Abstract
Main conclusion
Shoot tip necrosis is a physiological condition that negatively impacts the growth and development of in vitro plant shoot cultures across a wide range of species.
Abstract
Shoot tip necrosis is a physiological condition and disorder that can arise in plantlets or shoots in vitro that results in death of the shoot tip. This condition, which can spread basipetally and affect the emergence of axillary shoots from buds lower down the stem, is due to the cessation of apical dominance. STN can occur at both shoot multiplication and rooting stages. One of the most common factors that cause STN is nutrient deficiency or imbalance. Moreover, the presence or absence of plant growth regulators (auxins or cytokinins) at specific developmental stages may impact STN. The cytokinin to auxin ratio within an in vitro plant can be modified by varying the concentration of cytokinins used in the culture medium. The supply of nutrients to in vitro shoots or plantlets might also affect their hormonal balance, thus modifying the occurrence of STN. High relative humidity within culture vessels and hyperhydricity are associated with STN. An adequate supply of calcium as the divalent cation (Ca2+) can hinder STN by inhibiting the accumulation of phenolic compounds and thus programmed cell death. Moreover, the level of Ca2+ affects auxin transport and ethylene production, and higher ethylene production, which can occur as a result of high relative humidity in or poor ventilation of the in vitro culture vessel, induces STN. High relative humidity can decrease the mobility of Ca2+ within a plant, resulting in Ca2+ deficiency and STN. STN of in vitro shoots or plantlets can be halted or reversed by altering the basal medium, mainly the concentration of Ca2+, adjusting the levels of auxins or cytokinins, or modifying culture conditions. This review examines the literature related to STN, seeks to discover the associated factors and relations between them, proposes practical solutions, and attempts to better understand the mechanism(s) underlying this condition in vitro.
Electronic supplementary material
The online version of this article (10.1007/s00425-020-03449-4) contains supplementary material, which is available to authorized users.
Keywords: Boron, Calcium, Chloride, In vitro shoots, Mineral nutrient deficiency, Physiological disorder, Plant growth regulators
Introduction
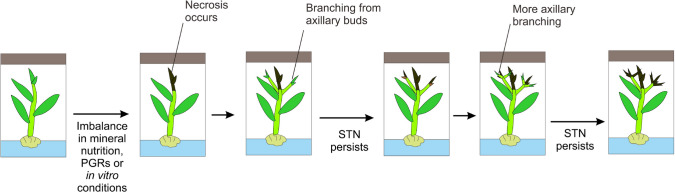
Shoot tip necrosis (STN) is a term that was originally coined by Sha et al. (1985). STN is sometimes also referred to as shoot tip abortion (Millington 1963), tip burn (McCown and Sellmer 1987), apical necrosis (Amo-Marco and Lledo 1996; Koubouris and Vasilakakis 2006; Machado et al. 2014), apex necrosis (Rugini et al. 1986), top necrosis (De Klerk and ter Brugge 2011), shoot tip damage/injury (Ahmed and Palta 2017b), or shoot die-back (Barghchi and Alderson 1996). STN occurs when the shoot tip of a plant, both ex vitro and in vitro, shows signs of browning and death during multiplication, elongation and/or rooting stages, despite growing in apparently ideal conditions (Vieitez et al. 1989; Bairu et al. 2009b). In vitro, STN can ultimately result in the inhibited growth of the entire plantlet or it can be localized at affected shoots. The affected area spreads basipetally down from the shoot tip to lower parts of shoots. However, shoot formation from basal axillary shoot buds is not necessarily inhibited, as was observed for pistachio (Pistachia vera L.) (Barghchi and Alderson 1996). STN is also not always fatal to the plant, and apical dominance can be assumed by the next closest axillary bud, at least in sweet chestnut (Castanea sativa Mill.) and oak (Quercus robur L.) (Vieitez et al. 1989). If growing axillary branches develop STN, then a “witches’ broom” pattern develops (Fig. 1; McCown and Sellmer 1987). On some occasions, the shoot tip can outgrow STN, leaving behind a scarred part of the stem with deformed leaves (McCown and Sellmer 1987). Sudha et al. (1998) observed axillary branching after STN in jivanthi (Holostemma annulare (Robx.) K. Schum., i.e., Holostemma ada-kodien Schult.) in vitro cultures. STN is problematic not only for stock cultures of in vitro plantlets, but also for commercial production (Sha et al. 1985).
Fig. 1.
Schematic diagram of shoot tip necrosis (STN). An imbalance in minerals, nutrients, plant growth regulators, or other in vitro conditions, lead to STN. This results in the blackening and death of the terminal shoot tip, the branching of axillary buds, and in some cases, STN in axillary shoots, leading to the formation of a “witches’ broom” pattern
The precise mechanism underlying STN still remains unclear, although some possible reasons have been proposed, including mineral deficiency or the presence of high concentrations of plant growth regulators (PGRs) in the medium. One of the most cited reasons is calcium (Ca) deficiency. Ca deficiency is also a reason for the tip-burn disorder in the leaves and stems of field-grown fruits such as strawberry (Fragaria × ananassa Duchesne) and vegetables (Mason and Guttridge 1974, 1975; Saure 1998) and its symptoms closely resemble those of STN. This review aims to examine the literature that exists on this physiological disorder, including an earlier review by Bairu et al. (2009b), while exploring new literature published over the past decade. One objective is to attempt to better identify some of the possible reasons for the occurrence of STN and to suggest practical solutions to alleviate this physiological disorder in vitro.
Shoot tips are a popular explant in plant tissue culture. On occasion, shoot tip explants necrose (e.g., Krishna et al. 2008). In this review, the necrosis of shoot tip explants, i.e., explant necrosis, is not considered to be STN, which relates exclusively to the shoot tip of a tissue-cultured in vitro plantlet.
Shoot tip necrosis: occurrence and alleviation
A wide range of plants display STN in in vitro cultures (Table 1; Suppl. Table 1). Among all published studies, the occurrence of STN is particularly prominent in trees and woody shrubs (58.9% and 21.9%, respectively, of studies in Suppl. Table 1; Suppl. Figure 1). Studies on pistachio represent the largest proportion (10.8%) of studies on STN in vitro, followed by pear (Pyrus spp.) (8.1%) (Suppl. Figure 2). The incidence of STN in micropropagation, especially at the rooting stage, is shown in Fig. 2. STN, at least according to the reported literature, has occurred most frequently in the Rosaceae (20.5%), followed by the Anacardiaceae (12.3%) (Suppl. Figure 3). We caution readers that relative values might simply indicate the popularity of a studied species and not necessarily the actual incidence of STN in plan species or families studied to date. For example, only a single report on STN exists for an orchid, hybrid Cymbidium (Guha and Usha Rao 2012), so the incidence for the Orchidaceae is in fact 100% of studies, but the relative incidence (relative to all other species studied in Table 1) is only 1.4%.
Table 1.
Shoot tip necrosis: observations and possible solutions*
| Scientific name and cultivar | Stage, medium and observed problems | Reason(s) provided for incidence of STN | Solution provided to halt, reduce, or prevent STN, and other observations | References |
|---|---|---|---|---|
| Azadirachta indica A. Juss | Optimum SMM: MS + 1.11 µM BA + 1.43 µM IAA + 81.43 µM AdS. STN = UQ | Basal medium micro- and macronutrient concentration | Addition of 0.42 mM Ca(NO3)2, 0.70 mM Na2SO4, and 0.57 mM K2SO4 | Arora et al. (2010) |
| Begonia homonyma Steud | Optimum SMM: MS + 15 µM BA + 5 µM NAA. STN (all on MS) = 18% (15 µM mTR + 5 µM NAA for 12 weeks → 2 µM mTR + 0.5 µM NAA for 6 weeks), 34% (15 µM TDZ + 5 µM NAA for 12 weeks → 2 µM TDZ + 0.5 µM NAA for 6 weeks), 44% (15 µM BA + 5 µM NAA for 12 weeks → 2 µM BA + 0.5 µM NAA for 6 week) | Use of PGRs. High BA conc. and/or use of BA as the CK | Use of mTR. Use half-strength MS rather than MS, reduce BA conc. to 0.5 µM and add 2 or 5 µM GA3: STN = 10–36% in various media defined in column 2 | Kumari et al. (2017) |
| Butea monosperma (Lam.) Taub | Optimum SGM: half-strength WPM + 5 mg/l BA (± 10 mg/l fructose). 90% STN in terminal 2–3 mm | No substantiated reason provided. Only theoretical observation with no supporting data | Addition of fructose eliminated STN in 95% of STN-positive cultures. Some phenolics were exuded from cut ends | Kulkarni and D’Souza (2000) |
| Castanea dentata (Marsh.) Borkh. cv. B’ville, Iowa #2, BDW | Optimum RIM: half-strength MS + AC. SEM: WPM salts + NN vitamins. SMM = 500 mg/l PVP 40 + 500 mg/l MES + 0.89 µM BA. STN = 25–67%, depending on genotype and treatment. STN reduced to 19–21% across three genotypes in replication trial | Wounding, developmental stage, genotype | Low concentration of BA (0.22 µM) at an advanced stage of root initiation reduced STN. When on SEM, wounding had no effect on STN (~ 30–38%, 13–30%, 20–25% for B’ville, Iowa #2, and BDW, respectively). STN increased to 67% and 88% in Iowa #2 and BDW, respectively, when cuttings were plated on SMM (no change for B’ville, at 38%) | Xing et al. (1997) |
| Castanea sativa Mill. clones 431, T-13, 812; Quercus robur L | Optimum RIM: half-strength MS + 3 mg/l IBA (7 day) or dip in 1 g/l IBA (20–60 s) for chestnut; half-strength Gresshoff and Doy (1972) basal + 0.5 g/l IBA (8 min) for oak. STN UQ (only axillary shoot development) | In SEM, when BA was removed, or in RIM, STN developed | Addition of 0.01 mg/l BA to RIM, but this reduced rooting in chestnut and oak, but axillary shoots developed marginally more (+ 1%) in chestnut clone T-13. When the cut surface of shoot tips was added to BA-impregnated agar, rooting was reduced in both trees, but axillary shoot development increased, the amount depending on the day of decapitation | Vieitez et al. (1989) |
| Castanea sativa Mill. cv. Garrone rosso, Clone 46 | Optimum RIM: MS + 0.044 µM BA + 5 µM IBA (8 day) then same medium without IBA. STN = 23% after 8 day, 77% after 26 day for Clone 46; UQ for Garrone rosso | Ca deficiency; lack of BA | Clone 46 formed > twofold more STN than Garrone rosso (68% vs 25%). A block of agar containing 3 mM CaCl2 and/or 5 µM BA that was placed around shoot tips eliminated or delayed STN | Piagnani et al. (1996) |
| Cercis canadensis var. mexicana | SMM: WPM + MS vitamins + 11.1 µM BA. STN UQ | Excessively high concentrations of 2iP (25–74 µM), TDZ (presumably 5 or 11–23 µM) and kinetin (concentration range NR) | General suggestions on how to improve shoot growth, but no specific details, or data, about how to improve STN | Mackay et al. (1995) |
| Corylus avellana L | Optimum SMM: half-strength Cheng (1975) basal + 25 µM BA (15 days) → same medium but + 0.5 or 2.5 µM BA (25 days); optimum RIM: half-strength liquid Cheng (1975) basal + 50 µM IBA (5 days) → same medium (solid) (15 days). STN = UQ | High IBA concentration | Reducing IBA from 50 µM at the rooting stage to 10 or 25 µM, or by reducing exposure period to IBA to 8 days | Pérez et al. (1985) |
| Cydonia oblonga Mill. rootstock clone C | Optimum SMM: MS + 5 µM BA. STN = 15% in SM of control cultures | Ca deficiency | Raising Ca2+ (in the form of Ca(NO3)2) from 3 to 18 mM reduced STN, but this also reduced shoot proliferation. Link between Ca deficiency and hyperhydricity unclear | Singha et al. (1990) |
| Cymbidium hybrid Via del Playa Yvonne | Optimum SMM: MS – MgSO4 + Na2SO4. STN = 5% (control), 35%, 60%, 80% (10, 15, 20 µM SNP, respectively) | Addition of SNP, a nitric oxide donor | Nitric oxide, a positive and negative regulator of stress, could not prevent STN | Guha and Usha Rao (2012) |
| Dalbergia latifolia Roxb | Optimum SMM: ¾ (macro) MS or WPM + 5 mg/l BA + 0.5 mg/l NAA. STN = UQ | Tended to find STN associated with leaf abscission, but not linked to poor aeration or high humidity | Doubling Ca2+ concentration in MS or WPM media did not reduce STN. Solution only provided to reduce leaf abscission by adjusting the NH4/NO3 ratio, but not STN | Lakshmi Sita and Raghava Swamy (1993) |
| Dipterocarpus alatus Roxb., D. intricatus Dyer | Optimum seedling establishment: MS or WPM + 0.1 µM BA. STN = UQ | Nitrogen level | Removal of NH4NO3 from WPM. High humidity likely not the cause because of high aeration of vessels | Linington (1991) |
| Dwarf rose (Rosa gymnocarpa Nutt.) cv. Starina | Optimum RIM: auxin-free MS. Lowest incidence of STN = 6% (on RIM). When 1 mg/l IAA was added, STN increased from 6–22% to 16–62% (range caused by the treatment) | Inclusion of auxin, specifically IAA | In the absence of auxin, 2.5–10 mg/l AgNO3 reduced the incidence of STN from 22% to 2–12%. In IAA-containing RIM, 1.5 × Ca2+ levels decreased STN from 58 to 28%. In IAA-containing RIM, 1.5 × Ca2+ levels + 2 × Mg2+ levels decreased STN from 58 to 24% | Podwyszyńska and Goszczyńska (1998) |
| Ensete ventricosum Welw. cv. Oniya | Optimum SMM: MS + 11 µM BA + 6 µM IAA | The term STN was not used | However, STN was induced since shoot tips were split vertically down the center for micropropagation. 40% of greenhouse-derived shoot tips died due to blackening (aka STN; 0% in in vitro shoot tips) | Diro and van Staden (2005)a |
| Gaultheria hispidula (L.) Muhl. ex Bigelow, Rhododendron cv. Chinsayii, Rhododendron dauricum L | Optimum SMM: Anderson (1984) basal + 15.9–16.3 mg/l 2iP (across three plants). STN = UQ | Presence of BA in medium at any concentration (0.1–10 mg/l). Use of 2iP did not induce STN | No suggestions | Norton and Norton (1985) |
| Haloxylon persicum (Bunge ex Boiss & Buhse) | Optimum SMM: MS + 0.5 µM TDZ. STN = 100% (0 or 2 µM TDZ), 86% (0.5 µM TDZ), 90% (1 µM TDZ); all with 10 µM Kin: 74% (2 mM CaCl2), 65% (4 mM CaCl2), 21% (2 mM CaCl2 + 0.1 mM H3BO3), 16% (4 mM CaCl2 + 0.1 mM H3BO3), 23% (2 mM CaCl2 + 0.2 mM H3BO3), 19% (4 mM CaCl2 + 0.2 mM H3BO3) | Low Ca2+ and | Addition of 4 mM CaCl2 + 0.1 mM H3BO3 + 10 µM Kin. Use of several sugars (sucrose and maltose (60–180 mM), or fructose and glucose (110–330 mM)) with 10 µM Kin did not reduce STN (range = 89–100% for all treatments), except for 120 mM sucrose (STN reduced to 84%) | Kurup et al. (2018) |
| Harpagophytum procumbens [(Burch) de Candolle ex Meissner] | Optimum SMM: half-strength MS + 6 mM Ca2+. STN = 27% (PGR-free MS), 25–35% (MS + 5 µM BA, mT or mTR), 33–62% (MS + 5 or 10 µM BA, mT or mTR + 2.5 µM IAA), 80% (half-strength MS), 20–133% (half-strength MS + 6–9 mM Ca2+ alone or in various combinations with 0.2–0.5 mM ) | High CK (BA) level. Addition of auxin (IAA) | Addition of 6–9 mM Ca2+ with or without 0.2–0.5 mM , or only 0.5 mM , and in IAA-containing medium, 5 or 10 µM mT or mTR reduced STN | Bairu et al. (2009a) |
| Harpagophytum procumbens [(Burch) de Candolle ex Meissner] | Optimum SMM: MS + 8.8 µM BA. STN = 88, 90, 86% (full-strength MS, NN and WPM); 29, 27, 27% (half-strength MS, NN and WPM); 14, 26, 28% (quarter-strength MS, NN and WPM); 18, 21, 25, 26% (1, 2, 3, 4% sucrose); 29, 37, 64, 76% (sucrose, glucose, fructose, maltose at 0.086 M); 19, 28% (2-week subculture; 4-week continuous culture) | High mineral content of MS, NN, or WPM. High sucrose concentration (> 3%). Use of non-sucrose sources or carbohydrates. Lack of subculture | Reducing basal media to half strength. Use of low sucrose concentration. Use of 2-week subcultures | Jain et al. (2009) |
| Harpagophytum procumbens [(Burch) de Candolle ex Meissner] | Same as Bairu et al. (2009a) | Active CKs may be converted to other inactive or irreversible forms of CKs, e.g., 9-glucosides | Selection of CK, and the choice of CK:auxin ration can influence endogenous level of CKs, and thus the outcome of STN | Bairu et al. (2011) |
| Harpagophytum procumbens [(Burch) de Candolle ex Meissner] | Optimum SMM: MS + 1.5 mg/l BA + 6.2 mg/l H3BO3. STN = 53% (SMM + 10 mg/l H3BO3), 13% (SMM + 10 mg/l H3BO3 + 5 mM Si in the form of sodium silicate solution) | No reason provided | Addition of Si as SiO2 | Lišková et al. (2016) |
| Hibiscus rosa-sinensis L. cv. Cassiopeia Wind Yellow, Caribbean Pink | Optimum SMM: MS + 2.2 µM BA. STN = UQ | Low Ca2+ level (independent of BA concentration) | STN only assessed visually, but photographic evidence provided | Christensen et al. (2008) |
| Juglans nigra L | Optimum SMM: half-strength DKW. STN = 11% or 17% in stage 1 (3–4 week culture) when Zea = 5 or 12.5 µM, respectively, (44% and 33% in stage 2, which is 5–8 week of culture). 53% STN on MS when 12.5 µM Zea was used, and measured in stage 2 | Use of BA at 25 µM or Zea at 12.5 or 25 µM. Basal medium (decreasing level of STN): half-strength DKW > DKW > MS > WPM (stage 1) or MS > half-strength DKW > DKW > WPM (stage 2) | Increasing TDZ from 0.5 to 1 µM or reducing BA from 25 to 12.5 µM BA improved percentage of spontaneous shoots, i.e., reduced STN | Bosela and Michler (2008) |
| Lavandula angustifolia Mill. cv Provence Blue | Optimum SMM: MS + 1 µM BA (40 day subculture). STN = 10% (1320 mg/l CaCl2), 21% (440 mg/l CaCl2) for a single subculture; 30% (1320 mg/l CaCl2), 51% (440 mg/l CaCl2) for a second subculture | Low Ca2+ level. Subcultures | Including CaCl2 at 1320 mg/l. Only subculture once | Machado et al. (2014) |
| Lens culinaris Medikus cv. Titore | Optimum SMM: MS + 0.4–0.8 mg/l. STN = 85%, 70%, 56% and 49% in MS + 0.2, 0.4, 0.6 and 0.8 mg/l BA, respectively (91%, 87%, 75% and 73% in B5; 0–3% in MS + 440 mg/l CaCl2; 18%, 16%, 5% and 5% in B5 + 750 mg/l CaCl2) | Low BA conc. or reduced levels of Ca2+ | Increasing BA conc. or adding 750 mg/l CaCl2 to basal medium | Ye et al. (2002) |
| Lonicera caerulea f. caerulea; L. caerulea f. edulis | SMM: 9/10 × MS + 8.9 µM BA, 2.4 µM pyridoxine HCl. STN = 17% on half-strength MS; 6% on 75% MS; 9% on MS | Insufficient micro- and macronutrients in MS; high day/night temperatures | In caerulea form, 0% STN at 24 °C/20 °C (6% at 26 °C/20 °C, 17% at 28 °C/21 °C). In edulis form, 1% STN at 24 °C/20 °C (23% at 26 °C/20 °C, 49% at 28 °C/21 °C) | Karhu (1997) |
| Macadamia tetraphylla L.A.S.Johnson | Optimum SMM: MS + 2 mg/l BA. RIM: SM + 3 mg/l IBA. STN in RIM: 40% at 3 mM Ca2+ (20%, 70%, 85% at 6, 12 and 24 mM). Mulwa and Bhalla (2000) reported 76% STN in RIM | Inadequate aeration, high humidity | Application of < 6 mM Ca2+ in RIM | Mulwa and Bhalla (2000); Bhalla and Mulwa (2003) |
| Malus × domestica (Borkh.); Camellia sinensis (L.) Kuntze; Populus tremula L. × P. alba L.; Gerbera jamesonii Bolus ex Hooker f | SMM/RIM: MS + 2.2 µM BA + 5.3 µM NAA. STN = 49, 53, 3 and 5% in shoots of apple, tea, poplar and gerbera, respectively | Lack of exogenous CK (BA) in medium; lack of endogenous hormones in plants | CK required in medium but details not provided | Kataeva et al. (1991) |
| Musa spp. cv. Grande Naine (GN; AAA), Dwarf Cavendish (DC; AAA), Nendran (AAB), Quintal Nendran (QN; AAB) | Optimum SMM: MS + 6.66 µM BA. STN = 27% and 29% in GN and DC after seven subcultures on SMM (18% and 19% at the rooting stage); 38% and 40% in Nendran and QN after five subcultures on SMM (26% and 27% at the rooting stage) | Low Ca2+ level | Reducing the culture period, modifying salt strength in basal medium, addition of various PGRs (Kin, NAA, and IBA), adjusting levels of sucrose, fructose, and AgNO3 did not improve STN levels. Addition of 50–100 mg/l CaCl2 for at least two subcultures after the fourth and sixth subculture (for bananas and plantains, respectively) allowed 91–97% of shoots (across all four cultivars) to be recovered (unclear if recovered shoots were free of STN) | Martin et al. (2007) |
| Paeonia suffruticosa Andr | SMM: WPM + 0.3% AC. STN UQ | Low Ca2+ level | Adding 6 mM CaCl2 to WPM | Wang and van Staden (2001) |
| Pimelea spicata R.Br | Optimum SMM: half-strength MS + 0.5 or 1.0 mg/l BA. STN = 38% (MS), 73% (MS + ventilation), 18% (half-strength MS), 56% (half-strength MS + ventilation), 32% (half-strength MS + 440 mg/l CaCl2) | Addition of Ca2+. Application of ventilation to culture flasks | Using half-strength MS; not ventilating flasks; not adding supplementary Ca2+ | Offord and Tyler (2009) |
| Pistacia integrima × P. atlantica rootstock UCB1 | Optimum SMM: MS + 0.5 mg/l BA. STN = 42% (1 × CaCl2, 1 × H3BO3), 29% (1 × CaCl2, 2 × H3BO3), 38% (1 × CaCl2, 3 × H3BO3), 21% (1.5 × CaCl2), 19% (1.5 × CaCl2, 2 × H3BO3), 32% (1.5 × CaCl2, 3 × H3BO3), 19% (2 × CaCl2), 17% (2 × CaCl2, 2 × H3BO3), 30% (2 × CaCl2, 3 × H3BO3) (all × levels relative to MS) | Low Ca2+and | Increasing CaCl2 level to 3 × MS level, and doubling MS level of H3BO3 reduced STN to 17%. High level of KNO3 (2280 mg/l) with 1320 or 1650 mg/l NH4NO3 eliminated STN from 10% at all other concentrations | Nezami et al. (2015) |
| Pistacia integrima × P. atlantica rootstock UCB1 | Optimum SMM: MS + 0.5 mg/l BA, Gamborg vitamins. STN = 41% (control, no CNTs), 37% (50 µg/l CNTs), 30% (100 µg/l CNTs), 23% (150 µg/l CNTs), 13% (200 µg/l CNTs) | CNTs promote or improve physiological processes | Use of 200 µg/l CNTs | Kermani et al. (2017) |
| Pistacia vera L. cv. Mateur | SMM: MS + 3 mg/l BA tested for STN after 5 week. STN = 100% in 28-day cultures | Ca Ca2+ and deficiency | First STN symptoms in 12 days, affecting the whole aerial portion by 16 days. 100, 500 or 1000 µM of B, as H3BO3, reduced STN but 200 µM increased STN. 500 and 1000 µM B stunted shoots. Ca2+ as 0.3 and 3 mM CaCl2, and 15 and 30 mM CG increased shoot number and length, but only 15 and 30 mM could reduce STN, but eliminate it. Shoots immersed in liquid medium + 15 mM Ca2+ prevented STN | Abousalim and Mantell (1994) |
| Pistacia vera L. cv. NR | SMM: unrooted shoots on MS + 4 mg/l BA after 4 week. STN = partly quantified | High humidity in culture jars slowing nutrient flow | Addition of 12 mM CaCl2 reduced STN the most from 2.7/cultured explant to 1.1/cultured explant, but 3–24 mM was an effective range. Ca acetate could also reduce STN but also caused shoot stunting. H3BO3 at 100–800 µM reduced STN from 2.6 (100 µM) to 0.4 (800 µM), but above 200 µM, shoot multiplication was reduced while shoot stunting occurred at 100 and 200 µM. No Ca- or B-free controls were used. Increasing ventilation of adding a liquid medium overlay did not reduce STN | Barghchi and Alderson (1996) |
| Pistacia vera L. cv. NR | Optimum SMM: DKW + 5 µM BA + 0.5 µM IBA + 0.01 g/l AA. STN = 25% (DKW), 45% (MS), 60% (WPM) | Use of CG, shoot density in flasks, flask ventilation, flask volume, bottom cooling | Improvements in STN when using bottom cooling (50% STN), reducing shoot number per flask from 7 to 5 (52% STN), or use of ventilated jars with larger volume (58% STN), relative to the control (75% STN) or the addition of 3 mM CG (80% STN) | García et al. (2011) |
| Pistacia vera L. cv. Ohadi, Kalleghochi | SMM: unrooted shoots on MS + 4 mg/l BA + 0.25 mg/l NAA tested for STN after 4–6 weeks; callus production and media browning also observed. STN = UQ | NAA inhibited CK production; callus that formed at base of shoots used nutrients; rooted shoots may absorb nutrients; insufficient Ca2+ uptake | No suggestions. STN initially detected in Barghchi and Alderson (1983) | Barghchi and Alderson (1985) |
| Platanus acerifolia (Ait.) Willd | Optimum SMM: MS + 1.33 µM BA + 0.27 µM IAA. STN = 57% (gelled medium), 69% (liquid medium), but wide variation (~ 20–69%) depending on the genotype | Use of liquid medium | Use of solid medium (gelled with 7 g/l agar) | Alegre et al. (2015) |
| Populus alba L. × P. tremula L.; P. trichocarpa Torr. & A.Gray ex. Hook. × P. deltoids W.Bartram ex Marshall | Optimum SMM: WPM + 0.5 mg/l MES + 0.02 mg/l TDZ. STN in transformation experiments = UQ | / ratio, especially < 0.8 mM in medium; medium pH < 4.9; Ca deficiency | Medium without TDZ could not form shoots; after 7 days, conc. decreased from 5.0 mM to 1.6 mM; use of 650 mg/l CG + 0.5 mg/l MES + 2.5 µg/l BA allowed shoot growth without STN | De Block (1990) |
| Portulaca grandiflora Hook | Optimum SMM: MS + 4 µM BA + 8 µM Kin. STN = 80–90% (0.1–0.4 mM B); ~ 75–100% (3, 6, 12, 18, 24 and 30 mM CG); 50–85% (3, 6, 9, 12, 24 and 30 mM CaCl2) | Insufficient Ca2+ and | Use of 18 mM CaCl2 reduced STN to 40% | Srivastava and Joshi (2013) |
| Prunus armeniaca L. cv. Helena, Lorna | Optimum SMM + RIM: QL + 1.78 µM BA + 0.2 µM IBA. STN was observed in the rooting phase (~ 65% for ‘Lorna’; ~ 75% for ‘Helena’) | No reason provided | Adding 0.2 mg/l BA reduced STN to ~ 5% in ‘Lorna’ (~ 25% for ‘Helena’), but this also reduced rooting efficiency. High rooting ability of both cultivars maintained with reduced STN when 5–20 mg/l BA added | Pérez-Tornero and Burgos (2000) |
| Prunus armeniaca Lam | Optimum SMM: WPM + 0.5 mg/l BA. STN = UQ, only weighted | Low and . Low mesos (Ca2+, Mg2+, K+) | Critical threshold for CaCl2·2H2O: 2.94x. If CaCl2·2 H2O > 2.94x interaction with KH2PO4, so it should be higher than 1.12x. Recommended level: > 45 mM. Considering STN and other growth factors, optimum range of is > 25 mM and ≤ 35 mM and optimum /Ca2+ ratio is ≤ 0.8 | Kovalchuk et al. (2017a, b, 2018) |
| Pyrus communis cvs. Old Home × Farmingdale 87, Horner 51, Winter Nelis; P. dimorphophylla; P. ussuriensis cv. Hang Pa Li | Optimum SMM: MS + 4.44 µM BA. STN = UQ, but genotype-dependent and characterized as a function of significant interactions between multiple factors | General trends: low mesos (Ca Ca2+, K+, Mg2+) and N caused STN. P. communis: low mesos + low Fe and N; P. dimorphophylla: high NH4NO3, mesos + Fe with low KNO3; P. ussuriensis: low NH4NO3, KNO3 and mesos + high Fe and micros caused STN | STN was reduced by increasing the mesos (P. communis), using low NH4NO3, KNO3 and high mesos (P. dimorphophylla), and using high KNO3 and low mesos (P. ussuriensis). STN frequently occurred simultaneously with other physiological problems such as callus induction, hyperhydricity, hypertrophy, fasciation and formation of hooked leaves | Reed et al. (2013) |
| Pyrus L. cv. Williams, Highland | Optimum SMM: half-strength MS or WPM + 10 µM BA + 55 µM ABA. After 4 weeks, STN was 23%, 31%, 14% and 14% in MS, ½MS, WPM and QL, respectively, for Williams (30%, 52%, 10% and 64% in Highland) | No substantiated reason provided. Level of STN differed with sampling time (2 vs 4 weeks) | Adjusting medium pH, levels of Ca2+, BA or GA3 had no visible effect on STN levels in both cultivars. WPM and low levels of Ca2+ should be used | Grigoriadou et al. (2000) |
| Pyrus sp. cv Punjab Beauty; rootstocks Patharnakh (P. pyrifolia [Burm F.] Nakai), Kainth (P. pashia Buch. Ham.), Shiara (P. serotina Rehd.); wild pear (P. pyrifolia) | Optimum SMM: MS + 4.44 mM BA + 2.46 mM IBA. STN = 79% (Punjab Beauty), 50% (Patharnakh), 25% (Kainth), 18% (Shiara), 7% (wild pear) | Addition of auxins (NAA, IBA), alone or in combination, increased STN in solid or liquid medium, but response was genotype dependent: 38% in control to 40–48% in wild pear, Kainth and Punjab Beauty; 23% in control to 25–30% in Patharnakh and Shiara | Response to Ca2+ and levels was genotype-dependent on half-strength MS. In wild pear, STN = 9% (control), 3% (3 mM Ca2+ + 100 µM ), 56–65% (6 or 9 mM Ca2+ + 100 µM ), 0% (1.5 mM Ca2+ + 100, 200, 500 or 1000 µM ). Except for 1.5 mM Ca2+ + 200 µM , all other Ca2+ and treatments increased STN in Punjab Beauty (from 72% in control to 73–82%). In Patharnakh, STN decreased from 42% (control) to 35–36%, but increased to 50% with 9 mM Ca2+ + 100 µM . These Ca2+ and treatments did not increase or decrease STN in Kainth and Shiara | Thakur and Kanwar (2011) |
| Pyrus sp. rootstocks Pyrodwarf, OHF | Optimum SMM: MS + 2.5 mg/l BA + 0.2 mg/l IBA. STN = 0–68% (OHF), 0–27% (Pyrodwarf) across 27 media with combinations of KNO3, NH4NO3, CaCl2, MgSO4, and KH2PO4, and three basal media (MS, QL, WPM) | ions and ions affected incidence of STN the most in OHF and Pyrodwarf, respectively | Neural network modeling and regression analysis were used to assess the severity of STN and to optimize medium components to reduce the incidence of STN. After model optimization, STN estimated to be 0% in OHF and 0.2% in Pyrodwarf | Jamshidi et al. (2016) |
| Quercus alba L., Q. robur L., Q. rubra L | Optimum SMM: WPM + 0.2 mg/l BA (2 weeks) then 0.1 mg/l BA (4 weeks). STN (Q. rubra only) = 22% (genotype 1), 44% (genotype 2), 3–5% (both genotypes on SMM + 3 mg/l AgNO3 | No reason provided | Addition of 3 mg/l AgNO3 | Vieitez et al. (2009) |
| Rhododendron ‘P.J.M. Hybrids’; Rubus sp. ‘Dirkson Thornless’; Hibiscus rosa-sinensis L | Basal medium: MS (Anderson (1975) for rhododendron) + no IBA (rhododendron) or + 10 µM IBA (hibiscus, blackberry). STN: 18% decrease in hibiscus, 39% decrease in rhododendron when 10 µM IBA used; in blackberry, 21% decrease when 1 µM IBA used, or 13% increase when 10 µM IBA used | Production of autoinhibitory exudate with polyphenols from cut surface | Addition of 10 µM IBA for hibiscus and rhododendron (1 µM IBA for blackberry). Objective was not to assess shoot or root growth, only STN (measured as length of shoot tip or stem blackening). In hibiscus, as BA was increased from 0 to 10 µM, STN increased | Compton and Preece (1988) |
| Rhododendron cv. Dopey, Hoppy and Sneezy; Disanthus cercidifolius Maxim.; Crataegus oxyacantha L. cv. Paul’s Scarlet | Optimum SMM: WPM + 2.5 µM 2iP (Rhododendron); LS + ½ MS (macro) + 3 µM BA (Disanthus); LS + 2.5 µM BA + 0.5 µM IBA (Crataegus). STN = 33–43% (depending on light filter applied) (Crataegus); 0% at 11 or 26 µmol m−2 s−1, 13% at 55 µmol m−2 s−1, and 64% at 106 µmol m−2 s−1 (Disanthus) | High light intensity (55 or 106 µmol m−2 s−1); reduced chlorophyll content; photolysis of endogenous auxin (theory) | STN not observed in any of the three Rhododendron cultivars, which grew equally well at all light intensities. Crataegus and Disanthus cultures should be grown at low light intensities (11 or 26 µmol m−2 s−1) | Marks and Simpson (1999) |
| Rosa clinophylla Thory | Optimum SMM: MS + 28.3 µM AA + 26 µM CA + 58.85 µM AgNO3. STN = 80% (control); Kn and AgNO3 treatments = UQ | Addition of CK (Kn) at 1.16–4.64 µM to SMM | Addition of 58.85 µM AgNO3 | Misra and Chakrabarty (2009) |
| Rosa hybrida cv Tineke | Optimum SMM: MS + 2 mg/l BA + 0.5 mg/l NAA. STN = 0% (0 µM ACC), 6% (10 µM ACC), 13% (25 µM ACC), 31% (50 µM ACC), 38% (100 µM ACC) in the absence of IAA; 38% (0 or 10 µM ACC), 56% (25 µM ACC), 69% (50 µM ACC), 81% (100 µM ACC) in the presence of 1 mg/l IAA | Increased ethylene in response to increased levels of ACC, in the presence or absence of IAA | IAA is likely not the most suitable auxin. The use of ethylene-inhibiting compounds (STS, SNP) improved apical shoot initiation (likely eliminated STN by removing ethylene) | Park et al. (2016) |
| Rubus idaeus L. cv. Allgold, Erika, Polka | SMM: unrooted shoot tips on MS + 0.6 mg/l BA after 30 and 60 d. STN = partly quantified | Browning (18–45% of explants in Allgold, 5–63% in Erika, 18–58% in Polka, depending on the medium); Ca deficiency | Lowest explant browning on medium with 1 mg/l CG (following Singha et al. 1990), resulting in 100% shoot initiation and survival (65–90% in controls (no CG), depending on the cultivar). AA at 50 and 100 mg/l reduced explant browning and STN in Allgold and Polka, but increased both phenomena in Erika. Explant browning and shoot initiation negatively correlated (R = 0.997); STN was also negatively correlated with shoot survival (R = 0.811) | Amalia et al. (2014) |
| Salix tarraconensis Pau ex Font Quer | Optimum SMM: MS + 4.9 µM 2iP. STN (SMM) = 0% in MS or WPM without 2iP; 23–37% (MS + 0.98–9.8 µM 2iP); 50% (WPM + 0.98 µM 2iP). STN (WPM-based rooting medium) = 7% (auxin-free control); 13–27% (1.14–5.71 µM IAA); 27–60% (0.98–4.9 µM IBA); 0–7% (1.07–5.37 µM NAA) | MS medium (relative to WPM). Presence of 2iP in basal medium. Inclusion of IAA and IBA as auxins in rooting medium | Use of a low concentration of 2iP (2.46 or 4.9 µM) in WPM for SMM for low levels of STN (7%). Use of NAA as the auxin for rooting | Amo-Marco and Lledo (1996) |
| Solanum tuberosum L. cv Dark Red Norland | Optimum SMM: MS + 0.5 mM myo-inositol. STN = UQ | Low Ca2+ content | When Ca2+ conc. was increased from 1 µM to 3000 µM, number of axillary shoots decreased from 21 to 1. STN increased when 5 mM EGTA was added to SMM, but decreased when 204 µM strontium was added | Ozgen et al. (2011) |
| Solanum tuberosum L. cv. Burbank, Dark Red Norland, Atlantic, Superior, Snowden | Same as Ahmed and Palta (2017a). STN (Acros/Fischer agar) = 29%/20% (250 μM CaCl2), 18%/13% (500 μM CaCl2), 0%/0% (2000 μM CaCl2) for cv. Atlantic, 29%/21% (250 μM CaCl2), 14%/0% (500 μM CaCl2), 7%/0% (2000 μM CaCl2) for cv. Snowden, 45%/25% (500 μM CaCl2), 17%/18% (1000 μM CaCl2), 0%/6% (2000 μM CaCl2) for cv. Burbank, 34%/23% (500 μM CaCl2), 6%/13% (1000 μM CaCl2), 0%/0% (2000 μM CaCl2) for cv. Superior, 12%/6% (500 μM CaCl2), 0%/7% (1000 μM CaCl2), 0%/0% (2000 μM CaCl2) for cv. Dark Red Norland, at 15 d; 79%/87% (250 μM CaCl2), 59%/38% (500 μM CaCl2), 6%/0% (2000 μM CaCl2) for cv. Atlantic, 29%/28% (250 μM CaCl2), 25%/17% (500 μM CaCl2), 29%/14% (2000 μM CaCl2) for cv. Snowden, 60%/31% (500 μM CaCl2), 38%/25% (1000 μM CaCl2), 22%/6% (2000 μM CaCl2) for cv. Burbank, 44%/33% (500 μM CaCl2), 11%/13% (1000 μM CaCl2), 12%/0% (2000 μM CaCl2) for cv. Superior, 24%/12% (500 μM CaCl2), 6%/7% (1000 μM CaCl2), 0%/0% (2000 μM CaCl2) for cv. Dark Red Norland, at 23 days | Ca deficiency | Inclusion of 250–2000 μM CaCl2 | Ahmed and Palta (2017b) |
| Solanum tuberosum L. cv. Dark Red Norland | Optimum SIM: MS + 0.56 mM myo-inositol. STN = 56% (60 μM CaCl2), 13% (1 μM NAA + 60 μM CaCl2), 0% (2 μM NAA + 60 μM CaCl2), 52% (250 μM CaCl2), 19% (300 μM LPE + 250 μM CaCl2), 14% (400 μM LPE + 250 μM CaCl2), and 14% (500 μM LPE + 250 μM CaCl2) at 15 days; 75% (60 μM CaCl2), 23% (1 μM NAA + 60 μM CaCl2), 13% (2 μM NAA + 60 μM CaCl2), 62% (250 μM CaCl2), 29% (300 μM LPE + 250 μM CaCl2), 20% (400 μM LPE + 250 μM CaCl2), and 24% (500 μM LPE + 250 μM CaCl2) at 25 days | Ca deficiency | Inclusion of 300–500 μM LPE (most effective at 400 μM) in Ca-deficient (250 μM CaCl2) medium. Inclusion of 1 or 2 μM NAA (most effective at 2 μM) in Ca-deficient (60 μM CaCl2) medium | Ahmed and Palta (2017a) |
| Solanum tuberosum L. cv. Russet Burbank, Superior, Norland | Optimum SMM: MS + modified levels of Ca2+ (0.3, 3 or 30 mM), as CaCl2 or Ca(NO3)2. STN = 72%, 62% or 48% (Russet Burbank, Superior, Norland, respectively). STN increased from 60 to 100%, 2% to 32%, and 3% to 15% when Parafilm® was used (vs. use of conventional plastic caps) in Russet Burbank, Superior, Norland, respectively | Low Ca2+ content. Use of Parafilm® | Increasing Ca2+ from 0.3 mM to 3 or 30 mM. STN reduced to 0–5%, 4–9% and 1–3% with 3 or 30 mM (Russet Burbank, Superior, Norland, respectively) | Sha et al. (1985) |
| Soymida febrifuga (Roxb.) A. Juss | Optimum SMM: MS + 2 mg/l BA + 0.2 mg/l NAA. STN = 100% (SMM); 7.5% (MS + 556 mg/l CAN + 1 mg/l vit B5); 5.7% (MS + 556 mg/l CAN + 1 mg/l vit B5 + 20 mg/l AC + 100 mg/l fructose); 3.3% (half-strength MS + 556 mg/l CAN + 1 mg/l vit B5 + 20 mg/l AC + 100 mg/l glucose); 1.9% (half-strength MS + 556 mg/l CAN + 1 mg/l vit B5 + 20 mg/l AC + 100 mg/l fructose) | Low Ca2+ content. Nutrient content of basal medium | Addition of CAN, vit B5, AC, glucose/fructose, usually together | Chiruvella et al. (2011) |
| Trichosanthes dioica Roxb | Optimum SMM: MS + 37.2 µM Kin. Optimum RIM: half-strength MS + 2.14 µM NAA. STN = 82% (SMM) vs 64% (RIM) at 42 days, but lower in earlier cultures (e.g., 16% (SMM) vs 6% (RIM) at 14 days) | Growth stage (rooting > shoot multiplication) | Supplementing SMM with 0.68 mM CaCl2 recovered 93% of shoot cultures with STN (18–38% recovery when 0.34, 1.02 or 1.36 mM CaCl2, or 8–24% when 0.32–1.28 mM H3BO3 was used) | Kishore et al. (2015) |
| Ulmus glabra Huds | Optimum SMM: WPM + 0.4 mg/l BA. STN = UQ | Use of MS (as opposed to WPM) | Use of 0.1 or 0.2 mg/l mT | Mirabbasi and Hosseinpour (2014) |
| Vitis vinifera L. cv. Arka Neelamani | Optimum SMM: MS + 1 µM IAA + 0.1 µM GA3. STN = 36.3%, but only 1–2 years after initial culture establishment | Cuttings with large leaf area or well developed root system. Choice of explant mass and position. Possibly low availability of Ca2+ and Mg2+ in shoot tips | STN cultures had higher root: shoot ratio, more roots, and stunted plants than non-STN cultures. STN cultures had Ca2+ and Mg2+ deficiency in the shoots, but higher levels in the roots, than non-STN cultures. STN ultimately did not negatively impact micropropagation. Solution: selection of explants with medium-sized leaves and density of > 4 plants/vessel | Thomas (2000) |
| Vitis vinifera L. cv. red globe | Optimum SMM: half-strength MS + 1 mg/l BA + 180 mg/l CaCl2 + 1.1 mg/l H3BO3. STN = 39% (2-w subculture), 68% (5-w subculture); 30% (half-strength MS + 1 mg/l BA), 39% (MS + 1 mg/l BA), 48% (half-strength MS + 2 mg/l BA), 51% (MS + 2 mg/l BA), 57% (half-strength MS + 3 mg/l BA), 67% (MS + 3 mg/l BA); (mg/l CaCl2 + mg/l H3BO3): 65% (120 + 1.1), 20% (180 + 1.1), 53% (240 + 1.1), 61% (120 + 2.2), 67% (120 + 3.3) | Low Ca2+ (negative correlation between Ca2+ content and STN; R2 = 0.9682). Infrequent subcultures | Adjusting/optimizing the level of BA, and addition of CaCl2 and H3BO3. Frequent (shorter) subcultures. Use of half-strength MS rather than MS | Surakshitha et al. (2019) |
Only studies for which data or other evidence was provided are shown; all other studies that claimed to have observed STN, but did not provide evidence or show data are discussed only in the text. Studies for which no data exist to support the claim of STN are not included in this table, but are instead discussed in the main text. Studies listed based on alphabetical botanical name of plant
2iP N6-(2-isopentenyl) adenine, AA ascorbic acid, AC activated charcoal, ACC 1-aminocyclopropane-1-carboxylic acid, AdS adenine sulfate, AgNO3 silver nitrate, B boron, BA N6-benzyladenine (BA is used throughout even though BAP (6-benzylamino purine) may have been used in the original (Teixeira da Silva 2012), B5 Gamborg et al. (1968) medium, Ca calcium, CA citric acid, CaCl2 calcium chloride, CAN calcium ammonium nitrate (H4CaN2O3), CG calcium gluconate, CK cytokinin, CNT carbon nanotube, cv cultivar, DKW Driver and Kuniyuki walnut medium (Driver and Kuniyuki 1984), EGTA ethylene glycol tetra acetic acid, GA3 gibberellic acid, H3BO3 boric acid, IAA indole-3-acetic acid, IBA indole-3-butyric acid, Kin kinetin (6-furfurylaminopurine), LPE lysophosphatidylethanolamine, LS Linsmaier and Skoog (1965) medium, MES 2-(N-morpholino)ethanesulfonic acid, mesos CaCl2·2H2O, KH2PO4, MgSO4, Mg magnesium, MS Murashige and Skoog (1962) medium, mT meta-topolin, mTR meta-topolin riboside, NAA α-naphthaleneacetic acid, NN Nitsch and Nitsch (1969), NR not reported, PGR plant growth regulator, PVP polyvinylpyrrolidone, QL Quoirin and Lepoivre (1977), RIM root induction medium, s second(s), SEM shoot elongation medium, SGM seed germination medium, SIM shoot induction medium, SMM shoot multiplication medium, SNP sodium nitroprusside, STN shoot tip necrosis, STS silver thiosulfate, TDZ thidiazuron (N-phenyl-N'-1,2,3-thiadiazol-5-ylurea), UQ unquantified, vit vitamin, WPM woody plant medium (Lloyd and McCown 1980), Zea zeatin (6-(4-hydroxy-3-methylbut-2-enylamino)purine)
aUnlike the majority of other studies where STN was observed after explants were plated or at different stages of in vitro multiplication, in this study, a form of STN was induced as a result of damage induced to shoot tips during explant preparation
Fig. 2.
Incidence of shoot tip necrosis (STN) in in vitro cultures of walnut (Juglans regia L.) Paradox rootstock during micropropagation in Driver and Kuniyuki walnut medium (DKW; Driver and Kuniyuki, 1984) (unpublished results). (1) if 3-week-old shoots were used, the incidence of STN was high (20–30%), most likely because tissue is soft (non-lignified), but the use of 4-week-old shoots, which are more lignified, have a lower incidence of STN, even reduced to 0%; (2) initial “Vlach” [a selection of Paradox (J. hindsii x J. regia)] material is from a 110-year-old mother tree, located near Modesto (CA, USA) for which in vitro cultures were originally established by John Driver in 1985; (3) walnut tends to be somewhat recalcitrant to rooting, so occasionally high concentrations of IBA (8–10 mg/l) are added to rooting medium. If IBA is transported to the shoot tip, especially soft shoots that may take up excessive amounts of IBA, this may result in the death to the shoot tip, a condition we coin as “IBA burn”, which is visually similar to STN. However, this does not take place if more mature shoots are used and this can be achieved by increasing the subculture interval from 3 to 4 weeks. Black arrows indicate STN. Scale bars indicate 3 cm (top), 2 cm (middle) and 2 cm (bottom)
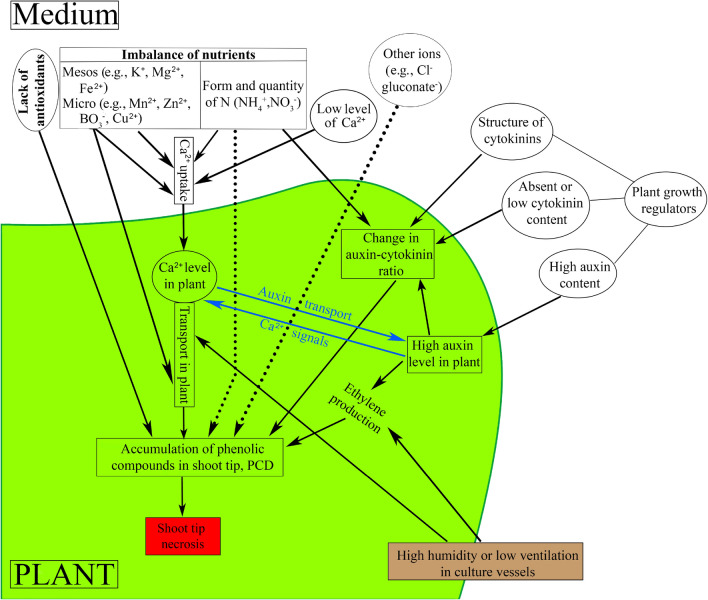
De Block (1990) found that STN was linked to Ca deficiency and associated with the use of Woody Plant Medium (WPM; Lloyd and McCown 1980). De Block (1990) also noted that the occurrence of STN might have been caused by a decrease in medium pH, possibly as a result of ammonium () uptake by shoots. Relative to Murashige and Skoog (1962) (MS) medium, WPM has almost the same Ca2+ content (≅ 3 mM in WPM), about a quarter the concentration of (20.61 vs 5.00 mM) and nitrate () (39.41 vs 9.71 mM), about two-thirds the content of K+ (20.05 vs 12.61 mM), about a quarter of the /Ca2+ ratio, but more than 1.5-times higher Ca2+/K+ ratio (Suppl. Table 2). MS was employed in 68.6% of the studies listed in Table 1 while 21.4% used WPM. This suggests that the use of these basal media is not recommended, especially for trees and woody shrubs. This is curious if one considers that WPM was designed specifically for Ericaceous woody plants. The most popular theory for the cause of STN is related to nutrient deficiency and imbalance. Another is the impact and imbalance of PGRs. These possibilities are explored in greater detail next.
Nutrient deficiencies
Calcium deficiency
The most commonly ascribed reason for STN is Ca deficiency (32.8% of studies in Table 1). Table 1 indicates that one of the most popular methods to relieve STN has been to increase Ca2+ concentration in the culture media (35.9% of studies in Table 1). In pistachio, Barghchi and Alderson (1985) suggested that STN was caused by Ca and boron (B) deficiency, but only on some shoots that had not rooted. Dolcet-Sanjuan and Claveria (1995) reduced STN by lowering the concentration of Ca2+ (as calcium chloride, CaCl2·2H2O) in medium to one-third of the level in MS, and by reducing the subculture period from 4–6 weeks to 3 weeks.
Kovalchuk et al. (2017a) used a CART (classification and regression tree analysis) decision tree to model the incidence of STN in wild apricot (Prunus armeniaca L.) shoot cultures based on previous response surface methodology (RSM). They noted that no STN developed in wild apricot shoots when CaCl2·2H2O was < 2.94 mM, which is the precise concentration of CaCl2·2H2O in WPM medium (Suppl. Table 2). Furthermore, the Reed et al. (2013) study of pear (one of several connected studies), which was based on MS medium, noted an increase in STN with low mesos (CaCl2·2H2O, MgSO4·7H2O, KH2PO4) but also the involvement of ammonium nitrate. Wang and van Staden (2001) doubled the concentration of CaCl2 in WPM to 6 mM to reduce the incidence of STN in tree peony (Paeonia × suffruticosa Andrews) cultures. Machado et al. (2014) found that the incidence of STN was halved when the level of CaCl2·2H2O was increased threefold from 440 to 1320 mg/l (from 3.96 mM to 11.89 mM) in true lavender (Lavandula angustifolia Mill.) shoot multiplication medium. Christensen et al. (2008) completely eliminated STN in Chinese hibiscus (Hibiscus rosa-sinensis L.) shoot cultures after increasing CaCl2 concentration in MS from 2.99 mM to 9 mM, independent of the N6-benzyladenine (BA) concentration used (0.22 or 2.2 µM). STN was observed in cultures of potato (Solanum tuberosum L.) ‘Dark Red Norland’ when insufficient (68 µM) CaCl2 was provided, resulting in a loss of apical dominance and enhanced axillary branching, a response that did not occur when there was sufficient (1360 µM) Ca2+ in medium (Busse et al. 2008). The level of CaCl2 was one of the factors that affected the level of STN in Indian lilac (Azadirachta indica A. Juss) cultures (Arora et al. 2010). In potato ‘Dark Red Norland’, Ozgen et al. (2011) ascribed the increase in STN, as a result of low Ca2+ levels in medium, to injury of the shoot tip and subsequent loss of apical dominance, thereby stimulating axillary shoot formation. In Indian redwood (Soymida febrifuga (Roxb.) A. Juss.) cultures, the simultaneous use of calcium nitrate and calcium pantothenate (vitamin B5) at intermediate concentrations could eliminate the incidence of STN (Chiruvella et al. 2011, 2014). Mubina et al. (2018) eliminated STN by doubling the MS-based levels of CaCl2 and KNO3 in chickpea (Cicer arietinum L.) shoot regeneration medium. Nutrient deficiencies or excesses sensu lato accounted for 9.4% of the studies in Table 1. Thirugnanasampandan et al. (2009) found that an adjustment of CaCl2 and MgSO4 in sarasaparilla (Smilax zeylanica Vent.) shoot regeneration medium prevented STN. In lentil (Lens culinaris Medikus), increasing Ca2+ (up to 750 mg/l, i.e., 6.75 mM) and BA concentration (0.2–0.6 mg/l, i.e., 0.89–2.66 µM) in MS and B5 (Gamborg et al. 1968) basal media decreased the incidence of STN (Ye et al. 2002). That decision that was based on earlier research by Parh et al. (1998). Wetzstein et al. (1989) noted STN during the acclimatization stage of pecan nut (Carya illinoensis (Wangenh.) K. Koch) and not in vitro, reducing its incidence by applying a foliar spray of 0.4% calcium nitrate.
Another way to increase Ca concentration in plant culture medium is using calcium gluconate (6.3% of studies in Table 1), usually from the start of shoot induction or multiplication stages (McCown and Sellmer 1987). The application of Ca-gluconate during in vitro culture of hybrid aspen (Populus alba × Populus tremula) and poplar (Populus trichocarpa × P. deltoides) eliminated STN in 50% of the shoots (De Block 1990). However, if 3 mM Ca-gluconate was combined with 0.5 mg/l (2.5 µM) 2-(N-morpholino)ethanesulfonic acid (pH 5.8), a buffer, then STN was totally eliminated. This may be because Ca-gluconate uptake into cells has a different pathway, via the glucose uptake system, and this form of Ca2+ does not involve the release of toxic chloride if CaCl2 is used, allowing medium pH to be stabilized and thus ion exchange and uptake to occur at an optimum pH range of 5.6–5.9 (Pasqua et al. 2002). However, the supply of additional Ca2+ via CaCl2 can also increase the concentration of chloride (Cl−) ions, similar to the use of NaCl, and this may be toxic to plant tissues (McCown and Sellmer 1987). In wild apricot, Pérez-Tornero and Burgos (2000) found that the addition of calcium nitrate or Ca-gluconate decreased the incidence of STN but also lowered rooting ability.
Shoot growth rate may be balanced by Ca2+ supply to shoots to avoid STN. This balance might depend on species and cultivars, the concentration of other nutrients in the medium that might modify Ca uptake, as well as the tissue or plant’s developmental stage. The form of Ca2+ may also affect STN since the same ion (Ca2+) content (Suppl. Table 2) can be supplied by different additives (salts or organic forms), but with different uptake mechanisms (Thor 2019) and thus various effects on STN (Table 1). The organic form has a dual uptake mechanism: (1) after dissociation of the Ca2+ ion through the highly regulated Ca2+ uptake system which is strongly affected by the culture conditions (pH, relative concentration of other cations and anions, etc.); (2) without dissociation, the organic form of calcium is taken up directly into the cytoplasm via the uptake system but the organic part is under completely different regulation (White and Broadly 2003).
Boron deficiency
Unlike Ca deficiency, where the effect of STN occurs on younger leaves in the growing meristem and develops basipetally, STN caused by B deficiency (6.3% of studies in Table 1) affects older leaves and spread upwards, or acropetally, as was reported in pistachio (Abousalim and Mantell 1994). Martinelli (1988) indicated the same problem in zebrawood (Pistacia integerrima J.L. Stewart ex Brandis) and Mt. Atlas mastic tree (Pistachia atlantica Desf.). Similarly, Parfitt and Almehdi (1994) found STN in hybrid pistachio UCB-1 (P. atlantica × P. integerrima), independent of the basal medium used, suggesting that the condition was not based on nutrients. Abousalim and Mantell (1994) confirmed these findings, noticing STN in P. vera cv. Mateur shoot cultures, but partially resolved this by adding calcium (Ca2+) or boron (). Barghchi and Alderson (1996) used the same approach (see details in Table 1) but could also reduce STN using liquid medium. There is an interaction between and Ca2+ uptake (Fox and Albrecht 1958): (1) a high concentration can improve the uptake of Ca2+; (2) boron helps the movement of Ca2+ in plants. However, Abdulnour et al. (2000) described that high concentrations could adversely affect Ca2+ uptake, even causing toxicity if levels were as high as 0.4 mM, as in the case of devil’s claw (Harpagophytum procumbens (Burch.) DC. ex Meisn.) (Bairu et al. 2009a). Boron deficiency often appears to occur in in vitro cultures of Pistachia species. However, the proper balance of nutrients should be assessed due to their interaction.
Nitrogen deficiency: nitrogen form and quantity
Grigoriadou et al. (2000) found that the occurrence of STN in pear was cultivar dependent and strongly related to the basal medium used. In their study, the application of Quoirin and Lepoivre medium (1977) resulted in the highest rate of STN (64%) in the case of ‘Highland’, while they observed that most shoots were affected by STN on half-strength MS medium in ‘Williams’ (31%). The former medium contains about a quarter the level of , a quarter of the / ratio, and only about 14% of the /Ca2+ ratio compared to MS medium. However, the rate of STN was only 10% in ‘Highland’ and 14% in ‘Williams’ when shoots were cultured on WPM, in which the / ratio is the same as in MS medium but the total level of N and the /Ca2+ ratio is only one-quarter of that in MS. In shoot cultures of wild apricot (Kovalchuk et al. 2017a), the use of RSM showed that some STN occurred in control shoot cultures in WPM. However, the influence of and was much stronger, i.e., when the concentration of these nutrients was low, STN was higher (Kovalchuk et al. 2017b). Ultimately, the recommended level of was > 45 mM (Kovalchuk et al. 2018). Excessive and in two pear rootstock cultures (12.3 and 13.2 mM for OHF; 22 and 20.9 mM for Pyrodwarf) resulted in STN (Jamshidi et al. 2016). In contrast, a shortage of N in dunns white gum (Eucalyptus dunnii Maiden) cultures resulted in STN, and the minimum level of N required was 27.7 g/kg (Oberschelp and Gonçalves 2018). The total N content and/or the / ratio differ in several media commonly used for the micropropagation of various plant species (Suppl. Table 2; Phillips and Garda 2019). These can cause variation in the growth and developmental responses of in vitro shoots. From the above results, the occurrence of STN appears to depend mainly on the quantity and form of N, the /Ca2+ ratio, and the quantity of mesos elements [mainly Ca2+, magnesium (Mg2+) and potassium (K+)] in medium (Reed et al. 2016; Kovalchuk et al. 2017a, b).
Interaction of other ions on STN: the ion-confounding effect
Unlike the above studies, which concluded that one of the main reasons for STN was Ca deficiency, some studies did not show any effect of Ca2+ on STN (4.7% of studies in Table 1). When Piagnani et al. (1996) applied CaCl2 at 3, 9 or 18 mM, this did not reduce the incidence of STN in two sweet chestnut cultivars. In fact, 18 mM CaCl2 reduced rooting. When Grigoriadou et al. (2000) increased the level of Ca2+, this did not decrease the incidence of STN in pear. Thomas (2000) observed that the balance of Ca2+ and Mg2+ ions in roots and shoots was responsible for STN. Unlike the trend in most of these studies, Offord and Tyler (2009) found that the addition of Ca2+ to half-strength MS medium almost doubled STN in an endangered Australian shrub, pink pimelea (Pimelea spicata R.Br.).
Recently, the implementation of knowledge-based design of experiment (DOE) techniques has been widely used for understanding and improving the performance of complex in vitro systems (for example, Wada et al. 2015; Kovalchuk et al. 2017a). Niedz and Evens (2016) reviewed the greatest advantage of DOE in simultaneously minimizing the quantity of data while maximizing data quality based on considering only low order interactions in multi-factor studies (“hierarchical ordering”) on the basis of “sparsity of effects” wherein just a few factors would drive the system efficiently (Box and Meyer 1986).
The use of DOE by Reed et al. (2013) enabled them to conduct a unique experiment to simultaneously study the effect of all macro- and microelements of MS medium on a wide range of physiological disorders in diverse pear germplasms. They divided mineral nutrients of MS medium into five independent groups with the advantage of reducing the required treatment numbers from 3125 (55) to just 43 treatments. Noticeably, their findings asserted that STN is a genotype-dependent disorder that is affected by an imbalance of nutrients in culture media. Therefore, deficiencies in mesos (CaCl2·2H2O, MgSO4·7H2O, and KH2PO4) or nitrogen (either NH4NO3 or KNO3) commonly contributed to STN. Wada et al. (2013, 2015) followed the same approach to improve the quality of many in vitro pear genotypes by readjusting nutrients in MS medium, such as increasing mesos (CaCl2, MgSO4, KH2PO4) with increased nitrogen, to eliminate all physiological disorders. In their studies, STN was more evident with lower Ca2+ content than MS-based concentrations although lower concentrations of some mesos, including in the MS medium control, may have accounted for the disorders, although no general trend was observed. In addition to the level of CaCl2, Arora et al. (2010) reported that other nutrients, principally Ca(NO3)2, Na2SO4, and K2SO4 in basal MS medium, also affected the level of STN in Indian lilac (Azadirachta indica A. Juss.) cultures.
The next challenge of tissue culture studies are ion-confounding problems (Niedz and Evens 2006, 2007), wherein salts are subjected as factors in an experimental design and analysis rather than ions by themselves, whilst ions drive the system. For instance, many authors have frequently tried to alleviate STN in different species by increasing the amount of MS-CaCl2 because this unique salt contains the Ca2+ ion. CaCl2 in MS medium releases 2.99 mM Ca2+ plus 6 mM Cl− into solution (Suppl. Table 2). Therefore, it is inconclusive to attribute the problem of STN exclusively to Ca2+ deficiency while the role of Cl− is completely overlooked. Numerous examples of this inconclusiveness can be found in the literature (Barghchi and Alderson 1996; Piagnani et al. 1996; Bairu et al. 2009a, 2009b; Ozgen et al. 2011; Machado et al. 2014; Poothong and Reed 2014; Surakshitha et al. 2019). Nevertheless, it has recently been proved that Cl− (> 4.67 mM) has a positive effect on reducing STN symptoms in pistachio (Nezami-Alanagh et al. 2019). To the best of our knowledge, the latter study was the first report of the beneficial effect of Cl− on controlling STN in plants.
Ca-gluconate has been reported as a way to alleviate STN in herbal medicinal plants (Srivastava and Joshi 2013), woody shrubs (Amalia et al. 2014), fruit trees (Abousalim and Mantell 1994; Pérez-Tornero and Burgos 2000), and other trees (De Block 1990; Pasqua et al. 2002). As far as we know, the only report to assess the individual role of the gluconate− ion (C6H11O7−) in plant growth and development was Nezami-Alanagh et al. (2017). Using artificial intelligence models, a significant negative influence of gluconate− concentration (range 0.0–6.02 mM) on two growth parameters (shoot length and total fresh weight) during pistachio micropropagation was determined. Thus, we strongly advise to cautiously use gluconate in medium formulations for plant micropropagation. Moreover, we also encourage the use of any method (statistical, response surface methodology, chaid or artificial intelligence) that allows the simultaneous study, on one hand, of the effect of a single ion, and on the other hand, of interactions between several factors.
Plant growth regulators affect STN
Another popular theory to explain the cause of STN is the effect of the level and type of PGRs in the medium. STN has been linked to the level of PGRs in 23.4% of the studies in Table 1. However, an increase in PGRs may alleviate some nutrient deficiencies (Preece 1995). This fortifies the notion that nutrient deficiency is the major cause of STN. STN in apple (Malus × domestica Borkh.) was attributed to low endogenous hormone content (Kataeva et al. 1991). According to Kataeva et al. (1991), in the absence of roots, where cytokinins (CKs) are mainly synthesized, endogenous CK concentrations in shoots decrease. This affects the synthesis of auxin in the shoot apical meristem, stimulating STN. In sweet chestnut and oak, the absence of CK (BA) in rooting medium, or the presence of a low concentration of BA, induced STN, although the application of BA to cut ends of shoots prior to rooting increased axillary shoot production (Vieitez et al. 1989). When Piagnani et al. (1996) added 5 µM BA to sweet chestnut shoot tips, STN was eliminated, but a mixture of 5 µM BA and 3 mM CaCl2 delayed STN. A CK × Ca2+ × interaction on STN was observed in grape (Vitis vinifera L.) cv. Red Globe where supplementary CaCl2 and H3BO3 were needed to suppress STN, even after the level of BA had been optimized (Surakshitha et al. 2019). Thomas (2000) observed that CK concentration had no signficant effect on STN. Surakshitha et al. (2019) did not observe this effect in grape; instead, the level of STN depended on BA concentration. When BA concentration was increased from 8.9 µM (0% STN) to 17.8 µM, cane apple (Arbutus unedo L.) cultures displayed 8.7% STN (Gomes et al. 2010). Pérez et al. (1985) reduced STN in filbert (Corylus avellana L.) by adding indole-3-butyric acid (IBA) to medium at a low concentration (10 or 25 µM), or by reducing the period of exposure to IBA. In apricot, dipping shoot tips in a solution of BA (1.78–3.11 µM, depending on the cultivar) prior to culture in rooting medium alleviated STN while kinetin had no effect (Pérez-Tornero and Burgos 2000). The mere presence of 2.5 µM BA in MS medium induced STN in moringa (Moringa oleifera Lam.) (Hassanein et al. 2018). In pistachio micropropagation, STN was significantly reduced when BA was added at high concentrations (5.77 < BA < 6.66 µM) to basal media (Nezami-Alanagh et al. 2019).
In contrast, in blackberry (Rubus sp. ‘Dirkson Thornless’), rhododendron (Rhododendron ‘P.J.M. Hybrids’) and Chinese hibiscus, when Compton and Preece (1988) increased BA concentration to 10 µM, STN increased (details in Table 1). Norton and Norton (1985) also noticed STN in Gaultheria sp. and Rhododendron sp. (Ericaceae) when any concentration of BA was used, although 17 other Ericaceae species did not show STN. As mentioned above, Podwyszyńska and Goszczyńska (1998) found that when indole-3-acetic acid (IAA) was present in medium, the incidence of STN increased in rooting cultures of dwarf rose (Rosa gymnocarpa Nutt. ‘Starina’). Lin et al. (2011) also observed STN in Korean pasque flower (Pulsatilla koreana) shoots on MS-based rooting medium containing BA and IAA. Serres et al. (1990) observed STN in American chestnut (Castanea dentata [Marsh.] Borkh.) in rooting medium containing IBA, and only the top node was affected, allowing lower axillary shoots to form shoots and thus not influencing explant survival. Bairu et al. (2009a) found that the inclusion of BA increased STN in devil’s claw, even more so when an auxin (IAA) was also added. However, the inclusion of meta-topolin (mT) or meta-topolin riboside (mTR; more background in Aremu et al. (2012)) could reduce—but not eliminate—the incidence of STN. Kinetin stimulated STN in Rosa clinophylla Thory cultures (Misra and Chakrabarty 2009). In buchu (Coleonema pulchellum I.Williams) shoot-inducing cultures, STN only occurred when thidiazuron (TDZ) was applied at 13.6 µM in MS basal medium, or in response to 300 µM casein hydrolysate or mebendazole, 40 µM glutamine, or 40 µM glutamine in combination with 4.5 µM TDZ (Baskaran et al. 2014). STN was also observed in grape ivy (Cissus rhombifolia Vahl, syn. Cissus alata Jacq.) shoot cultures grown in the presence of 4.5 µM TDZ, but not in response to 4.4 µM BA (Dewir et al. 2018). The use of 2 µM TDZ, or even the lack of TDZ, induced STN in 100% of white saxaul (Haloxylon persicum (Bunge ex Boiss and Buhse)) shoot cultures. The latter was also associated with stem fasciation, a common response to high concentrations of TDZ (Dewir et al. 2018). Intermediate concentrations (0.5 or 1 µM) of TDZ reduced the incidence of STN by 10–14% (Kurup et al. 2018). The incidence of STN was reduced when 0.1 or 0.2 mg/l (0.8 µM) mT was added to the shoot multiplication medium of Scots elm (Ulmus glabra Huds.) shoots (Mirabbasi and Hosseinpour 2014). When Marín et al. (2016) replaced BA with 5 µM mT in pistachio shoot culture medium, STN was reduced to 20% of cultures.
The application of 15 mg/l (40.7 µM) adenine sulfate prevented STN in nannaari (Hemidesmus indicus (L.) R.Br.) (Nagahatenna and Peiris 2007). When Naaz et al. (2014) added 100 mg/l (271.3 µM) adenine sulfate to BA-supplemented MS medium (WPM resulted in higher levels of STN), STN was reduced to 10% in jambolan (Syzygium cumini (L.) Skeels.) shoot cultures.
Several other studies assessed the ability of PGRs to reduce STN. Podwyszyńska and Goszczyńska (1998) significantly reduced the incidence of STN in dwarf rose rooting medium containing IAA by adding 2.5–10 mg/l (14.7–58.8 µM) silver nitrate (AgNO3), and by increasing the level of MS-based Ca2+ 1.5-fold (increasing the level of MS-based Mg2+ twofold was optional). AgNO3 is an effective ethylene inhibitor (Purnhauser et al. 1987). Vieitez et al. (2009) reduced the incidence of STN in northern red oak (Quercus rubra L.) cultures by supplementing medium with 3 mg/l (17.6 µM) AgNO3. Martínez et al. (2017) found that AgNO3 at 20 µM reduced the incidence of STN in evergreen oak (Quercus ilex L.) cultures. Park et al. (2016) found that the production of ethylene in rose (Rosa hybrida cv. Tineke) shoot multiplication medium increased the level of STN. They proved this by applying different levels of an ethylene promoter, 1-aminocyclopropane-1-carboxylic acid (ACC), to medium. Ahmed and Palta (2017a) reduced the incidence of STN in Ca2+-deficient (6.7 or 27.75 mg/l (60.3–250 µM) CaCl2) potato shoot induction medium by adding 1 or 2 μM NAA, or 300–500 μM lysophosphatidylethanolamine (a phospholipid). Curiously, Ahmed and Palta (2017b) found that agars with different levels of Ca2+ significantly affected the level of STN: Acros agar was Ca2+ deficient (22.92 mg/l (0.5718 mM)) while Fischer Scientific agar was slightly Ca2+ deficient (84.36 mg/l (2.1 mM)) relative to the control (MS Ca level = 3000 μM or 120.23 mg/l). However, supplementation with 27.75–221.96 mg/l (0.25–1.99 mM) CaCl2 reduced or eliminated STN in five potato cultivars (see details in Table 1). If auxin is used in excess, especially in juvenile pistachio cultures at the rooting stage, STN may develop (Fig. 2).
The ability of endogenous and exogenously added PGRs to alter the level of STN in response to PGR type and concentration, especially during the rooting phase, suggests their important role in STN. To limit or prevent STN, an adequate level of BA and TDZ should be applied, while the application of mT and/or its derivatives may be beneficial. Broadly, altering the type or level of exogenously applied PGRs in plant in vitro cultures might not impact STN exclusively, but might also impact many mechanisms, while different genera or species might respond differently (Cardoso et al. 2018). Auxins should not be used at excessive concentrations while ethylene production should be inhibited as much as possible. Excessive ethylene production in plant in vitro cultures can be avoided by applying auxins at a suitable concentration, by increasing aeration of culture vessels (Kumar et al. 1998), using aerated containers, or it can be inhibited by applying ethylene inhibitors such as AgNO3 (Teixeira da Silva 2013).
Other factors and interactions impacting the incidence of STN
Timing of measurements and subculture length
Grigoriadou et al. (2000) noted that the level of STN was much higher at 4 weeks than at 2 weeks, suggesting that sampling time influenced the quantitative outcome. This issue was not raised in most other studies on STN but is an important issue to consider when dealing with plant tissue cultures (Teixeira da Silva and Dobránszki 2013). Srivastava and Joshi (2013) found that STN was 62% after 2 weeks, but 90% after 4 weeks in rose moss (Portulaca grandiflora Hook.) cultures. The same time-dependent incidence of STN was observed in tissue cultures of five pear cultivars (Thakur and Kanwar 2011). The time-sensitive outcome of STN was also observed by Kishore et al. (2015) in pointed gourd (Trichosanthes dioica Roxb. var. Swarna Alaukik). They observed higher STN (83%) during shoot multiplication at 42 days than at 14 (16%), 21 (44%), 28 (61%), and 35 (72%) days on MS medium containing 3% sucrose, 0.8% agar, 0.02% carbendazim and 37.17 µM kinetin. Ahmed and Palta (2017a) observed 56% STN in Ca2+-deficient (60 μM CaCl2; 52% STN with 250 μM CaCl2) shoot induction medium of potato cv. Dark Red Norland when sampled at 15 days, but 75% STN after 25 days (62% STN with 250 μM CaCl2). In other words, reported STN levels were higher in older cultures. Similarly, Ahmed and Palta (2017b) found higher levels of STN in the majority of five potato cultivars (i.e., a genotype-specific response) when two Ca2+-deficient agar brands were used in shoot induction medium and sampled at 23 days relative to 15 days. Thakur and Kanwar (2011) also observed STN during in vitro rooting on semisolid and liquid medium in five pear cultivars: 6%, 28%, 39%, 49%, and 64% of cultures displayed STN at 14, 21, 28, 35, and 42 days (details in Table 1). Sudha et al. (1998) attributed a long culture period, in excess of 8 months, to the incidence of STN in arka (Holostemma annulare (Roxb.) K. Schum.). Amin and Jaiswal (1988) also attributed STN to excessive subculture length in guava (Psidium guajava L.) for cv. Chittidar during shoot tip (derived from mature plants) culture on MS medium with 4.4 µM BA. Papadatou et al. (1990), however, did not observe any STN when seedling-derived shoot tips of the same guava cultivar was used on Rugini olive medium (Rugini 1984) with 8.8 µM BA. Delaying the subculture period longer than 2 weeks induced STN in rose and miniature Chinese rose (Rosa chinensis minima (Sims) Voss.) (Hsia and Korban 1996). Ca2+ concentration that exceeded 6 mM negatively impacted Pistacia vera shoot growth and increased shoot chlorosis, but a reduction of the subculture period from 4–5 weeks to 3 weeks reduced the incidence of STN (Dolcet-Sanjuan and Claveria 1995). Tilkat et al. (2008) also found 3 weeks to be suitable for reducing STN in pistachio cultures. Alderson et al. (1987) suggested that increasing the frequency of subcultures, thus reducing the subculture period, could reduce the incidence of STN in dwarf Russian almond (Prunus tenella Batsch). A longer subculture length was also associated with hyperhydricity, which is frequently caused by the accumulation of ethylene in cultures (Park et al. 2004).
These results are not surprising. One cause of STN is the deficiency of nutrients, so the chance of nutrient deficiencies within a subculture increases over time as nutrients become depleted (Ramage and Williams 2002). The timing of sampling can influence the reported outcome of STN, although the likelihood of STN is higher in older cultures and may be related to changes in the nutrient content of tissue culture medium over time.
Genotype-specific responses
Mythili and Thomas (1999) successfully micropropagated two female cultivars (Swarna Alaukik and Swarna Rekha) and one male line of pointed gourd on MS medium but noted a decline in transferable nodes in Swarna Alaukik due to leaf chlorosis if subculture was delayed by 8 weeks. In contrast, no symptoms of STN were observed in pointed gourd accession IIVRPG-102 (Kumar et al. 2016), suggesting that STN could be a genotype-specific response or due to the presence of carbendazim, as was also reported by Kishore et al. (2015). Thakur and Kanwar (2011) observed STN between the 6th and 8th week at the shoot regeneration stage in three pear rootstocks (P. pyrifolia [Burm F.] Nakai, P. pashia Buch. Ham. and P. serotina Rehd.), and two scion cultivars ‘Patharnakh’ (P. pyrifolia [Burm F.] Nakai) and ‘Punjab Beauty’ (P. pyrifolia x P. communis), but the level of STN was dependent on genotype. Thakur and Kanwar (2011) found a genotype dependence in response to Ca and B supplementation. When 3 µM Ca2+ (up from 1.5 µM) and 200 µM were used, this completely alleviated the incidence of STN in the wild cultivar (from 9.12% to 2.60%) but had no significant effect nor did it prevent STN in the remaining four cultivars. In London plane tree (Platanus acerifolia (Ait.) Willd), Alegre et al. (2015) found a clear influence of genotype on the incidence of STN during shoot multiplication, with a wide range (~ 20–69%) of affected cultures that was genotype dependent. Thus, the susceptibility of a plant to develop STN might be both species and cultivar dependent.
Choice of basal medium
Bosela and Michler (2008) also noticed that the choice of basal medium affected the level of STN in Eastern black walnut (Juglans nigra L.). However, this was also dependent on the in vitro developmental stage and the CK used, with higher levels of STN observed in the presence of Driver and Kuniyuki walnut medium (DKW; Driver and Kuniyuki 1984) and zeatin. Similarly, in unpublished results, STN was observed in vitro cultures of walnut Paradox rootstock during micropropagation in DKW medium (Fig. 2). Shoots were first multiplied on DKW basal medium supplemented with 1 mg/l (4.4 µM) BA, 0.1 mg/l (0.49 µM) IBA and 30 g/l sucrose and subcultured every 3 weeks. Three-week-old shoots, in preparation for rooting, were first placed in the dark for 5 days at 24 °C. STN was observed in rooting medium consisting of DKW free of cytokinins (BA), but including 10 mg/l (44 µM) IBA and 50 mg/l (146 µM) sucrose. After 5 days in rooting medium, auxin-induced shoots were placed in a greenhouse and exposed to high relative humidity (> 95%). These induced shoots rooted and acclimatized concurrently ex vitro.
Curiously, García et al. (2011) observed quite the opposite in pistachio where DKW medium resulted in lower levels of STN than in MS or WPM media. They attributed STN to the three times higher levels of Ca2+ in DKW (relative to MS and WPM). Moreover, some authors previously recommended the inclusion of calcium gluconate to prevent STN (Abousalim and Mantell 1994). However, Nezami-Alanagh et al. (2017) fund that gluconate− had an adverse effect on in vitro pistachio plant growth. In high-bush blueberry (Vaccinium corymbosum L.), the use of MS medium induced STN, especially when 0.5 mg/l zeatin was used with higher concentrations (> 1 mg/l) of IBA, but when this was replaced by Anderson’s rhododendron medium (Anderson 1984), STN was eliminated (Ružić et al. 2012). Anderson’s rhododendron medium, relative to MS medium, contains about one-quarter the concentration of K+, and (Suppl. Table 2). Martin et al. (2007) tested several factors, including PGRs, carbohydrate sources, and AgNO3, in the media of subcultured necrotic shoots to try and improve the incidence of STN in in vitro banana (Musa spp.) cultures. Normal shoots were recovered only with the addition of 50–100 mg/l (0.45–0.9 mM) of CaCl2. When full-strength MS medium was used, STN was observed in Zeyheria montana Mart. cultures, but not when half- or quarter-strength MS was used (Cardoso and Teixeira da Silva 2013). Similarly, full-strength MS medium induced STN in Barbados nut (Jatropha curcas L.) cultures, but not half-strength MS (Daud et al. 2013), an outcome that Dangi et al. (2014) also observed for bahera (Terminalia bellerica (Gaertn.) Roxb.). Using basal CK medium that had diluted levels of MS micro- and macronutrients (Cellárová et al. 1992), Moura (1998) found 15% and 23% STN in shoot initiation and elongation stages, respectively, of leafy St. John’s wort (Hypericum foliosum Aiton). The use of WPM induced more STN than MS in the multiplication of wych elm (Ulmus glabra Huds.) shoots (Mirabbasi and Hosseinpour 2014). Consequently, the choice of appropriate basal medium can be a solution in itself. Further, altering the level of certain ions, especially Ca2+, can also help to reduce STN. However, changing a single medium constituent might affect the uptake or utilization of other nutrients, while agar source and type may affect micronutrients, as discussed elsewhere in this review. Thus, this solution should be viewed cautiously. Moreover, several species responded well to reduced MS salts.
Antioxidants
Amalia et al. (2014) also noticed some (unquantified) reduction in STN of raspberry (Rubus idaeus L.) shoots when 50 or 100 mg/l (0.284–0.568 mM) of ascorbic acid was used, but not as effectively as the use of 1 g/l Ca-gluconate. The reduction in STN was also genotype dependent. Misra et al. (2010) were also able to reduce STN in Barbados nut cultures by adding antioxidants, either 25 mg/l (81.3 µM) of reduced glutathione or 10 mg/l (56.7 µM) of ascorbic acid. Jaiswal et al. (2013) observed STN in Indian kino tree (Pterocarpus marsupium Roxb.) cultures. They eliminated STN by adding 568 µM ascorbic acid, 260 µM citric acid, 605 µM ammonium sulfate, and 217 µM adenine sulfate to MS basal medium. By adding 1% activated charcoal to root proliferation medium, Sánchez et al. (1997) reduced the incidence of STN from 89 to 30% in sweet chestnut clone 90,025 and from 38 to 13% in clone Pr5. The addition of antioxidants to basal medium during shoot multiplication might be an effective way to reduce or prevent STN.
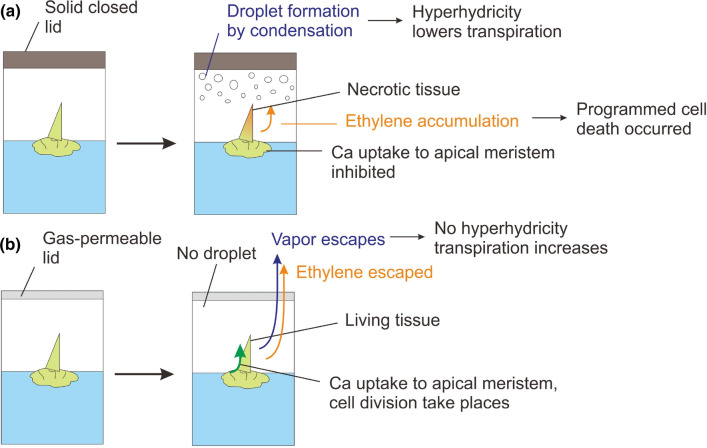
Humidity, aeration, and hyperhydricity: is there a link to STN?
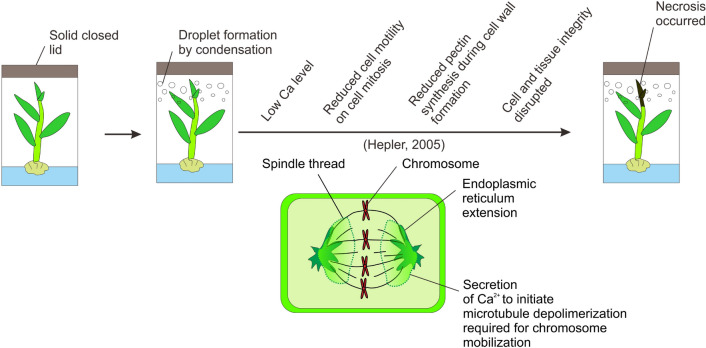
High humidity and weak ventilation in culture vessels can cause abnormalities, including hyperhydricity (Lai et al. 2005), or STN (Fig. 3a). These abnormalities may in turn be caused by increased ethylene production (Isah 2015). A decrease in humidity within culture vessels can be achieved by improving the ventilation of vessels, or by increasing the agar concentration in basal medium. The former can encourage gas exchange, thereby decreasing ethylene concentration within the vessel (reviewed in Isah 2015). In their summary, Bairu et al. (2009b) concluded that better aeration decreased STN. Barghchi and Alderson (1983, 1985, 1996), in addition to stating that STN was caused by Ca deficiency, also proposed that STN was linked to high humidity in a culture vessel. They found that high humidity reduced plantlet transpiration rate, causing a “low mobility of calcium ions in the xylem”, i.e., reduced nutrient flow to meristematic regions in growing shoot tips. Several authors found that high relative humidity and low transpiration caused by closed culture vessels decreased Ca2+ flow during transpiration, causing Ca deficiency (Sha et al. 1985; Singha et al. 1990; Abousalim and Mantell 1994) (Fig. 3a).
Fig. 3.
Schematic diagram depicting how high humidity and reduced transpiration in closed tissue culture vessels may induce shoot tip necrosis (STN). Such growth conditions can induce low levels of calcium (Ca) which in turn reduces cell motility and pectin synthesis, disrupting cell (cell wall or cell membrane) and tissue integrity, and reduce transpiration (Hepler 2005), potentially leading to STN. This biochemical hypothesis has still not yet been tested specifically for STN
Ca2+ transport is inhibited by apoplast flooding in which apoplastic air spaces are blocked as a result of water clogging (van den Dries et al. 2013). Bhalla and Mulwa (2003) noted that when Ca2+ in medium exceeded 6 mM in macadamia nuts (Macadamia F. Muell.), STN symptoms increased. They found that this was as a result of poor culture vessel aeration and high relative humidity and was not linked to Ca2+ level in the medium. McCown and Sellmer (1987) suggested that when culture vessels that increase gas exchange or reduce relative humidity are used, hyperhydricity as well as STN were reduced (Fig. 3b), while the use of Gelrite instead of agar improved shoot growth, but increased the incidence of hyperhydricity. Although Matu et al. (2006) did not specifically link aeration problems or hyperhydricity with the incidence of STN in staff tree (Maytenus senegalensis (Lam.) Exell) tissue culture, they described this condition as “a major problem”. They improved shoot growth by substituting Gelrite for agar as the gelling agent during shoot multiplication. Offord and Tyler (2009) noted that increased ventilation by employing vented lids for greater transpiration, STN in pink pimelea (Pimelea spicata R. Br.) cultures increased from 38 to 73% on MS medium and from 18 to 56% on half-MS medium, but hyperhydricity was observed in both ventilated and unventilated treatments. Compared to cultures on solid medium containing DKW macroelements, MS microelements, 3% sucrose and 0.44 µM BA, cultures of dahlia (Dahlia x hybrida) in liquid culture eliminated STN (De Klerk and ter Brugge 2011). Vibha et al. (2014) reached the same conclusion for North Indian rosewood (Dalbergia sissoo Roxb.) cultures, reducing hyperhydricity by adding ammonium sulfate to the medium. In quince (Cydonia oblonga Mill.), Singha et al. (1990) found that long culture periods and infrequent subcultures resulted in both STN and hyperhydricity, as well as leaf necrosis. However, the application of 3 to 18 mM Ca2+ and increasing agar concentration from 0.6% to 1.2% reduced the incidence of these two physiological disorders, but also lowered shoot proliferation and shoot fresh and dry weight. McCown and Sellmer (1987) suggested that some poplar genotypes developed hyperhydricity in response to media with high nitrogen (N) levels. Balla and Kirilla (2006) noted STN in in vitro cultures of peach interspecific rootstocks at the rooting phase. One possible reason was the development of hyperhydricity at temperatures exceeding 22 °C (Balla and Mansvelt 2012). Kataeva et al. (1991) found that the absence of BA in medium resulted in no hyperhydricity, but in high levels of STN, in unrooted apple and tea (Camellia sinensis (L.) Kuntze) shoots and in rooted poplar (Populus tremula L. × P. alba L.) and gerbera (Gerbera jamesonii Bolus ex Hooker f.) plantlets (Table 1). However, when BA was added at 4.4 µM into media for apple, hyperhydricity increased to 4% in cotton-covered vessels (18% in foil-covered vessels), even more at 22.1 µM (18% in cotton-covered vessels and 73% in foil-covered vessels), and even more at 22.1 µM with 5.3 µM NAA (58% in cotton-covered vessels and 80% in foil-covered vessels). Had the levels of STN in these four plant species been defined, this would have been an important assessment of the possible link between STN and hyperhydricity.
We recommend the use of culture vessels with improved ventilation and reduced hyperhydricity to reduce the accumulation of ethylene. This would improve Ca2+ flow, ultimately reducing the incidence of STN.
Other factors
Several studies in the literature have reported the incidence of STN in response to factors that are not linked to nutrients, PGRs, or other factors discussed previously.
Lall et al. (1997) observed that exposure of in vitro Mrs Flanagan’s impatiens (Impatiens flanaganiae Hemsl.) plantlets to high light intensity (280 µmol m−2·s−1) for 7 weeks induced necrosis in terminal parts, but it was not clear if this was STN. However, Marks and Simpson (1999) also noticed a similar pattern of increased STN in in vitro cultures of disanthus (Disanthus cercidifolius Maxim.) and Northern European hawthorn (Crataegus oxyacantha ‘Paul’s Scarlet’), but not of three Rhododendron cultivars. In their study, plants were exposed to moderate or high light intensity (55 or 106 µmol m−2 s−1) and tested against low light intensity (11 or 26 µmol m−2 s−1), in culture (Table 2).
Table 2.
Cause–effect (IF–THEN) rules created by neurofuzzy logic indicating the best combination of inputs to alleviate STN in pistachio in vitro cultures
| Rules | Membership degree | |||
|---|---|---|---|---|
| SubModel:1 | ||||
| 1 | IF | EDTA− is low and K+ is low | THEN | Low (1.00) |
| 2 | EDTA− is low and K+ is high | Low (1.00) | ||
| 3 | EDTA− is mid and K+ is low | Low (1.00) | ||
| 4 | EDTA− is mid and K+ is high | Low (1.00) | ||
| 5 | EDTA− is high and K+ is low | High (1.00) | ||
| 6 | EDTA− is high and K+ is high | High (1.00) | ||
| SubModel:2 | ||||
| 7 | IF | BA is low | THEN | High (1.00) |
| 8 | BA is high | Low (1.00) | ||
| SubModel:3 | ||||
| 9 | IF | IF Cl− is low | THEN | High (1.00) |
| 10 | IF Cl− is high | Low (1.00) | ||
| SubModel:4 | ||||
| 11 | IF | Genotype is Ghazvini | THEN | High (0.55) |
| 12 | Genotype is UCB-1 | Low (0.93) | ||
| SubModel:5 | ||||
| 13 | IF | Na+ is low | THEN | Low (0.78) |
| 14 | Na+ is mid | High (0.94) | ||
| 15 | Na+ is high | Low (1.00) | ||
Inputs with stronger effects have been highlighted by software (for additional details see Nezami-Alanagh et al. 2019)
In American chestnut genotypes B’ville, Iowa #2 and VDW, wounding of cuttings did not significantly affect the rate of STN, but promoted rooting, although the level of STN and rooting was intricately dependent on the level of auxin and cytokinin (Xing et al. 1997). Khalafalla and Daffalla (2008) found that scion length and rootstock age impacted the incidence of STN in grafted gum arabic (Acacia senegal (L.) Willd.) shoot tips. They registered 7% STN when scions were 2.5–3 cm, 27% when they were 1.5–2 cm, or 14% when rootstocks were 7 days old (0% STN when rootstocks were 14 days old). The incidence of STN in grape cv. Arka Neelamani also depended on the position of the explant on the stock plantlet and its initial weight (Thomas 2000).
The establishment of STN is also influenced by light intensity during shoot multiplication, and other factors such as rootstock age.
Possible mechanisms underlying STN
Programmed cell death, necrosis, and stress
It is possible that the underlying mechanisms to explain explant wounding and subsequent tissue browning may be similar. However, since we consider STN to be a physiological response of a living tissue on a developing in vitro plant, rather than a cut explant, we will hereafter only consider the possible factors that might affect STN. The use of chemicals such as antioxidants (e.g., Misra et al. 2010), reduction in light intensity since high light intensity stimulates polyphenol oxidase (Krishna et al. 2008), or the inhibition of phenylpropanoid biosynthesis (Jones and Saxena 2013) may be viable ways to alleviate STN, similar to tissue browning after explant cutting during the establisment of an in vitro culture. When oxidative stress can no longer be controlled, programmed cell death (PCD) develops (Gaspar et al. 2002), which may explain STN. Beckman (2000) further suggested that specialized cells induced cell suberization and lignification as a result of the accumulation of phenolic compounds, thereby strategically stimulating PCD. PCD develops as a function of H2O2, the “death signal”, and in response to other reactive oxygen species (Demidchik 2015). PCD affects several developmental events in plants, including sensescence where proteins, phospholipids and pigments may be degraded (Drury and Gallois 2006; Henmi et al. 2007; Misra et al. 2010; Kacprzyk et al. 2011). Ethylene, which can accumulate in closed tissue culture vessels in vitro, is a strong inducer of leaf senescence and a trigger for PCD (Santner et al. 2009; Trobacher 2009; Park et al. 2016). Moreover, ethylene initiates a signaling pathway, including calcium transport, during the development of aerenchyma, which also plays a role in PCD (Jones 2001). It is still unclear if PCD is involved in, related to, or the cause of STN.
Calcium and calcium signaling
Ca deficiency is one of the most commonly cited reasons for STN (Table 1). In closed tissue culture vessels, the high relative humidity and reduced transpiration induced low Ca2+ levels, because Ca2+ cannot translocate but must be actively transported (Hepler 2005). This does not permit pectin to be synthesized, impeding the formation of the shoot meristem due to compromised cell integrity and membrane permeability (Martin et al. 2007; Naaz et al. 2014) (Fig. 4). Moreover, Ca2+ serves as a universal secondary messenger in cellular signaling in plants, so the hormonal balance and Ca2+ supply during growth and development may affect each other. There is a correlation between Ca2+ and auxin signaling: auxin induces Ca2+ signals and vice versa, and Ca2+ controls the speed of transport of an auxin (Vanneste and Friml 2013). A high level of auxin might cause excessive ethylene production in jars and change the CK: auxin ratio. As was observed by Busse et al. (2008), STN in potato cultures, which resulted in a loss of apical dominance, was caused by low levels of Ca2+ in medium (Ozgen et al. 2011). This theory was confirmed by two experiments (Ozgen et al. 2011): the addition of a Ca2+ chelator, ethylene glycol tetra acetic acid (EGTA), to medium with sufficient Ca2+ (2720 µM) induced the precise same symptoms as low Ca2+ levels, namely STN and axillary shoot formation; in that condition, the supplemental addition of 204 µM strontium (Sr2+), which is a Ca2+ analog, restored apical dominance. Increasing Ca2+ in medium of several tree species has been shown to alleviate STN (McCown and Sellmer 1987). Ca-gluconate is an organic form of Ca that allows Ca2+ to be released into aqueous solutions, explaining why it has occasionally been used to alleviate STN, but it negatively affects shoot growth (Singha et al. 1990; Amalia et al. 2014). We suspect that the use of gas-permeable culture vessels, such as the Vitron or Miracle Pack (Teixeira da Silva et al. 2006), could reduce hyperhydricity, reduce the accumulation of ethylene, increase transpiration and consequently increase the transport of Ca2+ to the shoot apical meristem, although this hypothesis has yet to be tested on in vitro plant cultures displaying STN.
Fig. 4.
The impact of hyperhydricity, as a result of poorly ventilated culture vessels, may promote shoot tip necrosis (STN). Schematic representation of the possible mechanisms involved in STN when an in vitro plant culture is established in a closed vessel, causing ethylene to accumulate (a). Schematic representation of the possible methods to reduce hyperhydricity and ethylene accumulation, by employing gas-permeable culture vessels, to ensure the healthy growth of shoot tips in vitro by reducing or eliminating the incidence of STN (b)
Since the level of the endogenously accessible Ca2+ depends not only on its content in the medium but also on its uptake, it is reasonable to expect that the content of other ions in the medium such as Mg2+, K+, some microelements or , which can modify the uptake of Ca2+ from the medium based on nutrient interactions (Fageria 2001), may have an effect on STN. In this sense, Ca deficiency may be relative. The content of mesoelements such as Ca2+, Mg2+ and K+ in the medium modified the rate of the STN in different plant species (Reed et al. 2016; Kovalchuk et al. 2017a, b; details in Table 1). In wild apricot shoot culture, the /Ca2+ ratio should be optimally below 0.8, to minimize STN, specifically > 45 mM and 25 mM < ≤ 45 mM + /Ca2+ ≤ 0.8 for node 5 (Kovalchuk et al. 2018). In shoot cultures of different pear species, besides the role of various mesoelements like Ca2+, Mg2+, and K+, the roles of Fe2+ and the proper concentrations of nitrogen compounds were reported to be involved in the occurrence of STN (Reed et al. 2013, details in Table 1).
In addition, Subbaiah et al. (2000) found that activation of protease, which played a role in PCD induced by anoxia in maize (Zea mays L.) roots, was Ca2+ dependent. The role of Ca2+ in plant stress response and signaling has been detailed in a review by Robertson (2013). Bairu (2008) found that the main problem related to Ca deficiency was not the level of Ca2+ in medium but its limited transport in plantlets due to excess . Moreover, Ca2+ transport in plants through the xylem sap requires transpiration, which is inhibited by high humidity in the culture vessel, thus the limited mobility of Ca2+ can play a role in the development of STN (Hirschi 2004).
Plant growth regulators
As indicated above, ethylene is a likely inducer of PCD and thus may be a direct cause of STN in unventilated culture vessels. Table 1 indicates that PGRs have been heavily implicated in STN, mostly CKs during the shoot induction stage, but also the CK × auxin interaction during the root induction stage of shoots. For example, the absence or use of low concentrations of CKs was implicated as a reason for the presence of STN since roots are the main source of CKs (Chen et al. 1985), reducing cell division in the shoot apical meristem (Piagnani et al. 1996). The damage to shoot tips reduces the synthesis of auxin because shoot tips are the main site of auxin biosynthesis (Leopold 1975; Aloni et al. 2003; Hopkins and Hüner 2009). Exogenously added CKs can act with different efficiency depending on their structure, the plant species or even the cultivar (Dobránszki and Teixeira da Silva 2010). Application of the highly active mT or mTR (hydroxylated BA derivatives) can delay senescence and can eliminate abnormalities of in vitro cultures, including a reduction of hyperhydricity and STN (Aremu et al. 2012). Similarly, Kumari et al. (2017) found that when mTR was used in the shoot regeneration medium of dwarf wild begonia (Begonia homonyma Steud.), the occurrence of shoot necrosis was reduced to about a half of other regenerants cultured on medium with BA or TDZ. After subculture of regenerated shoots onto elongation medium, STN occurred again at a low frequency (18%) if previously regenerants had developed on medium with mTR. Bairu et al. (2011) studied the background effects of CK on STN, including an analysis of both endogenous and exogenous CKs and their derivatives, in devil’s claw. They found a higher content of total CKs in necrotic shoots than in normal shoots in all studied cases. However, they also detected larger quantities of deactivated forms of CKs such as 9-glucolides in BA-treated and necrotic shoots relative to normal and mT-treated shoots, suggesting that the occurrence of STN may be due to a change of active CKs to other deactivated products, possibly reversibly, but that can be toxic. N7 and N9 conjugates, which are the inactive forms of BA, are biologically inactive and chemically quite stable, but their conjugation is not fully irreversible (Werbrouck et al. 1996). These conjugates usually accumulate at the base of in vitro shoots, so the active form can be continuously released and cause disorders such as STN (Werbrouck et al. 1996; Strnad et al. 1997). Topolins are hydroxylated forms of BA with high activity in plant tissue culture but they have a different metabolism from that of BA and, therefore, side-effects caused by the release of the active form from inactive BA conjugates can be avoided (reviewed in: Dobránszki and Teixeira da Silva 2010).
Unmasking the effect of media ingredients on STN using artificial intelligence
In the 1990s, a wide range of statistical methods for the multivariate analysis of plant cell tissue data were employed, but those studies have some limitations (Gago et al. 2010; Gallego et al. 2011): (i) limited kind of data (qualitative or quantitative) can be analyzed using multivariate analysis, but not nominal or image data; (ii) limited application of linear tools such as ANOVA and regression, since biological responses present a high degree of intra- and inter-individual variation that interacts in a non-linear and non-deterministic way; and (iii) a slump in the use of statistical methods to predict or optimize plant tissue culture. In recent years, several parametric approaches such as response surface methodology (RSM), decision trees, Chi-square automatic interaction detector (CHAID), adaptive regression splines and artificial intelligence tools, based on machine learning systems, have been successfully applied to the design of plant tissue media as advanced techniques (Poothong and Reed 2014; Akin et al. 2016,2020). Other computer-based tools based on artificial intelligence tools for understanding the effect of media components on in vitro cultured plants were also explored (Gago et al. 2010; Gallego et al. 2011; Zielińska and Kępczyńska 2013).
Gallego et al. (2011) extensively reviewed the advantages of using artificial neural networks (ANNs) and fuzzy logic, to discover the cause–effect function of various factors on the response of in vitro plantlets. These artificial intelligence tools help researchers to obtain insight of the cause–effect relationships between factors studied (i.e., mineral nutrients) and a wide range of responses (i.e., growth parameters, physiological disorders, etc.). More recently, the combination of DOE with neurofuzzy logic provided them with a powerful tool to obtain a deeper understanding about the effects of culture media composition on different growth parameters (Nezami-Alanagh et al. 2018) and also several physiological disorders, including STN, during micropropagation of two pistachio rootstocks, UCB-1 and Ghazvini (Nezami-Alanagh et al. 2019). Noticeably, in the latter study, STN was successfully modeled with the help of neurofuzzy logic tools, being affected by complex interactions of ions, cytokinin (BA), and genotype. Those results indicated that in pistachio in vitro cultures, STN is caused by the effect of several factors (individually or in interaction), splitting these effects into five sub-models: (1) the complex interaction of ethylenediaminetetraacetic acid (EDTA−) × K+ as the strongest effect, followed by (2) BA, (3) Cl−, (4) genotype and (5) Na+ (Fig. 5). The cause–effect induced by STN-related “factors” can be easily understood by several ‘IF–THEN’ rules presented in Table 3, which can be summarized as follows: (i) the strongest effect of low-mid concentrations of EDTA− (0.06 < × < 0.39 mM), regardless of the K+ content (rules 5–6). Furthermore, the lowest STN values are also obtained on media including high amounts of BA (1.30 < × < 1.50 mg/l, i.e., 5.72 < × < 6.60 µM) and Cl− (9.10 < × < 17.96 mM (rules 8 and 10). ‘UCB-1′ shoots were more resistant than ‘Ghazvini’ rootstock with respect to STN (rules 11–12). Finally, Na+ influenced the appearance of STN in pistachio cultures, with lowest STN when a high concentration of Na+ is included in the medium (rule 15).
Fig. 5.
Graphical representation of critical factors affecting STN extracted by neurofuzzy logic. The inputs with stronger effects are marked in pink
In conclusion, these findings assert the importance of applying computer-based tools in order to: (i) create a well-sampled design space with the advantage of saving time and costs, (ii) the possibility of splitting salts to their fundamental ions without ion-confounding concerns, (iii) discovery of key factors that impact the measured parameters, and iv) optimization of new cost-effective culture media for healthy in vitro plant growth.
Conclusion
The literature on STN in in vitro plant micropropagation exceeds 100 studies. While many authors observed STN, in several cases finding practical solutions to eliminate this physiological condition (64 studies in Table 1), many other studies reported the presence of STN, or a reduction in STN, without providing any exact data. Readers should note that Table 1 and Suppl. Table 1 do not reflect the total of all studies that claimed the existence of STN, only those studies that provided quantitative evidence of this phenomenon. In addition, it is likely that many more studies in the plant tissue culture literature may have observed STN. However, STN tends to be observed as a negative finding, but may have either been referred to in other terms, or not reported at all because the publication of negative results is generally not encouraged in mainstream, including plant science, journals (Teixeira da Silva 2015). Thus, the values that we report may underrepresent the true extent of the occurrence of STN.
In summary, first and foremost, the in vitro factor with the most notable influence is nutrient deficiency, mainly Ca, B and N, although there is an interaction with other ions, the ion-confounding effect. The level and type of PGRs in a medium can also impact STN. Other factors that were found to induce STN were the timing of measurements and length of subculture, genotype, choice of basal medium, antioxidants, and possibly humidity, aeration and hyperhydricity.
In Fig. 6, we summarized the mechanisms by which STN can occur based on our current knowledge. The lack or imbalance of different nutrients, inappropriate PGR content, and the lack of antioxidants in medium, as well as high humidity and/or low ventillation in culture vessels are proven causes of STN mainly by affecting the uptake and transport of Ca from medium, and modifying the endogenous hormonal balance of in vitro plantlets. Ca deficiency in plants can occur either directly, if a low level of Ca2+ is added to the culture medium, or indirectly due to the presence and concentration of other nutrients by modifying Ca uptake based on nutrient interactions. Ca2+ can activate polyphenol oxidase (PPO) by changing its conformational state (Ruiz et al. 2003) and peroxidase (POD) by inducing cross-linking in the chains of polygalacturonan (Penel et al. 1999). Thus, Ca2+ can inhibit the accumulation of phenolic compounds by stimulating their oxidation and, as a result, it can decrease or inhibit STN, hindering the accumulation of phenolic coumpounds and PCD (continuous arrows). Boron acts in two ways when inhibiting STN. A high concentration of can stimulate the uptake of Ca2+ and helps Ca2+ movement within the plant (continuous arrows). The quantity and form of N ( or ), as well as the ratios of / and /Ca2+ affect the occurrence of STN, likely in part through the modification of the ratio of cytokinins to auxins (dotted arrows). The levels of Cl− and gluconate− in the medium can affect the level of STN as well, but the mechanisms by which they act are unknown (dotted arrows). The level and type of PGRs added to the medium also affect the occurrence of STN by changing the levels of ethylene, cytokinins, and auxin. The structure of cytokinins added to the culture medium affects the quantity of their active forms and thus the cytokinin to auxin ratio within the plant. A high level of auxin in a plant modifies Ca2+ signaling, while a modified level of Ca2+ in a plant modifies auxin transport (blue arrows). Moreover, a high level of auxin in a plant can act directly and generate the production of ethylene, thereby increasing STN. The addition of different antioxidants into the medium can prevent the accumulation of ethylene, thus inducing STN. If relative humidity is high, or if there is weak or no ventilation in culture vessels, this can lead to increased ethylene production and thus STN. High humidity also modifies Ca2+ transport within a plant causing its low mobility in the xylem and leading to Ca deficiency in the shoot tip (continuous arrows).
Fig. 6.
Schematic diagram showing the overall mechanisms of shoot tip necrosis (STN) in in vitro plants. A detailed description may be found in the text, in "Conclusion"
Several possible solutions to eliminating or reducing STN in vitro have been proposed, based on the main factors known for causing STN in vitro (Fig. 6). Despite these proposals, the mechanism(s) remains inconclusive, unexplored, and far from resolved, especially given the wide-ranging, and sometimes contradictory, responses and frequently observed genotype dependence. This review provides, despite not fully understanding the mechanism underlying STN, a more concise and updated summary of studies in STN in vitro with the hope that it will generate new ideas that would allow plant physiologists and molecular biologists to further explore the physiological and genetic mechanisms underlying this disorder.
Author contribution statement
JATdS and MMK conceived the idea and compiled the literature. JATdS wrote the first drafts. In subsequent versions and drafts, all authors contributed equally to ideas, writing, figures, supplements, revisions and corrections. All authors approve the published version and take responsibility for its content.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank The American Society for Horticultural Science for kindly providing a scanned copy of the Sha et al. (1985) paper. This research was financed by the Higher Education Institutional Excellence Programme (NKFIH-1150-6/2019) of the Ministry of Innovation and Technology in Hungary, within the framework of the Biotechnology thematic programme of the University of Debrecen. The study and submission for publication were approved by the University of Debrecen (BPTR/DEENK/0008/2019). Esmaeil Nezami-Alanagh thanks the Biotechnology Department at Imam Khomeini International University (IKIU) for their assistance in carrying out a part of the experiment work and also to Science and Technology Park of East-Azarbaijan, Islamic Republic of Iran, for financial support. The Spanish work on STN modeling was funded by Xunta de Galicia, Spain (CITACA Strategic Partnership, Reference: ED431E 2018/07 and REDES, Reference: ED431D-2017/19). The authors also thank Mr. Nicolas Manterola (Laboratorios Green Nova, Casablanca, Chile) for kindly providing the photographs used in Fig. 2. The authors thank the input, critique, and valuable suggestions made by six anonymous peer reviewers.
Funding
Open access funding provided by University of Debrecen.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest of relevance to this paper to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaime A. Teixeira da Silva, Email: jaimetex@yahoo.com
Adhityo Wicaksono, Email: adhitwicaksono@genbinesia.or.id, Email: adhityo.wicaksono@gmail.com.
References
- Abdulnour JE, Donnelly DJ, Barthakur NN. The effect of boron on calcium uptake and growth in micropropagated potato plantlets. Potato Res. 2000;43:287–295. doi: 10.1007/BF02358088. [DOI] [Google Scholar]
- Abousalim A, Mantell SH. A practical method for alleviating shoot-tip necrosis symptoms in in vitro shoot cultures of Pistacia vera cv. Mateur J Hortic Sci. 1994;69:357–365. doi: 10.1080/14620316.1994.11516465. [DOI] [Google Scholar]
- Ahmed ZFR, Palta JP. Hormone-like action of a natural lipid, lysophosphatidylethanolamine: a comparison with auxin. Acta Hortic. 2017;1187:107–114. doi: 10.17660/ActaHortic.2017.1187.13. [DOI] [Google Scholar]
- Ahmed ZFR, Palta JP. Significant variations in mineral composition among agar sources: implications in nutrition and abiotic stress studies that use in vitro culture. Acta Hortic. 2017;1187:115–122. doi: 10.17660/ActaHortic.2017.1187.14. [DOI] [Google Scholar]
- Akin M, Eyduran SP, Eyduran E, Reed BM. Analysis of macro nutrient related growth responses using multivariate adaptive regression splines. Plant Cell Tiss Org Cult. 2020;140:661–670. doi: 10.1007/s11240-019-01763-8. [DOI] [Google Scholar]
- Akin M, Eyduran E, Reed BM. Use of RSM and CHAID data mining algorithm for predicting mineral nutrition of hazelnut. Plant Cell Tiss Org Cult. 2016;128:303–316. doi: 10.1007/s11240-016-1110-6. [DOI] [Google Scholar]
- Alderson PG, Harbour MA, Patience PA. Micropropagation of Prunus tenella cv Firehill. Acta Hortic. 1987;212:463–468. doi: 10.17660/ActaHortic.1987.212.69. [DOI] [Google Scholar]
- Alegre J, Nisa M, Ramírez Martín N, Cuevas A, Ruiz-Galea M, Celestino C, Tello ML, Toribio M. Micropropagation of mature Platanus × hispanica trees by axillary shoot proliferation. Acta Hortic. 2015;1083:353–360. doi: 10.17660/ActaHortic.2015.1083.44. [DOI] [Google Scholar]
- Aloni R, Schwalm K, Langhans M, Ullrich CI. Gradual shifts in sites of free-auxin production during leaf-primordium develpoment and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta. 2003;216:841–853. doi: 10.1007/s00425-002-0937-8. [DOI] [PubMed] [Google Scholar]
- Amalia F, Debnath SC, Yeoung YR. Effects of calcium gluconate and ascorbic acid on controlling shoot necrosis during micropropagation of primocane-fruiting raspberry (Rubus idaeus L.) cultivars. Afr J Biotechnol. 2014;13:4361–4368. doi: 10.5897/AJB2014.14201. [DOI] [Google Scholar]
- Amin MN, Jaiswal VS. Micropropagation as an aid to rapid cloning of a guava cultivar. Sci Hortic. 1988;36:89–95. doi: 10.1016/0304-4238(88)90010-6. [DOI] [Google Scholar]
- Amo-Marco JB, Lledo MD. In vitro propagation of Salix tarraconensis Pau ex Font Quer, an endemic and threatened plant. Vitro Cell Dev Biol Plant. 1996;32:42–46. doi: 10.1007/BF02823012. [DOI] [Google Scholar]
- Anderson WC. Propagation of rhododendrons by tissue culture: I. Development of a culture medium for multiplication of shoots. Comb Proc Int Plant Prop Soc. 1975;25:129–135. [Google Scholar]
- Anderson WC. A revised tissue culture medium for shoot multiplication of Rhododendron. J Am Soc Hort Sci. 1984;109:343–347. [Google Scholar]
- Aremu AO, Bairu MW, Doležal K, Finnie JF, van Staden J. Topolins: a panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012;108:1–16. doi: 10.1007/s11240-011-0007-7. [DOI] [Google Scholar]
- Arora K, Sharma M, Srivastava J, Ranade SA, Sharma AK. Rapid in vitro cloning of a 40-year-old tree of Azadirachta indica A. Juss (Neem) employing nodal stem segments. Agrofor Syst. 2010;78:53–63. doi: 10.1007/s10457-009-9230-1. [DOI] [Google Scholar]
- Bairu MW, Jain N, Stirk WA, Doležal K, van Staden J. Solving the problem of shoot-tip necrosis in Harpagophytum procumbens by changing the cytokinin types, calcium and boron concentrations in the medium. S Afr J Bot. 2009;75:122–127. doi: 10.1016/j.sajb.2008.08.006. [DOI] [Google Scholar]
- Bairu MW, Novák O, Doležal K, van Staden J. Changes in endogenous cytokinin profiles in micropropagated Harpagophytum procumbens in relation to shoot-tip necrosis and cytokinin treatments. Plant Growth Regul. 2011;63:105–114. doi: 10.1007/s10725-010-9558-6. [DOI] [Google Scholar]
- Bairu MW, Stirk WA, van Staden J. Factors contributing to in vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tissue Organ Cult. 2009;98:239–248. doi: 10.1007/s11240-009-9560-8. [DOI] [Google Scholar]
- Bairu MW (2008) Characterization and control of micropropagation problems in aloe, devil’s claw and banana. PhD thesis, University of Kwa-Zulu Natal, Pietermaritzburg, South Africa, pp 167. https://researchspace.ukzn.ac.za/handle/10413/8315
- Balla I, Kirilla Z. Micropropagation of peach rootstocks and cultivars. Acta Hortic. 2006;725:511–516. doi: 10.17660/ActaHortic.2006.725.74. [DOI] [Google Scholar]
- Balla I, Mansvelt L. Micropropagation of peach rootstocks and cultivars. Methods Mol Biol. 2012;11013:137–148. doi: 10.1007/978-1-62703-074-8_10. [DOI] [PubMed] [Google Scholar]
- Barghchi M, Alderson PG. In vitro propagation of Pistacia vera L. from seedling tissues. J Hortic Sci. 1983;58:435–445. doi: 10.1080/00221589.1983.11515140. [DOI] [Google Scholar]
- Barghchi M, Alderson PG. In vitro propagation of Pistacia vera L. and the commercial cultivars Ohadi and Kalleghochi. J Hortic Sci. 1985;60:423–430. doi: 10.1080/14620316.1985.11515647. [DOI] [Google Scholar]
- Barghchi M, Alderson PG. The control of shoot tip necrosis in Pistacia vera L. in vitro. Plant Growth Regul. 1996;20:31–35. doi: 10.1007/BF00024054. [DOI] [Google Scholar]
- Baskaran P, Moyo M, van Staden J. In vitro plant regeneration, phenolic compound production and pharmacological activities of Coleonema pulchellum. S Afr J Bot. 2014;90:74–79. doi: 10.1016/j.sajb.2013.10.005. [DOI] [Google Scholar]
- Beckman CH. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol Mol Plant Pathol. 2000;57:101–110. doi: 10.1006/pmpp.2000.0287. [DOI] [Google Scholar]
- Bhalla PL, Mulwa RMS. Tissue culture and Macadamia propagation. Acta Hortic. 2003;616:343–346. doi: 10.17660/ActaHortic.2003.616.50. [DOI] [Google Scholar]
- Bosela MJ, Michler CH. Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinin types. Vitro Cell Dev Biol Plant. 2008;44:316–329. doi: 10.1007/s11627-008-9114-5. [DOI] [Google Scholar]
- Box GEP, Meyer RD. An analysis for unreplicated fractional factorials. Technometrics. 1986;28:11–18. doi: 10.1080/00401706.1986.10488093. [DOI] [Google Scholar]
- Busse JS, Ozgen S, Palta JP. Influence of root zone calcium on subapical necrosis in potato shoot cultures: localization of injury at the tissue and cellular levels. J Am Soc Hortic Sci. 2008;133:653–662. doi: 10.21273/JASHS.133.5.653. [DOI] [Google Scholar]
- Cardoso JC, Teixeira da Silva JA. Micropropagation of Zeyheria montana Mart. (Bignoniaceae), an endangered endemic medicinal species from the Brazilian cerrado biome. Vitro Cell Dev Biol Plant. 2013;49:710–716. doi: 10.1007/s11627-013-9558-0. [DOI] [Google Scholar]
- Cardoso JC, Gerald LTC, Teixeira da Silva JA (2018) Micropropagation in the twenty-first century. In: Loyola-Vargas VM, Ochoa-Alejo N (Eds) Plant cell culture protocols, methods in molecular biology. Vol. 1815, Humana Press, New York, pp. 17–46. 10.1007/978-1-4939-8594-4_2 [DOI] [PubMed]
- Cellárová E, Kimáková K, Brutovská R. Multiple shoot formation and phenotypic changes of R0 regenerants in Hypericum perforatum L. Acta Biotechnol. 1992;12:445–452. doi: 10.1002/abio.370120602. [DOI] [Google Scholar]
- Chen C-M, Ertl JR, Leisner SM, Chang C-C. Localization of cytokinin biosynthetic sites in pea plants and carrot roots. Plant Physiol. 1985;78:510–513. doi: 10.1104/pp.78.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TY. Adventitious bud formation in culture of Douglas fir (Pseudotsuga menziensii (Mirb.) Franco) Plant Sci Lett. 1975;5:97–102. doi: 10.1016/0304-4211(75)90049-8. [DOI] [Google Scholar]
- Chiruvella KK, Mohammed A, Dampuri G, Ghanta RG. In vitro shoot regeneration and control of shoot tip necrosis in tissue cultures of Soymida febrifuga (Roxb.) A. Juss Plant Tiss Cult Biotechnol. 2011;21:11–25. doi: 10.3329/ptcb.v21i1.9559. [DOI] [Google Scholar]
- Chiruvella KK, Mohammed A, Ghanta RG. Phenotypic aberrations during micropropagation of Soymida febrifuga (Roxb.) Adr. Juss. Not Sci Biol. 2014;6:99–104. doi: 10.15835/nsb619202. [DOI] [Google Scholar]
- Christensen B, Sriskandarajah S, Serek M, Müller R. In vitro culture of Hibiscus rosa-sinensis L.: influence of iron, calcium and BAP on establishment and multiplication. Plant Cell Tiss Org Cult. 2008;93:151–161. doi: 10.1007/s11240-008-9354-4. [DOI] [Google Scholar]
- Compton ME, Preece JE. Response of tobacco callus to shoot tip exudation from five species. HortScience. 1988;23:208–210. [Google Scholar]
- Dangi B, Khurana-Kaul V, Kothari SL, Kachhwaha S. Micropropagtion of Terminalia bellerica from nodal explants of mature tree and assessment of genetic fidelity using ISSR and RAPD markers. Physiol Mol Biol Plants. 2014;20:509–516. doi: 10.1007/s12298-014-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud N, Faizal A, Geelen D. Adventitious rooting of Jatropha curcas L. is stimulated by phloroglucinol and by red LED light. Vitro Cell Dev Biol Plant. 2013;49:183–190. doi: 10.1007/s11627-012-9486-4. [DOI] [Google Scholar]
- De Block M. Factors influencing the tissue culture and the Agrobacterium tumefaciens-mediated transformation of hybrid aspen and poplar clones. Plant Physiol. 1990;93:1110–1116. doi: 10.1104/pp.93.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Klerk G-J, ter Brugge J. Micropropagation of dahlia in static liquid medium using slow-release tools of medium ingredients. Sci Hortic. 2011;127:542–547. doi: 10.1016/j.scienta.2010.11.015. [DOI] [Google Scholar]
- Demidchik V. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot. 2015;109:212–228. doi: 10.1016/j.envexpbot.2014.06.021. [DOI] [Google Scholar]
- Dewir YH, Nurmansyah NY, Teixeira da Silva JA. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018;37:1451–1470. doi: 10.1007/s00299-018-2326-1. [DOI] [PubMed] [Google Scholar]
- Diro M, van Staden J. The type of explant plays a determining role in the micropropagation of Ensete ventricosum. S Afr J Bot. 2005;71:154–159. doi: 10.1016/S0254-6299(15)30127-7. [DOI] [Google Scholar]
- Dobránszki J, Teixeira da Silva JA. Micropropagation of apple—a review. Biotechnol Adv. 2010;28:462–488. doi: 10.1016/j.biotechadv.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Dolcet-Sanjuan R, Claveria E. Improved shoot-tip micropropagation of Pistacia vera L. and the beneficial effects of methyl jasmonate. J Am Soc Hortic Sci. 1995;120:938–942. doi: 10.21273/JASHS.120.6.938. [DOI] [Google Scholar]
- Driver JA, Kuniyuki AH. In vitro propagation of Paradox walnut rootstock. Hort Sci. 1984;19:507–509. [Google Scholar]
- Drury GE, Gallois P. Programmed cell death in plants and flowers. In: Teixeira da Silva JA, editor. Floric ornam biotechnol. Isleworth: Global Science Books; 2006. pp. 141–156. [Google Scholar]
- Fageria VD. Nutrient interactions in crop plants. J Plant Nutr. 2001;24:1269–1290. doi: 10.1081/PLN-100106981. [DOI] [Google Scholar]
- Fox RL, Albrecht WA. Calcium–boron interaction demonstrated by Lemna minor on clay suspensions elements. Res Bull Univ Miss Agr Expt Sta. 1958;663:15. [Google Scholar]
- Gago J, Landín M, Gallego PP. Artificial neural networks modeling the in vitro rhizogenesis and acclimatization of Vitis vinifera L. J Plant Physiol. 2010;167:1226–1231. doi: 10.1016/j.jplph.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Gago J, Martínez-Núñez L, Landín M, Flexas J, Gallego PP. Modeling the effects of light and sucrose on in vitro propagated plants: a multiscale system analysis using artificial intelligence technology. PLoS ONE. 2014;9:e85989. doi: 10.1371/journal.pone.0085989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego PP, Gago J, Landin M. Artificial neural networks technology to model and predict plant biology process. In: Suzuki K, editor. Artificial neural networks—methodological advances and biomedical applications. Rijeka: Intech; 2011. pp. 197–216. [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- García E, Lorente P, Marín JA, Andreu P, Arbeloa A. Factors affecting shoot-tip necrosis of Pistacia vera L. shoots cultured in vitro. Inf Téc Econ Agrar. 2011;107:315–323. [Google Scholar]
- Gaspar T, Franck T, Bisbis B, Kevers C, Jouve L, Hausman JF, Dommes J. Concepts in plant stress physiology: application to plant tissue cultures. Plant Growth Regul. 2002;37:263–285. doi: 10.1023/A:1020835304842. [DOI] [Google Scholar]
- Gomes F, Simões M, Lopes ML, Canhoto JM. Effect of plant growth regulators and genotype on the micropropagation of adult trees of Arbutus unedo L. (strawberry tree) New Biotechnol. 2010;27:882–892. doi: 10.1016/j.nbt.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Grigoriadou K, Leventakis N, Vasilakakis M. Effects of various culture conditions on proliferation and shoot tip necrosis in pear cultivars ‘Williams’s’ and ‘Highland’ grown in vitro. Acta Hortic. 2000;520:103–108. doi: 10.17660/ActaHortic.2000.520.10. [DOI] [Google Scholar]
- Guha S, Usha Rao I. Nitric oxide promoted rhizome induction in Cymbidium shoot buds under magnesium deficiency. Biol Plant. 2012;56:227–236. doi: 10.1007/s10535-012-0081-7. [DOI] [Google Scholar]
- Hassanein AM, Salem JM, Faheed FA, El-nagish A. Effect of anti-ethylene compounds on isoenzyme patterns and genome stability during long term culture of Moringa oleifera. Plant Cell Tiss Org Cult. 2018;132:201–212. doi: 10.1007/s11240-017-1326-0. [DOI] [Google Scholar]
- Henmi K, Yanagida M, Ogawa K. Roles of reactive oxygen species and glutathione in plant development. Int J Plant Dev Biol. 2007;1:185–193. [Google Scholar]
- Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WG, Hüner NPA. Hormones I: Auxins. In: Hopkins WG, Hüner NPA, editors. Introduction to plant physiology. 4. USA: Wiley; 2009. pp. 305–321. [Google Scholar]
- Hsia C-N, Korban SS. Factors affecting in vitro establishment and shoot proliferation of Rosa hybrida L. and Rosa Chinensis minima. Vitro Cell Dev Biol Plant. 1996;32:217–222. doi: 10.1007/BF02822690. [DOI] [Google Scholar]
- Isah T. Adjustments to in vitro culture conditions and associated anomalies in plants. Acta Biol Cracov Bot. 2015;57:9–28. doi: 10.1515/abcsb-2015-0026. [DOI] [Google Scholar]
- Jain N, Bairu MW, Stirk WA, van Staden J. The effect of medium, carbon source and explant on regeneration and control of shoot-tip necrosis in Harpagophytum procumbens. S Afr J Bot. 2009;75:117–121. doi: 10.1016/j.sajb.2008.08.005. [DOI] [Google Scholar]
- Jaiswal S, Arya S, Kant T. Role of various additives in controlling shoot tip necrosis of Pterocarpus marsupium Roxb.—a multipurpose leguminous tree. J Phytol Res. 2013;26:43–46. [Google Scholar]
- Jamshidi S, Yadollahi A, Ahmadi H, Arab MM, Eftekhari M. Predicting in vitro culture medium macro-nutrients composition for pear rootstocks using regression analysis and neural network models. Front Plant Sci. 2016;7:1–12. doi: 10.3389/fpls.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM. Programmed cell death in development and defense. Plant Physiol. 2001;125:94–97. doi: 10.1104/pp.125.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AMP, Saxena PK. Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: a novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE. 2013;8:e76802. doi: 10.1371/journal.pone.0076802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacprzyk J, Daly CT, McCabe PF. The botanical dance of death: programmed cell death in plants. Adv Bot Res. 2011;60:169–261. doi: 10.1016/B978-0-12-385851-1.00004-4. [DOI] [Google Scholar]
- Karhu ST. Axillary shoot proliferation of blue honeysuckle. Plant Cell Tiss Org Cult. 1997;48:195–201. doi: 10.1023/A:1005842022064. [DOI] [Google Scholar]
- Kataeva NV, Alexandrova IG, Butenko RG, Dragavtceva EV. Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tiss Org Cult. 1991;27:149–154. doi: 10.1007/BF00041283. [DOI] [Google Scholar]
- Kermani SA, Hokmabadi H, Jahromi MG. The evaluation of the effect of multiwall carbon nano tube (MWCNT) on in vitro proliferation and shoot tip necrosis of pistachio rootstock UCB-1 (Pistacia integrima × P. atlantica) J Nut. 2017;8:49–59. doi: 10.22034/jon.2017.530392. [DOI] [Google Scholar]
- Khalafalla MM, Daffalla HM. In vitro micropropagation and micrografting of gum arabic tree [Acacia senegal (L.) Wild] Int J Sustain Crop Prod. 2008;3:19–27. [Google Scholar]
- Kishore K, Patnaik S, Shukla AK. Optimization of method to alleviate in vitro shoot tip necrosis in Trichosanthes dioica Roxb. Ind J Biotechnol. 2015;14:107–111. [Google Scholar]
- Koubouris G, Vasilakakis M. Improvement of in vitro propagation of apricot cultivar ‘Bebecou’. Plant Cell Tiss Org Cult. 2006;85:173–180. doi: 10.1007/s11240-005-9066-y. [DOI] [Google Scholar]
- Kovalchuk IY, Mukhitdinova Z, Turdiyev T, Madiyeva G, Akin M, Eyduran E, Reed BM. Modeling some mineral nutrient requirements for micropropagated wild apricot shoot cultures. Plant Cell Tiss Org Cult. 2017;129:325–335. doi: 10.1007/s11240-017-1180-0. [DOI] [Google Scholar]
- Kovalchuk IY, Mukhitdinova Z, Turdiyev T, Madiyeva G, Akin M, Eyduran E, Reed BM. Nitrogen ions and nitrogen ion proportions impact the growth of apricot (Prunus armeniaca) shoot cultures. Plant Cell Tiss Org Cult. 2018;133:263–273. doi: 10.1007/s11240-018-1379-8. [DOI] [Google Scholar]
- Kovalchuk IY, Mukhitdinova ZR, Turdiyev TT, Madiyeva GA, Reed BM. Optimization of in vitro growth medium for a wild Kazakhstan apricot, Prunus armeniaca. Acta Hortic. 2017;1155:193–200. doi: 10.17660/ActaHortic.2017.1155.27. [DOI] [Google Scholar]
- Krishna H, Sairam RK, Singh SK, Patel VB, Sharma RR, Grover M, Nain L, Sachdev A. Mango explant browning: effect of ontogenic age, mycorrhization and pre-treatments. Sci Hortic. 2008;118:132–138. doi: 10.1016/j.scienta.2008.05.040. [DOI] [Google Scholar]
- Kulkarni KR, D’Souza L. Control of in vitro shoot tip necrosis in Butea monosperma. Curr Sci. 2000;78:125–126. [Google Scholar]
- Kumar PP, Lakshmanan P, Thorpe TA. Regulation of morphogenesis in plant tissue culture by ethylene. Vitro Cell Dev Biol Plant. 1998;34:94–103. doi: 10.1007/BF02822771. [DOI] [Google Scholar]
- Kumar S, Singh H, Pandey V, Singh BD. In vitro multiplication of pointed gourd (Trichosanthes dioica) through nodal explant culture, and testing the genetic fidelity of micropropagated plants using RAPD markers. Indian J Biotechnol. 2016;15:581–588. [Google Scholar]
- Kumari A, Baskaran P, van Staden J. In vitro regeneration of Begonia homonyma—a threatened plant. S Afr J Bot. 2017;109:174–177. doi: 10.1016/j.sajb.2016.12.027. [DOI] [Google Scholar]
- Kurup SS, Purayil FT, Alkhaili MMS, Tawfik NH, Cheruth AJ, Kabshawi M, Subramaniam S. Thidiazuron (TDZ) induced organogenesis and clonal fidelity studies in Haloxylon persicum (Bunge ex Boiss & Buhse): an endangered desert tree species. Physiol Mol Biol Plant. 2018;24:683–692. doi: 10.1007/s12298-018-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Lin H-M, Nalawade SM, Fang W, Tsay H-S. Hyperhydricity in shoot cultures of Scrophularia yoshimurae can be effectively reduced by ventilation of culture vessels. J Plant Physiol. 2005;162:355–361. doi: 10.1016/j.jplph.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Lakshmi Sita G, Raghava Swamy BV. Regeneration of plantlets from leaf disc cultures of rosewood: control of leaf abscission and shoot tip necrosis. Plant Sci. 1993;88:107–112. doi: 10.1016/0168-9452(93)90115-G. [DOI] [Google Scholar]
- Lall N, Bosa A, Nikolova RV. Morphological characteristics of impatiens flanaganiae Hemsl. grown under different light conditions. S Afr J Bot. 1997;63:216–222. doi: 10.1016/S0254-6299(15)30747-X. [DOI] [Google Scholar]
- Leopold AC. Part II. Growth regulation. In: Leopold AC, Kriedemann PE, editors. Plant growth and development. 2. USA: McGraw-Hill Book Company; 1975. pp. 109–222. [Google Scholar]
- Lin G-Z, Zhao X-M, Hong S-K, Lian Y. Somatic embryogenesis and shoot organogenesis in the medicinal plant Pulsatilla koreana Nakai. Plant Cell Tiss Org Cult. 2011;106:93–103. doi: 10.1007/s11240-010-9897-z. [DOI] [Google Scholar]
- Linington IM. In vitro propagation of Dipterocarpus alatus and Dipterocarpus intricatus. Plant Cell Tiss Org Cult. 1991;27:81–88. doi: 10.1007/BF00048211. [DOI] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. doi: 10.1111/j.1399-3054.1965.tb06874.x. [DOI] [Google Scholar]
- Lišková D, Kollárová K, Kučerová D, Vatehová Z, Zelko I, Lux A, van Staden J. Alternatives to improve long-term cultures of Harpagophytum procumbens in vitro. S Afr J Bot. 2016;104:55–60. doi: 10.1016/j.sajb.2015.10.008. [DOI] [Google Scholar]
- Lloyd G, McCown B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb Proc Intl Plant Prop Soc. 1980;30:421–427. [Google Scholar]
- Machado MP, da Silva ALL, Biasi LA, Deschamps C, Filho JCB, Zanette F. Influence of calcium content of tissue on hyperhydricity and shoot-tip necrosis of in vitro regenerated shoots of Lavandula angustifolia Mill. Braz Arch Biol Technol. 2014;57:636–643. doi: 10.1590/S1516-8913201402165. [DOI] [Google Scholar]
- Mackay WA, Tipton JL, Thompson GA. Micropropagation of Mexican redbud, Cercis canadensis var. mexicana. Plant Cell Tiss Org Cult. 1995;43:295–299. doi: 10.1007/BF00039959. [DOI] [Google Scholar]
- Marks TR, Simpson SE. Effect of irradiance on shoot development in vitro. Plant Growth Regul. 1999;28:133–142. doi: 10.1023/A:1006276724956. [DOI] [Google Scholar]
- Martin KP, Zhang CL, Slater A, Madassery J. Control of shoot necrosis and plant death during micro-propagation of banana and plantains (Musa spp.) Plant Cell Tiss Org Cult. 2007;88:51–59. doi: 10.1007/s11240-006-9177-0. [DOI] [Google Scholar]
- Martinelli A. Use of “in vitro” techniques for selection and cloning of different Pistacia species. Acta Hortic. 1988;227:436–437. doi: 10.17660/ActaHortic.1988.227.85. [DOI] [Google Scholar]
- Martínez MT, Corredoira E, Vieitez AM, Cernadas MJ, Montenegro R, Ballester A, Vieitez FJ, San José MC. Micropropagation of mature Quercus ilex L. trees by axillary budding. Plant Cell Tiss Org Cult. 2017;131:499–512. doi: 10.1007/s11240-017-1300-x. [DOI] [Google Scholar]
- Marín JA, García E, Lorente P, Andreu P, Arbeloa A. A novel approach for propagation of recalcitrant pistachio cultivars that sidesteps rooting by ex vitro grafting of tissue cultured shoot tips. Plant Cell Tiss Org Cult. 2016;124:191–200. doi: 10.1007/s11240-015-0871-7. [DOI] [Google Scholar]
- Mason GF, Guttridge CG. The role of calcium, boron and some divalent ions in leaf tipburn of strawberry. Sci Hortic. 1974;2:299–308. doi: 10.1016/0304-4238(74)90039-9. [DOI] [Google Scholar]
- Mason GF, Guttridge CG. The influence of relative humidity and nutrition on leaf tipburn of strawberry. Sci Hortic. 1975;3:339–349. doi: 10.1016/0304-4238(75)90048-5. [DOI] [Google Scholar]
- Matu ENN, Lindsey KLL, van Staden J. Micropropagation of Maytenus senegalensis (Lam.) Excell. S Afr J Bot. 2006;72:409–415. doi: 10.1016/j.sajb.2005.11.005. [DOI] [Google Scholar]
- McCown BH, Sellmer JC (1987) General media and vessels suitable for woody plant culture. In: Bonga JM, Durzan D (eds) Cell and tissue culture in forestry. Springer, The Netherlands, pp. 4–16. 10.1007/978-94-017-0994-1_2
- Millington WF. Shoot tip abortion in Ulmus americana. Am J Bot. 1963;50:371–378. doi: 10.1002/j.1537-2197.1963.tb07205.x. [DOI] [Google Scholar]
- Mirabbasi SM, Hosseinpour B. Prevention of shoot tip necrosis, hyperhydricity and callus production associated with in vitro shoot culture of Ulmus glabra. J Novel Appl Sci. 2014;3:683–689. [Google Scholar]
- Misra P, Chakrabarty D. Clonal propagation of Rosa clinophylla Thory. through axillary bud culture. Sci Hortic. 2009;119:212–216. doi: 10.1016/j.scienta.2008.07.028. [DOI] [Google Scholar]
- Misra P, Toppo DD, Gupta N, Chakrabarty D, Tuli R. Effect of antioxidants and associate changes in antioxidant enzymes in controlling browning and necrosis of proliferating shoots of elite Jatropha curcas L. Biomass Bioenergy. 2010;34:1861–1869. doi: 10.1016/j.biombioe.2010.07.027. [DOI] [Google Scholar]
- Moura M. Conservation of Hypericum foliosum Alton, an endemic Azorean species, by micropropagation. Vitro Cell Dev Biol Plant. 1998;34:244–248. doi: 10.1007/BF02822715. [DOI] [Google Scholar]
- Mubina N, Hoque M, Sarker R. In vitro regeneration and over expression of pea DNA helicase 45 (PDH45) gene into the local cultivars of chickpea (Cicer arietinum L.) through Agrobacterium-mediated genetic transformation. Plant Tissue Cult Biotechnol. 2018;28:125–140. doi: 10.3329/ptcb.v28i1.37204. [DOI] [Google Scholar]
- Mulwa RMS, Bhalla PL. In vitro shoot multiplication of Macadamia tetraphylla L. Johnson J Hortic Sci Biotechnol. 2000;75:1–5. doi: 10.1080/14620316.2000.11511192. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Mythili JB, Thomas P. Micropropagation of pointed gourd (Trichosanthes dioica Roxb.) Sci Hortic. 1999;79:87–90. doi: 10.1016/S0304-4238(98)00201-5. [DOI] [Google Scholar]
- Naaz A, Shahzad A, Anis M. Effect of adenine sulphate interaction on growth and development of shoot regeneration and inhibition of shoot tip necrosis under in vitro condition in adult Syzygium cumini L.—a multipurpose tree. Appl Biochem Biotechnol. 2014;173:90–102. doi: 10.1007/s12010-014-0797-2. [DOI] [PubMed] [Google Scholar]
- Nagahatenna DSK, Peiris SE. In vitro propagation of Hemidesmus indicus (L.) R. Br. (Iramusu) through nodal culture. Trop Agr Res. 2007;19:181–192. [Google Scholar]
- Nezami SR, Yadollahi A, Hokmabadi H, Eftekhari M. Control of shoot tip necrosis and plant death during in vitro multiplication of Pistachio rootstock UCB1 (Pistacia integrima × P.atlantica) J Nuts. 2015;6:27–35. doi: 10.22034/jon.2015.515646. [DOI] [Google Scholar]
- Nezami-Alanagh E, Garoosi G-A, Haddad R, Maleki S, Landín M, Gallego PP. Design of tissue culture media for efficient Prunusrootstock micropropagation using artificial intelligence models. Plant Cell Tiss Org Cult. 2014;117:349–359. doi: 10.1007/s11240-014-0444-1. [DOI] [Google Scholar]
- Nezami-Alanagh E, Garoosi G-A, Landín M, Gallego PP. Combining DOE with neurofuzzy logic for healthy mineral nutrition of Pistachio rootstocks in vitro culture. Front Plant Sci. 2018;9:1474. doi: 10.3389/fpls.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezami-Alanagh E, Garoosi G-A, Landín M, Gallego PP. Computer-based tools provide new insight into the key factors that cause physiological disorders of Pistachio rootstocks cultured in vitro. Sci Rep. 2019;9:9740. doi: 10.1038/s41598-019-46155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezami-Alanagh E, Garoosi G-A, Maleki S, Landín M, Gallego PP. Predicting optimal in vitro culture medium for Pistacia vera micropropagation using neural networks models. Plant Cell Tiss Org Cult. 2017;129:19–33. doi: 10.1007/s11240-016-1152-9. [DOI] [Google Scholar]
- Niedz RP, Evens TJ. A solution to the problem of ion confounding in experimental biology. Nat Methods. 2006;3:417–427. doi: 10.1038/nmeth0606-417. [DOI] [PubMed] [Google Scholar]
- Niedz RP, Evens TJ. Regulating plant tissue growth by mineral nutrition. Vitro Cell Dev Biol Plant. 2007;43:370–381. doi: 10.1007/s11627-007-9062-5. [DOI] [Google Scholar]
- Niedz RP, Evens TJ. Design of experiments (DOE)—history, concepts, and relevance to in vitro culture. Vitro Cell Dev Biol Plant. 2016;52:547–562. doi: 10.1007/s11627-016-9786-1. [DOI] [Google Scholar]
- Nitsch JP, Nitsch C. Haploid plants from pollen grains. Science. 1969;163:85–87. doi: 10.1126/science.163.3862.85. [DOI] [PubMed] [Google Scholar]
- Norton ME, Norton CR. In vitro propagation of Ericaceae: a comparison of the activity of the cytokinins N6-benzyladenine and N6-isopentenyladenine in shoot proliferation. Sci Hortic. 1985;27:335–340. doi: 10.1016/0304-4238(85)90038-X. [DOI] [Google Scholar]
- Oberschelp GPJ, Gonçalves AN. Analysis of nutrient deficiencies affecting in vitro growth and development of Eucalyptus dunnii Maiden. Physiol Mol Biol Plants. 2018;24:693–702. doi: 10.1007/s12298-018-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord CA, Tyler JL. In vitro propagation of Pimelea spicata R.Br (Thymelaeaceae), an endangered species of the Sydney region. Aust Plant Cell Tiss Org Cult. 2009;98:19–23. doi: 10.1007/s11240-009-9534-x. [DOI] [Google Scholar]
- Ozgen S, Busse JS, Palta JP. Influence of root zone calcium on shoot tip necrosis and apical dominance of potato shoot: simulation of this disorder by ethylene glycol tetra acetic acid and prevention by strontium. Hort Sci. 2011;46:1358–1362. doi: 10.21273/HORTSCI.46.10.1358. [DOI] [Google Scholar]
- Papadatou P, Pontikis C, Ephtimiadou E, Lydaki M. Rapid multiplication of guava seedlings by in vitro shoot tip culture. Sci Hortic. 1990;45:99–103. doi: 10.1016/0304-4238(90)90072-M. [DOI] [Google Scholar]
- Parfitt DE, Almehdi AA. Use of a high CO2 atmosphere and medium modifications for the successful micropropagation of pistachio. Sci Hortic. 1994;56:321–329. doi: 10.1016/0304-4238(94)90050-7. [DOI] [Google Scholar]
- Parh DK, Conner AJ, Jacobs JME, McNeil DL. Shoot-tip necrosis and alleviation during in vitro culture of Lens culinaris. SABRAO J Breed Genet. 1998;30:97–101. [Google Scholar]
- Park SW, Jeon JH, Kim HS, Park YM, Aswath C, Joung H. Effect of sealed and vented gaseous microenvironments on the hyperhydricity of potato shoots in vitro. Sci Hortic. 2004;99:199–205. doi: 10.1016/S0304-4238(03)00097-9. [DOI] [Google Scholar]
- Park JS, Naing AH, Kim CK. Effects of ethylene on shoot initiation, leaf yellowing, and shoot tip necrosis in roses. Plant Cell Tiss Org Cult. 2016;127:425–431. doi: 10.1007/s11240-016-1066-6. [DOI] [Google Scholar]
- Pasqua G, Manes F, Monacelli B, Natale L, Anselmi S. Effects of the culture medium pH and ion uptake in in vitro vegetative organogenesis in thin cell layers of tobacco. Plant Sci. 2002;162:947–955. doi: 10.1016/S0168-9452(02)00048-1. [DOI] [Google Scholar]
- Penel C, van Cutsem P, Greppin H. Interactions of a plant peroxidase with oligogalacturonides in the presence of calcium ions. Phytochemistry. 1999;51:193–198. doi: 10.1016/S0031-9422(98)00741-9. [DOI] [Google Scholar]
- Phillips GC, Garda M. Plant tissue culture media and practices: an overview. Vitro Cell Dev Biol Plant. 2019;55:242–257. doi: 10.1007/s11627-019-09983-5. [DOI] [Google Scholar]
- Piagnani C, Zocchi G, Mignani I. Influence of Ca2+ and 6-benzyladenine on chestnut (Castanea sativa Mill.) in vitro shoot-tip necrosis. Plant Sci. 1996;118:89–95. doi: 10.1016/0168-9452(96)04423-8. [DOI] [Google Scholar]
- Podwyszyńska M, Goszczyńska DM. Effect of inhibitors of ethylene biosynthesis and action, as well as calcium and magnesium on rose shoot rooting, shoot-tip necrosis and leaf senescence in vitro. Acta Physiol Plant. 1998;20:91–98. doi: 10.1007/s11738-998-0049-6. [DOI] [Google Scholar]
- Poothong S, Reed BM. Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci Hortic. 2014;165:132–141. doi: 10.1016/j.scienta.2013.10.040. [DOI] [Google Scholar]
- Preece J. Can nutrient salts partially substitute for plant growth regulators? Plant Tiss Cult Biotech. 1995;1:26–37. [Google Scholar]
- Purnhauser L, Medgyesy P, Czakó M, Dix PJ, Márton L. Stimulation of shoot regeneration in Triticum aestivum and Nicotiana plumbaginifolia Viv. tissue cultures using the ethylene inhibitor AgNO3. Plant Cell Rep. 1987;6:1–4. doi: 10.1007/BF00269725. [DOI] [PubMed] [Google Scholar]
- Pérez C, Rodríguez R, Tamés R. “In vitro” filbert (Corylus avellana L.) micropropagation from shoots and cotyledonary node segments. Plant Cell Rep. 1985;4:137–139. doi: 10.1007/BF00571300. [DOI] [PubMed] [Google Scholar]
- Pérez-Tornero O, Burgos L. Different media requirements for micropropagation of apricot cultivars. Plant Cell Tiss Org Cult. 2000;63:133–141. doi: 10.1023/A:1006430718024. [DOI] [Google Scholar]
- Quoirin M, Lepoivre P. Improved media for in vitro culture of Prunus sp. Acta Hortic. 1977;78:437–442. doi: 10.17660/ActaHortic.1977.78.54. [DOI] [Google Scholar]
- Ramage CM, Williams RR. Mineral nutrition and plant morphogenesis. Vitro Cell Dev Biol Plant. 2002;38:116–124. doi: 10.1079/IVP2001269. [DOI] [Google Scholar]
- Reed BM, DeNoma J, Wada S, Niedz R. Determining optimum in vitro mineral nutrition for diverse pear germplasm using response surface methodology. Acta Hortic. 2016;1113:79–84. doi: 10.17660/ActaHortic.2016.1113.11. [DOI] [Google Scholar]
- Reed BM, Wada S, DeNoma J, Niedz RP. Mineral nutrition influences physiological responses of pear in vitro. Vitro Cell Dev Biol Plant. 2013;49:699–709. doi: 10.1007/s11627-013-9556-2. [DOI] [Google Scholar]
- Robertson DN. Modulating plant calcium for better nutrition and stress tolerance. ISRN Bot. 2013;2013:952043. doi: 10.1155/2013/952043. [DOI] [Google Scholar]
- Rugini E. In vitro propagation of some olive (Olea europea sativa L.) cultivars with different rootability, and medium development using analytical data from developing shoots and embryos. Sci Hortic. 1984;24:12–134. doi: 10.1016/0304-4238(84)90143-2. [DOI] [Google Scholar]
- Rugini E, Tarini P, Mari F. In vitro control of shoot vitrification in almond (P. dulcis) and development of technique to eliminate apex necrosis and shoot base photo-oxidation in pistachio (Pistacia vera) HortScience. 1986;21:804. [Google Scholar]
- Ruiz JM, Rivero RM, López-Cantarero I, Romero L. Role of Ca2+ in the metabolism of phenolic compounds in tobacco leaves (Nicotiana tabacum L.) Plant Growth Regul. 2003;41:173–177. doi: 10.1023/A:1027358423187. [DOI] [Google Scholar]
- Ružić D, Vujović T, Libiakova G, Cerović R, Gajdošova A. Micropropagation in vitro of highbush blueberry (Vaccinium corymbosum L.) J Berry Res. 2012;2:97–103. doi: 10.3233/JBR-2012-030. [DOI] [Google Scholar]
- Sanchez MC, San-Jose MC, Ferro E, Ballester A, Vieitez AM. Improving micropropagation conditions for adult-phase shoots of chestnut. J Hortic Sci. 1997;72:433–443. doi: 10.1080/14620316.1997.11515531. [DOI] [Google Scholar]
- Santner A, Calderon-Villalobos LIA, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol. 2009;5:301–307. doi: 10.1038/nchembio.165. [DOI] [PubMed] [Google Scholar]
- Saure MC. Causes of the tipburn disorder in leaves of vegetables. Sci Hortic. 1998;76:131–147. doi: 10.1016/S0304-4238(98)00153-8. [DOI] [Google Scholar]
- Serres R, Read P, Hackett W, Nissen P. Rooting of American chestnut microcuttings. J Environ Hortic. 1990;8:86–88. doi: 10.24266/0738-2898-8.2.86. [DOI] [Google Scholar]
- Sha L, McCown BH, Peterson LA. Occurrence and cause of shoot-tip necrosis in shoot cultures. J Am Soc Hortic Sci. 1985;110:631–634. [Google Scholar]
- Singha S, Townsend EC, Oberly GH. Relationship between calcium and agar on vitrification and shoot-tip necrosis of quince (Cydonia oblonga Mill.) shoots in vitro. Plant Cell Tiss Org Cult. 1990;23:135–142. doi: 10.1007/BF00035834. [DOI] [Google Scholar]
- Srivastava A, Joshi AG. Control of shoot tip necrosis in shoot cultures of Portulaca grandiflora Hook. Not Sci Biol. 2013;5:45–49. doi: 10.15835/nsb519009. [DOI] [Google Scholar]
- Strnad M, Hanuš J, Vaňek T, Kamínek M, Ballantine JA, Fussell B, Hanke DE. Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus x canadensis Moench., cv. Robusta) Phytochemistry. 1997;45:213–218. doi: 10.1016/S0031-9422(96)00816-3. [DOI] [Google Scholar]
- Subbaiah CC, Kollipara KP, Sachs MM. A Ca2+-dependent cysteine protease is associated with anoxia-induced root tip death in maize. J Exp Bot. 2000;51:721–730. doi: 10.1093/jexbot/51.345.721. [DOI] [PubMed] [Google Scholar]
- Sudha CG, Krishnan PN, Pushpangadan P. In vitro propagation of Holostemma annulare (Robx.) K. Schum., a rare medicinal plant. Vitro Cell Dev Biol Plant. 1998;34:57–63. doi: 10.1007/BF02823124. [DOI] [Google Scholar]
- Surakshitha NC, Soorianathasundaram K, Ganga M, Raveendran M. Alleviating shoot tip necrosis during in vitro propagation of grape cv. Red Globe Sci Hortic. 2019;248:118–125. doi: 10.1016/j.scienta.2019.01.013. [DOI] [Google Scholar]
- Teixeira da Silva JA. Is BA (6-benzyladenine) BAP (6-benzylaminopurine)? Asian Austral J Plant Sci Biotech. 2012;6:121–124. [Google Scholar]
- Teixeira da Silva JA. The effect of ethylene inhibitors (AgNO3, AVG), an ethylene-liberating compound (CEPA) and aeration on the formation of protocorm-like bodies of hybrid Cymbidium (Orchidaceae) Front Biol. 2013;8:606–610. doi: 10.1007/s11515-013-1283-x. [DOI] [Google Scholar]
- Teixeira da Silva JA. Negative results: negative perceptions limit their potential for increasing reproducibility. J Negative Results BioMed. 2015;14:12. doi: 10.1186/s12952-015-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira da Silva JA, Dobránszki J. How timing of sampling can affect the outcome of the quantitative assessment of plant organogenesis. Sci Hortic. 2013;159:59–66. doi: 10.1016/j.scienta.2013.05.00. [DOI] [Google Scholar]
- Teixeira da Silva JA, Giang DTT, Tanaka M. Photoautotrophic micropropagation of Spathiphyllum. Photosynthetica. 2006;44:53–61. doi: 10.1007/s11099-005-0158-z. [DOI] [Google Scholar]
- Thakur A, Kanwar JS. Effect of phase of medium, growth regulators and nutrient supplementations on in vitro shoot-tip necrosis in pear. NZ J Crop Hort Sci. 2011;39:131–140. doi: 10.1080/01140671.2011.559254. [DOI] [Google Scholar]
- Thirugnanasampandan R, Mutharaian VN, Bai VN. In vitro propagation and free radical studies of Smilax zeylanica Vent. Afr J Biotechnol. 2009;8:395–400. [Google Scholar]
- Thomas P. Microcutting leaf area, weight and position on the stock shoot influence root vigour, shoot growth and incidence of shoot tip necrosis in grape plantlets in vitro. Plant Cell Tiss Org Cult. 2000;61:189–198. doi: 10.1023/A:1006425807853. [DOI] [Google Scholar]
- Thor K. Calcium-nutrient and messenger. Front Plant Sci. 2019;10:440. doi: 10.3389/fpls.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilkat E, Onay A, Yildirim H, Çetin Ozen H. Micropropagation of mature male pistachio Pistacia vera L. J Hortic Sci Biotechnol. 2008;83:328–333. doi: 10.1080/14620316.2008.11512387. [DOI] [Google Scholar]
- Trobacher CP. Ethylene and programmed cell death in plants. Botany. 2009;87:757–769. doi: 10.1139/B09-041. [DOI] [Google Scholar]
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith (eds) (2018) International code of nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 10.12705/Code.2018
- van den Dries N, Giannì S, Czerednik A, Krens FA, de Klerk G-JM. Flooding of the apoplast is a key factor in the development of hyperhydricity. J Exp Bot. 2013;64:5221–5230. doi: 10.1093/jxb/ert315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Calcium: the missing link to auxin action. Plants. 2013;2:650–675. doi: 10.3390/plants2040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibha JB, Shekhawat NS, Mehandru P, Dinesh R. Rapid multiplication of Dalbergia sissoo Roxb.: a timber yielding tree legume through axillary shoot proliferation and ex vitro rooting. Physiol Mol Biol Plant. 2014;20:81–87. doi: 10.1007/s12298-013-0213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieitez AM, Corredoira E, Ballester A, Muñoz F, Durán J, Ibarra M. In vitro regeneration of the important North American oak species Quercus alba, Quercus bicolor and Quercus rubra. Plant Cell Tiss Org Cult. 2009;98:135–145. doi: 10.1007/s11240-009-9546-6. [DOI] [Google Scholar]
- Vieitez AM, Sánchez C, San-José C. Prevention of shoot-tip necrosis in shoot cultures of chestnut and oak. Sci Hortic. 1989;41:151–159. doi: 10.1016/0304-4238(89)90059-9. [DOI] [Google Scholar]
- Wada S, Niedz RP, DeNoma J. Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. Vitro Cell Dev Biol Plant. 2013;49:356–365. doi: 10.1007/s11627-013-9508-x. [DOI] [Google Scholar]
- Wada S, Niedz RP, Reed BM. Determining nitrate and ammonium requirements for optimal in vitro response of diverse pear species. Vitro Cell Dev Biol Plant. 2015;51:19–27. doi: 10.1007/s11627-015-9662-4. [DOI] [Google Scholar]
- Wang H, van Staden J. Establishment of in vitro cultures of tree peonies. S Afr J Bot. 2001;67:358–361. doi: 10.1016/S0254-6299(15)31141-8. [DOI] [Google Scholar]
- Werbrouck SPO, Strnad M, Van Onckelen HA, Debergh PC. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol Plant. 1996;98:291–297. doi: 10.1034/j.1399-3054.1996.980210.x. [DOI] [Google Scholar]
- Wetzstein HY, Ault JR, Merkle SA. Further characterization of somatic embryogenesis and plantlet regeneration in pecan (Carya illinoensis) Plant Sci. 1989;64:193–201. doi: 10.1016/0168-9452(89)90024-1. [DOI] [Google Scholar]
- White PJ, Broadly MR. Calcium in plants. Ann Bot. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Satchwell MF, Powell WA, Maynard CA. Micropropagation of American chestnut: increasing rooting rate and preventing shoot-tip necrosis. Vitro Cell Dev Biol Plant. 1997;33:43–48. doi: 10.1007/s11627-997-0039-1. [DOI] [Google Scholar]
- Ye G, McNeil DL, Conner AJ, Hill GD. Multiple shoot formation in lentil (Lens culinaris) seeds. N N Z J Crop Hort Sci. 2002;30:1–8. doi: 10.1080/01140671.2002.9514193. [DOI] [Google Scholar]
- Zielińska A, Kępczyńska E. Neural modeling of plant tissue cultures: a review. BioTechnologia. 2013;94:253–268. doi: 10.5114/bta.2013.46419. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.