Abstract
A 53‐year‐old man diagnosed with disease stage IIIB pulmonary adenocarcinoma underwent chemotherapy and radiotherapy in the first‐line setting. After disease progression, he received targeted therapy because of subsequent detection of EGFR exon 19 del mutation. Following an increase in his adrenal metastases, a combination of immunotherapy and antiangiogenic therapy (sintilimab plus bevacizumab) was commenced. After one month, imaging showed that the adrenal metastases had shrunk and a progression‐free survival (PFS) of 6.0 months was achieved. In this case, we showed that the PD1 inhibitor sintilimab plus bevacizumab was effective in a refactory advanced EGFR‐mutant NSCLC with positive PD‐L1 expression.
Key points
Our case report provides clinical evidence of the durable response of a patient with advanced EGFR‐mutant lung adenocarcinoma to a combination of immunotherapy and anti‐angiogenic agent, sintilimab and bevacizumab, as subsequent‐line therapy. Sintilimab and bevacizumab combination therapy was well‐tolerated and effective, resulting in dramatic tumor reduction and improvement in clinical symptoms.
Keywords: EGFR‐mutant, NSCLC, PD‐1, resistance, sintilimab
Our case reports an advanced EGFR‐mutant lung adenocarcinoma patient achieved PR and a PFS of 6 months to a combination of immunotherapy and anti‐angiogenic agent, sintilimab and bevacizumab, as subsequent‐line therapy. Sintilimab and bevacizumab combination therapy was well‐tolerated and effective, resulting in dramatic tumor reduction and improvement in clinical symptoms.
Introduction
Lung cancer continues to be the most common malignant tumor and the leading cause of cancer death worldwide. Despite the discovery of EGFR (epidermal growth factor receptor) gene alterations and development of targeted therapies which has revolutionized the treatment of NSCLC, the mortality of lung cancer has continued to increase over time. 1 , 2 More importantly, most of patients will inevitably experience acquired resistance within less than one year. 3
On the other hand, there has been some recent breakthroughs in cancer immunotherapy for the treatment of solid tumor patients. The activation of PD‐1 (programmed cell death protein‐1)/PD‐L1 (programmed death ligand‐1) pathway can prevent tumors from immune surveillance. Nivolumab and pembrolizumab as anti‐PD‐1 antibody and atezolizumab as PD‐L1 antibody, the immune checkpoint inhibitor (ICI), have proved their efficacy and safety in patients with pretreated advanced NSCLC in clinical trials. 4 , 5 , 6 However, one of the greatest challenges is that patients harboring EGFR mutations may have very little opportunity to benefit from immunotherapy. 5
Here, we report a patient with advanced NSCLC where chemotherapy and EGFR target therapy failed and who was administered bevacizumab in combination with sintilimab, a new anti‐PD‐1 antibody, which has shown antitumor effects and tolerability in preclinical in vitro and phase I clinical trials. The patient in this study achieved a partial response (PR) with a progression‐free survival (PFS) of six months.
Case report
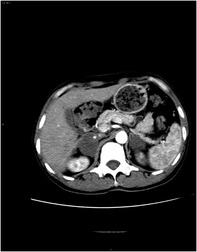
A 53‐year‐old man with a history of smoking (30 pack‐years) presented to the hospital for physical examination in March 2017. Chest computed tomography (CT) scan showed space‐occupying lesions of the lower left lung. A CT‐guided percutaneous pneumocentesis biopsy was performed and histological adenocarcinoma was diagnosed. There was no opportunity for surgery at that time because he was already at stage IIIB (cT3N2M0) of diagnosis, with mediastinal lymph node metastasis. He subsequently underwent four courses of chemotherapy (pemetrexed 800 mg D1 plus cisplatin 30 mg D1‐4) and achieved a partial response (PR) based on the Response Evaluation Criteria In Solid Tumors 1.1 (RECIST), during March 2017 to June 2017. Subsequently, primary lung lesions and mediastinal lymph node metastases received intensity modulated radiation therapy (60 Gy/30 F) and the patient also received oral TS‐1 treatment. In October 2017, polymerase chain reaction (PCR) test of the tumor showed EGFR exon 19del, but targeted therapy was refused at the time of genotyping. Unfortunately, he was found to have PD (progressive disease) with enlarged primary lung lesions and left hilar nodes in January 2018 after a PFS of 9.6 months receiving first‐line chemotherapy. Based on this, he received gefitinib (250 mg once a day) as the second‐line of treatment. In May 2018, the patient was evaluated and confirmed to have PD following the emergence of right adrenal and liver metastasis, despite a shrunken lung lesion and PFS which had lasted 4.1 months. In consideration of the benefits of gefitinib, treatment with gefitinib was continued until July 2018. On 3 July 2018, the patient underwent rebiopsy of the right adrenal through CT‐guided percutaneous puncture and the histology still indicated adenocarcinoma, and notably PD‐L1 expression 15% + by IHC on Roche Vantana Benchmark IHC (immunohistochemistry) platform with antibody of SP142. Next‐generation sequencing (NGS, Burning Rock, Guangzhou, People's Republic of China) test for a large panel containing 520 oncogenic genes was used for molecular testing of the adrenal biopsy sample, which confirmed the presence of MET (MET proto‐oncogene, receptor tyrosine kinase) gene amplification (copy number 10.3) (Fig 1), in addition to the baseline EGFR exon 19 del with an allele frequency of 47.5%, and tumor mutation burden (TMB) was calculated (7.9 mutations/Mb). The patient was subsequently switched to bevacizumab (375 mg) combined with gefitinib (250 mg once a day) from July to October 2018. Unfortunately, the disease continued to progress with increases in left adrenal lesions and other metastases (Fig 2).
Figure 1.
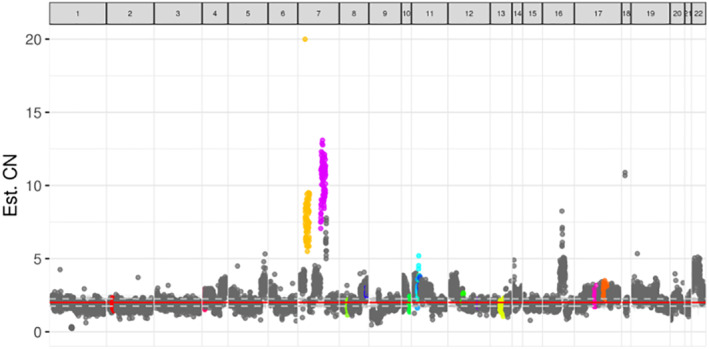
Distribution of gene copy number (purple blot: MET gene amplification). ( ) ALK; (
) ALK; ( ) BRCA1; (
) BRCA1; ( ) BRCA2; (
) BRCA2; ( ) CCND1; (
) CCND1; ( ) CDK4; (
) CDK4; ( ) EGFR; (
) EGFR; ( ) ERBB2; (
) ERBB2; ( ) FGF19; (
) FGF19; ( ) FGF3; (
) FGF3; ( ) FGF4; (
) FGF4; ( ) FGFR1; (
) FGFR1; ( ) FGFR2; (
) FGFR2; ( ) FGFR3; (
) FGFR3; ( ) KRAS; (
) KRAS; ( ) MET; (
) MET; ( ) MYC; (
) MYC; ( ) others.
) others.
Figure 2.
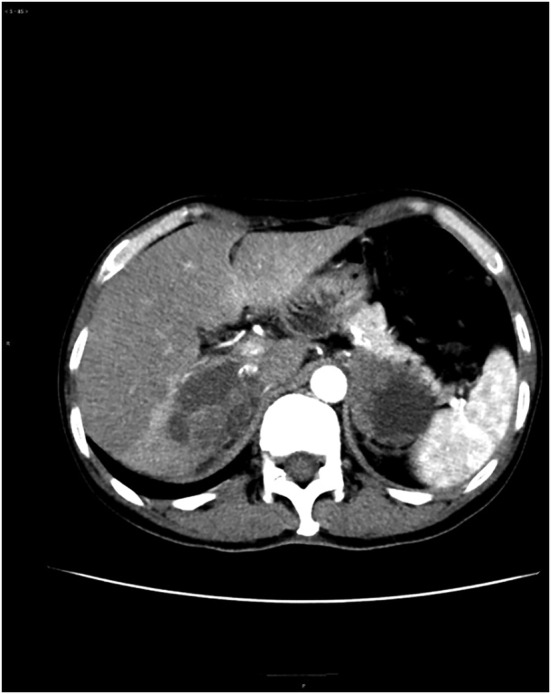
Bilateral adrenal metastases before sintilimab plus bevacizumab treatment (27 June 2018).
Due to the poor response to bevacizumab plus gefitinib therapy and PD‐L1 expression 15% + (IHC) on adrenal puncture sample, sintilimab combined with bevacizumab treatment was commenced on 11 November 2018, at a dose of 200 mg and 400 mg, respectively, every three weeks for four times and a PR was obtained after four weeks (Fig 3). During the sintilimab plus bevacizumab treatment, the patient developed grade 3 rashes which can reoccur spontaneously. Myelosuppression, hypertension, diarrhea, or other adverse events were not apparent. Symptoms of dry cough and pain were improved during ICI plus angiogenesis inhibitor treatment. A PFS of 6.0 months was achieved.
Figure 3.
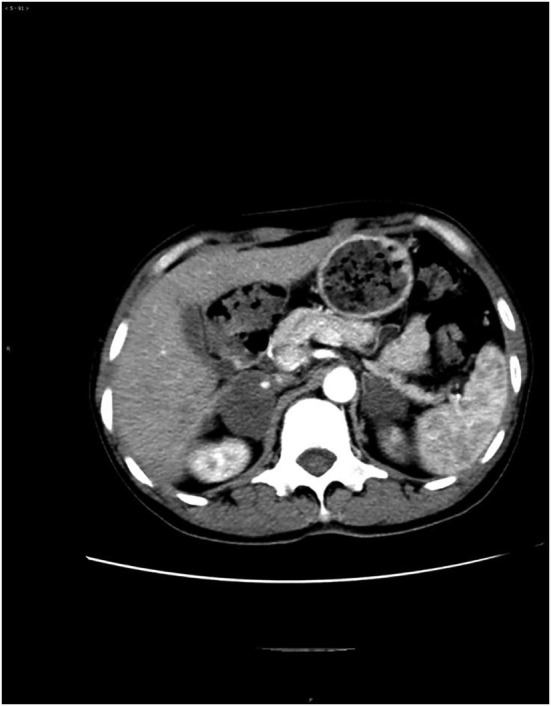
Bilateral adrenal metastases one month after sintilimab plus bevacizumab treatment (20 December 2018).
Unfortunately, thereafter, the patient had progression of bilateral adrenal metastases (Fig 4), his general condition worsened, and he had a dry cough, expectoration and pain. Even if we had attempted to use sintilimab combined with anlotinib or docetaxel, it would not have stopped the disease from deteriorating. The patient died in June 2019.
Figure 4.
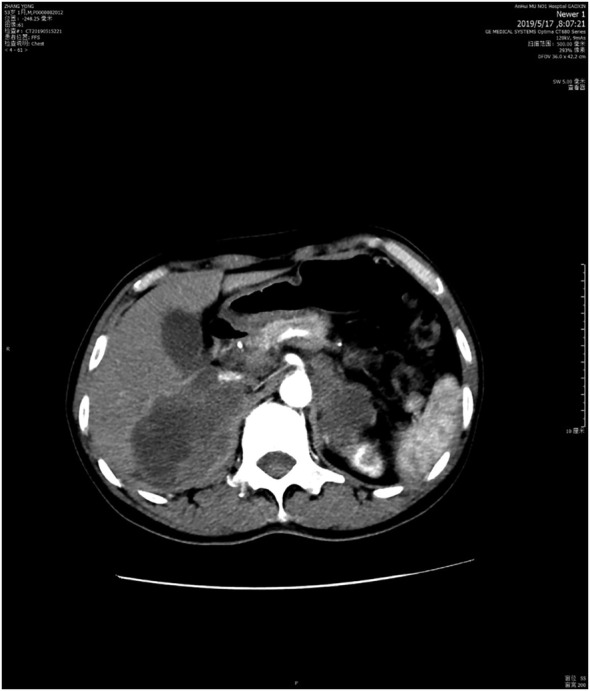
Bilateral adrenal metastases six months after sintilimab plus bevacizumab treatment (15 May 2019).
Discussion
Over the past decade, many studies have shown that EGFR tyrosine kinase inhibitors (TKIs) are preferred for the treatment of advanced NSCLC with EGFR mutation. 7 , 8 Despite initial efficacy, almost all patients will inevitably develop resistance to these agents. 9 The molecular mechanisms underlying acquired resistance to EGFR TKIs are very complex. 10 , 11 , 12
In this case, after our patient progressed following first‐line treatment, targeted therapy was applied because the patient harbored EGFR exon 19 del, but only 4.1 months of PFS was obtained. We considered that the patient's rapid resistance to targeted therapy may have resulted from secondary mutations, or a combination of other mutations. Therefore, molecular testing of the adrenal biopsy sample by NGS was therefore carried out, which confirmed the presence of MET gene amplification and EGFR exon 19 del. We thought MET amplification may have led to acquired resistance to gefitinib, as previous studies have noted that MET amplification is a major cause of acquired resistance to the first and third generation EGFR‐TKIs, such as erlotinib, gefitinib and osimertinib. 13 , 14 , 15 Crizotinib is effective for patients with MET amplification, 16 but the clinical efficacy of combination therapy of crizotinib and EGFR‐TKIs in patients who acquired MET amplification during treatment with EGFR‐TKIs remains controversial. Based on this, and the poor response to gefitinib, we added bevacizumab, an antiangiogenic drug, when the tumor progressed. However, the response to bevacizumab plus gefitinib therapy was poor.
Recently, ICIs, such as pembrolizumab have been recommended for first‐line treatment in advanced NSCLC patients, whose tumor cell PD‐L1 expression is ≥50% (IHC) and PS (performance status) 0–1. In addition, pembrolizumab and other ICIs combined with platinum or bevacizumab have become one of the first‐line treatment of choice for NSCLC without oncogenic drivers, irrespective of PD‐L1 expression. 17 , 18 The emergence of immunotherapy brings a new choice for lung cancer with poor prognosis.
As the PD‐L1 expression was 15% + (IHC) on adrenal puncture sample of this patient, we chose treatment with sintilimab combined with bevacizumab. Sintilimab, a fully human IgG4 monoclonal antibody which can bind to PD‐1 to block the interaction of PD‐1 with PD‐L1 and PD‐L2, has demonstrated its antitumor activity and safety in classical Hodgkin's lymphoma. In phase IA clinical trials in solid tumors it has also been shown to have similar pharmacokinetic characteristics to nivolumab and pembrolizumab. 19 In our case, the treatment with sintilimab plus bevacizumab conferred six months of PFS in the EGFR‐mutant patient with advanced NSCLC who had undergone multiline therapies, which demonstrate its powerful antitumor efficacy. In addition, the toxicity during treatment is mild and controllable. However, it has been suggested that patients with EGFR mutations treated with PD‐1/L1 inhibitors (pembrolizumab, nivolumab, and atezolizumab) as second‐line therapy receive very limited benefit from immunotherapy. 5 , 20 , 21 On the other hand, positive results are still being reported following immunotherapy treatment in EGFR‐mutated patients. The ATLANTIC study demonstrated that an objective response was achieved in 12.2% patients who had EGFR+/ALK+ NSCLC with tumor cell PD‐L1 expression ≥25%, treated with durvalumab (anti‐PD‐L1). 22
Additionally, preclinical and clinical trials have demonstrated synergistic and increased efficacy of antiangiogenic therapy combined with immunotherapy in the treatment of pretreated advanced NSCLC, owing to the immunosuppressive activity of proangiogenic factors, 23 and it has also been suggested that immunotherapies can be antiangiogenic. 24 Taken together, it suggests that the combination of these two therapies can act synergistically on the target tumors. Based on this, several trials are currently investigating combinations of angiogenesis inhibitors and ICIs in patients with NSCLC. Preliminary data from such combination therapies are promising. In the study by Reck et al. on IMpower150, there was an improved overall survival with ABCP (atezolizumab plus bevacizumab plus carboplatin plus paclitaxel) versus BCP (bevacizumab plus carboplatin plus paclitaxel) observed in patients with sensitizing EGFR mutations. 25 A phase I study evaluated the safety and efficacy of switching to nivolumab maintenance therapy, as monotherapy or combined with bevacizumab in patients with advanced NSCLC who did not progress on first‐line chemotherapy. The results showed improved median PFS of patients treated with nivolumab + bevacizumab compared with nivolumab monotherapy, and the toxicity was well tolerated. 26 Herbst et al. evaluated the safety and tolerability of pembrolizumab combined with ramucirumab in patients with various solid tumors including advanced NSCLC and found that the objective response rate was 30%, while the disease control rate was 85%. The treatment‐related AE was well tolerated. 27 Based on these results above, in our case, a possible explanation for the achievement of clinical benefit from immunotherapy might be the treatment of sintilimab combined with bevacizumab.
After a PFS of six months, the patient had disease progression without significant change in gene mutation profile, but there was positive downregulation of PD‐L1 (5%) after acquired resistance to immunotherapy. The PD‐L1 expression of tumor tissue may represent a predictive biomarker to ICI immunotherapies and the persistence of PD‐L1 positive tumor tissue in this patient after immunotherapy resistance might reflect a mechanism of immunotherapy escape. In NSCLC, it has been proven that PD‐L1 overexpression is correlated with poor prognosis in patients. 28 A previous study noted the importance of PD‐L1‐expressing cells promoting dysfunction in tumor‐responding CD8+ T cells from pleural effusions in lung cancer patients. 29 Similar to our results, a previous study has demonstrated that after treatment with nivolumab for six months, patients with PD‐L1 negative circulating tumor cells (CTCs) all obtained a clinical benefit, while patients with PD‐L1 positive CTCs all had PD. 30
In addition to this, other mechanisms of ICI immunotherapy acquired resistance also include blockade of antigen presentation and new immune escape mutations in tumors (JAK/STAT, B2M). 31 , 32 Clinical trials of a combination of immunotherapy with metabolic inhibitors, chemotherapy, and cancer vaccines are underway to provide patients with durable disease control. 33 Choosing or combining with another immunotherapeutic agent after resistance is also an option, but the preliminary results of clinical trials have so far been unsatisfactory. 34 In our case, after disease progression following immunotherapy, a combination of sintilimab with cytotoxic chemotherapy, docetaxel and the antiangiogenic drug, anlotinib, were instigated, yet the efficacy was limited.
Although the patient eventually died of disease progression, we have reason to believe that sintilimab plus bevacizumab is a potential option for NSCLC patients with oncogene drivers who progressed in targeted therapy. High expression of PD‐L1 and/or immune cells infiltration might be predictive biomarkers for choosing PD1 inhibition to treat EGFR‐mutant NSCLC, and this merits further investigation.
Contributor Information
Xuchao Zhang, Email: zhxuchao3000@126.com.
Yingying Du, Email: duyingying@126.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67 (1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (6): 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol 2014; 11 (8): 473–81. [DOI] [PubMed] [Google Scholar]
- 4. Gettinger SN, Horn L, Gandhi L et al Overall survival and long‐term safety of nivolumab (anti‐programmed death 1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2015; 33 (18): 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387 (10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 6. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387 (10030): 1837–46. [DOI] [PubMed] [Google Scholar]
- 7. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non–small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362 (25): 2380–8. [DOI] [PubMed] [Google Scholar]
- 8. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13 (3): 239–46. [DOI] [PubMed] [Google Scholar]
- 9. Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non‐small cell lung cancer. Clin Cancer Res 2008; 14 (10): 2895–9. [DOI] [PubMed] [Google Scholar]
- 10. Engelman JA, Zejnullahu K, Mitsudomi T. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316 (5827): 1039–43. [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi S, Boggon TJ, Dayaram T et al EGFR mutation and resistance of non–small‐cell lung cancer to gefitinib. N Engl J Med 2005; 352 (8): 786–92. [DOI] [PubMed] [Google Scholar]
- 12. Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor‐tyrosine kinase inhibitors and a potential treatment strategy. Cell 2018; 7 (11): 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bean J, Brennan C, Shih JY et al MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007; 104 (52): 20932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu HA, Arcila ME, Rekhtman N et al Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19 (8): 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Planchard D, Loriot Y, André F et al EGFR‐independent mechanisms of acquired resistance to AZD9291 in EGFR T790M‐positive NSCLC patients. Ann Oncol 2015; 26 (10): 2073–8. [DOI] [PubMed] [Google Scholar]
- 16. Zou HY, Li Q, Lee JH et al An orally available small‐molecule inhibitor of c‐Met, PF‐2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 2007; 67 (9): 4408–17. [DOI] [PubMed] [Google Scholar]
- 17. Proto C, Ferrara R, Signorelli D et al Choosing wisely first line immunotherapy in non‐small cell lung cancer (NSCLC): What to add and what to leave out. Cancer Treat Rev 2019; 75: 39–51. [DOI] [PubMed] [Google Scholar]
- 18. Remon J, Besse B, Soria JC. Successes and failures: What did we learn from recent first‐line treatment immunotherapy trials in non‐small cell lung cancer? BMC Med 2017; 15 (1): 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoy SM. Sintilimab: First Global Approval. Drugs 2019; 79 (3): 341–6. [DOI] [PubMed] [Google Scholar]
- 20. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373 (17): 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389 (10066): 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garassino MC. Durvalumab as third‐line or later treatment for advanced non‐small‐cell lung cancer (ATLANTIC): An open‐label, single‐arm, phase 2 study. Lancet Oncol 2018; 19 (4): 521–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garber K. Promising early results for immunotherapy‐antiangiogenesis combination. J Natl Cancer Inst 2014; 106 (11): dju392. [DOI] [PubMed] [Google Scholar]
- 24. Manegold C, Dingemans AMC, Gray JE et al The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 2017; 12 (2): 194–207. [DOI] [PubMed] [Google Scholar]
- 25. Reck M, Mok TSK, Nishio M et al Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med 2019; 7 (5): 387–401. [DOI] [PubMed] [Google Scholar]
- 26. Rizvi NA, Antonia SJ, Shepherd FA et al Nivolumab (anti‐PD‐1; BMS‐936558, ONO‐4538) maintenance as monotherapy or in combination with bevacizumab (BEV) for non‐small cell lung cancer (NSCLC) previously treated with chemotherapy: Metastatic non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2014; 90 (5): S32. [Google Scholar]
- 27. Herbst RS, Bendell JC, Isambert N et al A phase 1 study of ramucirumab (R) plus pembrolizumab (P) in patients (pts) with advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma, non‐small cell lung cancer (NSCLC), or urothelial carcinoma (UC): Phase 1a results. J Clin Oncol 2016; 34 (15_suppl): 3056–6. [Google Scholar]
- 28. Zhou Z‐J, Zhan P, Song Y. PD‐L1 over‐expression and survival in patients with non‐small cell lung cancer: A meta‐analysis. Transl Lung Cancer Res 2015; 4 (2): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prado‐Garcia H, Romero‐Garcia S, Puerto‐Aquino A, Rumbo‐Nava U. The PD‐L1/PD‐1 pathway promotes dysfunction, but not “exhaustion”, in tumor‐responding T cells from pleural effusions in lung cancer patients. Cancer Immunol Immunother 2017; 66 (6): 765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicolazzo C, Raimondi C, Mancini M et al Monitoring PD‐L1 positive circulating tumor cells in non‐small cell lung cancer patients treated with the PD‐1 inhibitor Nivolumab. Sci Rep 2016; 6 (1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nowicki TS, Hu‐Lieskovan S, Ribas A. Mechanisms of resistance to PD‐1 and PD‐L1 blockade. Cancer J 2018; 24 (1): 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma P, Hu‐Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168 (4): 707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018; 118 (1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saleh K, Khalifeh‐Saleh N, Kourie HR. Is it possible to rechallenge with PD‐1/PD‐L1 inhibitors after progression. Immunotherapy 2018; 10 (5): 345–7. [DOI] [PubMed] [Google Scholar]


