Abstract
Background
Image‐guided radiotherapy (IGRT) is an advanced radiotherapy technique to improve the precision and accuracy of treatment delivery. A recent randomized controlled trial (RCT) for prostate cancer patients treated with radiotherapy via either IGRT or routine care reported statistically significantly worse overall survival (OS) for those patients treated with IGRT. This raised the concern regarding the effectiveness of IGRT in definitive concurrent chemoradiotherapy (dCCRT) for locally advanced lung cancer (LALC).
Methods
Eligible LALC patients diagnosed between 2011 and 2016 were identified via the Taiwan Cancer Registry. We used propensity score (PS) weighting to balance observable potential confounders between groups. The hazard ratio (HR) of death and other outcomes were compared between IGRT and non‐IGRT. We also evaluated OS in various subgroups.
Results
Our primary analysis consisted of 797 patients in whom covariates were well balanced after PS weighing. The HR for death when IGRT was compared with non‐IGRT was 0.96 (95% confidence interval 0.79–1.15, P = 0.65). There were also no significant differences for most of the other outcomes or subgroup analyses.
Conclusions
In this updated nonrandomized study, we found that OS of LALC patients treated with dCCRT was not statistically different between those treated with IGRT versus non‐IGRT. The results should be interpreted with caution given the nonrandomized design. Studies regarding toxicity, local control, or designed as RCT are needed to clarify the role of IGRT.
Key points
Significant findings of the study
The OS of LALC patients treated with dCCRT was not statistically different between those treated with IGRT versus those without IGRT, although the observed HR for death was less than unity (ie, in favor of IGRT).
What this study adds
In this updated nonrandomized study using real world data with additional potential confounders, our study provided a reasonable tentative evidence in the lack of RCT as suggested in the literature.
Keywords: Concurrent chemoradiotherapy, image‐guided radiotherapy, lung cancer
In this updated nonrandomized study, we found that overall survival of locally advanced lung cancer patients treated with definitive concurrent chemoradiotherapy was not statistically different between those treated with IGRT versus non‐IGRT. The results should be interpreted with caution given the nonrandomized design. Randomized controlled trials are needed to clarify this finding.
Introduction
Lung cancer is the major cause of cancer mortality around the world. 1 Definitive concurrent chemoradiotherapy (dCCRT) is the standard of care for unresectable locally advanced small cell lung cancer (SCLC) or non‐small cell lung cancer (NSCLC) with good performance status 2 , 3 although consolidative immunotherapy had been recommended for NSCLC since September 2017. 4
Image‐guided radiotherapy (IGRT) is an advanced auxiliary technique used to improve the accuracy of radiotherapy delivery. 5 , 6 IGRT uses images (mainly x‐ray) to verify and adjust the position of the radiotherapy target. Conceptually, this improvement in radiotherapy delivery accuracy improves the clinical outcome, as advocated in the literature or textbooks, 5 , 6 and as also reported in our previous nonrandomized study utilizing data from the Taiwan Cancer Registry (TCR) for lung cancer patients diagnosed during 2007–2010. 7
However, a recent randomized study in 2018 for prostate cancer patients treated with conventional fractionated radiotherapy via either IGRT or routine care (no daily IGRT) reported a statistically significant worse overall survival for those treated with IGRT, although side effects and disease control were improved. 8 Theoretically, the extra‐radiotherapy dose due to xray‐IGRT may have contributed to the increased risk of other cancer (10% vs. 5%) or cardiovascular mortality (6/236 vs. 1/234) observed in this study and led to the impaired overall survival. 8 This raises a concern regarding the effectiveness of IGRT for other scenarios such as dCCRT for lung cancer.
Since 2011, additional prognostic factors such as body mass index (BMI), use of alcohol or betel nuts or smoking, and performance status (PS) have been prospectively collected in the TCR. Because these potential confounders were not adjusted in our previous study due to data limitation at that time, here we aimed to investigate the effectiveness of IGRT for locally advanced lung cancer (LALC) patients treated with dCCRT in this updated analysis with consideration of the above potential confounders.
Methods
Data source
The database with personal identifiers removed in our retrospective cohort study comes from the Health and Welfare Data Science Center (HWDC), including the Taiwan Cancer Registry (TCR), death registration and reimbursement data for the whole Taiwan population provided by the Bureau of National Health Insurance (NHI). The TCR is a high‐quality database 9 that provides complete information such as patient, disease, treatment characteristics, and prognostic factor details. This study was approved by the Research Ethics Committee, National Health Research Institutes (CRREC‐108‐080).
Study population and study design
The study flow chart (Fig 1) was designed to conform to the STROBE statement, 10 and depicted our main study population. We included nonoperated LALC adult (age ≥ 18) patients diagnosed between 2011 and 2016 who received curative concurrent systemic therapy and external beam radiotherapy 50–70 Gy using conventional fractionation via image‐guided radiotherapy (IGRT) or non‐IGRT, but excluded patients with other cancer(s). The explanatory variable of interest (IGRT vs. non‐IGRT), the primary outcome of interest (overall survival [OS]) and other supplementary outcomes (incidence of lung cancer mortality [ILCM], other cancer mortality [IOCM] and cardiovascular mortality [ICVM]) were determined via the recordings in the TCR or the death registry. We defined the date of diagnosis as the index date and calculated the OS or other endpoints from the index date to the date of death or 31 December 2018 (censoring date of the death registry). The covariates (see next section “Other explanatory covariates”) were modified from our experiences in clinical care and TCR studies 7 , 11 , 12 to adjust for potential nonrandomized treatment selection.
Figure 1.
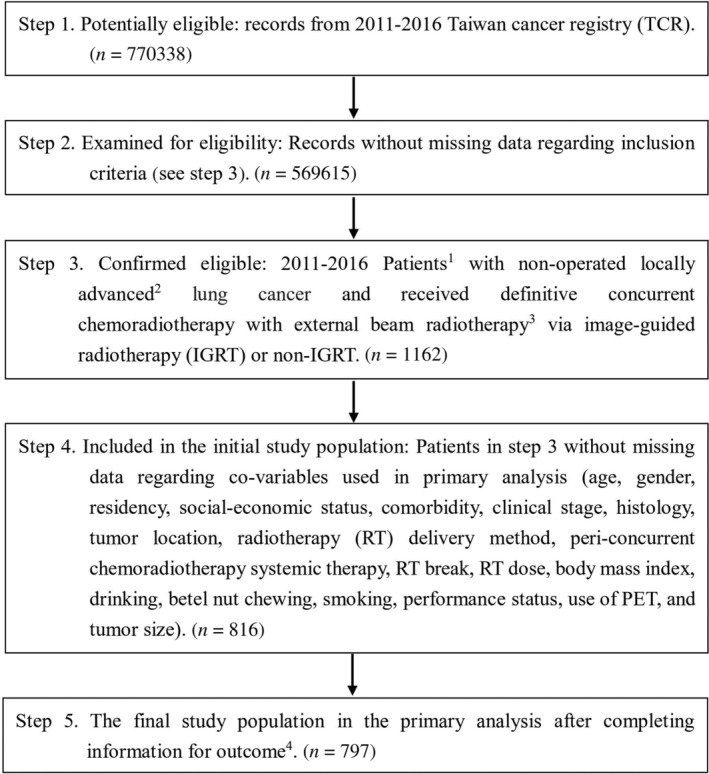
STROBE study flowchart and the number of individuals at each stage of the study. 1, We only included those treated (class 1–2) by any single institution to ensure data consistency. 2, The seventh American Joint Committee on Cancer staging clinical stage 3A and 3B. 3, 50–70 Gy in 1.8–2 Gy/fraction, within ± 10% in dose and treatment duration. 4, Without missing information in the TCR and death registry regarding survival status, and cause of death.
Other explanatory covariates
In this study, the included covariates were patient demographics (age, gender, residency), patient characteristics (socioeconomic status, comorbidity, drinking, betel nut chewing, smoking, body mass index [BMI]), disease characteristics (clinical stage, tumor size, histology, tumor location), treatment characteristics (radiotherapy [RT] delivery, RT dose, peri‐CCRT systemic therapy, RT break), and prognostic factor (performance status, use of positron emission tomography [PET]) were defined as follows. Age was classified as at least 65 years old or not. Patient residency region was classified as northern Taiwan or elsewhere. Socioeconomic status was classified as higher (income greater than minimum wage) or not. Comorbidity was classified as with or without via Carlson comorbidity score. The drinking, betel nut chewing, smoking, and use of PET covariates were classified as yes or no. Clinical stage was classified as 3A or 3B. Histology was classified as small cell lung carcinoma (SCLC) or non‐small cell lung carcinoma (NSCLC). Tumor location was classified as lower or upper/middle lobe. RT delivery was classified as 3D conformal radiotherapy (3DCRT) or intensity‐modulated radiotherapy (IMRT). Peri‐CCRT systemic therapy was classified as with or without. RT break was defined as more than one week or not. Performance status was classified as Eastern Cooperative Oncology Group 0–1 or 2.
Statistical and subgroup analyses
In the primary analysis (PA), we adopted the propensity‐score (PS) method to balance the measured potential confounders. 13 , 14 , 15 We evaluated the probability of receiving IGRT (vs. non‐IGRT) with a logistic regression model based on all the above covariates, used the logit of the probability as the PS, and then assessed the balance of covariates between groups via the standardized difference (SDif). 16 , 17 We compared the hazard ratio (HR) of death between the IGRT and non‐IGRT groups during the entire follow‐up period via a PS weighting approach with overlap weights as suggested in studies. 18 , 19 We used Cox proportional hazards model in the weighted sample for point estimation and used the bootstrap method to estimate the 95% confidence interval (95% CI). 18 , 20 , 21 We further adopted the E‐factor to evaluate the impact of potential unmeasured confounding factor(s) on OS. 22 We took a competing risk approach to compare ILCM, IOCM, and ICVM between groups. 23
In the subgroup analyses (SA), we used alternative approach (PS matching) as also advocated in the literature. 18 We constructed additional 1:1 PS matching for four subgroups separately and compared the hazard ratio (HR) of death between the IGRT and non‐IGRT groups via a robust variance estimator. 18 In the first subgroup analysis (SA‐1), we performed PS matching for patients in the primary analysis. The treatment for SCLC and NSCLC were quite different as reflected by the different treatment guidelines. 2 , 3 In addition, the optimal treatment is less clear for a subset of patients with NSCLC with mutant epidermal growth factor receptor (EGFR), as reported in our previous first nonrandomized population‐based study utilizing TCR. 24 Therefore, we did an additional SA for SCLC (SA‐2), NSCLC with wild‐type EGFR (SA‐3) or NSCLC with mutant EGFR (SA‐4). In SA‐5, we included three additional potential covariates addressed by reviewers during revision to examine the robustness of our findings. These covariates included c‐T stage (T1‐2 vs. T3‐4), c‐N stage (N0‐1 vs. N2‐3), and treatment era (early = 2011–2013 vs. late = 2014–2016).
We performed the statistical analyses using the software SAS 9.4 (SAS Institute, Cary, NC) and R (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria) version 3.5.3.
Results
Identification of the study population used in the primary analysis
As shown in Fig 1, 797 eligible nonoperated LALC adult patients who received IGRT or non‐IGRT in 2011–2016 were used as our primary study population and were divided into the IGRT group (n = 192) versus the non‐IGRT group (n = 605). The patient characteristics are described in Table 1. Some covariates were not balanced before PS weighting but all covariates were well balanced (standardized differences <0.25) after PS weighting. 17
Table 1.
Patient characteristics of the study population in the primary analysis
| IGRT (n = 192) | non‐IGRT (n = 605) | Standardized difference (rounded)* | |||||
|---|---|---|---|---|---|---|---|
| Number or mean (sd) ‡ | (%) ‡ | Number or mean (sd) ‡ | (%) ‡ | Before PSW | After PSW | ||
| Age | <65 | 95 | (49) | 319 | (53) | 0.07 | ≈0 |
| ≥65 | 97 | (51) | 286 | (47) | |||
| Gender | Female | 45 | (23) | 116 | (19) | 0.10 | ≈0 |
| Male | 147 | (77) | 489 | (81) | |||
| Residency | Non‐north | 128 | (67) | 290 | (48) | 0.39 | ≈0 |
| North | 64 | (33) | 315 | (52) | |||
| Social economic status | No more than minimum wage | 50 | (26) | 163 | (27) | 0.02 | ≈0 |
| Higher | 142 | (74) | 442 | (73) | |||
| Comorbidity | Without | 104 | (54) | 345 | (57) | 0.06 | ≈0 |
| With † | 88 | (46) | 260 | (43) | |||
| Clinical stage | 3A | 56 | (29) | 193 | (32) | 0.06 | ≈0 |
| 3B | 136 | (71) | 412 | (68) | |||
| Histology | NSCLC | 155 | (81) | 510 | (84) | 0.09 | |
| SCLC | 37 | (19) | 95 | (16) | |||
| Tumor location | Lower | 48 | (25) | 179 | (30) | 0.10 | |
| Upper/middle | 144 | (75) | 426 | (70) | |||
| RT delivery | 3DCRT | 3 | (2) | 62 | (10) | 0.38 | ≈0 |
| IMRT | 189 | (98) | 543 | (90) | |||
| Peri‐CCRT Systemic therapy | Without | 110 | (57) | 323 | (53) | 0.08 | |
| With | 82 | (43) | 282 | (47) | |||
| RT break | ≤1 week | 139 | (72) | 473 | (78) | 0.13 | ≈0 |
| >1 week | 53 | (28) | 132 | (22) | |||
| RT dose | 61.98 (6.12) | 61.61 (5.99) | 0.06 | ≈0 | |||
| BMI | 23.60 (3.84) | 24.16 (3.82) | 0.15 | ≈0 | |||
| Drinking | No | 126 | (66) | 367 | (61) | 0.10 | ≈0 |
| Yes | 66 | (34) | 238 | (39) | |||
| Betel nut chewing | No | 159 | (83) | 479 | (79) | 0.09 | ≈0 |
| Yes | 33 | (17) | 126 | (21) | |||
| Smoking | No | 57 | (30) | 144 | (24) | 0.13 | ≈0 |
| Yes | 135 | (70) | 461 | (76) | |||
| Performance Status | 0–1 | 179 | (93) | 574 | (95) | 0.07 | ≈0 |
| 2 | 13 | (7) | 31 | (5) | |||
| Use of PET | No | 106 | (55) | 323 | (53) | 0.04 | ≈0 |
| Yes | 86 | (45) | 282 | (47) | |||
| Tumor size (mm) | 56.36 (24.42) | 56.64 (24.05) | 0.07 | ≈0 | |||
3DCRT, 3D conformal radiotherapy; BMI, body mass index; CCRT, concurrent chemoradiotherapy; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; NSCLC, non‐small cell lung cancer; PET, positron emission tomography; PSW, propensity‐score weighting; RT, radiotherapy; SCLC, small cell lung cancer; sd, standard deviation.
Modified Carlson comorbidity score ≥ 1.
Rounded.
Primary analysis
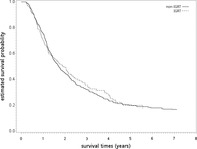
After a median follow‐up of 21 months (range 3–95), death was observed for 141 patients in the IGRT group and for 458 patients in the non‐IGRT group. The overlap weights adjusted OS curve are shown in Fig 2. The five‐year OS rates for both groups were 19.61% (IGRT) and 19.96% (non‐IGRT), respectively. When IGRT was compared to non‐IGRT, the HR of death was 0.96 (95% confidence interval [95% CI]: 0.79–1.15, P = 0.65). The observed HR could be explained by an unmeasured confounder associated with the selection of treatment (IGRT or non‐IGRT) and survival by a risk ratio of 1.20 (E‐value) fold each, but weaker confounding factors could not do so. The results were also not significantly different for ILCM (HR = 0.92, P = 0.55) and IOCM (HR = 2.23, P = 0.37). The crude ICVM was around 1% (7/605) in the non‐IGRT group and even lower in the IGRT group (exact number not reported per HWDC policy).
Figure 2.
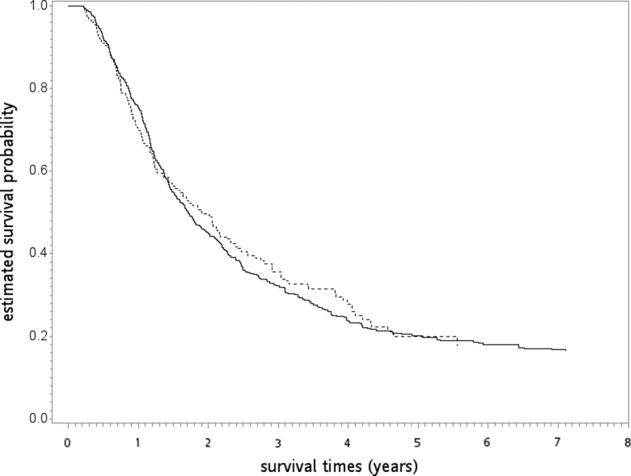
The overlap weights adjusted overall survival curve (in years) in the primary analysis.  , non‐IGRT;
, non‐IGRT;  , IGRT.
, IGRT.
Subgroup analyses (SA)
In the SA‐1 to SA‐5, we were still able to construct balanced subpopulations after PS‐matching except failed to balance all covariates in the SA‐4 (NSCLC with mutant EGFR), probably due to the relatively small number of cases (Tables 2, 3, 4, 5, 6). There was also no statistically significant difference for OS when IGRT was compared with non‐IGRT (SA‐1: HR = 0.99, P = 0.90; SA‐2: HR = 0.99, P = 0.97; SA‐3: HR = 1.26, P = 0.47; SA‐4: HR = 0.90, P = 0.75; SA‐5: HR = 1.03, P = 0.80).
Table 2.
SA‐1: Patient characteristics of the PS‐matched subgroup in the supplementary analysis
| IGRT (n = 192) | non‐IGRT (n = 192) | Standardized difference (rounded)* | ||||
|---|---|---|---|---|---|---|
| Number or mean (sd) § | (%) § | Number or mean (sd) § | (%) § | |||
| Age | <65 | 95 | (49) | 106 | (55) | 0.12 |
| ≥65 | 97 | (51) | 86 | (45) | ||
| Gender | Female | 45 | (23) | 47 | (24) | 0.02 |
| Male | 147 | (77) | 145 | (76) | ||
| Residency | Non‐north | 128 | (67) | 134 | (70) | 0.07 |
| North | 64 | (33) | 58 | (30) | ||
| Social economic status | No more than minimum wage | 50 | (26) | 60 | (31) | 0.12 |
| Higher | 142 | (74) | 132 | (69) | ||
| Comorbidity | Without | 104 | (54) | 106 | (55) | 0.02 |
| With † | 88 | (46) | 86 | (45) | ||
| Clinical stage | 3A | 56 | (29) | 62 | (32) | 0.07 |
| 3B | 136 | (71) | 130 | (68) | ||
| Histology | NSCLC | 155 | (81) | 155 | (81) | 0 |
| SCLC | 37 | (19) | 37 | (19) | ||
| Tumor location | Lower | 48 | (25) | 47 | (24) | 0.01 |
| Upper/middle | 144 | (75) | 145 | (76) | ||
| RT delivery | 3DCRT | ‡ | ‡ | ‡ | ‡ | 0.05 |
| IMRT | ‡ | ‡ | ‡ | ‡ | ||
| Peri‐CCRT Systemic therapy | Without | 110 | (57) | 108 | (56) | 0.02 |
| With | 82 | (43) | 84 | (44) | ||
| RT break | ≤1 week | 139 | (72) | 139 | (72) | 0 |
| >1 week | 53 | (28) | 53 | (28) | ||
| RT dose | 61.98 (6.12) | 61.53 (6.03) | 0.07 | |||
| BMI | 23.60 (3.84) | 23.79 (3.66) | 0.05 | |||
| Drinking | No | 126 | (66) | 122 | (64) | 0.04 |
| Yes | 66 | (34) | 70 | (36) | ||
| Betel nut chewing | No | 159 | (83) | 157 | (82) | 0.03 |
| Yes | 33 | (17) | 35 | (18) | ||
| Smoking | No | 57 | (30) | 60 | (31) | 0.03 |
| Yes | 135 | (70) | 132 | (69) | ||
| Performance status | 0–1 | 179 | (93) | 184 | (96) | 0.12 |
| 2 | 13 | (7) | 8 | (4) | ||
| Use of PET | No | 106 | (55) | 103 | (54) | 0.03 |
| Yes | 86 | (45) | 89 | (46) | ||
| Tumor size (mm) | 56.36 (24.42) | 54.49 (24.75) | 0.08 | |||
3DCRT, 3D conformal radiotherapy; BMI, body mass index; CCRT, concurrent chemoradiotherapy; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; NSCLC, non‐small cell lung cancer; PET, positron emission tomography; PSW, propensity‐score weighting; RT, radiotherapy; SCLC, small cell lung cancer; sd, standard deviation.
Modified Carlson comorbidity score ≥ 1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Rounded.
Table 3.
SA‐2: SCLC group
| IGRT (n = 37) | non‐IGRT (n = 37) | Standardized difference (rounded)* | ||||
|---|---|---|---|---|---|---|
| Number or mean (sd) § | (%) § | Number or mean (sd) § | (%) § | |||
| Age | <65 | 20 | (54) | 21 | (57) | 0.05 |
| ≥65 | 17 | (46) | 16 | (43) | ||
| Gender | Female | 4 | (11) | 3 | (8) | 0.09 |
| Male | 33 | (89) | 34 | (92) | ||
| Residency | Non‐north | 25 | (68) | 25 | (68) | 0 |
| North | 12 | (32) | 12 | (32) | ||
| Social economic status | No more than minimum wage | 9 | (24) | 9 | (24) | 0 |
| Higher | 28 | (76) | 28 | (76) | ||
| Comorbidity | Without | 25 | (68) | 24 | (65) | 0.06 |
| With † | 12 | (32) | 13 | (35) | ||
| Clinical stage | 3A | 10 | (27) | 11 | (30) | 0.06 |
| 3B | 27 | (73) | 26 | (70) | ||
| Tumor location | Lower | 8 | (22) | 6 | (16) | 0.14 |
| Upper/middle | 29 | (78) | 31 | (84) | ||
| Peri‐CCRT Systemic therapy | Without | 18 | (49) | 18 | (49) | 0 |
| With | 19 | (51) | 19 | (51) | ||
| RT break | ≤1 week | 20 | (54) | 22 | (59) | 0.11 |
| >1 week | 17 | (46) | 15 | (41) | ||
| RT dose | 60.20 (6.29) | 61.15 (4.72) | 0.17 | |||
| BMI | 24.54 (4.15) | 24.44 (3.64) | 0.03 | |||
| Drinking | No | 17 | (46) | 14 | (38) | 0.17 |
| Yes | 20 | (54) | 23 | (62) | ||
| Betel nut chewing | No | 30 | (81) | 30 | (81) | 0 |
| Yes | 7 | (19) | 7 | (19) | ||
| Smoking | No | ‡ | ‡ | ‡ | ‡ | 0.20 |
| Yes | ‡ | ‡ | ‡ | ‡ | ||
| Performance status | 0–1 | 34 | (92) | 34 | (92) | 0 |
| 2 | 3 | (8) | 3 | (8) | ||
| Use of PET | No | 25 | (68) | 29 | (78) | 0.25 |
| Yes | 12 | (32) | 8 | (22) | ||
| Tumor size (mm) | 65.46 (31.35) | 63.00 (27.12) | 0.08 | |||
All patients were treated by intensity‐modulated radiotherapy. BMI, body mass index; CCRT, concurrent chemoradiotherapy; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; PET, positron emission tomography; PSW, propensity‐score weighting; RT, radiotherapy; SCLC, small cell lung cancer; sd, standard deviation.
Modified Carlson comorbidity score ≥ 1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Rounded.
Table 4.
SA‐3: NSCLC wild‐type EGFR group
| IGRT (n = 41) | non‐IGRT (n = 41) | Standardized difference (rounded)* | ||||
|---|---|---|---|---|---|---|
| Number or mean (sd) § | (%) § | Number or mean (sd) § | (%) § | |||
| Age | <65 | 20 | (49) | 22 | (54) | 0.10 |
| ≥65 | 21 | (51) | 19 | (46) | ||
| Gender | Female | 11 | (27) | 10 | (24) | 0.06 |
| Male | 30 | (73) | 31 | (76) | ||
| Residency | Non‐north | 21 | (51) | 21 | (51) | 0 |
| North | 20 | (49) | 20 | (49) | ||
| Social economic status | No more than minimum wage | 13 | (32) | 11 | (27) | 0.11 |
| Higher | 28 | (68) | 30 | (73) | ||
| Comorbidity | Without | 16 | (39) | 16 | (39) | 0 |
| With † | 25 | (61) | 25 | (61) | ||
| Clinical stage | 3A | 9 | (22) | 9 | (22) | 0 |
| 3B | 32 | (78) | 32 | (78) | ||
| Tumor location | Lower | 13 | (32) | 9 | (22) | 0.22 |
| Upper/middle | 28 | (68) | 32 | (78) | ||
| RT delivery | 3DCRT | ‡ | ‡ | ‡ | ‡ | 0.10 |
| IMRT | ‡ | ‡ | ‡ | ‡ | ||
| Peri‐CCRT Systemic therapy | Without | 23 | (56) | 22 | (54) | 0.05 |
| With | 18 | (44) | 19 | (46) | ||
| RT break | ≤1 week | 34 | (83) | 33 | (80) | 0.06 |
| >1 week | 7 | (17) | 8 | (20) | ||
| RT dose | 61.24 (6.62) | 61.18 (5.81) | 0.01 | |||
| BMI | 23.54 (4.05) | 23.48 (3.86) | 0.02 | |||
| Drinking | No | 32 | (78) | 31 | (76) | 0.06 |
| Yes | 9 | (22) | 10 | (24) | ||
| Betel nut chewing | No | ‡ | ‡ | ‡ | ‡ | 0.19 |
| Yes | ‡ | ‡ | ‡ | ‡ | ||
| Smoking | No | 14 | (34) | 12 | (29) | 0.11 |
| Yes | 27 | (66) | 29 | (71) | ||
| Performance status | 0–1 | ‡ | ‡ | ‡ | ‡ | 0 |
| 2 | ‡ | ‡ | ‡ | ‡ | ||
| Use of PET | No | 19 | (46) | 15 | (37) | 0.20 |
| Yes | 22 | (54) | 26 | (63) | ||
| Tumor size (mm) | 51.51 (21.63) | 50.61 (19.18) | 0.04 | |||
BMI, body mass index; CCRT, concurrent chemoradiotherapy; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; PET, positron emission tomography; PSW, propensity‐score weighting; RT, radiotherapy; SCLC, small cell lung cancer; sd, standard deviation.
Modified Carlson comorbidity score ≥ 1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Rounded.
Table 5.
SA‐4: NSCLC mutant EGFR group
| IGRT (n = 30) | non‐IGRT (n = 30) | Standardized difference (rounded)* | ||||
|---|---|---|---|---|---|---|
| Number or mean (sd) § | (%) § | Number or mean (sd) § | (%) § | |||
| Age | <65 | 12 | (40) | 16 | (53) | 0.27 |
| ≥65 | 18 | (60) | 14 | (47) | ||
| Gender | Female | 19 | (63) | 20 | (67) | 0.07 |
| Male | 11 | (37) | 10 | (33) | ||
| Residency | Non‐north | 21 | (70) | 17 | (57) | 0.28 |
| North | 9 | (30) | 13 | (43) | ||
| Social economic status | No more than minimum wage | 8 | (27) | 10 | (33) | 0.15 |
| Higher | 22 | (73) | 20 | (67) | ||
| Comorbidity | Without | 20 | (67) | 17 | (57) | 0.21 |
| With † | 10 | (33) | 13 | (43) | ||
| Clinical stage | 3A | 12 | (40) | 9 | (30) | 0.21 |
| 3B | 18 | (60) | 21 | (70) | ||
| Tumor location | Lower | 5 | (17) | 3 | (10) | 0.20 |
| Upper/middle | 25 | (83) | 27 | (90) | ||
| Peri‐CCRT Systemic therapy | Without | 8 | (27) | 11 | (37) | 0.22 |
| With | 22 | (73) | 19 | (63) | ||
| RT break | ≤1 week | 26 | (87) | 25 | (83) | 0.09 |
| >1 week | 4 | (13) | 5 | (17) | ||
| RT dose | 62.52 (5.62) | 62.15 (4.69) | 0.07 | |||
| BMI | 24.30 (3.87) | 24.19 (3.37) | 0.03 | |||
| Drinking | No | 26 | (87) | 24 | (80) | 0.18 |
| Yes | 4 | (13) | 6 | (20) | ||
| Betel nut chewing | No | ‡ | ‡ | ‡ | ‡ | 0.15 |
| Yes | ‡ | ‡ | ‡ | ‡ | ||
| Smoking | No | 23 | (77) | 21 | (70) | 0.15 |
| Yes | 7 | (23) | 9 | (30) | ||
| Use of PET | No | 14 | (47) | 13 | (43) | 0.07 |
| Yes | 16 | (53) | 17 | (57) | ||
| Tumor size (mm) | 49.13 (18.48) | 47.17 (22.27) | 0.10 | |||
All patients were performance status score 0–1 and treated by intensity‐modulated radiotherapy. BMI, body mass index; CCRT, concurrent chemoradiotherapy; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; PET, positron emission tomography; PSW, propensity‐score weighting; RT, radiotherapy; SCLC, small cell lung cancer; sd, standard deviation.
Modified Carlson comorbidity score ≥ 1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Rounded.
Table 6.
SA‐5: Patient characteristics of the PS‐matched subgroup (included three additional potential covariates)
| IGRT (n = 191) | non‐IGRT (n = 191) | Standardized difference (rounded)* | ||||
|---|---|---|---|---|---|---|
| Number or mean (sd)§ | (%) § | Number or mean (sd)§ | (%) § | |||
| Age | <65 | 94 | (49) | 96 | (50) | 0.02 |
| ≥65 | 97 | (51) | 95 | (50) | ||
| Gender | Female | 45 | (24) | 46 | (24) | 0.01 |
| Male | 146 | (76) | 145 | (76) | ||
| Residency | Non‐north | 127 | (66) | 121 | (63) | 0.07 |
| North | 64 | (34) | 70 | (37) | ||
| Social economic status | No more than minimum wage | 50 | (26) | 48 | (25) | 0.02 |
| Higher | 141 | (74) | 143 | (75) | ||
| Comorbidity | Without | 103 | (54) | 107 | (56) | 0.04 |
| With † | 88 | (46) | 84 | (44) | ||
| Clinical stage | 3A | 56 | (29) | 49 | (26) | 0.08 |
| 3B | 135 | (71) | 142 | (74) | ||
| Histology | NSCLC | 154 | (80) | 154 | (80) | 0 |
| SCLC | 37 | (20) | 37 | (20) | ||
| Tumor location | Lower | 48 | (25) | 46 | (24) | 0.02 |
| Upper/middle | 143 | (75) | 145 | (76) | ||
| RT delivery | 3DCRT | ‡ | ‡ | ‡ | ‡ | 0.05 |
| IMRT | ‡ | ‡ | ‡ | ‡ | ||
| Peri‐CCRT Systemic therapy | Without | 109 | (57) | 112 | (59) | 0.03 |
| With | 82 | (43) | 79 | (41) | ||
| RT break | ≤1 week | 139 | (73) | 142 | (74) | 0.04 |
| >1 week | 52 | (27) | 49 | (26) | ||
| RT dose | 62.01 (6.12) | 62.03 (5.71) | 0.004 | |||
| BMI | 23.58 (3.84) | 23.34 (3.68) | 0.07 | |||
| Drinking | No | 125 | (65) | 129 | (68) | 0.04 |
| Yes | 66 | (35) | 62 | (32) | ||
| Betel nut chewing | No | 158 | (83) | 164 | (86) | 0.09 |
| Yes | 33 | (17) | 27 | (14) | ||
| Smoking | No | 57 | (30) | 58 | (30) | 0.01 |
| Yes | 134 | (70) | 133 | (70) | ||
| Performance status | 0–1 | 178 | (93) | 181 | (95) | 0.07 |
| 2 | 13 | (7) | 10 | (5) | ||
| Use of PET | No | 106 | (56) | 106 | (56) | 0 |
| Yes | 85 | (44) | 85 | (44) | ||
| Tumor size (mm) | 56.23 (24.41) | 56.34 (25.43) | 0.005 | |||
| c‐T stage | T1‐2 | 50 | (26) | 54 | (28) | 0.05 |
| T3‐4 | 141 | (74) | 137 | (72) | ||
| c‐N stage | N0‐1 | 19 | (10) | 15 | (8) | 0.07 |
| N2‐3 | 172 | (90) | 176 | (92) | ||
| Treatment era | Early | 76 | (40) | 80 | (42) | 0.04 |
| Late | 115 | (60) | 111 | (58) | ||
3DCRT, 3D conformal radiotherapy; BMI, body mass index; CCRT, concurrent chemoradiotherapy; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; NSCLC, non‐small cell lung cancer; PET, positron emission tomography; PSW, propensity‐score weighting; RT, radiotherapy; SCLC, small cell lung cancer; sd, standard deviation.
Modified Carlson comorbidity score ≥ 1.
The exact numbers were not reported because of a Health and Welfare Data Science Center (HWDC) database center policy to avoid numbers in single cells (≤2).
Rounded.
Discussion
In this updated nonrandomized study using real world data with additional potential confounders, we found that the OS of LALC patients treated with dCCRT was not statistically different between those treated with IGRT versus those without IGRT. However, the observed HR for death in our study was less than unity (ie, in favor of IGRT), in contrast to the HR 2.12 (P = 0.042) observed in the RCT for prostate cancer. 8 However, it should be noted that OS was the second but not primary outcome of this RCT, 8 so the significance regarding the difference in OS or other outcomes may be false positive 25 and should be interpreted with caution. Furthermore, although this RCT 8 failed to show superiority in its primary endpoint (recurrence‐free survival, HR 0.81 P = 0.33), post hoc analyses did show a benefit to IGRT in biochemical progression‐free, clinical progression‐free interval, and rectal toxicity.
From the viewpoint of evidence‐based medicine, our finding was not a high level evidence 15 , 26 and should be cautiously interpreted due to the nonrandomized study design. 15 Although OS was obviously the most important endpoint in cancer treatment, it may not be the most relevant endpoint for IGRT, and our study lacked the data on local control or toxicity which might be better endpoints for IGRT. However, when we searched the clinical trial registry (https://clinicaltrials.gov/) in March 2020 using keywords “(image‐guided radiation therapy) or (image‐guided radiotherapy) or (IGRT) | lung cancer | Phase 2, 3, Not Applicable”, we found no RCT regarding IGRT for LALC patients treated with dCCRT. Therefore, our study provided a reasonable tentative evidence in the lack of RCT as suggested in the literature. 15
We also looked for relevant studies by searching PubMed in May 2020 using the following keywords “(IGRT) or (image‐guided radiation therapy) or (image*) and (guid*) and (radiotherapy) or (radiation therapy) and survival and (lung cancer)”and found two subsequent institutional studies from the United States in addition to our previous study. 7 , 27 , 28 Kilburn et al. used data of 169 NSCLC patients and found similar OS (P = 0.63) although better locoregional control and less grade III acute toxicity for IGRT. 27 Deek et al. retrospectively reviewed 91 patients with NSCLC and reported daily KV image IGRT was associated with longer OS (P = 0.01) as well as less locoregional failure when compared to weekly MV image. 28 In contrast, our current study was a population based study from Asia with a much larger number of cases (797 vs. [91 or 169] in the previous studies).
There were several limitations in our study. First, the treatment assignment in our study (IGRT or not) was not randomized and potential unmeasured confounders are always a limitation of a nonrandomized study as seen in our previous study 7 and the current study. Although we had included additional covariates in the current study, there were still possible unmeasured confounders. For example, use of invasive mediastinal staging, organ at risk dose, the planning target volume (PTV) margin or volume, dose calculation algorithms, or systematic therapy details might be different for those treated with IGRT or not and if imbalanced between groups may impact our results, but this information was not available in the TCR. Furthermore, accessibility (IGRT may not be available in some institutes [nor provided by the TCR in HWDC]) issues may also be possible because IGRT is a relatively recent technology. Therefore, we reported E‐value to quantify the potential impact of unmeasured confounder(s) as suggested in the literature. Second, locoregional control or side effects but not OS might be more relevant endpoints as reported in previous studies. 27 , 28 However, we did not include these endpoints as we felt the recording of relapse pattern in TCR was relatively immature for lung cancer. Third, the implication regarding IGRT for current practice may be unclear in the era of immunotherapy because consolidative immunotherapy (durvalumab) has been the standard of care for locally advanced NSCLC treated with definitive CCRT since 2017. 29 Finally, the intervention (IGRT) in our study was not homogenous. To our knowledge, several different forms of xray‐based IGRT are available in Taiwan including, but not limited to, cone beam computed tomography, KV imaging, and MV imaging, which could not be differentiated in the TCR. However, it is unclear whether these various technologies lead to different clinical benefits, 30 although, to our knowledge, the modern xray‐free magnetic resonance image guided IGRT 31 was not available in Taiwan for our study population. The quality of IGRT may also be a concern 32 but could not be assessed in our study due to study limitations.
In conclusion, in this updated nonrandomized study using real world data with additional potential confounders, we found that the OS of LALC patients treated with dCCRT was not statistically different between those treated with IGRT versus those without IGRT, with the hazard ratio less than unity ie, in favor of IGRT. The results should be interpreted with caution given the nonrandomized design. Studies regarding toxicity, local control, or designed as RCT are needed to clarify the role of IGRT.
Disclosure
The authors have not published or submitted the manuscript elsewhere. The authors declare there are no conflicts of interest.
Acknowledgments
The data analyzed in this study were provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. We are grateful to the Health Data Science Center at the China Medical University Hospital and the Ministry of Science and Technology (MOST 109‐2314‐B‐039‐014‐) for providing administrative, technical and funding support.
References
- 1. International Agency for Research on Cancer, World Health Organization . 2020. The Global Cancer Observatory. https://gco.iarc.fr. Accessed May 30 2020.
- 2. National Comprehensive Cancer Network (NCCN) . 2020. Guidelines for Small Cell Lung Cancer, version 3.2020. http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed April 1 2020.
- 3. National Comprehensive Cancer Network (NCCN) . 2020. Guidelines for non‐small cell lung cancer, version 3.2020. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed April 1 2020.
- 4. National Comprehensive Cancer Network (NCCN) . 2017. Guidelines for non‐small cell lung cancer, version 9.2017. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed September 28 2017.
- 5. Bujold A, Craig T, Jaffray D, Dawson LA. Image‐guided radiotherapy: Has it influenced patient outcomes? Semin Radiat Oncol 2012; 22: 50–61. [DOI] [PubMed] [Google Scholar]
- 6. Simpson RD, Mell LK, Mundt AJ, Atwood TF. Image‐guided radiation therapy In: Halperin EC, Wazer DE, Perez CA, Brady LW. (eds). Perez & Brady's Principles and Practice of Radiation Oncology, 7th edn Lippincott Williams & Wilkins, Philadelphia, PA: 2018; 288–307. [Google Scholar]
- 7. Hsia TC, Tu CY, Fang HY, Liang JA, Li CC, Chien CR. Cost and effectiveness of image‐guided radiotherapy for non‐operated localized lung cancer: A population‐based propensity score‐matched analysis. J Thorac Dis 2015; 7: 1643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Crevoisier R, Bayar MA, Pommier P et al Daily versus weekly prostate cancer image guided radiation therapy: Phase 3 multicenter randomized trial. Int J Radiat Oncol Biol Phys 2018; 102: 1420–9. [DOI] [PubMed] [Google Scholar]
- 9. Chiang CJ, Wang YW, Lee WC. Taiwan's Nationwide cancer registry system of 40 years: Past, present, and future. J Formos Med Assoc 2019; 118: 856–8. [DOI] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M et al The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–9. [DOI] [PubMed] [Google Scholar]
- 11. Kuo Y‐H, Fang H‐Y, Lin Y‐S et al Effectiveness of image‐guided radiotherapy for locally advanced esophageal squamous cell carcinoma patients treated with definitive concurrent chemoradiotherapy. Thorac Cancer 2020; 11: 113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsia TC, Tu CY, Chen HJ et al A population‐based study of primary chemoradiotherapy in clinical stage III non‐small cell lung cancer: Intensity‐modulated radiotherapy versus 3D conformal radiotherapy. Anticancer Res 2014; 34: 5175–80. [PubMed] [Google Scholar]
- 13. Jagsi R, Bekelman JE, Chen A et al Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys 2014; 90: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum PR. Chapter 5 between observational studies and experiments In: Observation and Experiment. Harvard University Press, Cambridge, MA: 2017. [Google Scholar]
- 15. Booth CM, Karim S, Mackillop WJ. Real‐world data: Towards achieving the achievable in cancer care. Nat Rev Clin Oncol 2019; 16: 312–25. [DOI] [PubMed] [Google Scholar]
- 16. Ali MS, Groenwold RH, Belitser SV et al Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: A systematic review. J Clin Epidemiol 2015; 68: 112–21. [DOI] [PubMed] [Google Scholar]
- 17. Garrido MM, Kelley AS, Paris J et al Methods for constructing and assessing propensity scores. Health Serv Res 2014; 49: 1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33: 1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao H, Li L, Greene T. Propensity score weighting analysis and treatment effect discovery. Stat Methods Med Res 2019; 28: 2439–54. [DOI] [PubMed] [Google Scholar]
- 20. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75: 45–9. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016; 35: 5642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haneuse S, VanderWeele TJ, Arterburn D. Using the E‐value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019; 321: 602–3. [DOI] [PubMed] [Google Scholar]
- 23. Bolch CA, Chu H, Jarosek S, Cole SR, Elliott S, Virnig B. Inverse probability of treatment‐weighted competing risks analysis: An application on long‐term risk of urinary adverse events after prostate cancer treatments. BMC Med Res Methodol 2017; 17: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsia TC, Liang JA, Li CC, Chien CR. Comparative effectiveness of concurrent chemoradiotherapy versus EGFR‐tyrosine kinase inhibitors for the treatment of clinical stage IIIb lung adenocarcinoma patients with mutant EGFR. Thorac Cancer 2018; 9: 1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghadjar P, Fiorino C, Rosenschöld PMA, Pinkawa M, Zilli T, van der Heide UA. ESTRO ACROP consensus guideline on the use of image guided radiation therapy for localized prostate cancer. Radiother Oncol 2019; 141: 5–13. [DOI] [PubMed] [Google Scholar]
- 26. Djulbegovic B, Guyatt GH. Progress in evidence‐based medicine: A quarter century on. Lancet 2017; 390: 415–23. [DOI] [PubMed] [Google Scholar]
- 27. Kilburn JM, Soike MH, Lucas JT et al Image guided radiation therapy may result in improved local control in locally advanced lung cancer patients. Pract Radiat Oncol 2016; 6: e73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deek MP, Kim S, Yue N et al Modern radiotherapy using image guidance for unresectable non‐small cell lung cancer can improve outcomes in patients treated with chemoradiation therapy. J Thorac Dis 2016; 8: 2602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antonia SJ, Villegas A, Daniel D et al Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377: 1919–29. [DOI] [PubMed] [Google Scholar]
- 30. Goyal S, Kataria T. Image guidance in radiation therapy: Techniques and applications. Radiol Res Pract 2014; 2014: 705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pollard JM, Wen Z, Sadagopan R, Wang J, Ibbott GS. The future of image‐guided radiotherapy will be MR guided. Br J Radiol 2017; 90: 20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tiong A, Lao L, MacKean J, Goonetilleke M, Kron T. Faculty of Radiation Oncology Position Paper on the use of image‐guided radiation therapy. J Med Imaging Radiat Oncol 2016; 60: 772–80. [DOI] [PubMed] [Google Scholar]


