Abstract
Background
The intestinal microbiota is an important factor in modulating immune‐mediated tumor cell destruction. Alterations in the microbiome composition have been linked to reduced efficacy of immune checkpoint inhibitor (ICI) therapies. Therefore, antibiotic treatment (ATB), which modifies the diversity of the gut bacteria populations, could lead to a reduced efficacy of ICI treatments.
Methods
This was a retrospective cohort study. Patients with advanced non‐small cell lung cancer (NSCLC) treated with anti‐programmed cell death ligand‐1 (PD‐L1) alone, or in combination in three different countries in Latin America were included. After identification, patients were placed into three groups: Non‐ATB exposed (no‐ATB), exposed within 30 days of the first dose of ICI (pre‐ICI ATB) and patients receiving ATB concomitantly with ICI (ICI‐ATB). Progression‐free survival (PFS), overall survival (OS) and response rates to treatment with ICI were assessed.
Results
A total of 140 patients were included, of which 32 patients (23%) received ATB treatment. The most common ATB types were fluoroquinolones and B‐lactams. No differences in survival according to antibiotic type were identified. Median OS in patients not exposed to ATB was 40.6 months (95% CI: 32–67.7), compared with 20.3 months (95% CI: 12.1‐non‐reached [NR]) for patients with pre‐ICI ATB treatment and 24.7 months (95% CI: 13‐NR) for patients treated with ATB concomitantly with ICI. There were no significant differences in terms of PFS, or response rates across all treatment groups.
Conclusions
Antibiotic treatment was associated with reduced OS in Hispanic patients with NSCLC treated with ICIs.
Keywords: Antibiotics, immunotherapy, lung cancer

Antibiotic treatment has been shown to deter outcomes in patients treated with immune checkpoint inhibitors. The effect of antibiotics is studied for the first time in Latin American patients undergoing ICI therapy. Patients treated with antibiotics prior or during ICI therapy had a significantly shorter overall survival.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of several neoplasms. Non‐small cell lung cancer (NSCLC), the first cause of cancer related deaths worldwide, has been a prime example of the widespread implementation of these drugs in the treatment of both locally‐advanced and metastatic stages as first, second or further lines. 1 , 2 , 3 , 4 , 5 , 6 Despite the improved overall survival (OS) rates observed with anti PD‐L1 ICIs in lung cancer, there are a significant number of patients who do not reap a clinical benefit from this therapeutic strategy. Several factors such as innate resistance mechanisms including the absence of PD‐L1 expression, 7 low tumor mutational burden (TMB), 8 STK11/LKB1 mutations, 9 among others can deter the expected response to these agents. 7 On the other hand, synergistic associations can potentiate the effect of these agents. Combining pembrolizumab, 5 , 10 as well as atezolizumab/bevacizumab with chemotherapy has been reported to be highly effective in a subset of patients with metastatic disease. 11 , 12 Sequential administration of durvalumab after concomitant chemotherapy and radiotherapy for irresectable locally advanced NSCLC also prolongs survival outcomes with a tolerable toxicity profile. 13 , 14 Although multiple combination therapies aiming to improve clinical outcomes are currently in development, there are still patients who experience lack of response or even hyperprogression. 15 , 16 The study of immune antitumor response has been helpful in establishing possible mechanisms that result in a negative ICI response. 17
In recent years, high throughput DNA sequencing technologies have enabled the deep characterization of commensal organisms, such as bacteria, protozoa, virus and fungi that coexist in all individuals. These studies have shed a light on the genomic features and the composition of coexisting organisms, known as the human microbiome or microbiota. 18 International research initiatives have set the goal to characterize the metagenome of the microbiome and elucidate its role in both health and disease processes. 19 Microbiota composition participates in several diseases, including obesity, 20 type II diabetes 21 and Alzheimer's disease. 22 However, its role in the modulation of the host immune system is one of the most relevant research areas in development. The microbiome serves as a training ground for both innate and adaptive immune response elements, and could play a role in the development and regulation of autoinflammatory and autoimmune diseases. 23 , 24 As an example, specific taxa and bacterial strands, such as Saccharomyce cerevisiae, have been found in patients with rheumatoid arthritis and lupus. 24 Hence, therapeutic modulation of the human microbiome has been proposed for the treatment of different disease entities. Diverse strategies such as dietary modifications, 25 antibiotic treatment (ATB) 26 and even fecal transplants have been tested in a variety of settings. 27 , 28 , 29 , 30 , 31
A high biodiversity and proper balance between microbiota species is essential for a commensal and symbiotic relationship, whereas the disruption of these factors could lead to a nonwarranted immune response. Considering that ICI effectiveness relies on the activation of immune effectors to induce anticancer responses, the microbiome composition could influence significantly the outcomes with these treatments. Preclinical models of tumor bearing mice treated with both anti‐CTLA‐4 and anti‐PD‐1/PD‐L1 agents revealed that enrichment of the subject's microbiome with specific strands, including Bacteriodes fragilis, Burkholderia cepacia, Bifidobacterium, Faecalibacterium and others was associated with an increase in interferon γ levels, dendritic cells maturation and CD8+T cell activation. 32 , 33 Impressively, mice undergoing microbiome transplants obtained from responders and nonresponders to anti‐PD‐L1 therapy, mimicked responses observed in fecal donors. 34 These preclinical findings were rapidly translated into the clinical setting. A study conducted in patients with metastatic melanoma treated with anti‐PD‐L1 ICI identified that a higher diversity of colonic microbiome at treatment baseline was associated with improved clinical benefit. In addition, the enrichment in members of the Bacteroides order and Faecalibaterium genus was strongly associated with tumor responses. 35 , 36 In this scenario, disrupting the composition of the microbiota can potentially alter the efficacy of immunotherapy for patients with cancer. In the current study, we investigated the relationship between antibiotic administration as a surrogate marker and indirect indicator of microbiome disruption, and clinical outcomes of patients with metastatic NSCLC who underwent ICI treatment.
Methods
Study design and patient inclusion
A multicenter multinational retrospective cohort study was conducted in referral cancer centers in Mexico, Colombia, Costa Rica and Peru between June 2013 and January 2018. Adult patients (over 18‐years‐old), with metastatic or inoperable non‐small cell lung cancer (NSCLC) who received ICIs at any line of treatment were included. Clinical charts were retrospectively reviewed to collect demographic and clinical variables, treatment types, responses and survival outcomes. Additionally, we compiled information regarding bacterial infections that required ATB. Patients were assigned to three groups based on whether ATB was given, and the temporal relationship of this intervention with ICI administration. The first group was comprised of patients who received antibiotics 30 days prior to the first dose of ICI (pre‐ICI ATB). The second group consisted of patients who received antibiotics during ICI treatment (ICI‐ATB). The third group included patients who did not receive ATB prior or during the course of treatment with ICIs (non‐ATB [no‐ATB]). Data was deidentified and collected in a centralized database within the p‐Platform of the Latin American Consortium for the Investigation of Lung Cancer (CLICaP). Serial radiologic evaluations were evaluated to assess treatment responses according to the RECIST criteria, and adverse effects were categorized in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 37 OS, defined as time from immunotherapy start until death by any cause or loss to follow up, and progression free survival (PFS), defined as time from immunotherapy start until radiological evidence of progressive disease or lost to follow‐up, were estimated.
Ethical considerations
This was a retrospective noninterventional study. Patients data was deidentified and managed according to local regulations. The study was approved by the local ethics and research board.
Statistical analysis
Descriptive statistics were used to group and analyze clinical variables. Continuous variables were summarized as arithmetic means, medians and ranges. Categorical variables were reported as proportions. Inferences were carried using appropriate hypothesis testing and corresponding statistical tests as well as 95% confidence intervals. Survival analysis and curves were conducted using Kaplan‐Meier methodology. In order to assess confounding factors that could influence survival or response, regression analyses that included logistic and Cox models were used. Statistical analyses were performed using SPSS version 23.0 (SSPSS, Inc., Chicago, IL, US) and R version 3.6.1 (The R Foundation, Vienna, Austria).
Results
Patient and treatment characteristics
A total of 140 patients were included in the analysis; 14 patients (10%) received antibiotics in the 30 days prior to ICI initiation (pre‐ICI ATB), 18 patients (12.9%) required antibiotic administration during ICI treatment (ICI‐ATB), whereas the remaining 108 subjects (77.1%) were not exposed to antimicrobial medications prior nor during the course of treatment (no‐ATB). Clinical characteristics are summarized in Table 1. In the pre‐ICI ATB group the most commonly administered antibiotics were piperacillin/tazobactam in four patients (28.6%, [95% CI: 5–52.2%]) and ciprofloxacin in three (21.4%, [95% CI: 0–42.9%]) with the most common indications being pneumonia in six patients (42.9%, [95% CI: 16.9–68.8%]) and urinary tract infections in four individuals (28.6%, [95% CI: 5–52.2%]). In the ICI‐ATB group, the most commonly used antibiotics were meropenem in four patients (22.2%, [95% CI: 3–41.4%]) and piperacillin/tazobactam in three (16.6%, [95% CI: 0–33.9%]), six cases were prescribed ATB due to pneumonia (33.3%, [95% CI: 11.6–55.1%]) and in three cases this was secondary to catheter‐related infections (16.6%, [95% CI: 0–33.8%]). All patients in the pre‐ICI ABT group received short courses of treatment (7–14 days), whereas in the ICI ATB group, one patient received 21 days of treatment.
Table 1.
Patient characteristics
| Variable | All patients (n = 140) | Pre‐ICI ATB (n = 14) | ATB ICI (n = 18) | No ATB (n = 108) |
|---|---|---|---|---|
| Median age | 63 years, (range: 33–86) | 69 years, (range:51–84) | 63 years, (range: 41–78) | 63 years, (range: 33–86) |
| Female % | 40.1% | 42.9% | 33.3% | 41.7% |
| ECOG | ||||
| 0 | 45 (32.1%) | 7 (50%) | 7 (38.9%) | 32 (29.6%) |
| 1 | 91 (65%) | 7 (50%) | 9 (50%) | 73 (67.6%) |
| 2 | 4 (2.9%) | 0 | 2 (11.1%) | 3 (2.8%) |
| Exposure to tobacco | ||||
| Never smokers | 40 (28.6%) | 3 (21.4%) | 4 (23.3%) | 33 (30.6%) |
| Ever smokers | 100 (71.4%) | 11 (78.6%) | 14 (77.7%) | 75 (69.4%) |
| Histology | ||||
| Nonsquamous cell carcinoma | 130 (92.9%) | 11 (78.6%) | 17 (94.4%) | 99 (91.6%) |
| Squamous cell carcinoma | 10 (7.1%) | 3 (31.5%) | 1 (5.6%) | 9 (8.4%) |
| Sites of metastasis | ||||
| Central nervous system | 44 (31.4%) | 2 (14.3%) | 4 (22.2%) | 38 (35.2%) |
| Bone | 81 (57.9%) | 9 (64.3%) | 9 (50%) | 71 (65.7%) |
| Liver | 33 (23.6%) | 2 (14.3%) | 6 (33.3%) | 26 (24.1%) |
| Pleural | 42 (30%) | 4 (26.6%) | 5(27.8%) | 33 (30.6%) |
| Lymph nodes | 111 (79.3%) | 12 (85.7%) | 11(61.1%) | 87 (80.6%) |
| Adrenal glands | 29 (20.7%) | 2 (14.3%) | 6 (33.3%) | 21 (19.4%) |
| Antibiotic name or group | ||||
| B Lactam | 8 (57.3%) | 13 (72.1%) | ||
| Ciprofloxacin | 3 (21.4%) | 2 (11.1%) | ||
| Clindamycin | 0 | 1 (5.6%) | ||
| Clarithromycin | 1 (7.1%) | 1 (5.6%) | ||
| Nitrofurantoin | 1 (7.1%) | 1 (5.6%) | ||
| Doxycycline | 1 (7.1%) | 0 | ||
| Number of previous lines before ICI | ||||
| ICI as first‐line | 35 (25%) | 4 (28.6%) | 6 (33%) | 25 (23.1%) |
| 1 | 45 (32.1%) | 6 (42.8%) | 6 (33%) | 33 (30.6%) |
| 2 | 39 (27.9%) | 4 (28.6%) | 4 (22%) | 31 (28.7%) |
| 3 or more | 21 (15%) | 0 | 2 (11%) | 19 (17.6%) |
Treatment with ICI included anti PD‐(L) monoclonal antibodies given as monotherapy, or in combination with chemotherapy as first, second or further line of treatment. Details of ICI treatment agent for each group are presented in Table 2. ICIs were administered as first‐line therapy in 24% of patients in the no‐ATB group compared with 28.6% in the pre‐ICI ATB and 27.7% in the ICI‐ATB group. The proportion of ICI given as second‐line treatment was evenly distributed between groups: 32.4%, 43% and 27.8%, respectively. No statistically significance differences were found for all comparisons among these groups (P = 0.813). In order to determine imbalances between groups, a logistic regression on variables that could potentially impact outcomes was conducted. Sex, age, performance score, type of ICI, number of therapeutic line, nor metastatic site were associated with an increase probability of receiving antibiotic medications.
Table 2.
Immune checkpoint inhibitors and combination medications administered to each group
| ICI | ATB before ICI (n = 14) | ATB parallel to ICI (n = 18) | No ATB (n = 108) | Median number of cycles |
|---|---|---|---|---|
| Nivolumab | 4 (28.7%) | 11 (61.2%) | 64 (59.3%) | 3 (1–6) |
| Pembrolizumab | 6 (42.9%) | 4 (22.3%) | 23 (21.3%) | 2 (1–7) |
| Docetaxel/pembrolizumab | 1 (5.5%) | 6 (5.5%) | 4 (2–9) | |
| Durvalumab | 1 (7.1%) | 2 (11%) | 3 (2.8%) | 4 (1–6) |
| Avelumab | 1 (7.1%) | 5 (4.6%) | 2 (1–5) | |
| Ipilimumab/nivolumab | 1 (7.1%) | 5 (4.6%) | 3 (1–6) | |
| Platin/pemetrexed/nivolumab | 1 (7.1%) | 2 (1.9%) | 2 (1–3) |
Treatment outcomes
In the entire study population, the median OS from the start of ICI treatment was 9.2 months (95% CI: 4.1–19.7) (Fig 1 ). In patients exposed to antibiotics, we observed a detrimental effect on OS. Median OS was 40.6 months (95% CI: 32–67.7) in the no‐ATB group, compared with 20.3 months (95% CI: 12.1‐non‐reached [NR]) for pre‐ICI ATB and 24.7 months (95% CI: 13‐NR) in the ICI‐ATB group. In a direct comparison between patients treated with antibiotics priori, or during ICI treatment (pre‐ICI ATB and ICI‐ATB groups) and patients in the no‐ATB group, use of antibiotics was significantly associated with decreased OS (Fig 2). No differences in OS were observed between patients in the pre‐ICI ATB group compared with the ICI‐ATB group (P = 0.4) (Fig.3).
Figure 1.
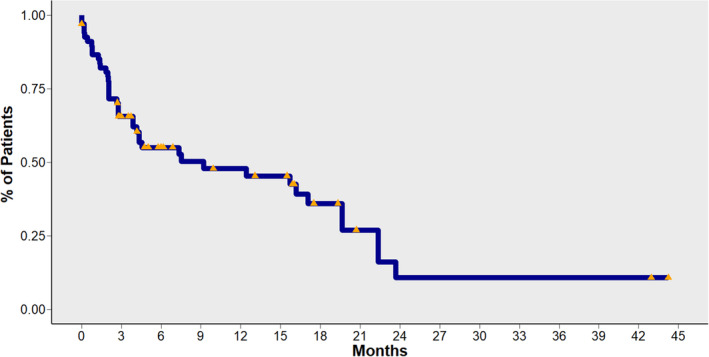
Overall survival (OS) for patients included in the cohort.
Figure 2.
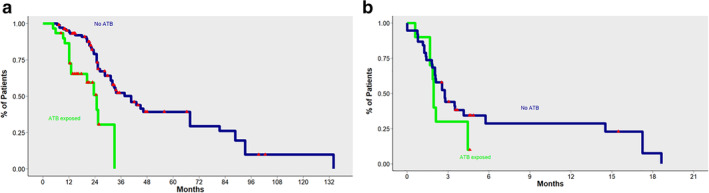
(a) Overall survival (OS) for patients exposed to antibiotics (pre‐ICI ATB and ICI‐ATB) compared with patients not exposed to antibiotics (non‐ATB: [no‐ATB]). (b) Progression‐free survival (PFS) for patients exposed to antibiotics (pre‐ICI ATB and ICI‐ATB) compared with patients not exposed to antibiotics (no‐ATB).
Figure 3.
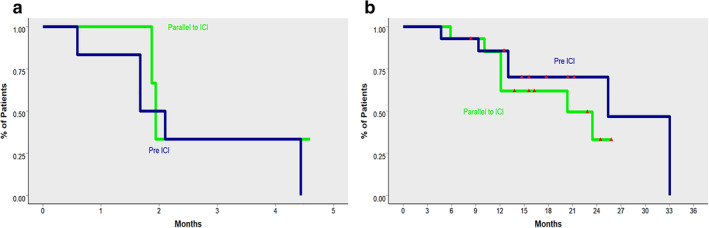
(a) Overall survival (OS) depending on timing of exposure to antibiotics in relationship with ICI administration. (b) Progression‐free survival (PFS) depending on timing of exposure to antibiotics in relationship with ICI administration. Figures have been reversed. Figure A corresponds to Progression free survival (PFS) as denoted by the relatively few months represented in the X axis. Figure B on the other hand represents Overall survival (OS) [Correction added on 17 August 2020, after first online publication: the figure legends for fig 3a and b have been transposed.]
The median progression‐free survival (PFS) was 2.6 months (95% CI: 2.0–3.6) across ICI treatments and lines of therapy (Fig 4 ). There were no significant differences in the PFS between groups, according to ATB exposure. Median PFS in the no‐ATB group was 2.7 months (95% CI: 2.0–3.7), 1.9 months (95% CI: 1.9‐NR) in the pre‐ICI ATB group, and 1.9 months (95% CI: 1.7‐NR) in the ICI‐ATB group (P = 0.4). Overall, response rate for the no‐ATB group was 26.9% (complete 2.9% and partial 24%) compared to 25.9% (complete 3.7% and partial 21.3%) without significant differences between groups (P = 0.481).
Figure 4.
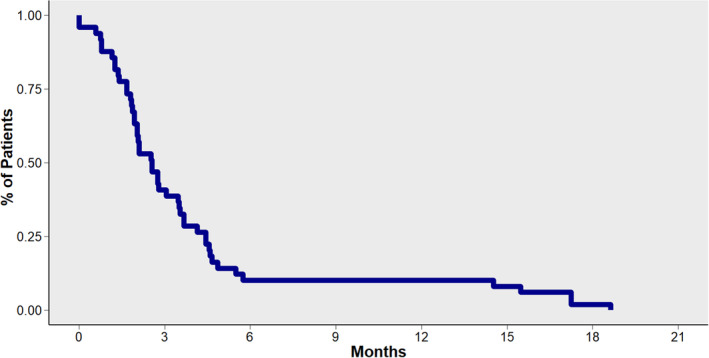
Progression‐free survival for patients included in the cohort.
Clinical practice points
Several studies have ascertained the role of ATB during, or prior to, ICI therapy in Caucasian and Asian populations; nonetheless, this effect has not previously been studied in patients from Latin America. Subjects from Latin America have a diverse environment, including different exposures to environmental bacteria and dietary habits which could impact microbiota and indirectly affect ICI efficacy. In this study, the effect of ATB prior to or during ICI therapy was assessed. Among a group of 140 Latin American patients who received ICI treatment for advanced or metastatic NSCLC, a total of 23% received antibiotics (n = 14 patients received them 30 days prior to ICI initiation, and n = 18 patients received them concomitantly with ICI treatment). Among the patients studied, those who received antibiotics either prior or concomitantly with ICI treatment had a significantly decreased OS compared with patients without antibiotic exposure; nonetheless PFS and response rate were not significantly affected. As a result of this study, clinicians in the Latin America setting should consider treatment choice in patients who are at high risk of infection, particularly considering the costs and access challenges for ICI treatment in limited resource settings.
Discussion
In this retrospective cohort study, we studied the effect of ATB on survival outcomes of NSCLC patients treated with ICIs. In our study, we observed a significant detrimental effect of antibiotic exposure in OS of included patients. Similar results were observed in different studies. Derosa and colleagues reported the largest study on this issue, including 360 patients treated with ICIs for either renal cell carcinoma (34%) or NSCLC (66%). Among patients with metastatic NSCLC, 20% had been exposed to antibiotics within 30 days of ICI initiation. Results of this study showed that prior exposure to antibiotics had an independent negative impact in OS, with a median OS in nonantibiotic exposed patients of 24.6 months compared with 7.9 months in patients treated with antibiotics (HR 4.4, P < 0.01). In addition, prior treatment with antibiotics was also detrimental in terms of PFS (HR 1.5, P = 0.03). 45 In a smaller study conducted by Pinato et al. a total of 119 patients with NCSLC treated with ICIs were included. About 15% of patients had received prior antimicrobial treatment and among these 35% concomitantly with ICIs. Concomitant treatment with ICIs and antibiotics was significantly associated with a reduction in OS (median OS 26 months compared to 2.5 months [P < 0.001]). This effect on OS was not replicated in patients treated with antibiotics prior to ICI initiation, nor did ATB impact on PFS estimates. 43 In another cohort of 30 patients harboring nonsquamous NSCLC, antibiotic exposure was determined at 30 days prior, or after, the initiation of ICI therapy and a statistically significance of a reduction of around 7.5 months for OS was observed in this subgroup of patients. Contrary to the previous report and the current study, this negative effect also impacted median PFS. 44
Our study contributes to previously published data by exploring the role of antibiotic use in ICI outcomes in a Latin‐American population. Although we included a relatively small number of patients exposed to antibiotics, the magnitude of the effect observed on OS suggests a relevant size effect. Compared with the study published by Derosa and colleagues, in the present study we did not observe a difference in PFS. 43 One possible explanation for this difference is the small sample size in our study, which limits the capacity of observing minor differences in PFS between groups.
In the present study, most patients received broad‐spectrum antibiotics such as B‐lactams and quinolones, known to impact significantly in the human microbiome composition. In the case of piperacillin/tazobactam, even short treatments lasting four to eight days resulted in the reduction of aerobic bacterial strands such as bifidobacteria, eubacteria and lactobacilli. 38 , 39 Carbapenem antibiotics such as meropenem and ertapenen have also been shown to reduce the population of Bacterorides species. 40 Fluoroquinolones, one of the broadest and most active antibacterial agents, can reduce the fecal Enterobacteriaceae population, and on occasions, lead to complete eradication of this species. In addition, it can cause depletion of anaerobic bacteria such as clostridia, bifidobacteria and bacteroides spp. 41 , 42 In our study we did not characterize the microbiota composition of the patients; hence the effect of antibiotics in the specific strains of bacteria in patients living in Latin‐American countries remains to be explored. However, in conjunction with preclinical studies in other populations, a relationship between this species depletion could be evaluated as a candidate for explaining the negative effects of antibiotics on ICI response rates. Furthermore, it is also worth mentioning that this taxon is also diminished in NSCLC compared to healthy controls. 43 Furthermore, the heterogeneous antibiotic administration routes (oral or intravenous), antibacterial spectrum (broad vs. narrow), dosage and duration could be factors that could influence microbiota disruption and are difficult to adjust as confounders in analysis.
Although the impact of antibiotic use and outcomes to ICI treatment in NSCLC has not been validated in randomized trials, several recommendations could be inferred from our study and others in the field. First, clinicians should limit patient exposure to broad‐spectrum ATB, known to have a negative impact on microbiota diversity (such as B‐lactams and fluoroquinolones) and, when feasible, resort to lesser disruptive antibiotics such as nitrofurantoin and doxycycline. 46 Further research is required to study different strategies to boost the immune response against cancer cells with ICIs through the effective modulation of the host's microbiota, and to prevent gastrointestinal toxicities such as autoimmune colitis. Several approaches are currently being tested in the clinical setting, which include the supplementation of probiotics and fecal transplants (NCT03705442, NCT03772899).
This study had several limitations. Due to the retrospective design of the study, data quality is subjected to reported variables, and confounders may be present. However, to reduce bias, data was curated by each investigator in a standardized dataset and prognostic variables were statistically tested to ensure that adverse prognostic features were well balanced between groups. A second limitation of our study was the small sample size of patients treated with antibiotics. However, this is the largest study held in Latin‐America, in which microbiome composition may differ from other world regions. In addition, due to a significant number of patients treated with ICI beyond the second‐line, the survival estimates of patients in the present study could be diminished relative to other publications.
In conclusion, ATB with broad‐spectrum medications could result in shorter OS in patients treated with ICI in Latin‐American countries. Limitation or selection of appropriate antibiotic medications should be considered when dealing with an active infection in ICI treated patients.
Disclosure
Dr Arrieta reported receiving grants or personal fees from the National Council for Science and Technology in Mexico (CONACyT), Pfizer, AstraZeneca, Boehringer Ingelheim, Lilly, Merck, Bristol‐Myers Squibb, and Roche. Dr Zatarain‐Barrón reported receiving grants from CONACyT. Dr Cardona reported receiving grants or personal fees from Roche, Boehringer Ingelheim, AstraZeneca, Pfizer, Celldex, Bristol‐Myers Squibb, Merck Sharp & Dohme, and AbbVie and reported being cofounder of the Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia. Dr Recondo is a consultant and on the advisory board for Roche, Amgen and Pfizer, and received travel grants from AstraZeneca and Pfizer.
Acknowledgments
We would like to acknowledge all who are involved with the Latin American Consortium for Lung Cancer Investigation (CLICaP) for their continued support in order to improve research for the Latin American region. This study was funded by grant No. 131‐2018 from the Latin American Consortium for Lung Cancer Investigation (CLICaP).
Contributor Information
Andrés F. Cardona, Email: andres.cardona@clinicadelcountry.com, Email: a_cardonaz@yahoo.com.
Oscar Arrieta, Email: ogar@unam.mx.
References
- 1. Jain P, Jain C, Velcheti V. Role of immune‐checkpoint inhibitors in lung cancer. Ther Adv Respir Dis 2018; 12: 1753465817750075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruiz‐Patiño A, Arrieta O, Cardona AF et al Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non‐small cell lung cancer (NSCLC) compared with chemotherapy (Quijote‐CLICaP). Thorac Cancer 2020; 11 (2): 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horn L, Spigel DR, Vokes EE et al Nivolumab versus docetaxel in previously treated patients with advanced non‐small‐cell lung cancer: Two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 35: 3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 6. Vokes EE, Ready N, Felip E et al Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol 2018; 29: 959–65. [DOI] [PubMed] [Google Scholar]
- 7. Nowicki TS, Hu‐Lieskovan S, Ribas A. Mechanisms of resistance to PD‐1 and PD‐L1 blockade. Cancer J 2018; 24: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee M, Samstein RM, Valero C et al Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum Vaccin Immunother 2019;16 (1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skoulidis F, Arbour KC, Hellmann MD et al Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non‐squamous non‐small cell lung cancer. J Clin Oncol 2019; 37: 102–2. [Google Scholar]
- 10. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 11. Socinski MA, Jotte RM, Cappuzzo F et al Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–301. [DOI] [PubMed] [Google Scholar]
- 12. West H, McCleod M, Hussein M et al Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2019; 20: 924–37. [DOI] [PubMed] [Google Scholar]
- 13. Antonia SJ, Villegas A, Daniel D et al Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377: 1919–29. [DOI] [PubMed] [Google Scholar]
- 14. Antonia SJ, Villegas A, Daniel D et al Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–50. [DOI] [PubMed] [Google Scholar]
- 15. Frelaut M, Le Tourneau C, Borcoman E. Hyperprogression under immunotherapy. Int J Mol Sci 2019; 20: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shields BD, Mahmoud F, Taylor EM et al Indicators of responsiveness to immune checkpoint inhibitors. Sci Rep 2017; 7: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint blockade‐based combination therapies. Cancer Cell 2018; 33: 581–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cani PD. Human gut microbiome: Hopes, threats and promises. Gut 2018; 67: 1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NIH HMP Working Group , Peterson J, Garges S et al The NIH human microbiome project. Genome Res 2009; 19: 2317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol 2010; 26: 5–11. [DOI] [PubMed] [Google Scholar]
- 21. Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract 2018; 146: 111–8. [DOI] [PubMed] [Google Scholar]
- 22. Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer's disease. J Alzheimers Dis 2017; 58: 1–15. [DOI] [PubMed] [Google Scholar]
- 23. De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol 2019; 195: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157: 121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomova A, Bukovsky I, Rembert E et al The effects of vegetarian and vegan diets on gut microbiota. Front Nutr 2019; 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bass NM, Mullen KD, Sanyal A et al Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362: 1071–81. [DOI] [PubMed] [Google Scholar]
- 27. Kim KO, Gluck M. Fecal microbiota transplantation: An update on clinical practice. Clin Endosc 2019; 52: 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui B, Feng Q, Wang H et al Fecal microbiota transplantation through mid‐gut for refractory Crohn's disease: Safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015; 30: 51–8. [DOI] [PubMed] [Google Scholar]
- 29. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta‐analysis. J Crohns Colitis 2014; 8: 1569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willmann M, Vehreschild MJGT, Biehl LM et al Distinct impact of antibiotics on the gut microbiome and resistome: A longitudinal multicenter cohort study. BMC Biol 2019; 17: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016; 65: 1906–15. [DOI] [PubMed] [Google Scholar]
- 32. Sivan A, Corrales L, Hubert N et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science 2015;350:1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vétizou M, Pitt JM, Daillère R et al Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science 2015; 350: 1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matson V, Fessler J, Bao R et al The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gopalakrishnan V, Spencer CN, Nezi L et al Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaput N, Lepage P, Coutzac C et al Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017; 28: 1368–79. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. [Cited 31 Jan 2020.] Available from URL: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf
- 38. Nord CE, Brismar B, Kasholm‐Tengve B, Tunevall G. Effect of piperacillin/tazobactam treatment on human bowel microflora. J Antimicrob Chemother 1993; 31 (Suppl. A): 61–5. [DOI] [PubMed] [Google Scholar]
- 39. Kundrapu S, Sunkesula VCK, Jury LA et al Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis 2016; 16: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pletz MWR, Rau M, Bulitta J et al Ertapenem pharmacokinetics and impact on intestinal microflora, in comparison to those of ceftriaxone, after multiple dosing in male and female volunteers. Antimicrob Agents Chemother 2004; 48: 3765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Lastours V, Fantin B. Impact of fluoroquinolones on human microbiota. Focus on the emergence of antibiotic resistance. Future Microbiol 2015; 10: 1241–55. [DOI] [PubMed] [Google Scholar]
- 42. Bhalodi AA, van Engelen TSR, Virk HS, Wiersinga WJ. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother 2019; 74: i6–i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinato DJ, Howlett S, Ottaviani D et al Antibiotic treatment prior to immune checkpoint inhibitor therapy as a tumor‐agnostic predictive correlate of response in routine clinical practice. J Clin Oncol 2019; 37: 147–7. [Google Scholar]
- 44. Huemer F, Rinnerthaler G, Westphal T et al Impact of antibiotic treatment on immune‐checkpoint blockade efficacy in advanced non‐squamous non‐small cell lung cancer. Oncotarget 2018; 9: 16512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Derosa L, Hellmann MD, Spaziano M et al Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non‐small‐cell lung cancer. Ann Oncol 2018; 29: 1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haas K, Notay M, Rodriguez W et al 383 Doxycycline effects on the gut and skin microbiomes and lipidome in acne. J Invest Dermatol 2018; 138: S65. [Google Scholar]


