Abstract
RNA-based medicine is receiving growing attention for its diverse roles and potential therapeutic capacity. The largest obstacle in its clinical translation remains identifying a safe and effective delivery system. Studies investigating RNA therapeutics in pulmonary diseases have rapidly expanded and drug administration by inhalation allows the direct delivery of RNA therapeutics to the target site of action while minimizing systemic exposure. In this review, we highlight recent developments in pulmonary RNA delivery systems with the use of nonviral vectors. We also discuss the major knowledge gaps that require thorough investigation and provide insights that will help advance this exciting field towards the bedside.
Keywords: aerosol, mRNA, nonviral vector, pulmonary delivery, respiratory diseases, siRNA
Potential of RNA Therapy for the Treatment or Prevention of Lung Diseases
The diverse roles of RNA in the body have led to the emergence of different approaches to harnessing RNA for therapeutic use. RNA therapeutics can be broadly divided into three functional classes: (i) inhibition of gene expression [e.g., small interfering RNA (siRNA; see Glossary), microRNA (miRNA), and antisense oligonucleotide (ASO)]; (ii) protein encoding (e.g., mRNA); and (iii) protein targeting (e.g., RNA aptamer s) [1]. Despite their diverse mechanisms of action, it is no secret that the biggest barrier to all types of RNA therapeutic is delivery; that is, to bring therapeutic RNA molecules into the target cells effectively in a safe and reproducible manner. With the US Food and Drug Administration (FDA) approval of the first two siRNA therapeutics, patisiran and givosiran, both of which target hepatic disorders, the field of RNA therapy is ready to look for applications beyond the liver [2,3].
There is an increasing number of studies that report the potential of RNA in treating a range of lung diseases including asthma [4], cystic fibrosis (CF) [5], lung cancer [6], and respiratory infections [7]. Inhalation of aerosol is an efficient way to deliver RNA to the lung by maximizing local concentration while minimizing systemic exposure. ALN-RSV01, designed to treat respiratory syncytial virus (RSV) infection, was the first siRNA candidate to be delivered through the pulmonary route in clinical trials in 2008 [8,9]. Since then, several clinical trials on inhaled RNA therapy have been initiated (Box 1 ). However, no inhaled RNA therapeutic has yet been approved for use in clinics.
Box 1. Inhaled RNA Therapies in Clinical Trials.
ALN-RSV01 (Clinical Trial No i : NCT00496821, NCT00658086, and NCT01065935)
ALN-RSV01 is a naked siRNA targeting the RSV nucleocapsid protein for the treatment of the associated viral respiratory infection. It was the first siRNA investigated for pulmonary delivery in clinical trials. RSV causes significant illness in immunocompromised patients following lung transplantation, and bronchiolitis obliterans syndrome (BOS) is the major cause of morbidity and mortality in these patients [101]. The Phase I clinical trial (NCT00496821) started in 2007 and demonstrated that ALN-RSV01 was well tolerated following intranasal administration. The Phase IIb clinical trial (NCT01065935) showed that aerosolized ALN-RSV01 was effective in reducing the incidence of new or progressive BOS in lung transplant patients with RSV infection following inhalation. Although ALN-RSV01 failed to progress to a Phase III trial, it marked an important milestone of inhaled RNA therapy [8,101., 102., 103.].
Excellair
Excellair is an siRNA targeting spleen tyrosine kinase (Syk), which is involved in the inflammatory response in the lung epithelium [104]. It was investigated for the treatment of asthma by inhalation. The Phase I trial began in 2009. There was little information published about the outcome of the study, although it was reported that the drug was well tolerated in patients with asthma. The Phase II trial was discontinued in 2015 [105].
Eluforsen (Previously Known as QR-010) (Clinical Trial No: NCT02532764 and NCT02564354)
Eluforsen is a single-stranded RNA ASO targeting CF transmembrane conductance regulator (CFTR) for inhalation to patients with F508del CF. The Phase I clinical trial (NCT02564354) initiated in 2015 showed that CFTR activity was restored after intranasal administration of eluforsen. The Phase Ib clinical trial (NCT02532764) was completed in 2017 and demonstrated that inhaled eluforsen was safe, well tolerated, and improved respiratory symptoms in patients with F508del CF [106,107]. However, no further clinical development is planned for this candidate.
MRT5005 (Clinical Trial No: NCT03375047)
MRT5005 is the first inhaled mRNA candidate for CF and delivers mRNA encoding fully functional CFTR protein. The Phase I/II clinical trial was initiated in May 2018 to test the safety and tolerability of MRT5005. Patients with CF received mRNA encoding fully function CFTR protein through nebulization. Interim results were encouraging, showing that MRT5005 was well tolerated at low and mid-dose levels (8–16 mg) with no serious adverse events reported at any dose level (up to 24 mg). There was a marked improvement of lung function in patients after single dose of MRT5005 at the mid-dose levelii. In early 2020, the FDA granted Fast Track and Rare Pediatric Disease designations for MRT5005 for the treatment of CFiii, iv.
Alt-text: Box 1
Naked RNA for Inhalation
RNA is a negatively charged, hydrophilic macromolecule that is incapable of permeating the cell membrane. It is vulnerable to degradation before reaching the target sites due to the abundance of RNase in the body. Therefore, it has to rely on delivery vectors to protect it from premature degradation and facilitate its cell entry. Interestingly, it has been known for over a decade that naked RNA, including both siRNA and mRNA, can be transfected in the lung following pulmonary delivery, as shown in many in vivo studies [10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24.]. Given that the lung comprises various cell types with distinct functions, it is crucial to understand which cell types are susceptible to naked RNA transfection for effective clinical translation. To address this issue, Ng et al. carried out a comprehensive investigation on the distribution and activity of naked siRNA in the lung of mice following intratracheal administration [21]. The silencing activity of naked siRNA was most prominent in lung epithelial cells, dendritic cells, and alveolar macrophages. Similar observations were reported by other groups in which the activity of naked siRNA was primarily found in lung epithelial cells but not the endothelial cells [23], making it attractive for use in lung conditions affecting these cell types without systemic exposure, for example in RSV infection and CF. Ng et al. stressed that chemical modification is crucial for naked siRNA to induce effective gene silencing by improving metabolic stability and reducing immunostimulation [21]. Using modified naked mRNA, Tiwari et al. successfully developed a mRNA-based approach to express neutralizing antibodies in the lung via intratracheal delivery to prevent RSV infection in mice [19]. The authors also compared naked mRNA with the use of polyethyleneimine (PEI, a synthetic cationic polymer discussed later) derivatives as delivery vectors and noticed that the transfection efficiency of naked mRNA was either better than, or comparable to, these polymers. Despite the promising effect of pulmonary naked RNA delivery, the exact mechanism of how naked RNA crosses the cell membrane barrier in the lung remains unclear, although it has been suggested that the pulmonary surfactant has a significant role in facilitating RNA uptake [25,26]. Some studies also showed that the use of delivery vectors could significantly improve RNA transfection compared with naked RNA in the airways [27., 28., 29., 30., 31.].
The development of safe and effective inhaled delivery systems in parallel is paramount currently. With the recent success of siRNA in the clinic and the intensive investigation of mRNA in clinical trials, including mRNA vaccines against coronavirus disease 2019 (COVID-19) [32,33], we believe that these two RNA candidates are likely to be the first to enter the clinic for treating lung diseases. Therefore, in this review, we focus on the pulmonary delivery of siRNA and mRNA. We discuss and highlight the recent advances of nonviral vector-based pulmonary RNA delivery systems development, gather what we have learnt from these studies, and identify the major gaps of knowledge. With these, we provide insights and direction to move the field forward.
RNA Delivery Vectors for Pulmonary Delivery
Many RNA delivery vectors have been developed for pulmonary delivery and their major functions are to facilitate the uptake of RNA by target cells and to protect RNA from premature degradation. Selected recent studies with different RNA delivery vectors that have demonstrated in vivo transfection in animal models are summarized in Table 1 . We highlight some important studies in each category and discuss them in more detail herein.
Table 1.
Selected Studies of Pulmonary siRNA and mRNA Delivery in Animalsa
| Delivery vector | RNA type | Disease | Animal model | Refs |
|---|---|---|---|---|
| Lipid based | ||||
| Lipid nanoparticles (LNPs): comprising ionizable cationic lipid, phosphatidylcholine, cholesterol, and PEG | mRNA | Healthy | BALB/c mice | [40] |
| Polymer based | ||||
| Chitosan-coated PLGA nanoparticles: enhancing mucus penetration through a chitosan coating | mRNA | CF | Cftr–/– mice | [45] |
| Chitosan-derivative: piperazine-substituted chitosans that are water soluble | siRNA | Healthy | Nude mice | [46] |
| siRNA/Polymer powder: inhalable powder of siRNA complexed with PEI or chitosan prepared by spray–freeze drying and supercritical fluid | siRNA | Lung cancer | C57BL/6, BALB/c mice | [47,48,51] |
| Tf-PEI: PEI functionalised with transferrin (Tf) for targeting activated T cells | siRNA | Asthma | BALB/c mice | [50] |
| Hyperbranched poly(beta amino esters) (hPBAEs): polyplexes of biodegradable polymer and mRNA for nebulization; first inhalable mRNA formulation in in vivo study | mRNA | Healthy | C57BL/6 mice | [52] |
| Functional polyesters: synthetic amine A13 modified polyester series is promising for siRNA delivery to lung cancer cells | siRNA | Lung cancer | Nude mice | [27,53] |
| Peptide based | ||||
| Disulfide-constrained cyclic amphipathic peptide: siRNA release and intracellular delivery facilitated by reduction of disulfide bond of peptide in cytosol | siRNA | Healthy | C57BL/6 mice | [28] |
| siRNA/HMG/OR micelle ternary complexes: comprising oligoarginine (OR) micelles and high-mobility group (HMG) for alveolar macrophages targeting | siRNA | Asthma | BALB/c mice | [30] |
| PEGylated KL4 peptide: inhalable powder formulation of PEGylated KL4/mRNA complexes prepared by spray–drying and spray–freeze drying; first inhalable powder formulation with in vivo bioactivity | mRNA | Healthy | BALB/c mice | [31] |
| Hybrid based | ||||
| Lipid–polymer hybrids | ||||
| Lipidoid–polymer hybrid nanoparticles (LPNs): PLGA nanoparticles coated with lipidoid to control siRNA release | siRNA | Healthy | BALB/c mice | [60] |
| Self-assembled micelle inhibitory RNA (SMAiRNA): comprising hydrophilic polymer/lipid bi-conjugated siRNA | siRNA | Lung fibrosis | C57BL/6J mice | [61] |
| Lipid–peptide hybrids | ||||
| Liposomes-targeting peptide-siRNA (LPR): DOTMA/DOPE liposomes with peptides targeting lung epithelial cells | siRNA | Healthy | C57BL/6 mice | [62,63] |
| Polymer–peptide hybrids | ||||
| Virus-inspired polymer for endosomal release (VIPER): methacrylate-based polymer conjugated with melittin peptide to enhance endosomal escape | siRNA | Healthy | BALB/c mice | [64] |
| Self-assembled peptide-poloxamine nanoparticles: poloxamine nanoparticles functionalized with multiple moieties | mRNA | CF | B6CF mice | [65] |
| Functionalized nanoparticles | ||||
| Gold nanoparticles: functionalized gold nanoparticles with targeting peptides | siRNA | Lung cancer | B6 albino, nude mice | [67,74] |
| Surfactant-coated nanogel system: enhances intracellular delivery by coating dextran nanogel with surfactant protein B | siRNA | Healthy, ALI | BALB/c mice | [29,69] |
| Exosomes | ||||
| Exosomes: serum-derived exosomes targeting lung macrophages | siRNA | ALI | C57BL/6J mice | [73] |
Abbreviations: ALI, acute lung injury; CFTR, CF transmembrane conductance regulator; DOTMA, N-[1-(2,3-dioleyloxy)propyl]-n,n,n-trimethylammonium chloride; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane chloride salt; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid).
Lipid-Based Delivery Systems
Due to their high transfection efficiency, ease of synthesis, and low batch variability, lipids were popular as transfection agents during the early years of gene therapy studies for various routes of administration [34,35]. The transfection efficiency and toxicity of lipid-based systems are affected by the composition of lipids and the ratio of lipids to RNA. Typically, cationic lipids, such as N-[1-(2,3-dioleyloxy)propyl]-n,n,n-trimethylammonium chloride (DOTMA) and 1,2-dioleoyl-3-trimethylammonium-propane chloride salt (DOTAP), are used to form lipoplexes with, or encapsulate, RNA [36]. The addition of neutral helper lipids, such as dioleoyl phosphatidylethanolamine (DOPE) and cholesterol, in the lipoplexes provide the ability to facilitate RNA complexation, increase stability of the lipoplexes, and reduce toxicity [37,38]. One major problem associated with lipid-based systems for pulmonary delivery is their poor structural stability because they readily fuse with pulmonary surfactants in the airways [39], leading to premature release of RNA before cellular uptake. Lipid nanoparticles (LNPs), which comprise cationic lipids, cholesterol, and polyethylene glycol (PEG), have been developed to improve the structural stability of lipid-based systems [35,40] (Table 1). With proper design and engineering, they can encapsulate RNA efficiently. LNPs are already in use in the clinic for parenteral injection of siRNA [41] and also are in an ongoing clinical trial to deliver mRNA (MRT5005) to the lung through nebulization for the treatment of CF, with encouraging early results (Box 1).
Polymer-Based Delivery System
There are two main categories of polymers: natural and synthetic polymers. Natural polymers have the advantages of excellent biocompatibility, biodegradability, and safety profiles [42]. Derived from the shells of crustaceans, the natural polymer chitosan is commonly investigated for pulmonary delivery due to its mucoadhesive and mucopermeable properties, enabling it to cross the mucus layer in the airways efficiently [43]. It can be used to form polyplexes with RNA or as a coating layer on the surface of nanoparticles [44,45] (Table 1). However, chitosan is limited by its poor solubility at physiological pH and relatively low transfection efficiency [42]. To overcome these problems, water-soluble chitosan derivatives, such as piperazine-substituted chitosan, were developed and found to be efficient for pulmonary siRNA delivery in healthy mice [46] (Table 1). Furthermore, inhalable chitosan/siRNA dry powder formulations were successfully prepared by supercritical drying [47] and spray–freeze drying [48] (Table 1). Both studies demonstrated a gene-silencing effect of the powder formulations in lung tissues following intratracheal administration in mouse models of lung cancer, taking these delivery systems one step closer to clinical application.
Among the synthetic polymers, PEI is extensively studied due to its high cationic charge density, good aqueous solubility, and wide pH-buffering capacity [49]. Its high versatility allows it to be functionalized to achieve specific targeting. For example, transferrin-PEI was used to target activated T cells in the lung, particularly T helper 2 cells, as potential therapy for asthma by reducing airway inflammation [50] (Table 1). Inhalable powder formulations have also been investigated for PEI delivery system. Okuda et al. used a spray–freeze drying technique to produce inhalable PEI/siRNA dry powder with good pulmonary gene-silencing activity in mice with lung metastasis [51] (Table 1). However, the relatively high toxicity of this nonbiodegradable polymer remains a considerable concern, even with a low-molecular-weight PEI, rendering it difficult to be translated for clinical application. Thus, biodegradable synthetic polymers were developed to address this issue. For example, in a recent study, hyperbranched poly(beta amino esters) (hPBAEs) [52] were used to deliver mRNA through nebulization, with promising gene expression observed in the lung epithelium of mice without local or systemic toxicity after repeated dosing (Table 1). To enhance RNA delivery efficiency for lung cancer therapy, Yan et al. used a combinatorial library of functional polyester and high-throughput screening to identify matching cancer cells for specific targeting [27,53] (Table 1). After screening a library of >500 polyester candidates, synthetic amine A13 modified polyester PE4K-A13 was found to be potent for siRNA delivery to mice with lung tumors [27] (Table 1).
Peptide-Based Delivery Systems
Cell-penetrating peptides (CPPs) have attracted increasing attention for RNA delivery due to their versatility and cell entry ability [54]. The design of peptide sequence can be inspired by natural peptides, proteins with known functions, or by computational simulation [55,56]. The sequence of amino acids determines the properties of peptides, such as structure, charges, solubility, and polarity, which further affect the interaction with RNA, cellular uptake, toxicity, and transfection efficiency. One of the drawbacks of CPPs is the lack of cell specificity, which can be addressed by introducing cell-targeting sequences [57]. Furthermore, natural L- amino acids are susceptible to protease degradation; although strategies such as replacing L- amino acids with D- analogs has been proposed, this approach may lower the efficiency of the peptides [58]. Although many CPPs appeared to be promising candidates for delivering RNA to the lung, only a few have shown in vivo transfection. Welch et al. developed disulfide-constrained cyclic amphipathic peptides that form complexes with siRNA with good transfection efficiency because the disulfide bond reduction in the cytosol facilitated the release of the cargo as well as proteolytic clearance. Efficient gene knockdown was also observed in lung tissues of healthy mice following pulmonary delivery [28] (Table 1). Choi et al. developed a peptide-based vector that comprises micelles of oligoarginine peptides as the siRNA carrier and high mobility group (HMG) peptide ligand that targets the activated alveolar macrophages, with promising biological effects in an asthma model [30] (Table 1). Recently, Qiu et al. developed KL4 peptide, a surfactant protein B mimic, for delivering both siRNA and mRNA to the lung with good transfection efficiency [25,31]. The PEGylated KL4 peptide was also used to formulate mRNA as inhalable dry powder by spray–drying and spray–freeze drying techniques, with efficient gene expression observed in the lung of healthy mice [31] (Table 1).
Hybrid Delivery Systems
To overcome the limitations of a single class of delivery vector, hybrid delivery systems, which are defined as the combination of two or more delivery vectors into a single entity, have been investigated and developed. This formulation strategy aims to increase the strengths of these delivery vectors while decreasing their disadvantages, with lower toxicity compared with their precursors [59].
Lipid–Polymer Hybrids
The combination of lipids with polymers intends to address the problems of poor structural stability associated with lipids and the low biocompatibility of polymers. Thanki et al. developed lipid–polymer nanoparticles (LPNs) comprising a poly(lactic-co-glycolic acid) (PLGA) matrix core coated with lipidoid, with siRNA localized in both the core and the shell [60]. This design compensated the low siRNA-loading capacity of PLGA by introducing cationic lipidoids while controlling the rate of siRNA release through degradation of the polymer. Enhanced lung retention upon pulmonary administration of the siRNA-loaded LPNs was observed in animal studies, suggesting their potential for controlled siRNA delivery in the lung (Table 1). Yoon et al. developed self-assembled micelle inhibitory RNA (SMAiRNA) nanoparticles that comprised bioconjugated siRNAs with a hydrophilic polymer on one end and a lipid on the other end [61] (Table 1). This hybrid system demonstrated effective gene silencing in animal models of pulmonary fibrosis with good stability and low toxicity.
Lipid–Peptide Hybrids
Another common hybrid strategy is to combine lipids with peptides, with the latter serve as a hydrophilic group and facilitate cellular uptake and transportation. For example, cationic liposomes comprising DOTMA/DOPE were blended with epithelial-targeting peptides to deliver siRNA to the lung of healthy mice, leading to successful silencing of the epithelial sodium channel at the airway epithelium [62,63] (Table 1). The results demonstrated the potential application of this hybrid system for CF therapy.
Polymer–Peptide Hybrids
Similar to lipid–peptide hybrids, the major aim of combining polymers with peptides is to enhance the cellular transport efficiency. Polymers are usually conjugated covalently with the peptides. Feldmann et al. developed a polymer system called VIPER (virus-mediated polymer for endosomal escape), which comprised a cationic methacrylated-based copolymer for siRNA binding and a membrane lytic peptide melittin, to facilitate endosomal escape [64] (Table 1). The hybrid system demonstrated more effective gene-silencing effects in the lung of healthy mice compared with the unmodified polymer system. Guan et al. developed a multimodular synthetic peptide with anchor, cationic, and targeting moieties that can form complexes with biocompatible poloxamine-based copolymers and mRNA via self-assembly [65] (Table 1). These ternary complexes showed excellent mRNA expression in the lungs of mice with CF with negligible toxicity, making them an attractive gene delivery system for CF and other lung diseases.
Functionalized Nanoparticles
In addition to the aforementioned conventional delivery vectors, inorganic metal-based nanoparticles have also been evaluated for the pulmonary delivery of RNA. However, these nanoparticles cannot act as transfection vectors by themselves due to their lack of RNA-binding ability. Therefore, they are usually functionalized on the surface to enhance the transfection efficiency. For example, gold nanoparticles are attractive delivery vectors because of their ease of synthesis and conjugation as well as their superior stability [66]. Gold nanoparticles were conjugated with siRNA through gold–thiol bonds and functionalized with M2pep, a peptide that selectively targets tumor associated macrophages [67] (Table 1). Specific gene-silencing effects were observed in targeted macrophages following intratracheal administration in a mouse lung tumor model. It has been suggested that pulmonary surfactant facilitates the delivery of polymer-based delivery systems in the lung [68]. To take advantage of this phenomenon, pulmonary surfactant and surfactant protein B-coated dextran-based nanoparticles were developed for siRNA delivery, with successful gene-silencing effects observed in healthy mice and in a model of acute lung injury (ALI), respectively, following pulmonary administration [29,69] (Table 1).
Exosomes
Exosomes are extracellular vesicles secreted in many cell types. They are involved in communication through the transfer of substances, such as lipids, proteins, and nucleic acids, between cells [70,71]. They have many desirable features for RNA delivery, including high biocompatibility with low inherent toxicity and immunogenicity [72]. Zhang et al. presented a new approach to delivering small RNA to the lung with the use of exosomes. siRNA was loaded into serum-derived exosomes via calcium-mediated transfection. Following intratracheal administration, the siRNA-loaded exosomes were successfully taken up by lung macrophages to achieve specific gene silencing in a mouse model of ALI [73] (Table 1).
Lessons Learnt from these Studies
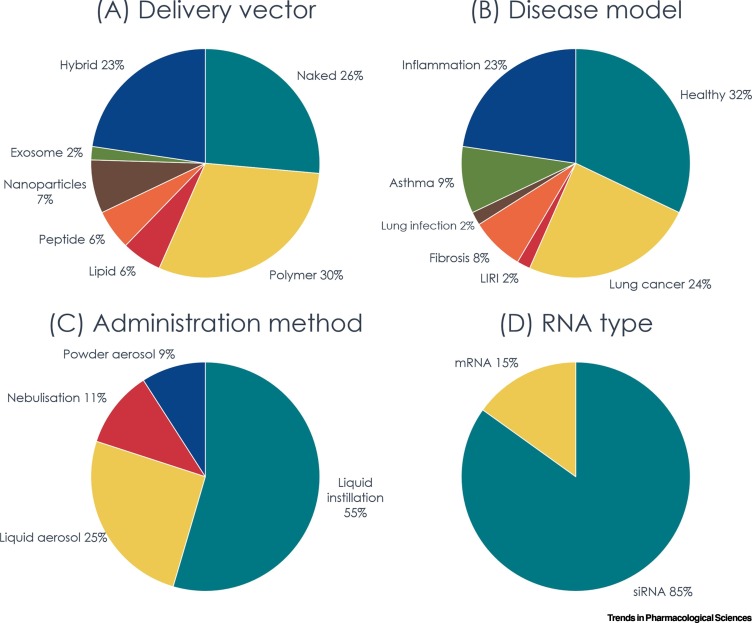
We carried out a survey of studies published between 2015 and early 2020 that reported siRNA or mRNA transfection following pulmonary delivery in animals (Figure 1 ). A PubMed search was performed using the search terms ‘siRNA’ or ‘mRNA’, ‘pulmonary delivery’, ‘intratracheal’, ‘inhalation’, ‘nebulization’, and with the filters ‘last 5 years’ (publication date) and ‘other animals’ (species) . In total, 53 articles were included in the survey [10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23.,27., 28., 29., 30., 31.,36,38,40,45., 46., 47., 48.,50., 51., 52., 53.,60., 61., 62., 63., 64., 65.,67,69,73., 74., 75., 76., 77., 78., 79., 80., 81., 82., 83., 84., 85., 86., 87.]. Each article was categorized manually according to the type of RNA delivery vector used or naked RNA; the animal model (disease or healthy); the method of administration to animal; and the type of RNA (siRNA or mRNA). While polymer was the most commonly used RNA delivery vector due to its high versatility and ease of preparation, the hybrid delivery system is gaining popularity, many of which include a targeting peptide to improve specificity. With the successful transfection of naked RNA in the lung and the technological advances in chemical modifications that greatly enhance the stability and reduce the immune activation of RNA [88,89], many researchers opt for the use of naked RNA to transfect lung tissues due to its simplicity (Figure 1A). This approach can also eliminate the risk of toxicity and immunogenicity associated with the delivery vectors. It is particularly popular when researchers are interested in examining the biological function of a particular protein rather than its therapeutic potential, such as the role of Yes-associated protein (YAP), ribosomal protein S3 (RPS3), and PI3K/SGK1 pathway in ALI [13,14,18].
Figure 1.
Survey of Recently Published Studies on Pulmonary Small Interfering RNA (siRNA) or mRNA Delivery in Animals.
Studies published between 2015 and early 2020 that reported siRNA or mRNA transfection following pulmonary delivery in animals were surveyed using an article search on PubMed with the search terms ‘siRNA’ or ‘mRNA’, ‘pulmonary delivery’, ‘intratracheal’, ‘inhalation’, ‘nebulization’, and with the filters ‘last 5 years’ (publication date) and ‘other animals’ (species) applied (N = 53). The studies are classified by (A) type of delivery vector; (B) disease model used; (C) administration method used in animal studies; and (D) RNA type. The classification was done independently by Y.Q. and M.C. and was cross-validated by J.K.W.L. Abbreviation: LIRI, lung ischemia–reperfusion injury.
Nevertheless, the successful use of naked RNA should not lead us to discard the use of delivery vectors for clinical applications. The transfection efficiency of naked RNA may not be robust enough for therapeutic use. Also, as mentioned earlier, the cellular uptake mechanism of naked RNA in the airways is still unclear. It has been speculated that lung lining fluid and pulmonary surfactants have a critical role in facilitating the transportation of RNA into cells [68]. The composition of lining fluids could vary substantially among patients and pathological conditions. Indeed, it has already been observed that pulmonary surfactant protein and lipid composition change significantly as a result of aging [90]. Lung diseases, such as CF, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease (COPD), are also associated with surfactant deficiency and altered lipid compositions [91., 92., 93.]. Consequently, the transfection efficiency of naked RNA could be easily influenced by the age and disease status of the patients, leading to problems of reproducibility. Moreover, naked RNA lacks active targeting ability. Given that the lung tissues contain a variety of cell types, it would be desirable to use a delivery vector with a targeting moiety to enhance specificity while reducing transfection in unintended cell types.
The potential of RNA therapeutics to treat a range of lung diseases prompts us to examine the possibility of developing a universal delivery platform that can be adopted by different RNA therapeutics for different conditions. While such a system would accelerate the clinical translation of inhaled RNA therapy, delivery barriers could be unique to a specific physiological condition. In that case, it would be more effective to have a carefully designed system to overcome these specific sets of barriers. For example, patients with CF have thick and excessive mucus in the airway; hence, a delivery vector with excellent mucus penetration ability would be favorable. Moreover, in preclinical studies, healthy animals are sometimes used to evaluate the efficiency of a delivery vector, with the use of reporter or housekeeping genes as the RNA target (Figure 1B). This approach may run into a similar problem in that a delivery vector may perform differently in a healthy animal versus a disease model due to the different delivery barriers in the airways. Furthermore, a non-disease RNA target (which is generally used to evaluate the efficiency of a delivery vector) does not provide any information regarding the pharmacodynamics properties of the proposed therapy. Even if a disease model is used, there is still the challenge of the clinical translation because many lung disease models have limitations that prevent their direct translation to human disease [94., 95., 96., 97.]. For instance, asthma is a complex condition that is observed exclusively in humans. The most common model used in preclinical studies is murine allergic airway inflammation. However, the distribution of lung inflammation in mice is different from human asthma, and the animals develop tolerance after repeated allergen exposure [97]. Therefore, a collaborative approach between formulation scientists, pharmacologists and clinicians will facilitate the development of a clinically relevant delivery system for inhaled RNA therapy.
Crucial Steps Involved in the Development of Inhaled RNA Delivery Systems
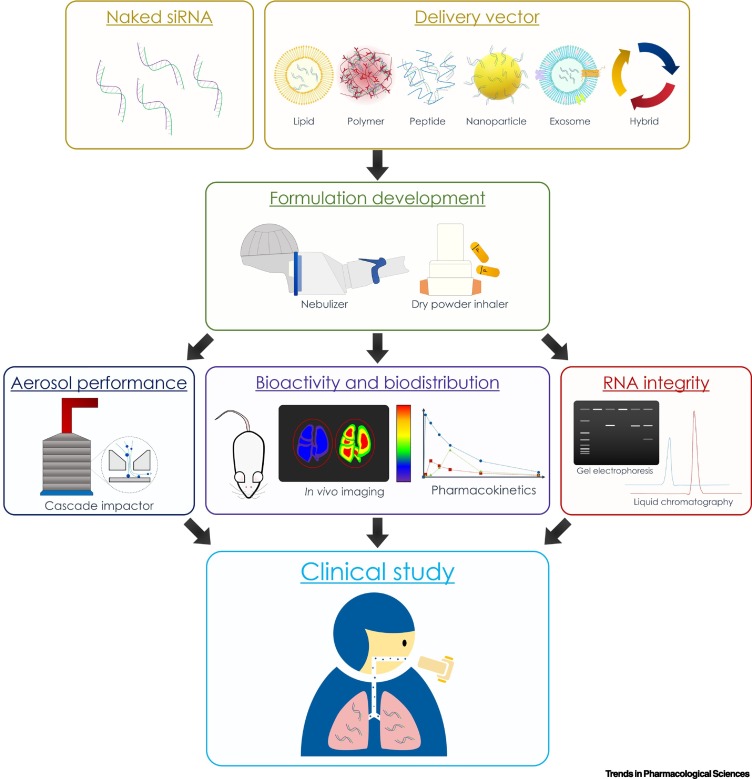
Many studies have focused on the development of RNA delivery vectors, but the translation of these vectors into suitable dosage forms for clinical application is currently lacking. Here, we summarize the critical steps for the development of successful pulmonary RNA delivery system (Figure 2 , Key Figure) and discuss the areas/factors that should be focused on.
Figure 2.
Key Figure. Summary of Crucial Steps Involved in the Development of Inhaled RNA Delivery Systems.
Firstly, either naked RNA is used or a delivery vector is designed to facilitate the uptake of RNA therapeutics to the targeted cells. A formulation (liquid or powder aerosol) is then developed with the identification of a suitable inhalation device for clinical use. The formulation is thoroughly characterized and evaluated for its aerosol performance, RNA integrity after aerosolization, and the biological activity and pharmacokinetic profile of the inhaled formulation in suitable animal models. Lastly, the potential candidates are identified for clinical study.
In general, it is desirable to use delivery vectors that exhibit cell-targeting properties with the ability to overcome the specific barriers associated with the disease concerned. Delivery vectors that are nonbiodegradable or with a low RNA-loading capability would be less attractive. A thorough understanding of the excretion pathway is required to ensure that the delivery vectors would not accumulate in the body, especially when synthetic or nonbiodegradable materials are used. Vectors with high loading capacity could avoid the use of excessive excipients, thereby minimizing the risk of toxicity. Moreover, to evaluate the in vivo efficacy of the delivery system, RNA is often administered intratracheally to the lung of animals either as large droplets instilled by pipette or as a fine spray aerosolized by a microsprayer or similar device (Figure 1C). Clearly, these administration methods are impractical for human use. For clinical practice, RNA drugs can be delivered either as liquid aerosols through nebulization or as dry powder for inhalation (Figure 2). While the former can deliver high doses of liquid formulations over a period of time (typically ~10–20 min, depending on the dose), the latter is more portable and convenient to use. However, there are stringent requirements for particles to be suitable for inhalation in clinical settings and animal studies cannot reflect the ‘inhalability’ of a formulation. Surprisingly, only a few studies have evaluated the aerosol performance of RNA formulations designed for inhalation [31,48,51] (Box 2 ). A good understanding of the aerosol properties of the formulation could boost the chance of successful clinical translation (Figure 2). Moreover, nebulizers or dry powder inhalers are needed to generate aerosol for inhalation. Given that both devices could damage the fragile RNA molecules (especially single-stranded mRNA) due to the high shear stress during aerosolization, it is essential to examine the RNA integrity, in terms of physical structure and biological activity, in the aerosolized particles (Figure 2).
Box 2. Evaluation of Aerosol Performance of Inhaled Formulations.
Aerodynamic Diameter
The most important factor that affects the aerosol performance of an inhaled formulation is particle size, which is most suitably expressed in aerodynamic diameter. Aerodynamic diameter is defined as the diameter of a sphere of density 1 g/cm3 that has the same settling velocity in still air as the particle of interest. It is generally accepted that particles with aerodynamic diameter between 1 and 5 μm are optimal for lung deposition [108]. Larger particles tend to deposit at the back of the throat and get swallowed subsequently, whereas smaller particles are likely to be exhaled. This criterion applies to both liquid and powder aerosol [109]. The aerodynamic particle size distribution of an aerosol is often described in terms of mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) [109].
Cascade Impactor
The method of choice for measuring particle size distribution of inhaled products is the cascade impactor (CI), which operates on the principle of inertia impaction [110]. CI comprises multiple stages and separates particles according to their aerodynamic diameters. Large particles with high inertia are unable to follow the airstream and impact on earlier stages, whereas small particles remain in the airstream and flow to the next stage, where the process is repeated. The two parameters commonly reported from CI are emitted dose and fine particle dose. The former refers to the total dose that has exited the dispersion device, while the latter represents the amount of aerosol with an aerodynamic diameter below a certain threshold (typically 5 μm) [111]. These can also be expressed in fractions relative to the loaded dose or recovered dose.
An important feature with the use of CI for measuring particle size is that a dispersion device (a nebulizer for liquid dosage forms or a dry powder inhaler for solid dosage forms) needs to be connected to generate the aerosol [112]. Given that the choice of device can have a dramatic impact on the aerosol properties of formulations, it is crucial to identify a suitable device to maximize the aerosol performance of a given formulation. There are different designs of CI, but only three are currently listed in both European Pharmacopoeia and United States Pharmacopoeia: the Andersen Cascade Impactor (ACI), the Next Generation Impactor (NGI), and the Multi-Stage Liquid Impinger (MSLI) [112].
Alt-text: Box 2
It is unclear whether the RNA dose is optimized in in vivo preclinical studies because the dose–response relationship of RNA following pulmonary delivery is often not reported. Dose optimization is critical for clinical translation not only for maximizing the therapeutic efficacy, but also for the practicality of administration. The amount of excipient (which may be included in the formulation to improve stability or to enhance aerosol performance) needs to be considered carefully because this can also affect the final liquid volume or powder mass to be administered. When RNA is delivered through nebulization, a high dose would increase the volume and, hence, the administration time, leading to the increased risk of RNA degradation due to prolonged exertion of shear stress on the RNA molecules. For dry powder formulations, there is a constraint on the amount of powder that could be inhaled by a patient each time. Currently, TOBI® Podhaler® (tobramycin inhalation powder) approved for treating Pseudomonas aeruginosa infection in patients with CF, has the highest inhaled dose of ~30 mg per actuation [98]. The RNA dose has to be taken into consideration during the development of an effective delivery system that is fit for purpose.
Another area that requires attention during development of a pulmonary RNA delivery system is understanding the biodistribution and pharmacokinetic profile of the inhaled RNA formulation (Figure 2). Many studies focus on the evaluation of gene expression in the lung tissues as a whole. While naked RNA is shown to manipulate gene expression primarily in the lung epithelial cells and macrophages [21], the sites where nanoparticulate RNA delivery vectors exert their biological activity are less clear. For delivery vectors that rely on nonspecific cellular uptake mechanisms, RNA could be effectively taken up by various cell types and absorbed into the systemic circulation. Therefore, it is critical that biodistribution as well as pharmacokinetic profiles are thoroughly investigated. Furthermore, the long-term toxicity of the delivery system also needs to be carefully evaluated.
Concluding Remarks and Future Perspectives
RNA therapeutics have great potential in lung diseases. Here, we have discussed nonviral delivery systems developed for siRNA and mRNA therapeutics in the lung and briefly summarized the current preclinical and clinical state of the field. Currently, it appears that the development of mRNA therapeutics is lagging behind that of siRNA therapeutics (Figure 1D), possibly because of the relatively poor stability of the long single-stranded RNA molecule. With technological advances in RNA modification that improve the stability, specificity, and safety of RNA therapeutics [88,89] and the recent success of siRNA therapeutics, we believe that both siRNA and mRNA will enter the clinic for the treatment of respiratory diseases in the near future. However, some crucial questions remain to be addressed before a successful RNA inhalation delivery system can be realized (see Outstanding Questions).
In light of the current COVID-19 pandemic, development of an inhaled version of mRNA vaccine is an area that deserves more attention. Currently, there are several clinical studies (Clinical Trial Noi.- NCT03164772; NCT03908671; NCT02662634; NCT03076385; and NCT03345043) that demonstrate the safety and efficacy of mRNA vaccines for the treatment of and protection against lung cancer and influenza, respectively [99,100]. mRNA vaccines for COVID-19 are being explored in several different clinical trials (Clinical Trial No.: NCT04283461; NCT04470427; NCT04405076; NCT04449276; NCT04480957; NCT04380701; and NCT04368728). These mRNA vaccine candidates are designed to be administered through parenteral injection. If any of these are successful, we believe that an inhaled version of the successful mRNA vaccine will be an area to explore because it will provide a non-invasive route of administration with the possibility of self-administration, especially dry powder formulations, which show superior stability. The challenges of manufacture and scale-up, including the production of RNA, delivery vectors, and the loading of RNA into the vectors without losing their physicochemical properties and biological activities, also need to be overcome. Overall, a safe and efficient RNA delivery system to the lung remains the key to successful clinical translation.
Outstanding Questions.
What is the exact cellular uptake mechanism of naked RNA in the airways? What are the factors governing its uptake?
What are the delivery barriers to different RNA delivery systems in different respiratory diseases?
Is it possible to have a universal pulmonary delivery platform that can be used to deliver different types of RNA therapeutics?
How can we protect the integrity of RNA molecules against shear and thermal stresses effectively during the aerosolization and drying process?
How do we improve the translation of inhaled RNA therapy from animal studies to clinical applications?
Alt-text: Outstanding Questions
Acknowledgments
J.K.W.L. and Y.Q. are supported by the Research Grant Council, Hong Kong (GRF17301918, GRF17300319) and Seed Funding for Strategic Interdisciplinary Research Scheme, The University of Hong Kong.
Glossary
- Aerosol
suspension of solid particles or liquid droplets in gas. In humans, drug delivery via the pulmonary route has to be administered in the form of an aerosol.
- Antisense oligonucleotide (ASO)
short single-stranded DNA or RNA (~20 nucleotides in length) that binds to a target mRNA through complementary base pairing, activating RNase H that leads to degradation of mRNA, thereby preventing the translation of mRNA into protein.
- Cell-penetrating peptide (CPP)
short cationic or amphipathic, natural or synthetic peptide (usually <30 amino acids) that is developed to deliver large cargoes, such as proteins, peptides, and nucleic acids, into cells by promoting cellular uptake.
- Chemical modification
strategy to improve the stability and/or reduce immunogenicity of RNA by modifying the structure of RNA while maintaining the biological activity of the molecules (e.g., methylation of the ribose 2'-OH group; alteration of the bases; modification of phosphodiester backbone, etc.).
- Intratracheal administration
introduction of substances directly into the trachea, either through the oral cavity via intubation or through a surgical procedure that creates an incision in the trachea (tracheotomy).
- MicroRNA (miRNA)
a short RNA (~ 21–25 nucleotides in length) that is partially complementary to multiple messenger RNA (mRNA), preventing the translation of mRNA into protein through the RNA interference mechanism.
- mRNA vaccine
mRNA that encodes the target antigen to elicit immune responses in the body.
- Naked RNA
RNA that is not associated with any delivery vectors or transfection agents, such as polymers and lipids.
- Nebulization
conversion of liquid medications into a spray of fine droplets that can be breathed in by the patient.
- Nonviral vector
agent or vehicle that transports nucleic acids into the cells without involving the use of viruses.
- Parenteral
nonoral route of drug administration; usually refers to injection or infusion of drug directly into the body, bypassing the skin and mucous membranes.
- RNA aptamer
singled-stranded RNA oligonucleotide that serves as ligand and binds to specific targets (e.g., proteins and nucleotides) with high affinity and specificity.
- Small interfering RNA (siRNA)
short double-stranded RNA (~21–23 nucleotides in length with two 3'-overhang nucleotides) in which the antisense strand binds to the target mRNA through complementary base pairing, preventing the translation of mRNA into protein via RNAi.
- Transfection
a process of introducing nucleic acids into cells artificially.
Resources
iwww.clinicaltrials.goviihttps://investors.translate.bio/news-releases/news-release-details/translate-bio-announces-interim-results-phase-12-clinical-trialiiihttps://investors.translate.bio/news-releases/news-release-details/translate-bio-receives-fda-fast-track-designation-mrt5005ivhttps://investors.translate.bio/news-releases/news-release-details/translate-bio-provides-mrt5005-program-updatesReferences
- 1.DeWeerdt S. RNA therapies explained. Nature. 2019;574:S2–S3. [Google Scholar]
- 2.Ledford H. Gene-silencing technology gets first drug approval after 20-year wait. Nature. 2018;560:291–293. doi: 10.1038/d41586-018-05867-7. [DOI] [PubMed] [Google Scholar]
- 3.Scott L.J. Givosiran: first approval. Drugs. 2020;80:335–339. doi: 10.1007/s40265-020-01269-0. [DOI] [PubMed] [Google Scholar]
- 4.Keil T.W.M., et al. T-cell targeted pulmonary siRNA delivery for the treatment of asthma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christopher Boyd A., et al. New approaches to genetic therapies for cystic fibrosis. J. Cyst. Fibros. 2020;19:S54–S59. doi: 10.1016/j.jcf.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Naghizadeh S., et al. Overcoming multiple drug resistance in lung cancer using siRNA targeted therapy. Gene. 2019;714 doi: 10.1016/j.gene.2019.143972. [DOI] [PubMed] [Google Scholar]
- 7.Asha K., et al. Advancements in nucleic acid based therapeutics against respiratory viral infections. J. Clin. Med. 2018;8:6. doi: 10.3390/jcm8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb J., et al. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J. Heart Lung Transplant. 2016;35:213–221. doi: 10.1016/j.healun.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Zamora M.R., et al. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- 10.Del Sorbo L., et al. Intratracheal administration of small interfering RNA targeting Fas reduces lung ischemia-reperfusion injury. Crit. Care Med. 2016;44:e604–e613. doi: 10.1097/CCM.0000000000001601. [DOI] [PubMed] [Google Scholar]
- 11.Zeyer F., et al. mRNA-mediated gene supplementation of toll-like receptors as treatment strategy for asthma in vivo. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0154001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J., et al. Ribosomal protein S3 gene silencing protects against experimental allergic asthma. Br. J. Pharmacol. 2017;174:540–552. doi: 10.1111/bph.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng W., et al. Insulin ameliorates pulmonary edema through the upregulation of epithelial sodium channel via the PI3K/SGK1 pathway in mice with lipopolysaccharide induced lung injury. Mol. Med. Rep. 2019;19:1665–1677. doi: 10.3892/mmr.2019.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi L., et al. Yes-associated protein (YAP) signaling regulates lipopolysaccharide-induced tissue factor expression in human endothelial cells. Surgery. 2016;159:1436–1448. doi: 10.1016/j.surg.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Habibovic A., et al. DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N., et al. Small interfering RNA targeting NF-kappaB attenuates lipopolysaccharide-induced acute lung injury in rats. BMC Physiol. 2016;16 doi: 10.1186/s12899-016-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geyer A., et al. Fluorescence- and computed tomography for assessing the biodistribution of siRNA after intratracheal application in mice. Int. J. Pharm. 2017;525:359–366. doi: 10.1016/j.ijpharm.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Dong J., et al. Ribosomal protein S3 gene silencing protects against cigarette smoke-induced acute lung injury. Mol. Ther. Nucleic Acids. 2018;12:370–380. doi: 10.1016/j.omtn.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari P.M., et al. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-06508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng T., et al. Herpes virus entry mediator (HVEM) expression promotes inflammation/organ injury in response to experimental indirect-acute lung injury. Shock. 2019;51:487–494. doi: 10.1097/SHK.0000000000001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng B., et al. Intratracheal administration of siRNA triggers mRNA silencing in the lung to modulate T cell immune response and lung inflammation. Mol. Ther. Nucleic Acids. 2019;16:194–205. doi: 10.1016/j.omtn.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T., et al. Establishment of an evaluation method for gene silencing by serial pulmonary administration of siRNA and pDNA powders: naked siRNA inhalation powder suppresses luciferase gene expression in the lung. J. Pharm. Sci. 2019;108:2661–2667. doi: 10.1016/j.xphs.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Xu S., et al. Blockade of endothelial, but not epithelial, cell expression of Pd-L1 following severe shock attenuates the development of indirect acute lung injury in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L801–L812. doi: 10.1152/ajplung.00108.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kormann M.S., et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y., et al. From pulmonary surfactant, synthetic KL4 peptide as effective siRNA delivery vector for pulmonary delivery. Mol. Pharm. 2017;14:4606–4617. doi: 10.1021/acs.molpharmaceut.7b00725. [DOI] [PubMed] [Google Scholar]
- 26.Guagliardo R., et al. Nanocarrier lipid composition modulates the impact of pulmonary surfactant protein B (SP-B) on cellular delivery of siRNA. Pharmaceutics. 2019;11:431. doi: 10.3390/pharmaceutics11090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan Y., et al. Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors. Biomaterials. 2017;118:84–93. doi: 10.1016/j.biomaterials.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch J.J., et al. Functional delivery of siRNA by disulfide-constrained cyclic amphipathic peptides. ACS Med. Chem. Lett. 2016;7:584–589. doi: 10.1021/acsmedchemlett.6b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Backer L., et al. Hybrid pulmonary surfactant-coated nanogels mediate efficient in vivo delivery of siRNA to murine alveolar macrophages. J. Control. Release. 2015;217:53–63. doi: 10.1016/j.jconrel.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Choi M., et al. Targeted delivery of Chil3/Chil4 siRNA to alveolar macrophages using ternary complexes composed of HMG and oligoarginine micelles. Nanoscale. 2020;12:933–943. doi: 10.1039/c9nr06382j. [DOI] [PubMed] [Google Scholar]
- 31.Qiu Y., et al. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Linares-Fernandez S., et al. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Jackson L.A., et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. Published online July 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foldvari M., et al. Non-viral gene therapy: gains and challenges of non-invasive administration methods. J. Control. Release. 2016;240:165–190. doi: 10.1016/j.jconrel.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garbuzenko O.B., et al. Combinatorial treatment of idiopathic pulmonary fibrosis using nanoparticles with prostaglandin E and siRNA(s) Nanomedicine. 2017;13:1983–1992. doi: 10.1016/j.nano.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caillaud M., et al. Small interfering RNA from the lab discovery to patients' recovery. J. Control. Release. 2020;321:616–628. doi: 10.1016/j.jconrel.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Kanehira Y., et al. Intratumoral delivery and therapeutic efficacy of nanoparticle-encapsulated anti-tumor siRNA following intrapulmonary administration for potential treatment of lung cancer. Pharm. Dev. Technol. 2019;24:1095–1103. doi: 10.1080/10837450.2019.1633345. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Mouton C., et al. The lord of the lungs: the essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 2019;144:230–243. doi: 10.1016/j.ejpb.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Pardi N., et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulkarni J.A., et al. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28:146–157. doi: 10.1089/nat.2018.0721. [DOI] [PubMed] [Google Scholar]
- 42.Serrano-Sevilla I., et al. Natural polysaccharides for siRNA delivery: nanocarriers based on chitosan, hyaluronic acid, and their derivatives. Molecules. 2019;24:2570. doi: 10.3390/molecules24142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., et al. Chitosan-based nanomaterials for drug delivery. Molecules. 2018;23:2661. doi: 10.3390/molecules23102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragelle H., et al. Chitosan-based siRNA delivery systems. J. Control. Release. 2013;172:207–218. doi: 10.1016/j.jconrel.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Haque A., et al. Chemically modified hCFTR mRNAs recuperate lung function in a mouse model of cystic fibrosis. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-34960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capel V., et al. Water-soluble substituted chitosan derivatives as technology platform for inhalation delivery of siRNA. Drug Deliv. 2018;25:644–653. doi: 10.1080/10717544.2018.1440668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihara D., et al. Histological quantification of gene silencing by intratracheal administration of dry powdered small-interfering RNA/chitosan complexes in the murine lung. Pharm. Res. 2015;32:3877–3885. doi: 10.1007/s11095-015-1747-6. [DOI] [PubMed] [Google Scholar]
- 48.Miwata K., et al. Intratracheal administration of siRNA dry powder targeting vascular endothelial growth factor inhibits lung tumor growth in mice. Mol. Ther. Nucleic Acids. 2018;12:698–706. doi: 10.1016/j.omtn.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey A.P., Sawant K.K. Polyethylenimine: a versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;68:904–918. doi: 10.1016/j.msec.2016.07.066. [DOI] [PubMed] [Google Scholar]
- 50.Xie Y., et al. Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. J. Control. Release. 2016;229:120–129. doi: 10.1016/j.jconrel.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okuda T., et al. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J. Control. Release. 2018;279:99–113. doi: 10.1016/j.jconrel.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Patel A.K., et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31 doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan Y., et al. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E5702–E5710. doi: 10.1073/pnas.1606886113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh T., et al. Versatility of cell-penetrating peptides for intracellular delivery of siRNA. Drug Deliv. 2018;25:1996–2006. doi: 10.1080/10717544.2018.1543366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kardani K., et al. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert Opin. Drug Deliv. 2019;16:1227–1258. doi: 10.1080/17425247.2019.1676720. [DOI] [PubMed] [Google Scholar]
- 56.Habault J., Poyet J.L. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules. 2019;24:927. doi: 10.3390/molecules24050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalmouni M., et al. Cancer targeting peptides. Cell. Mol. Life Sci. 2019;76:2171–2183. doi: 10.1007/s00018-019-03061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalafatovic D., Giralt E. Cell-penetrating peptides: design strategies beyond primary structure and amphipathicity. Molecules. 2017;22:1929. doi: 10.3390/molecules22111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gigante A., et al. Non-viral transfection vectors: are hybrid materials the way forward? Medchemcomm. 2019;10:1692–1718. doi: 10.1039/c9md00275h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thanki K., et al. Mechanistic profiling of the release kinetics of siRNA from lipidoid-polymer hybrid nanoparticles in vitro and in vivo after pulmonary administration. J. Control. Release. 2019;310:82–93. doi: 10.1016/j.jconrel.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Yoon P.O., et al. Self-assembled micelle interfering RNA for effective and safe targeting of dysregulated genes in pulmonary fibrosis. J. Biol. Chem. 2016;291:6433–6446. doi: 10.1074/jbc.M115.693671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manunta M.D.I., et al. Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex: a therapeutic strategy for cystic fibrosis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tagalakis A.D., et al. Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung. Thorax. 2018;73:847–856. doi: 10.1136/thoraxjnl-2017-210670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feldmann D.P., et al. In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. J. Control. Release. 2018;276:50–58. doi: 10.1016/j.jconrel.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guan S., et al. Self-assembled peptide-poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 2019;14:287–297. doi: 10.1038/s41565-018-0358-x. [DOI] [PubMed] [Google Scholar]
- 66.Lytton-Jean A.K., et al. Five years of siRNA delivery: spotlight on gold nanoparticles. Small. 2011;7:1932–1937. doi: 10.1002/smll.201100761. [DOI] [PubMed] [Google Scholar]
- 67.Conde J., et al. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells. Adv. Funct. Mater. 2015;25:4183–4194. doi: 10.1002/adfm.201501283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hidalgo A., et al. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015;95:117–127. doi: 10.1016/j.ejpb.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Merckx P., et al. Surfactant protein B (SP-B) enhances the cellular siRNA delivery of proteolipid coated nanogels for inhalation therapy. Acta Biomater. 2018;78:236–246. doi: 10.1016/j.actbio.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 70.Guiot J., et al. Exosomal miRNAs in lung diseases: from biologic function to therapeutic targets. J. Clin. Med. 2019;8:1345. doi: 10.3390/jcm8091345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crenshaw B.J., et al. In: Nanomedicines. Farrukh M.A., editor. IntechOpen; 2018. Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. [DOI] [Google Scholar]
- 72.Ha D., et al. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang D., et al. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol. Ther. 2018;26:2119–2130. doi: 10.1016/j.ymthe.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conde J., et al. RNAi-based glyconanoparticles trigger apoptotic pathways for in vitro and in vivo enhanced cancer-cell killing. Nanoscale. 2015;7:9083–9091. doi: 10.1039/c4nr05742b. [DOI] [PubMed] [Google Scholar]
- 75.Xu C., et al. Pulmonary codelivery of doxorubicin and siRNA by pH-sensitive nanoparticles for therapy of metastatic lung cancer. Small. 2015;11:4321–4333. doi: 10.1002/smll.201501034. [DOI] [PubMed] [Google Scholar]
- 76.Jeong E.J., et al. The spacer arm length in cell-penetrating peptides influences chitosan/siRNA nanoparticle delivery for pulmonary inflammation treatment. Nanoscale. 2015;7:20095–20104. doi: 10.1039/c5nr06903c. [DOI] [PubMed] [Google Scholar]
- 77.Lipka J., et al. Biokinetic studies of non-complexed siRNA versus nano-sized PEI F25-LMW/siRNA polyplexes following intratracheal instillation into mice. Int. J. Pharm. 2016;500:227–235. doi: 10.1016/j.ijpharm.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 78.Wu Y.F., et al. Multi–functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016;6 doi: 10.1038/srep21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jarzebinska A., et al. A single methylene group in oligoalkylamine-based cationic polymers and lipids promotes enhanced mRNA delivery. Angew. Chem. Int. Ed. Eng. 2016;55:9591–9595. doi: 10.1002/anie.201603648. [DOI] [PubMed] [Google Scholar]
- 80.Xu C., et al. The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and Survivin siRNA. Biomater. Sci. 2016;4:1646–1654. doi: 10.1039/c6bm00601a. [DOI] [PubMed] [Google Scholar]
- 81.Ding L., et al. Pulmonary delivery of polyplexes for combined PAI-1 gene silencing and CXCR4 inhibition to treat lung fibrosis. Nanomedicine. 2018;14:1765–1776. doi: 10.1016/j.nano.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Okuda T., et al. Biodistribution/biostability assessment of siRNA after intravenous and intratracheal administration to mice, based on comprehensive analysis of in vivo/ex vivo/polyacrylamide gel electrophoresis fluorescence imaging. Int. J. Pharm. 2019;565:294–305. doi: 10.1016/j.ijpharm.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Li Z., et al. Increased survival by pulmonary treatment of established lung metastases with dual STAT3/CXCR4 inhibition by siRNA nanoemulsions. Mol. Ther. 2019;27:2100–2110. doi: 10.1016/j.ymthe.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., et al. Treatment of acute lung injury and early- and late-stage pulmonary fibrosis with combination emulsion siRNA polyplexes. J. Control. Release. 2019;314:12–24. doi: 10.1016/j.jconrel.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 85.Garbuzenko O.B., et al. Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 2019;9:8362–8376. doi: 10.7150/thno.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feldmann D.P., et al. The impact of microfluidic mixing of triblock micelleplexes on in vitro / in vivo gene silencing and intracellular trafficking. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa6d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi M., et al. A new combination therapy for asthma using dual-function dexamethasone-conjugated polyethylenimine and vitamin D binding protein siRNA. Gene Ther. 2017;24:727–734. doi: 10.1038/gt.2017.83. [DOI] [PubMed] [Google Scholar]
- 88.Ku S.H., et al. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 2016;104:16–28. doi: 10.1016/j.addr.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 89.Youn H., Chung J.K. Modified mRNA as an alternative to plasmid DNA (pDNA) for transcript replacement and vaccination therapy. Expert. Opin. Biol. Ther. 2015;15:1337–1348. doi: 10.1517/14712598.2015.1057563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moliva J.I., et al. Molecular composition of the alveolar lining fluid in the aging lung. Age (Dordr.) 2014;36 doi: 10.1007/s11357-014-9633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gunasekara L., et al. Pulmonary surfactant dysfunction in pediatric cystic fibrosis: mechanisms and reversal with a lipid-sequestering drug. J. Cyst. Fibros. 2017;16:565–572. doi: 10.1016/j.jcf.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 92.Gunther A., et al. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur. Respir. J. 1999;14:565–573. doi: 10.1034/j.1399-3003.1999.14c14.x. [DOI] [PubMed] [Google Scholar]
- 93.Agudelo C.W., et al. Decreased surfactant lipids correlate with lung function in chronic obstructive pulmonary disease (COPD) PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0228279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmes A.M., et al. Animal models of asthma: value, limitations and opportunities for alternative approaches. Drug Discov. Today. 2011;16:659–670. doi: 10.1016/j.drudis.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Singh A.P., et al. Mouse models in squamous cell lung cancer: impact for drug discovery. Expert Opin. Drug Discov. 2018;13:347–358. doi: 10.1080/17460441.2018.1437137. [DOI] [PubMed] [Google Scholar]
- 96.Tashiro J., et al. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front Med. (Lausanne) 2017;4:118. doi: 10.3389/fmed.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aun M.V., et al. Animal models of asthma: utility and limitations. J. Asthma Allergy. 2017;10:293–301. doi: 10.2147/JAA.S121092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vazquez-Espinosa E., et al. Tobramycin inhalation powder (TOBI Podhaler) for the treatment of lung infection in patients with cystic fibrosis. Expert Rev. Anti-Infect. Ther. 2016;14:9–17. doi: 10.1586/14787210.2016.1118344. [DOI] [PubMed] [Google Scholar]
- 99.Sebastian M., et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 2019;68:799–812. doi: 10.1007/s00262-019-02315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bahl K., et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xing Y., Proesmans M. New therapies for acute RSV infections: where are we? Eur. J. Pediatr. 2019;178:131–138. doi: 10.1007/s00431-018-03310-7. [DOI] [PubMed] [Google Scholar]
- 102.DeVincenzo J., et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antivir. Res. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 103.DeVincenzo J., et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watts J.K., Corey D.R. Clinical status of duplex RNA. Bioorg. Med. Chem. Lett. 2010;20:3203–3207. doi: 10.1016/j.bmcl.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao W., et al. Oligonucleotide therapy for obstructive and restrictive respiratory diseases. Molecules. 2017;22:139. doi: 10.3390/molecules22010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sermet-Gaudelus I., et al. Antisense oligonucleotide eluforsen improves CFTR function in F508del cystic fibrosis. J. Cyst. Fibros. 2019;18:536–542. doi: 10.1016/j.jcf.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drevinek P., et al. Antisense oligonucleotide eluforsen is safe and improves respiratory symptoms in F508DEL cystic fibrosis. J. Cyst. Fibros. 2020;19:99–107. doi: 10.1016/j.jcf.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Lam J.K., et al. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliv. Rev. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ali M. In: Handbook of Non-Invasive Drug Delivery Systems. Kulkarni V.S., editor. Elsevier; 2010. Pulmonary drug delivery; pp. 209–246. [Google Scholar]
- 111.Agu R.U., Ugwoke M.I. In vitro and in vivo testing methods for respiratory drug delivery. Expert Opin. Drug Deliv. 2011;8:57–69. doi: 10.1517/17425247.2011.543896. [DOI] [PubMed] [Google Scholar]
- 112.Roberts D.L., Mitchell J.P. Measurement of aerodynamic particle size distribution of orally inhaled products by cascade impactor: how to let the product specification drive the quality requirements of the cascade impactor. AAPS PharmSciTech. 2019;20 doi: 10.1208/s12249-018-1276-9. [DOI] [PubMed] [Google Scholar]