable than in our cohort. However, the BM status differed from that of our study in that it excluded patients who had neurological symptoms or who needed corticosteroids. 22 This may be the reason for the differences in the results between the two studies. Our study may more accurately represent the patient population.
One possible reason for the decreased efficacy of anti‐PD‐1/PD‐L1 Ab monotherapy for intracranial lesions as compared with extracranial lesions is the nature of the brain microenvironment; the blood brain barrier (BBB) generally reduces drug penetration, and also regulates the penetration of immune cells into the brain. 23 BMs may contain less tumor‐infiltrating lymphocytes than primary lung cancer. 24 In addition, it was suggested that the brain microenvironment may be particularly immunosuppressive as compared with the extra‐brain microenvironment. PD‐L1 expression of BMs was reported to be lower than that of matched primary tumors in lung cancer patients. 24 In another study, the expression levels of MHC class I and II molecules were low, and the number of antigen‐presenting cells in the brain were few. 25 Therefore, the reasons highlighted in the aforementioned studies are indicative of a decreased efficacy of anti‐PD‐1/PD‐L1 Ab for intracranial tumors as compared with extracranial tumors. However, our study showed that some intracranial tumors lesions responded to anti‐PD‐1/PD‐L1 Ab monotherapy. Further studies are needed to reveal the mechanism of difference between responders and nonresponders for intracranial lesions.
This retrospective study has several limitations, the most significant being the small sample size; intracranial response was evaluated in only 15 patients. Patient characteristics varied, and the influence of selection bias cannot be ignored. The PD‐L1 tumor proportion score (TPS) may represent an important factor, which potentially influenced the results. However, PD‐L1 TPS was evaluated in only 48 patients and PD‐L1 expression was evaluated through the use of the 28–8 antibody in 103 patients; PD‐L1 was not evaluated in 77 patients.
In conclusion, this study showed that anti‐PD‐1/PD‐L1Ab monotherapy was not effective for NSCLC patients with active BMs. Further studies on immunotherapy are needed for patients with active BMs.
Disclosure
MN has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer‐ingelheim, MSD, Novartis; and received research funding from MSD, Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol Myers Squibb, Taiho Pharmaceutical, Eli Lilly, AstraZeneca, Pfizer, and Astellas, and had consulting/advisory roles with Novartis, Daiichi Sankyo Healthcare, Taiho Pharmaceutical, Bristol Myers Squibb, Boehringer‐Ingelheim, Ono Pharmaceutical, Eli Lilly, Chugai Pharmaceutical, AstraZeneca, Merck Serono, MSD, and Pfizer. AG has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo Healthcare, KYORIN Pharmaceutical, Accuray Japan, Boehringer‐Ingelheim, MSD, AstraZeneca, Kyowa Kirin, Otsuka Pharmaceutical, and Eisai, and received research funding from Nippon Kayaku. MS has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer‐Ingelheim, and MSD, and received research funding from Taiho Pharmaceutical, Chugai Pharmaceutical, and Boehringer‐Ingelheim. NY has received honoraria from Ono Pharmaceutical, Taiho Pharmaceutical, MSD, Novartis, and Bayer Yakuhin, and had consulting/advisory roles with Chugai Pharmaceutical. KU has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, AstraZeneca, and Boehringer‐Ingelheim, and had consulting/advisory roles with AstraZeneca and Chugai Pharmaceutical. SK has received honoraria from Chugai Pharmaceutical Co., Ltd., MSD K.K., Bristol‐Myers Squibb, ONO Pharmaceutical, and AstraZeneca. All remaining authors report no conflicts of interest.
Supporting information
Table S1 Intracranial and extracranial response in the active BM group.
Acknowledgments
We would like to thank Editage (www.editage.com)for English language editing.
Contributor Information
Takehiro Tozuka, Email: t-tozuka@nms.ac.jp.
Makoto Nishio, Email: mnishio@jfcr.or.jp.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics 2019. CA Cancer J Clin 2019; 69 (1): 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Takano N, Ariyasu R, Koyama J et al Improvement in the survival of patients with stage IV non‐small‐cell lung cancer: Experience in a single institutional 1995‐2017. Lung Cancer 2019; 131: 69–77. [DOI] [PubMed] [Google Scholar]
- 3. Saad AG, Yeap BY, Thunnissen FB et al Immunohistochemical markers associated with brain metastases in patients with nonsmall cell lung carcinoma. Cancer 2008; 113 (8): 2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnholtz‐Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol 2004; 22 (14): 2865–72. [DOI] [PubMed] [Google Scholar]
- 5. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005; 23 (25): 6207–19. [DOI] [PubMed] [Google Scholar]
- 6. Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J Clin Oncol 1988; 6 (9): 1474–80. [DOI] [PubMed] [Google Scholar]
- 7. D'Antonio C, Passaro A, Gori B et al Bone and brain metastasis in lung cancer: Recent advances in therapeutic strategies. Ther Adv Med Oncol 2014; 6 (3): 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non‐small‐cell lung cancer after a diagnosis of brain metastases. Curr Oncol 2013; 20 (4): e300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lockman PR, Mittapalli RK, Taskar KS et al Heterogeneous blood‐tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010; 16 (23): 5664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demeule M, Régina A, Jodoin J et al Drug transport to the brain: Key roles for the efflux pump P‐glycoprotein in the blood‐brain barrier. Vascul Pharmacol 2002; 38 (6): 339–48. [DOI] [PubMed] [Google Scholar]
- 11. Edelman MJ, Belani CP, Socinski MA et al Outcomes associated with brain metastases in a three‐arm phase III trial of gemcitabine‐containing regimens versus paclitaxel plus carboplatin for advanced non‐small cell lung cancer. J Thorac Oncol 2010; 5 (1): 110–6. [DOI] [PubMed] [Google Scholar]
- 12. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373 (2): 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373 (17): 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387 (10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 15. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389 (10066): 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375 (19): 1823–33. [DOI] [PubMed] [Google Scholar]
- 17. Mok TSK, Wu YL, Kudaba I et al Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019; 393 (10183): 1819–30. [DOI] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely‐available easy‐to‐use software “EZR” (easy R) for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gadgeel SM, Lukas RV, Goldschmidt J et al Atezolizumab in patients with advanced non‐small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer 2019; 128: 105–12. [DOI] [PubMed] [Google Scholar]
- 20. Mansfield AS, Herbst RS, Castro G Jr et al Outcomes with pembrolizumab (pembro) monotherapy in patients (pts) with PD‐L1–positive NSCLC with brain metastases: Pooled analysis of KEYNOTE‐001, −010, −024, and −042. Ann Oncol 2019; 30 (Suppl 5): mdz260.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendriks LEL, Henon C, Auclin E et al Outcome of patients with non‐small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol 2019; 14 (7): 1244–54. [DOI] [PubMed] [Google Scholar]
- 22. Goldberg SB, Gettinger SN, Mahajan A et al Pembrolizumab for patients with melanoma or non‐small‐cell lung cancer and untreated brain metastases: Early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol 2016; 17 (7): 976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mrass P, Weninger W. Immune cell migration as a means to control immune privilege: Lessons from the CNS and tumors. Immunol Rev 2006; 213: 195–212. [DOI] [PubMed] [Google Scholar]
- 24. Mansfield AS, Aubry MC, Moser JC et al Temporal and spatial discordance of programmed cell death‐ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 2016; 27 (10): 1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest 2010; 120 (5): 1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Intracranial and extracranial response in the active BM group.


