Abstract
Purpose
The prevalence of Mycobacterium tuberculosis (M. tb) and the status of M. bovis BCG vaccination may affect host immune responses to M. tb antigens. Understanding of the predominant local M. tb strain and immune signatures induced by its strain-specific antigens may contribute to an improved diagnosis of tuberculosis (TB). The aim of this study was to determine immune responses to M. tb antigen which was identified from the hyper-virulent Beijing/K strain in South Korea.
Materials and Methods
Pulmonary TB patients (n=52) and healthy subjects (n=92) including individuals with latent TB infection (n=31) were recruited, and QuantiFERON-TB Gold In-Tube tests were performed. The Beijing/K-antigen specific immune signatures were examined by diluted whole blood assays and multiplex bead arrays in a setting where nationwide BCG vaccination is employed.
Results
Statistical analyses demonstrated that three [C-X-C motif chemokine (CXCL10), interleukin (IL)-6, interferon (IFN)-α] of 17 cytokines/chemokines distinguished active cases from healthy controls following stimulation with the Beijing/K-specific antigen. IFN-α also differentiated between active diseases and latent TB infection (p<0.01), and the detection rate of TB was dramatically increased in combination with IL-6 and CXCL10 at the highest levels of specificity (95–100%).
Conclusion
Our data indicate that immune signatures to the M. tb Beijing/K-specific antigen can provide useful information for improved TB diagnostics. The antigen may be developed as a diagnostic marker or a vaccine candidate, particularly in regions where the M. tb Beijing/K strain is endemic.
Keywords: Mycobacterium tuberculosis, M. bovis BCG, Beijing/K, CXCL10, IL-6, IFN-α
INTRODUCTION
Tuberculosis (TB) infections with Mycobacterium tuberculosis (M. tb) remain a leading cause of ill health and death despite global efforts to reduce the burden of TB.1,2,3 The worldwide incidence of TB has continued to decline from 170 in 2000 to 130 in 2018 per 100000 people, and the absolute number of TB deaths has fallen by 27% among human immunodeficiency virus– negative individuals.4 However, successful treatment outcome rates for multidrug-resistant (MDR) TB has reached only 56% as of 2018. Therefore, early and accurate diagnosis, as well as prompt initiation of treatment, are priorities for controlling TB.4,5
The burden of new TB cases in Asia accounts for 62% of all TB cases worldwide,4 and three countries, including India (27%), China (9%), and Indonesia (8%), in this region contributed to almost half of the global total number of cases in 2018.4 The M. tb Beijing lineage has been reported throughout the region, with frequent genetic variations in the genome. It has been linked with an increase in MDR-TB with highly transmissible and virulent features.6,7 Mice infected with the Beijing M299 strain have been found to show irreversible necrotic lung lesions with enhanced inflammatory responses.8 Thus, understanding the characteristics of the Beijing stain is an essential requirement for improving the state of epidemiological control.
In South Korea, TB is still a major public health concern, with an incidence of 66 cases per 100000 populations, and MDR/rifampicin-resistant-TB in new cases was estimated to be 3.2%.9 The incidence rate of TB has been declining in past years, although transmission has been still ongoing within communities, such as schools or workplaces.10 About 80% of clinical isolates of pulmonary TB patients have been reported to carry Beijing genotype,11,12,13 and Beijing/K strains were firstly designated as the causative factor of a major cluster of high school TB outbreaks.14 Beijing/K strains are also frequently isolated from patients with drug resistance in South Korea.12 The Beijing/K strain is hyper-virulent and rapidly replicates with severe pathologies at early time points during infection, compared with M. tb H37Rv in mice.15 Whole-genome sequencing of the Beijing/K strain has revealed a 5.7-kb insertion region in the genome, which is disrupted by IS6110 insertion, unlike the H37Rv strain.16 Within the insertion, MTBK_24820 (GenBank accession no. AIB49026.1) was predicted as a member of the proline-proline-glutamic acid (PPE) family which is orthologous to the PPE39 protein of M. tb H37Rv.17 MTBK_24790 (GenBank accession no. AIB49023.1) and MTBK_24800 (Genbank accession no. AIB49024.1) were also identified as ESAT-6-like proteins.18,19,20 The three proteins are arranged in a row within the cluster, indicating that Esx family proteins containing the PPE family may play an immune-pathogenic role in hosts infected with the Beijing/K strain.
There is no gold standard for the diagnosis of latent tuberculosis infection (LTBI), although the following two methods are currently used in parallel: tuberculin skin test and interferon (IFN)-γ release assay (IGRA). Tuberculin skin test has been a recognized a conventional immunodiagnostic test for the last century, but its major drawback is a low specificity in BCG-vaccinated individuals and nontuberculous mycobacteria-sensitized persons, resulting in false-positive reactions.21 A recently developed IGRA, the QuantiFERON®-TB Gold In-Tube test (QFT-GIT; Qiagen, Hilden, Germany) offers superior sensitivity and specificity in active TB patients, compared with the tuberculin skin test.22,23 However, the QFT-GIT test cannot reflect the accurate status of disease progression with repeated conversions or reversions and has a limitation in that children under 4 years old yield indeterminate results.24,25,26 These shortcomings suggest an urgent requirement for improved methods using additional antigens or biomarkers in early diagnosis of M. tb infection for successful TB control.
In this study, we aimed to determine immune signatures that may serve as potential biomarkers for identifying M. tb infection, focusing on new antigen candidates for future diagnostics or a vaccine component. We previously demonstrated the protective efficacy of M. tb Beijing/K strain-derived PPE protein (MTBK_24820) in mice:17 mice infected with MTBK_24820 showed reduced bacterial loads and enhanced multifunctional T-cell activity, which indicate that the MTBK_24820 could potentially be used as a future vaccine component.17 Here, we evaluated 17 different cytokines and chemokines produced by type 1 or 2 T helper cells. The diagnostic potential of immune responses to MTBK_24820 was assessed in a population with BCG vaccination.
MATERIALS AND METHODS
Enrolment and characteristics of study participants
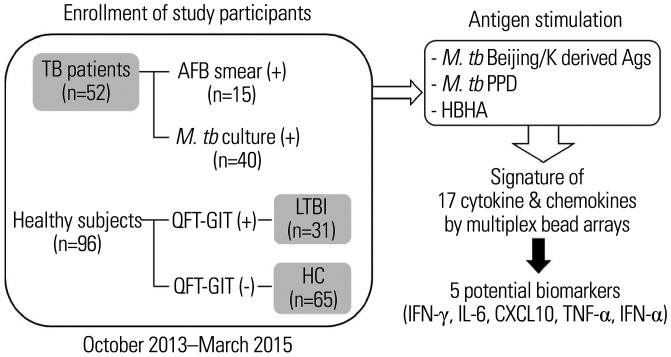
A total of 52 active TB patients and 96 healthy controls were enrolled between October 2013 and March 2015 at Ajou University Hospital (Fig. 1). The diagnosis of active pulmonary TB was made when M. tb was identified in the culture of a clinical specimen, or in the case of negative culture results, when suggestive clinical and radiologic features were reinforced by response to anti-TB therapy. About 29% (15 of 52) of the patients showed positive results of acid-fast bacilli (AFB) staining at early diagnosis, whereas M. tb was identified in about 77% (40 of 52) of patients by culture. Beijing or Beijing/K genotype was confirmed in 68% or 50% of the clinical isolates tested, from patients with AFB positive or culture positive results.19 Seven patients had extrapulmonary TB, such as pleural effusion, cervical lymphadenopathy, colitis, and meningitis. TB lymphadenitis was diagnosed by the presence of M. tb or by observing typical pathologic findings in a lymph node tissue specimen and demonstration of an appropriate response to anti-TB medication. TB pleural effusion was diagnosed by the presence of M. tb or by observation of typical pathologic findings in pleural tissues or compatible findings in cellular and biochemical analyses of pleural fluid, with an accompanying appropriate response to anti-TB medication. The diagnosis of meningeal TB was supported by cerebrospinal fluid biochemical findings. Chest computed tomography was performed in all TB patients and was utilized as an adjunctive tool for diagnosis of TB, especially in cases with negative culture results. The diagnosis of active TB was initially made by the physician in charge of the patients and was subsequently verified by the principal investigators. After completion of the enrollment, the patients were continuously followed up until August 2019 (at least 4.5 years), and finally, the diagnosis was independently reviewed and verified by one radiologist and two other respiratory medicine specialists, after which a final decision was made by consensus.
Fig. 1. Schematic diagram of the study design and subject enrolment. The study participants included 52 active TB patients, 31 latent TB infected subjects, and 65 non-infected healthy controls (gray boxes). Whole blood obtained from each subject was stimulated with M. tb Beijing/K-derived antigens, M. tb PPD, and HBHA, respectively. Potential biomarkers (i.e., IFN-γ, IL-6, CXCL10, TNF-α, and IFN-α) for TB were selected based on the immune signature through multiplex bead arrays. TB, tuberculosis; M. tb, Mycobacterium tuberculosis; QFT-GIT, QuantiFERON®-TB Gold In-Tube; LTBI, latent TB infection; HC, healthy control. AFB, acid-fast bacilli; PPD, purified protein derivatives; HBHA, heparin-binding haemagglutinin; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine; TNF, tumor necrosis factor.
BCG scars were confirmed in most patients (49 of 52). The mean age of the patients was 43 years, and age-matched healthy controls were recruited from volunteers via routine health examinations performed at the same hospital. The healthy control group was examined for M. tb infection, and individuals with LTBI (n=31) and naïve controls (n=65) were differentiated according to the outcome of QFT-GIT testing (Fig. 1). Individuals with immunosuppressive conditions who had cancer, diabetes, human immunodeficiency virus infection, or chronic renal failure were excluded. Written informed consent was obtained from all of the study participants, and all experiments were performed in accordance with relevant guidelines. The study protocol was approved by the Ajou University Hospital Ethics Review Committee (AJIRB-GEN-GEN-13-025, AJIRB-MED-SMP-12-068).
Preparation of blood samples
For QFT-GIT assay, 1 mL of blood was collected into each of three QFT-IT tubes (Qiagen, Hilden, Germany) precoated with saline (Nil) or an M. tb-specific peptide cocktail composed of ESAT-6, CFP-10, TB7.7 (Rv2654), and phytohemagglutinin (PHA; Mitogen). The tubes were incubated upright at 37℃ for 24 h, and harvested plasma samples were frozen at −80℃ until analysis. For diluted whole-blood assay, blood was collected in heparinized tubes (BD Vacutainer, Plymouth, UK) and was diluted with RPMI media (1 in 5; Invitrogen, Grand Island, NY, USA).
Preparation of antigens
Recombinant MTBK_24820 protein was prepared as previously described.17 MTBK_24820 was constructed using a pYUB1062 vector with NdeI and HindIII (New England Biolabs, Ipswich, MA, USA) sites. The constructed plasmid was overexpressed in Escherichia coli by the addition of 1 mM isopropyl-b-d-thiogalactopyranoside (BioWorld, Dublin, OH, USA). The protein was purified using Ni-NTA agarose resin (Qiagen, Venlo, Netherlands) and MonoQ anion exchange columns on an ÄKTA-FPLC system (GE Healthcare Biosciences, Pittsburgh, PA, USA) (Supplementary Fig. 1, only online). Protein concentrations were measured using a BCA assay (Thermo Fisher Scientific, Inc., Rockford, IL, USA) and stored at −80℃ until ready for use.
QFT-GIT testing
QFT-GIT assays were performed with harvested plasma according to the manufacturer's instructions (Qiagen). Briefly, 50 µL of conjugate and plasma, respectively, were added to a QFT-GIT enzyme-linked immunosorbent assay (ELISA) plate and incubated for 2 h at room temperature. After washing the plate six times, 100 µL of substrate solution was added and incubated. Absorbance was measured at 450 nm using a VersaMax ELISA reader (Molecular Devices, Sunnyvale, CA, USA), and the concentration of IFN-γ was calculated and interpreted by the analysis on the QuantiFERON®-TB Gold analysis software.
Diluted whole blood assays
Diluted whole blood assays were conducted as previously described using MTBK_24820 as the M. tb Beijing/K-specific antigen.27 The protein antigen was separately purified and mixed in a tube for whole blood assay experiments. PHA and M. tb purified protein derivatives (PPD) were used as positive controls for immunocompetence and reactivity to mycobacterial antigens, respectively. RPMI1640 medium was used as a negative control. Diluted blood was added into each well including antigens at 5 µg/mL. After 6 days of incubation at 37℃, the culture supernatant was harvested, and cytokine/chemokine responses were determined by multiplex bead array.
Multiplex bead arrays
Cytometric bead array was performed according to the manufacturer's instructions (BD Biosciences, San Jose, CA, USA) as described in a previous study.28 Harvested culture supernatant was analyzed with the following 17 different cytokines and chemokines: interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-22, IFN-α, IFN-γ, tumor necrosis factor (TNF)-α, IFN-γ-induced (IP)-10 (C-X-C motif chemokine, CXCL10), monocyte chemoattractant protein (MCP)-1 (chemokine ligand 2, CCL2), and sCD40L. Next, 25 µL specimens of samples and capture beads conjugated with specific antibodies were mixed and incubated with detection antibodies. The fluorescence of each complex was acquired using a FACS Verse flow cytometer (BD Biosciences) and analyzed using the FCAP Array™ software (BD Biosciences). The values of negative controls were subtracted from those induced by each antigen (Supplementary Fig. 2, only online).
Statistical analysis
Data were analyzed using Prism 6 (GraphPad Software, La Jolla, CA, USA). Differences among the study groups of cytokine/chemokine production were compared using the Mann-Whitney U test or Kruskal-Wallis one-way analysis of variance test with Dunn's multiple comparison. Values of *p<0.05, **p<0.01, ***p<0.001 were considered to be significant. The diagnostic accuracies of antigen-specific cytokine/chemokine features were assessed by the analysis of the area under the receiver operating characteristic curves (AUCs). Cut-off values of each analyte for the estimation of sensitivity and specificity were selected based on Youden's index.29
RESULTS
Analysis of sequences of M. tb Beijing/K-derived antigen
MTBK24820 is a PPE39 protein identified from M. tb Beijing/K strain, which consists of 622 amino acids. Based on sequence analyses on MTBK_24820 (BLAST search request identification no. S3HGJHAB015), a rate of about 60% for similarity was observed in the N-terminus of MTBK_24820 (259 amino acids) on alignment with a portion of PPE42, which has been tested as a new TB vaccine in clinical trials (Fig. 2).30 Its sequences in the N-terminus region are similar with sequences of M. bovis BCG as well (Fig. 2). However, most sequences of MTBK_24820 were disrupted or varied in M. bovis BCG. The protective efficacy of MTBK_24820 in mice infected with M. tb may be associated with the homologous sequences in the vaccine candidate of TB or the only existing TB vaccine, M. bovis BCG.17
Fig. 2. Sequence homology between M. tb Beijing/K-derived MTBK_24820 and corresponding sequences in vaccine candidate antigens. Amino acid sequences of MTBK_24820 were highly conserved with equivalent sequences in M. bovis BCG, and PPE42, respectively. The asterisk (*) represents identical amino acids between the antigens and comparing sequences in other strains. M. tb, Mycobacterium tuberculosis.
Immune signature in response to MTBK_24820 in active TB, LTBI, and controls
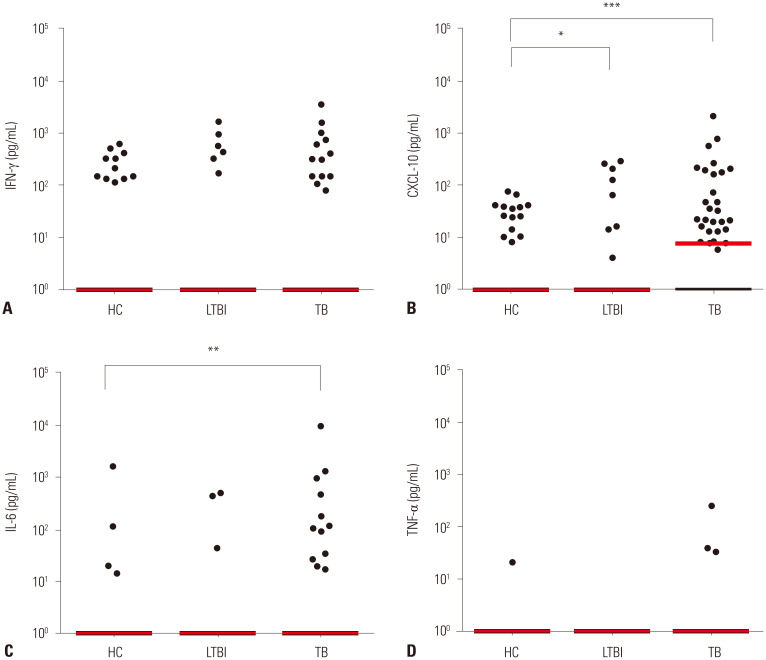
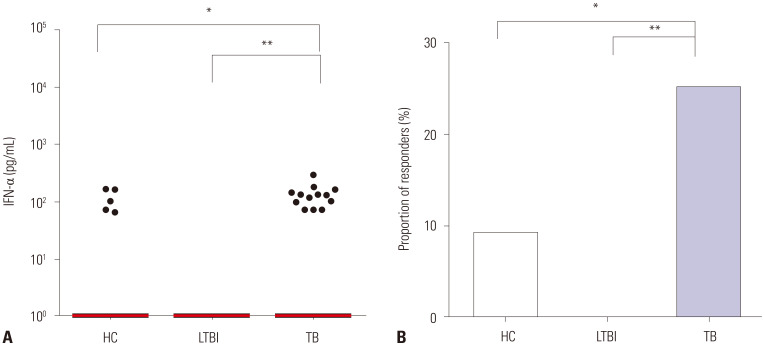
Among the 17 analytes tested, three analytes (CXCL10, IL-6, and IFN-α) identified M. tb infection or differentiated disease status (active TB vs. LTBI) in response to MTBK_24820 antigen (Figs. 3 and 4). MTBK_24820 did not differentiate among the study groups measuring the representative cytokine IFN-γ production (p>0.05) (Fig. 3A). However, active TB disease was clearly distinguished by CXCL10 (p<0.001) (Fig. 3B). MTBK_24820-specific CXCL10 responses also differentiated between healthy subjects and LTBI (p<0.05) (Fig. 3B). MTBK_24820 stimulation induced significantly higher IL-6 production in active TB patients than the healthy controls (p<0.01) (Fig. 3C). No significant difference in TNF-α responses was observed among the groups (p>0.05) (Fig. 3D). The MTBK_24820-specific IFN-α responses were significantly higher in active TB patients than in healthy controls (p<0.05) (Fig. 4A), and the IFN-α responses were different between active TB and LTBI in response to MTBK_24820 (p<0.01) (Fig. 4A). The proportion of IFN-α responders was about three-fold higher among active TB cases than in healthy subjects (p<0.05) (Fig. 4B).
Fig. 3. Immune profiles of MTBK_24820-specific cytokine/chemokine responses in diagnostic groups. (A) IFN-γ, (B) CXCL10, (C) IL-6, and (D) TNF-α were measured in response to MTBK_24820 in active TB (n=52), LTBI (n=31), and healthy control (n=65) groups. (B and C) Significantly higher CXCL10 and IL-6 responses were also found in active TB patients than in the healthy controls in response to MTBK_24820. Disease status (active TB vs. LTBI) was differentiated by MTBK_24820-specific CXCL10. (A and D) IFN-γ and TNF-α responses were not significantly changed in the three groups, compared with other cytokines. The horizontal lines represent medians (*p<0.05, **p<0.01, ***p<0.001; Kruskal-Wallis and Dunn's multiple comparison tests). HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine; TNF, tumor necrosis factor.
Fig. 4. IFN-α production induced by MTBK_24820 in diagnostic groups. (A) MTBK_24820 elicited significantly higher IFN-α responses in active TB patients than in healthy controls. (B) The proportions of IFN-α responders to MTBK_24820 are represented in each group. The horizontal lines indicate medians (*p<0.05, **p<0.01, ***p<0.001; Kruskal-Wallis and Dunn's multiple comparison tests). HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IFN, interferon.
Diagnostic value of MTBK_24820 in cytokine/chemokine production
In response to MTBK_24820, CXCL10, IL-6 and IFN-α production differentiated between active TB and healthy controls or between active TB and LTBI (Figs. 3 and 4). All of the analytes involved in distinguishing between study groups demonstrated sufficient diagnostic accuracy (0.55<AUC<0.65) (Table 1). The sensitivities and specificities for detecting M. tb infection were increased to 65.3% and 95.4% by the combination of CXCL10, IL-6, and IFN-α responses in MTBK_24820 stimulation (Table 2). Among the discriminative markers for disease status (active TB vs. LTBI), IFN-α had much higher sensitivity and specificity with combined responses with CXCL10, when compared with single cytokine responses (Table 2).
Table 1. Diagnostic Accuracy of MTBK_24820-Specific Immune Responses.
| Antigen | Group | Analyte | Sensitivity (%) | Specificity (%) | Cut-off (pg/mL) | AUC (95% CI) |
|---|---|---|---|---|---|---|
| MTBK_24820 | Active TB vs. control | CXCL10 | 53.9 | 78.5 | > 3.5 | 0.59 (0.48–0.69) |
| IL-6 | 23.1 | 95.4 | > 15.6 | 0.59 (0.48–0.69) | ||
| IFN-α | 25.0 | 93.9 | > 69.4 | 0.59 (0.48–0.69) | ||
| Active TB vs. LTBI | CXCL10 | 53.9 | 77.4 | > 5.0 | 0.63 (0.51–0.78) | |
| IFN-α | 25.0 | 100.0 | > 36.9 | 0.63 (0.51–0.74) |
AUC, area under the curve; CI, confidence interval; TB, tuberculosis; LTBI, latent TB infection; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine.
Table 2. Improved Diagnostic Accuracy Achieved by Combining Detection of MTBK_24820-Specific Immune Responses.
| Antigen | Group | Analyte | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| MTBK_24820 | Active TB vs. control | IL-6 | 23.1 | 95.4 |
| IL-6+IFN-α | 38.5 | |||
| IL-6+IFN-α+CXCL10 | 65.3 | |||
| Active TB vs. LTBI | IFN-α | 25.0 | 100.0 | |
| IFN-α+CXCL10 | 63.5 |
TB, tuberculosis; LTBI, latent TB infection; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine.
Immune signature depending on AFB staining results and Beijing/K genotyping
In this study, the majority of TB patients did not have positive AFB smear results; only 15 of the 52 TB patients enrolled were positive by AFB smear tests. M. tb growth was further confirmed by culture in 40 patients (Fig. 1). Of the 37 AFB smear negative patients, about 62% (23/37) of the patients showed positive responses to CXCL10, IL-6, or/and IFN-α upon MTBK_24820 stimulation (data not shown). Based on molecular genotyping, the Beijing types accounted for 68% (19/28 tested), and most isolates (14/19) were revealed as belonging to the Beijing/K strain.
DISCUSSION
Insufficient accuracy of current TB diagnostics and variable efficacy of the BCG vaccine suggest a need for the development of better strategies for identifying relevant biomarkers in TB. Geographically, dominant strains of M. tb have been considered as sources of new vaccine components, and recent developments achieved with highly sensitive multiplex detection technology have provided means with which to measure a number of analytes in limited volumes of a clinical sample. Using this multiplex detection technology in this study, we assessed host immune responses to antigen derived from Beijing/K strains which are hyper-virulent and responsible for TB outbreaks in South Korea. CXCL10, IL-6, and IFN-α were revealed as biomarker candidates among the 17 cytokines and chemokines tested. Each cytokine response had sufficient diagnostic values (AUC=0.59–0.63), and the sensitivity of single cytokine detection was increased with one or two additional cytokine combinations with the maintenance of high specificity.
The protective efficacy of MTBK_24820 in our previous study was strengthened by its sequential similarity with PPE42,17 which has been successfully tested in phase II clinical trials of TB vaccines:30,31 specifically, ID93 adjuvant with GLA-SE is a subunit vaccine including PPE42 and Esx proteins. Its safety, immunogenicity, and preliminary efficacy in preventing TB infection were confirmed in M. tb Beijing/K-strain-infected mice in a previous study.32 In this study, MTBK_24820-specific CXCL10, IL-6, and IFN-α differentiated M. tb infection from healthy controls, and active TB from LTBI. This suggests that MTBK_24820 may be utilized as a diagnostic antigen, as well as a vaccine candidate.
From the time of infection to active disease progression, many different cytokines and chemokines are released by immune cells and play roles in protecting against M. tb through both innate and adaptive immunity. In response to M. tb infection, TNF-α is secreted primarily by alveolar macrophages and initiates innate cytokine/chemokine responses with IL-6 and type I IFN, while phagocyte activation during early infection induces adaptive immunity.33 After the M. tb antigen is expressed by antigen-presenting cells, IFN-γ, which is expressed on M. tb-specific T-cells, activates macrophages and contributes to granuloma formation. CXCL10 is also expressed on M. tb-specific T-cells and plays a role in the early recruitment of T-cells to the lungs, resulting in granuloma formation.33 IFN-α, which belongs to the type I IFN category, is induced by M. tb-infected macrophages.34,35 Although controversial, a role for IFN-α in TB has been reported in the literature. IFN-α expression was previously shown to be related to strain virulence: the messenger RNA expression of IFN-α was significantly higher in the lungs of hypervirulent HN878-infected mice than the H37Rv strain-infected, whereas the downregulation of IFN-γ and TNF-α, which are related to Th1 immunity, was observed.36 Additional treatment with purified IFN-α/β increased lung bacterial burden with reduced survival, and induction of type I IFNs in relation to hypervirulent strains caused failure of Th1 type immunity.36 On the other hand, intramuscular BCG vaccination together with IFN-α boosting has been shown to elicit a reduction in bacterial load, as well as IFN-γ, IL-12, TNF-α, and IL-17 responses.37 In this study, the Beijing/K-specific IFN-α responses were significantly higher in TB patients than subjects with LTBI, suggesting that IFN-α may be more closely associated with pathogenicity than protection in relation to the Beijing/K strain. Several individuals in the healthy and LTBI groups showed positive IFN-α responses; the immune follow-up of these individuals might further reveal the pathogenic or protective role of IFN-α.
Besides the Beijing/K-derived antigen, we also tested M. tb PPD and heparin-binding haemagglutinin (HBHA) proteins as potential diagnostic antigens. None of the cytokine/chemokine responses to these antigens showed significant differences among the groups. All analytes were present at high concentrations in all groups (Supplementary Fig. 3, only online). This may due to cross-reactivity with M. bovis BCG in South Korea where BCG vaccination is received by >97% of the population. M. tb PPD and HBHA-induced cytokines including IFN-γ might differentiate between active TB and LTBI or healthy controls in countries where BCG vaccination is rare,38,39 whereas the antigens cannot be used as diagnostics in populations with universal BCG vaccination. The antigens might be useful for confirming the immunogenicity of BCG vaccination rather than the diagnosis of TB in this context.
In this study, about 23% of active TB cases were probable TB without M. tb identification by culture. Active TB was diagnosed from clinical and other diagnostic findings in the culture-negative patients; however, the possibility of misdiagnosis may exist, irrespective of the effort made to classify. In addition, QFT-GIT tests were not performed for diagnosis of active TB, which limits the diagnostic efficacy between different testing methods using the Beijing/K and QFT-GIT antigens.
Although the sensitivity and specificity of the detection should be improved, multi-cytokine signatures to Beijing/K antigens may accelerate the discovery of improved diagnostics and new vaccine components in places where the Beijing/K and Beijing/K-homologous strains of M. tb are endemic. This would also help with the early diagnosis in AFB smear-negative TB patients prior to confirmation with M. tb in culture. Continuous efforts for finding biomarkers using predominant M. tb strains should be made in cohorts with drug-resistance and outbreaks associated with the Beijing/K strain infection.
ACKNOWLEDGEMENTS
This study was supported by a National Research Foundation of Korea grant (NRF-2018R1D1A1A02049260) funded by the Ministry of Education in South Korea.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sang-Nae Cho, Yun-Gyoung Hur, and Kwang Joo Park.
- Data curation: Yun-Gyoung Hur and Kwang Joo Park.
- Formal analysis: Yun-Gyoung Hur and Kwang Joo Park.
- Funding acquisition: Yun-Gyoung Hur.
- Investigation: Kwang Joo Park, Yun-Gyoung Hur, Young Sun Kim, and Ahreum Kim.
- Methodology: Kwang Joo Park and Yun-Gyoung Hur.
- Project administration: Yun-Gyoung Hur.
- Resources: Sang-Nae Cho, Yun-Gyoung Hur, and Kwang Joo Park.
- Supervision: Yun-Gyoung Hur.
- Validation: Yun-Gyoung Hur and Kwang Joo Park.
- Visualization: Yun-Gyoung Hur and Ahreum Kim.
- Writing—original draft: Ahreum Kim and Yun-Gyoung Hur.
- Writing—review & editing: Yun-Gyoung Hur, Kwang Joo Park, Sang-Nae Cho, and Hazel M Dockrell.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Preparation of recombinant MTBK_24820. The recombinant protein was overexpressed and purified in Escherichia coli using Ni-NTA affinity chromatography, followed by an anion exchange purification system. Purified MTBK_24820 was confirmed by SDS-PAGE. Black arrows represent the eluted fraction of target protein in FPLC collector.
Immune signatures of MTBK_24820-specific cytokine/chemokine responses in diagnostic groups using multiplex bead arrays. Culture supernatant samples stimulated with MTBK_24820 were analyzed with the following 17 different cytokines and chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-22, IFN-α, IFN-γ, TNF-α, IP-10 (CXCL10), MCP-1 (CCL2), and sCD40L. Median responses are marked by red lines (*p<0.05, **p< 0.01, ***p<0.001, by one-way ANOVA with subsequent Kruskal-Wallis test). HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine; TNF, tumor necrosis factor.
Immune profiles induced by M. tb PPD and HBHA antigens in diagnostic groups using multiplex bead arrays. Culture supernatant samples stimulated with (A) M. tb PPD and (B) HBHA were analyzed with the following 17 different cytokines and chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-22, IFN-α, IFN-γ, TNF-α, IP-10 (CXCL10), MCP-1 (CCL2), and sCD40L. Median responses are marked by red lines. M. tb, Mycobacterium tuberculosis; PPD, purified protein derivatives; HBHA, heparin-binding haemagglutinin; HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine; TNF, tumor necrosis factor.
References
- 1.Raviglione MC, Uplekar MW. WHO's new Stop TB strategy. Lancet. 2006;367:952–955. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 2.GBD Tuberculosis Collaborators. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2018;18:261–284. doi: 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations. The sustainable development goals report 2019. [accessed on 2020 July 23]. Available at: https://unstats.un.org/sdgs/report/2019/The-Sustainable-Development-Goals-Report-2019.pdf.
- 4.World Health Organization. Global tuberculosis report 2019. [accessed on 2019 November 11]. Available at: https://www.who.int/tb/publications/factsheet_global.pdf?ua=1.
- 5.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 6.Merker M, Blin C, Mona S, Duforet-Frebourg N, Lecher S, Willery E, et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015;47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Castillo JG, Pino C, Niño LF, Rozo JC, Llerena-Polo C, Parra-López CA, et al. Comparative genomic analysis of Mycobacterium tuberculosis Beijing-like strains revealed specific genetic variations associated with virulence and drug resistance. Infect Genet Evol. 2017;54:314–323. doi: 10.1016/j.meegid.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Almeida FM, Ventura TL, Amaral EP, Ribeiro SC, Calixto SD, Manhães MR, et al. Hypervirulent Mycobacterium tuberculosis strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57BL/6 mice. PLoS One. 2017;12:e0173715. doi: 10.1371/journal.pone.0173715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Tuberculosis profiles: Republic of Korea. [accessed on 2020 July 23]. Available at: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&lan=%22EN%22&iso2=%22KR%22&main_tabs=%22est_tab%22.
- 10.Go U, Park M, Kim UN, Lee S, Han S, Lee J, et al. Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Other Mycobact Dis. 2018;11:28–36. doi: 10.1016/j.jctube.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi GE, Jang MH, Song EJ, Jeong SH, Kim JS, Lee WG, et al. IS6110-restriction fragment length polymorphism and spoligotyping analysis of Mycobacterium tuberculosis clinical isolates for investigating epidemiologic distribution in Korea. J Korean Med Sci. 2010;25:1716–1721. doi: 10.3346/jkms.2010.25.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang HY, Wada T, Iwamoto T, Maeda S, Murase Y, Kato S, et al. Phylogeographical particularity of the Mycobacterium tuberculosis Beijing family in South Korea based on international comparison with surrounding countries. J Med Microbiol. 2010;59(Pt 10):1191–1197. doi: 10.1099/jmm.0.022103-0. [DOI] [PubMed] [Google Scholar]
- 13.Shamputa IC, Lee J, Allix-Béguec C, Cho EJ, Lee JI, Rajan V, et al. Genetic diversity of Mycobacterium tuberculosis isolates from a tertiary care tuberculosis hospital in South Korea. J Clin Microbiol. 2010;48:387–394. doi: 10.1128/JCM.02167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Bai GH, Lee H, Kim HJ, Lew WJ, Park YK, et al. Transmission of Mycobacterium tuberculosis among high school students in Korea. Int J Tuberc Lung Dis. 2001;5:824–830. [PubMed] [Google Scholar]
- 15.Jeon BY, Kwak J, Hahn MY, Eum SY, Yang J, Kim SC, et al. In vivo characteristics of Korean Beijing Mycobacterium tuberculosis strain K1 in an aerosol challenge model and in the Cornell latent tuberculosis model. J Med Microbiol. 2012;61(Pt 10):1373–1379. doi: 10.1099/jmm.0.047027-0. [DOI] [PubMed] [Google Scholar]
- 16.Han SJ, Song T, Cho YJ, Kim JS, Choi SY, Bang HE, et al. Complete genome sequence of Mycobacterium tuberculosis K from a Korean high school outbreak, belonging to the Beijing family. Stand Genomic Sci. 2015;10:78. doi: 10.1186/s40793-015-0071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim A, Hur YG, Gu S, Cho SN. Protective vaccine efficacy of the complete form of PPE39 protein from Mycobacterium tuberculosis Beijing/K strain in mice. Clin Vaccine Immunol. 2017;24:e00219-17. doi: 10.1128/CVI.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WS, Kim H, Kwon KW, Cho SN, Shin SJ. Immunogenicity and vaccine potential of InsB, an ESAT-6-like antigen identified in the highly virulent Mycobacterium tuberculosis Beijing K strain. Front Microbiol. 2019;10:220. doi: 10.3389/fmicb.2019.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park PJ, Kim AR, Salch YP, Song T, Shin SJ, Han SJ, et al. Characterization of a novel antigen of Mycobacterium tuberculosis K strain and its use in immunodiagnosis of tuberculosis. J Microbiol. 2014;52:871–878. doi: 10.1007/s12275-014-4235-5. [DOI] [PubMed] [Google Scholar]
- 20.Hur YG, Chung WY, Kim A, Kim YS, Kim HS, Jang SH, et al. Host immune responses to antigens derived from a predominant strain of Mycobacterium tuberculosis. J Infect. 2016;73:54–62. doi: 10.1016/j.jinf.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005;172:631–635. doi: 10.1164/rccm.200502-196OC. [DOI] [PubMed] [Google Scholar]
- 24.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- 25.Pai M. Spectrum of latent tuberculosis-existing tests cannot resolve the underlying phenotypes. Nat Rev Microbiol. 2010;8:242. doi: 10.1038/nrmicro2236-c1. [DOI] [PubMed] [Google Scholar]
- 26.Bergamini BM, Losi M, Vaienti F, D'Amico R, Meccugni B, Meacci M, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics. 2009;123:e419–e424. doi: 10.1542/peds.2008-1722. [DOI] [PubMed] [Google Scholar]
- 27.Hur YG, Crampin AC, Chisambo C, Kanyika J, Houben R, Ndhlovu R, et al. Identification of immunological biomarkers which may differentiate latent tuberculosis from exposure to environmental nontuberculous mycobacteria in children. Clin Vaccine Immunol. 2014;21:133–142. doi: 10.1128/CVI.00620-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams A, Steffens F, Reinecke C, Meyer D. The Th1/Th2/Th17 cytokine profile of HIV-infected individuals: a multivariate cytokinomics approach. Cytokine. 2013;61:521–526. doi: 10.1016/j.cyto.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 30.Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines. 2018;3:34. doi: 10.1038/s41541-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrager LK, Harris RC, Vekemans J. Research and development of new tuberculosis vaccines: a review. F1000Res. 2018;7:1732. doi: 10.12688/f1000research.16521.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha SB, Kim WS, Kim JS, Kim H, Kwon KW, Han SJ, et al. Pulmonary immunity and durable protection induced by the ID93/GLA-SE vaccine candidate against the hyper-virulent Korean Beijing Mycobacterium tuberculosis strain K. Vaccine. 2016;34:2179–2187. doi: 10.1016/j.vaccine.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and chemokines in Mycobacterium tuberculosis infection. Microbiol Spectr. 2016;4:TBTB2-0018-2016. doi: 10.1128/microbiolspec.TBTB2-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 35.Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J Immunol. 2011;187:2540–2547. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivas-Santiago CE, Guerrero GG. IFN-α boosting of Mycobacterium bovis Bacillus Calmette Güerin-vaccine promoted Th1 type cellular response and protection against M. tuberculosis infection. Biomed Res Int. 2017;2017:8796760. doi: 10.1155/2017/8796760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hougardy JM, Schepers K, Place S, Drowart A, Lechevin V, Verscheure V, et al. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007;2:e926. doi: 10.1371/journal.pone.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SG, Lecher S, Blitz R, Locht C, Dockrell HM. Broad heparin-binding haemagglutinin-specific cytokine and chemokine response in infants following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:2511–2522. doi: 10.1002/eji.201142297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparation of recombinant MTBK_24820. The recombinant protein was overexpressed and purified in Escherichia coli using Ni-NTA affinity chromatography, followed by an anion exchange purification system. Purified MTBK_24820 was confirmed by SDS-PAGE. Black arrows represent the eluted fraction of target protein in FPLC collector.
Immune signatures of MTBK_24820-specific cytokine/chemokine responses in diagnostic groups using multiplex bead arrays. Culture supernatant samples stimulated with MTBK_24820 were analyzed with the following 17 different cytokines and chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-22, IFN-α, IFN-γ, TNF-α, IP-10 (CXCL10), MCP-1 (CCL2), and sCD40L. Median responses are marked by red lines (*p<0.05, **p< 0.01, ***p<0.001, by one-way ANOVA with subsequent Kruskal-Wallis test). HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine; TNF, tumor necrosis factor.
Immune profiles induced by M. tb PPD and HBHA antigens in diagnostic groups using multiplex bead arrays. Culture supernatant samples stimulated with (A) M. tb PPD and (B) HBHA were analyzed with the following 17 different cytokines and chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-22, IFN-α, IFN-γ, TNF-α, IP-10 (CXCL10), MCP-1 (CCL2), and sCD40L. Median responses are marked by red lines. M. tb, Mycobacterium tuberculosis; PPD, purified protein derivatives; HBHA, heparin-binding haemagglutinin; HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IFN, interferon; IL, interleukin; CXCL10, C-X-C motif chemokine; TNF, tumor necrosis factor.