Abstract
Background
GS-3K8 and GINST, both of which are modified ginseng extracts, have never been examined in terms of their effectiveness for the prevention of acute respiratory illness (ARI) in humans. We conducted a pilot study to assess the feasibility of performing a large-scale, randomized, controlled trial.
Methods
This study was a randomized, double-blind, placebo-controlled, pilot study at a single center from October 2014 to March 2015. The 45 healthy applicants were randomly divided into the GS-3K8 (n = 15), GINST (n = 15), and placebo groups (n = 15). The study drug was administered as a capsule (500 mg/cap and 3000 mg/day). GS-3K8 contained 6.31 mg/g of Rg1, 15.05 mg/g of Re, 30.84 mg/g of Rb1, 15.02 mg/g of Rc, 12.44 mg/g of Rb2, 6.97 mg/g of Rd, 1.59 mg/g of Rg3, 3.25 mg/g of Rk1, and 4.84 mg/g of Rg5. GINST contained 7.54 mg/g of Rg1, 1.87 mg/g of Re, 5.42 mg/g of Rb1, 0.29 mg/g of Rc, 0.36 mg/g of Rb2, 0.70 mg/g of Rd, and 6.3 mg/g of compound K. The feasibility criteria were the rates of recruitment, drug compliance, and successful follow-up. The primary clinical outcome measure was the incidence of ARI. The secondary clinical outcome measures were the duration of symptoms.
Results
The rate of recruitment was 11.3 participants per week. The overall rate of completed follow-up was 97.8%. The mean compliance rate was 91.64 ± 9.80%, 95.28 ± 5.75%, and 89.70 ± 8.99% in the GS-3K8, GINST, and placebo groups, respectively. The incidence of ARI was 64.3% (9/14; 95% confidence interval [CI], 31.4–91.1%), 26.7% (4/15; 95% CI, 4.3–49.0%), and 80.0% (12/15; 95% CI, 54.8–93.0%) in the GS-3K8, GINST, and placebo groups, respectively. The average days of symptoms were 3.89 ± 4.65, 9.25 ± 7.63, and 12.25 ± 12.69 in the GS-3K8, GINST, and placebo groups, respectively.
Conclusion
The results support the feasibility of a full-scale trial. GS-3K8 and GINST appear to have a positive tendency toward preventing the development of ARI and reducing the symptom duration. A randomized controlled trial is needed to confirm these findings.
Keywords: Acute respiratory illness, Clinical trial, GINST, GS-3K8, Pilot study
1. Introduction
Acute respiratory illness (ARI) is mainly caused by respiratory viruses, and its progress is self-limiting [1]. However, it leads to reduced functioning and work productivity [2]. ARI is also associated with a marked economic burden to the health-care system and society because of medical costs and lost work time [3]. According to the Korean Health Insurance Review and Assessment Service, the cost of medical care for ARI was approximately 920 million dollars per year from 2007 to 2011 and approximately 1.4 billion dollars per year from 2012 to 2016 [4].
Infections caused by respiratory viruses, particularly influenza and respiratory syncytial virus, remain a significant cause of death in community-dwelling older adults [5]. Influenza vaccination may have limited efficacy in this population because of an age-associated decrease in both humoral and cell-mediated immune function [6]. In addition, the use of amantadine and rimantadine is limited by their side effects and the development of resistance [7]. Although neuraminidase inhibitors do not have these limitations, the cost of these drugs has been the greatest barrier to their use in institutional settings [7].
Ginseng products, including Korean ginseng and American ginseng, are available over the counter and are often used for the prevention and treatment of ARI [8], [9]. Recently, we showed the preventive effects of Korean ginseng on the development of ARI [8]. However, there is insufficient evidence to conclude that standard ginseng extracts decrease the incidence of ARI [1]. Therefore, modified ginseng extracts that have changes in the composition or absorbability of ginsenosides, which are mainly attributed to the pharmacological effects of ginseng, are needed [10].
Ginsenoside Rb1, one of the protopanaxadiol (PPD)-type ginsenosides, suppresses viral infection and the proliferation of various viruses in vitro [11], [12], [13]. PPD-type ginsenosides Rb1, Rb2, and Rc are transformed into compound K by the intestinal microflora [14]. GINST is a modified ginseng extract that hydrolyzes these ginsenosides into pectinase and enhances the absorption of component K [15]. GS-3K8 is another modified ginseng extract with enhanced PPD-type ginsenoside content [16]. There has been no large-scale, randomized, controlled study on the preventive effects of GS-3K8 and GINST against ARI. Therefore, we conducted a pilot study on GS-3K8 and GINST to determine the feasibility of a larger study.
2. Materials and methods
2.1. Study design
This pilot study was conducted as a randomized, double-blind, placebo-controlled trial to assess the feasibility of a large, randomized, controlled trial to compare GS-3K8 and GINST with placebo. The trial was conducted at the Clinical Trial Center for Functional Foods at Jeonbuk National University Hospital, which is a 1,200-bed, university-affiliated teaching hospital and the largest referral center in Jeollabuk-do, a province of Korea. Volunteers were informed about the study, including the study protocol, after which the study physician obtained informed consent from each participant. The screening examination was conducted within 4 weeks of the study initiation. The participants who passed the screening process were randomly allocated into one of the three groups (GS-3K8, GINST, or placebo) with an allocation ratio of 1:1:1. Random numbers were generated using the block randomization method in the Microsoft Office Excel 2007 program (Microsoft Corporation, Redmond, WA, USA) for sequence generation. The randomization sequence and allocation were concealed from all study participants, research staff, investigators, and pharmacists until the completion of the study.
At the first visit, the enrolled participants were instructed to ingest two capsules three times per day (after every meal) for 12 weeks. All participants were educated regarding ARI and instructed to contact the investigators and study physician at the onset of a cold. The participants were instructed not to consume any ginseng products other than those provided for the study or other functional foods or dietary supplements. During the study period, the participants who developed ARI received symptomatic treatment under the direction of the study physician. They were instructed not to take any other cold medication unless advised by the study physician. Every 6 weeks, the participants were asked to report any adverse events or adverse drug reactions, changes in lifestyle or eating patterns, and symptoms of ARI and assess their drug compliance. Laboratory tests were performed at the first visit and 12-week visit. During the 12-week intervention period and 6-week follow-up period, ARI-related symptoms were assessed weekly via telephone. If any symptoms were reported to the study physician, telephone interviews were then conducted daily until the symptoms ended.
2.2. Study population
The participants were recruited through media advertisements, and those volunteers were screened via a telephone interview for the inclusion and exclusion criteria. The participants were eligible to participate in this study if they were in good general health and aged between 39 and 65 years. The participants were excluded based on the following criteria: (1) vaccinated against influenza in the previous 6 months, (2) upper respiratory tract infection at the time of screening, (3) underlying medical conditions such as human immunodeficiency virus infection, malignancy, and cardiovascular, pulmonary, hepatic, renal, neurological, psychiatric, autoimmune, or hematologic diseases, (4) alcohol abuse or drug addiction, (5) gastrointestinal diseases, such as Crohn's disease, that may interrupt the absorption of the test drug or gastrointestinal surgery, except herniotomy and appendectomy, (6) taking immunomodulators such as immunosuppressants or immunostimulants, (7) hypersensitive to ginseng or currently taking ginseng, (8) participation in another clinical study within 2 months of the screening test, (9) abnormal hepatic or renal test, (10) psychological conditions making it difficult to participate in a study, and (11) pregnant or lactating.
2.3. Study drugs
GS-3K8, the PPD saponin–enriched ginseng extract used in this study, was obtained from the International Ginseng & Herb Research Institute (Geumsan, Republic of Korea) and prepared as described previously with slight modifications [16]. The preparation method of GS-3K8 was as follows: Ginseng material was prepared from mixtures of ginseng main root and rootlet (root:rootlet = 4:6). Ginseng mixture (1 kg) was extracted with 10 L of water at 80°C for 6 hours. Each extraction was repeated three times. The obtained water extract was concentrated up to 80% of extraction volume by ultrafiltration system with a 3-kDa hollow fiber cartridge. Inner fraction of the ginseng extract obtained by ultrafiltration system was further dried in a freeze dryer for the preparation of PPD saponin–enriched ginseng extract and then named as GS-3K8. GS-3K8 contained 6.31 mg/g of Rg1, 15.05 mg/g of Re, 30.84 mg/g of Rb1, 15.02 mg/g of Rc, 12.44 mg/g of Rb2, 6.97 mg/g of Rd, 1.59 mg/g of Rg3, 3.25 mg/g of Rk1, and 4.84 mg/g of Rg5. GINST, a pectinase-processed Panax ginseng extract, was obtained from ILHWA Co. Ltd., (Guri, Republic of Korea) and prepared as described previously with slight modifications [15]. The preparation method of GINST was as follows: Dried ginseng (1 kg) was extracted in 5 L of 50% ethanol in water and concentrated with a vacuum concentrator. The dry ginseng extract was incubated with an enzyme solution containing 2.4% pectinase (Sigma-Aldrich, St Louis, MO, USA) at 55ºC for 24 hours. GINST contained 7.54 mg/g of Rg1, 1.87 mg/g of Re, 5.42 mg/g of Rb1, 0.29 mg/g of Rc, 0.36 mg/g of Rb2, 0.70 mg/g of Rd, and 6.3 mg/g of compound K [17]. The placebo capsule was composed primarily of maltodextrin, soybean oil, palm oil, lecithin, rice bran wax, cacao color, and annatto extract and was matched to the other capsules with regard to energy content, flavor, appearance, and dosage. The study drug capsules were packaged indistinguishably and labeled with the participant's number. The compositions of the study drugs are shown in Table 1. The participants were instructed to bring all remaining supplements at each visit, and good compliance was defined as 75% or greater. The study drug was supplied to the participants two times, at the first visit and 6-week visit.
Table 1.
Composition of the test drugs.
| Component, mg (%) | GS-3K8 | GINST | Placebo |
|---|---|---|---|
| Protopanaxadiol saponin–enriched ginseng extract | 162 (32.53) | - | - |
| Pectinase-processed ginseng extract | - | 160 (31.94) | - |
| Maltodextrin | - | - | 160 (32.00) |
| Soybean oil | 238 (47.79) | 243 (48.50) | 241 (48.10) |
| Palm oil | 40 (8.03) | 40 (7.98) | 30 (6.00) |
| Rice bran wax | 50 (10.04) | 50 (9.98) | 48 (9.60) |
| ER 290 | 8 (1.61) | 8 (1.60) | - |
| Lecithin | - | - | 11 (2.30) |
| Annatto extract | - | - | 3 (0.60) |
| Cacao color | - | - | 7 (1.40) |
| Total | 498 (100) | 501 (100) | 500 (100) |
2.4. Outcome measures
All outcome measures were defined a priori. The primary outcome measures for this pilot study were the recruitment rate, follow-up rate, and drug compliance rate. The three feasibility objectives would be considered successful if we achieved a recruitment rate of ≥11 participants per week, completed follow-ups for at least 95% of all recruited participants, and a mean compliance rate of 90% or greater. Completed follow-up was defined as the percentage of randomized participants who completed all assessments during the 18-week study period. Compliance was defined as follows: percent compliance = 100 × (number of capsules dispensed – number of capsules returned)/number of capsules prescribed. The secondary outcome measures were the clinical outcome measures that would be used in a large-scale clinical study. The primary clinical outcome was the incidence of ARI, and the secondary clinical outcome was the duration of ARI-related symptoms.
2.5. Safety assessment and tolerability
Safety was assessed through the number of adverse events and adverse drug reactions that were measured using the results of laboratory tests and vital signs. Tolerability was evaluated through the side effects of GS-3K8, GINST, and placebo capsules. Specifically, the participants were asked about gastrointestinal symptoms, such as nausea, vomiting, loss of appetite, gastrointestinal distress, and intolerance to certain tastes, at each visit.
2.6. Ethical approval
The protocol for this study was approved by the Institutional Review Board of Jeonbuk National University Hospital (IRB number: 2014-02-011) and registered at www.clinicaltrials.gov (NCT03028077).
2.7. Sample size and data analysis
Because preliminary data were lacking regarding the ability of GK-3K8 or GINST to prevent ARI in healthy people, a pilot trial was performed to generate preliminary data for the design of a large-scale clinical trial. Continuous variables were expressed as the mean value and standard deviation, and categorical variables were expressed as the percentage. The cumulative risk of ARI was estimated using the Kaplan–Meier method. Hazard ratios and 95% confidence intervals (CIs) comparing the incidence rate between GS-3K8 and placebo or between GINST and placebo were estimated using Cox proportional hazards regression models. Calculations of sample size were based on the ability to detect a 20% difference in the event rates of ARI between the GS-3K8 and placebo groups with 80% power using a two-sided p value of 0.017 by Bonferroni correction. SPSS software (version 19.0; IBM, Chicago, Illinois, USA) was used for all statistical analyses.
3. Results
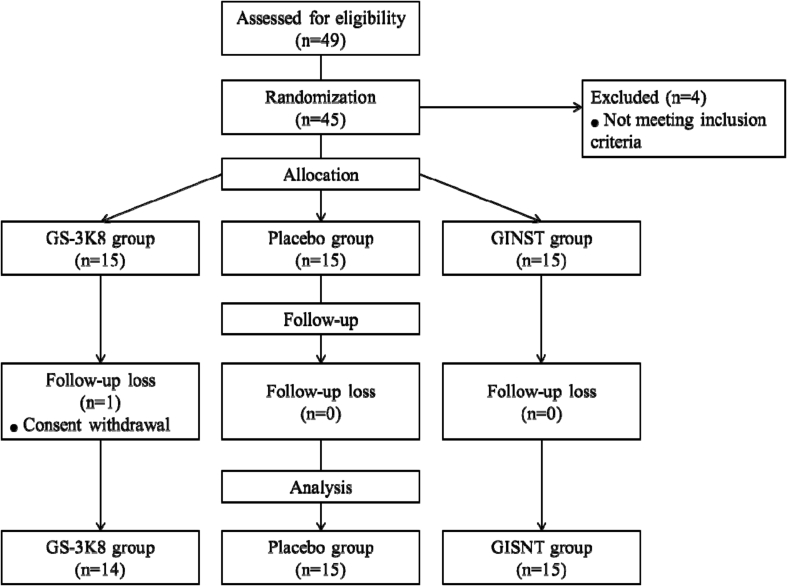
Fig. 1 describes the management of the participants in this pilot study. The participants were recruited at the Clinical Trial Center for Functional Foods at Jeonbuk National University Hospital, Jeollabuk-do, Republic of Korea, in October 2014. The rate of recruitment in this feasibility study was 11.3 participants per week. Initially, 49 participants were enrolled in the study; however, four participants did not meet the inclusion criteria and were excluded. The remaining 45 participants were randomly allocated to the GS-3K8 (n = 15), GINST (n = 15), and placebo (n = 15) groups. The participants in each group took the study drug during the 12-week intervention period and were assessed for ARI occurrence during the next 6 weeks (follow-up period). During the study period, one participant in the GS-3K8 group withdrew consent, and the remaining 44 of the 45 randomized participants were followed up for the entire study period, yielding a follow-up rate of 97.8%.
Fig. 1.
Flow chart showing the number of participants assessed for eligibility, randomization, follow-up, and analysis.
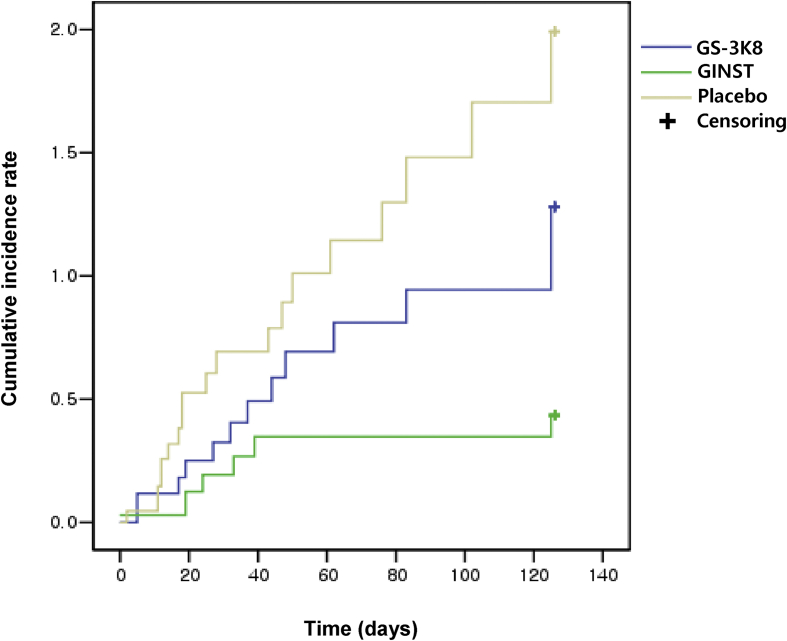
The demographic characteristics of the participants are presented in Table 2, and Table 3 presents the clinical events, that is, primary and secondary clinical outcomes, observed in this study. The incidence of ARI was 64.3% (9/14; 95% CI, 31.4–91.1%) in the GS-3K8 group, 26.7% (4/15; 95% CI, 4.3–49.0%) in the GINST group, and 80.0% (12/15; 95% CI, 54.8–93.0%) in the placebo group. The average number of days of symptoms were 3.89 ± 4.65, 9.25 ± 7.63, and 12.25 ± 12.69 in the GS-3K8, GINST, and placebo groups, respectively. The Kaplan–Meier plots in Fig. 2 show the cumulative incidence of ARI in the GS-3K8, GINST, and placebo groups. The hazard ratio of ARI was 0.59 (95% CI, 0.28–1.22) in the GS-3K8 group and 0.25 (95% CI, 0.01–0.64) in the GINST group. Statistical comparisons between the groups for clinical outcomes were not conducted because this pilot study was not powered for such comparisons and any statistical conclusions from such comparisons would be misleading.
Table 2.
Demographic characteristics of the study participants.
| Variables | GS-3K8 group (n = 15) | GINST group (n = 15) | Placebo group (n = 15) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 1 (6.7) | 2 | 0 |
| Female | 14 (93.3) | 13 | 15 (100) |
| Age, mean, y | 54.47 ± 3.40 | 55.00 ± 2.95 | 53.00 ± 3.66 |
| Height, mean, cm | 159.07 ± 6.86 | 159.27 ± 8.88 | 158.13 ± 5.08 |
| Body weight, mean, kg | 60.89 ± 6.93 | 64.26 ± 10.32 | 58.82 ± 6.28 |
| BMI, mean, kg/m2 | 24.09 ± 2.30 | 25.24 ± 2.73 | 23.58 ± 2.78 |
| Alcohol drinking, n (%) | |||
| Yes | 5 (33.3) | 7 (46.7) | 7 (46.7) |
| No | 10 (66.7) | 8 (53.3) | 8 (53.3) |
| Smoking, n (%) | |||
| Yes | 0 | 0 | 1 (6.7) |
| No | 15 (100) | 15 (100) | 14 (93.3) |
BMI, body mass index.
Table 3.
Number of clinical events observed in each group.
| Variables | GS-3K8 group (n = 14) | GINST group (n = 15) | Placebo group (n = 15) |
|---|---|---|---|
| Primary clinical outcome, n (%; 95% CI) | |||
| ARI | 9 (64.3; 31.4–91.1) | 4 (26.7; 4.3–49.0) | 12 (80.0; 54.8–93.0) |
| Secondary clinical outcomes, n, mean days ± SD | |||
| Symptoms and duration | |||
| Sore throat | 0 | 1, 2 | 5, 3.80 ± 2.49 |
| Coryza | 7, 2.57 ± 1.13 | 3, 5.00 ± 3.00 | 8, 6.50 ± 3.25 |
| Nasal congestion | 0 | 0 | 6, 4.00 ± 2.76 |
| Sneezing | 6, 2.33 ± 1.03 | 0 | 3, 7.67 ± 7.23 |
| Hoarseness | 2, 1.00 ± 0.00 | 1, 5 | 5, 10.00 ± 9.25 |
| Myalgia | 1, 1 | 0 | 2, 2.00 ± 0.00 |
| Otalgia | 0 | 0 | 0 |
| Fever | 0 | 1, 1 | 1, 1 |
| Headache | 1, 1 | 1, 1 | 4, 6.00 ± 6.06 |
| Cough | 3, 7.00 ± 6.93 | 3, 9.33 ± 9.45 | 7, 10.43 ± 12.63 |
| Total | 9, 3.89 ± 4.65 | 4, 9.25 ± 1.22 | 12, 12.25 ± 12.69 |
ARI, acute respiratory illness; CI, confidence interval; SD, standard deviation.
Fig. 2.
Kaplan–Meier estimates of the cumulative risk of acute respiratory illness in the GS-3K8 and GINST groups compared with the placebo group.
The mean compliance rate was 91.64 ± 9.80% in the GS-3K8 group, 95.28 ± 5.75% in the GINST group, and 89.70 ± 8.99% in the placebo group (Table 4). The percentage of participants achieving 75% or greater compliance was 92.9% in the GS-3K8 group and 100% in the GINST and placebo group. There were no adverse drug reactions reported in the GS-3K8, GINST, or placebo groups. Five adverse events were observed: one case of herpes zoster in the GS-3K8 group, one case each of herpes zoster and radial styloid tenosynovitis in the GINST group, and one case each of vaginitis and rhinitis in the placebo group. Gastrointestinal intolerance and taste intolerance were not observed in the GS-3K8, GINST, or placebo groups. During the study period, vital signs were measured and laboratory tests were performed for the safety assessment, and the results are shown in Table 5, Table 6.
Table 4.
Drug compliance and tolerability.
| Variables | GS-3K8 group (n = 14) | GINST group (n = 15) | Placebo group (n = 15) |
|---|---|---|---|
| Compliance | |||
| Capsules prescribed, mean ± SD | 498.14 ± 6.49 | 499.20 ± 8.13 | 502.80 ± 8.44 |
| Capsule intake, mean ± SD | 456.21 ± 46.36 | 475.73 ± 31.04 | 451.20 ± 47.59 |
| Compliance rate, %, mean ± SD | 91.64 ± 9.80 | 95.28 ± 5.75 | 89.70 ± 8.99 |
| Achieved ≥75% compliance, n (%) | 13 (92.9) | 15 (100) | 15 (100) |
| Tolerability | |||
| Gastrointestinal intolerance, n | 0 | 0 | 0 |
| Taste intolerance, n | 0 | 0 | 0 |
SD, standard deviation.
Table 5.
Change in laboratory findings during the study.
| Laboratory test | GS-3K8 group (n = 14) |
GINST group (n = 15) |
Placebo group (n = 15) |
|||
|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Baseline | 12 weeks | Baseline | 12 weeks | |
| Hematology | ||||||
| WBC | 5.72 ± 1.30 | 5.27 ± 0.98 | 5.36 ± 1.09 | 4.71 ± 0.99 | 4.70 ± 0.87 | 4.60 ± 1.46 |
| Hemoglobin | 13.87 ± 1.16 | 13.68 ± 1.14 | 13.67 ± 1.07 | 13.93 ± 0.98 | 13.53 ± 0.86 | 13.62 ± 0.70 |
| Platelets | 269.27 ± 58.97 | 268.14 ± 55.38 | 255.80 ± 49.36 | 256.73 ± 38.96 | 248.47 ± 37.90 | 249.73 ± 54.23 |
| Chemistry | ||||||
| ALP | 80.73 ± 16.54 | 87.57 ± 21.67 | 68.00 ± 19.49 | 68.47 ± 17.60 | 62.80 ± 14.96 | 65.87 ± 18.15 |
| GGT | 24.80 ± 26.43 | 23.07 ± 19.03 | 17.93 ± 16.33 | 16.33 ± 6.11 | 15.93 ± 4.06 | 14.67 ± 3.02 |
| AST | 23.67 ± 4.05 | 27.64 ± 8.74 | 26.73 ± 6.98 | 24.20 ± 4.04 | 25.27 ± 5.22 | 24.93 ± 4.30 |
| ALT | 19.00 ± 5.36 | 23.43 ± 8.40 | 23.40 ± 11.26 | 20.67 ± 6.99 | 22.27 ± 11.62 | 21.60 ± 6.96 |
| Total bilirubin | 0.81 ± 0.23 | 0.79 ± 0.17 | 0.82 ± 0.20 | 0.82 ± 0.33 | 0.88 ± 0.25 | 0.80 ± 0.26 |
| Total protein | 7.63 ± 0.33 | 7.53 ± 0.38 | 7.21 ± 0.32 | 7.38 ± 0.36 | 7.29 ± 0.34 | 7.33 ± 0.46 |
| Albumin | 4.44 ± 0.14 | 4.38 ± 0.17 | 4.35 ± 0.20 | 4.43 ± 0.21 | 4.31 ± 0.18 | 4.39 ± 0.22 |
| BUN | 14.67 ± 4.37 | 14.86 ± 4.04 | 13.00 ± 2.39 | 13.73 ± 2.52 | 12.13 ± 3.20 | 13.40 ± 4.07 |
| Creatinine | 0.64 ± 0.14 | 0.58 ± 0.13 | 0.61 ± 0.20 | 0.59 ± 0.18 | 0.56 ± 0.09 | 0.54 ± 0.05 |
| Total cholesterol | 188.73 ± 34.17 | 193.07 ± 28.24 | 203.93 ± 33.84 | 199.80 ± 27.02 | 187.40 ± 24.16 | 183.20 ± 21.99 |
| Triglycerides | 126.33 ± 58.08 | 109.29 ± 36.59 | 118.93 ± 47.62 | 123.13 ± 41.95 | 100.80 ± 36.28 | 99.07 ± 35.78 |
| Glucose | 86.33 ± 5.38 | 84.07 ± 6.89 | 88.67 ± 8.68 | 84.33 ± 6.78 | 86.33 ± 10.15 | 85.53 ± 6.14 |
| Urinalysis | ||||||
| Specific gravity | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 |
| pH | 6.03 ± 0.92 | 5.86 ± 0.84 | 6.47 ± 1.04 | 6.43 ± 0.65 | 6.37 ± 0.92 | 6.13 ± 0.93 |
All values are presented as mean ± standard deviation.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transaminase; BUN, blood urea nitrogen; GGT, gamma-glutamyltransferase; WBC, white blood cell.
Table 6.
Change in vital signs during the study.
| Vital sign | GS-3K8 group (n = 14) |
GINST group (n = 15) |
Placebo group (n = 15) |
|||
|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Baseline | 12 weeks | Baseline | 12 weeks | |
| Systolic BP | 124.00 ± 7.64 | 127.07 ± 6.63 | 124.87 ± 7.57 | 126.87 ± 8.03 | 116.20 ± 13.96 | 115.20 ± 13.51 |
| Diastolic BP | 79.27 ± 8.54 | 83.71 ± 7.33 | 76.80 ± 8.35 | 79.33 ± 7.96 | 77.73 ± 12.42 | 74.47 ± 10.15 |
| Heart rate | 77.67 ± 10.89 | 72.71 ± 6.52 | 74.00 ± 10.95 | 69.27 ± 8.53 | 75.47 ± 8.43 | 71.20 ± 6.90 |
| Body temperature | 36.42 ± 0.17 | 36.42 ± 0.15 | 36.33 ± 0.16 | 36.34 ± 0.15 | 36.34 ± 0.17 | 36.33 ± 0.20 |
All values are presented as mean ± standard deviation.
BP, blood pressure.
4. Discussion
We designed this pilot study to determine the feasibility of a large-scale clinical trial in the future. Korean Red Ginseng is often taken as a dietary supplement; therefore, we evaluated the success of the study based on the recruitment rate, compliance rate, and completed follow-up rate. The results of this study suggest that a full-scale randomized clinical trial is feasible.
The compliance rate was approximately 90% in this study, and most participants taking the study drugs achieved good compliance (consumption of ≥75% of the study drug). The reasons for this high compliance are that the study participants were interested in health supplements such as Korean Red Ginseng is readily available in everyday life, and the participants did not experience drug intolerance or adverse drug reactions in this study. A similar previous study in our center has a compliance rate of more than 90% [8]. However, the definition of good compliance in this study is somewhat arbitrary because the use of categories such as “good” and “poor” compliance is not supported, as noted by the appropriateness of the cutoff for specific medications in [18].
We achieved our target follow-up rate and successfully followed up 97.8% (44/45) of the randomized participants. The recruitment rate in this study was modest, with 11.3 participants per week. However, most studies on the effects of ginseng on the prevention of ARI did not report the recruitment rates. A previous large-scale randomized controlled trialperformed in our center recruited 12.5 participants per week, and another large-scale RCT on Panax quinquefolius recruited 36.6 participants per week [8], [19]. The results from this pilot study suggest that a sample of 345 participants (115 in each group) would be required to progress to a full-scale RCT in the future, which allows for a 5% loss of participants during the follow-up period. If a full-scale RCT was conducted in a single center, recruitment would take 7–8 months. However, if the number of centers was doubled, we estimate that full recruitment would be achieved in approximately 4 months.
The protective effects of Panax ginseng against infection caused by viruses, such as respiratory syncytial virus, rhinovirus, and influenza virus causing upper respiratory tract infections, have been shown previously in several experimental data [20]. Some RCTs have documented the clinically favorable effects of standard ginseng extract, including Panax quinquefolius or Panax ginseng, on ARI [8], [21]. Recently, a study on Panax ginseng at our institution showed a reduction in the incidence and symptom duration of ARI [8]. However, in other research, Panax quinquefolius did not show a protective effect against ARI [5]. In addition, previous studies of Panax quinquefolius or Panax ginseng on ARI in humans have shown insufficient evidence on their effectiveness in preventing ARI [22].
GINST is a modified ginseng extract with an increased content of compound K formed by fermentation of PPD-type ginsenosides Rb1, Rb2, and Rc by pectinase [14]. By increasing the content of compound K by fermentation, GINST showed excellent pharmacokinetic parameters in a human study rather than the usual nonfermented ginseng extract [15]. GINST showed higher antiviral activity against a variety of influenza viruses than nonfermented ginseng extract [23]. GS-3K8 also showed an antiviral activity against influenza virus A (H1N1) as a modified ginseng extract with a high proportion of Rb1/Rg1 by increasing the content of PPD-type ginsenosides via ultrafiltration [24].
Therefore, we conducted a pilot-scale human participant study using GS-3K8 and GINST, which are made from standard ginseng extracts by changing the composition or absorbability of ginsenosides and their enzymatic or heat-processed metabolites such as compound K. This study did not test a formal hypothesis, but this was a preliminary study for a future full-scale RCT on GS-3K8 and GINST. Therefore, statistical analysis of the secondary outcome measures was not performed. However, this pilot study found a positive trend toward a reduction in the development of ARI and of symptom duration among the participants in the GS-3K8 and GINST groups compared with those in the placebo group. The feasibility criteria were met, indicating that it would be possible to perform a large-scale RCT. Thus, based on the preliminary results obtained during this pilot study, the usefulness of GS-3K8 and GINST for the prevention of ARI should be further evaluated by using a larger randomized controlled trial.
No adverse drug reactions to GS-3K8, GINST, or the placebo occurred during the intervention or follow-up periods, and no drug intolerance was observed. Five adverse events developed: one in the GS-3K8 group, two in the GINST group, and two in the placebo group. However, there was no causality with the study drugs. During the study period, both the vital signs and laboratory findings remained within the normal range for all participants in the three study groups.
In conclusion, the feasibility results support a full-scale RCT to determine the efficacy of GS-3K8 and GINST in people with ARI, which would need to include at least 345 participants. This study provided encouraging results regarding the effects of GSK-3K8 and GINST on the development and symptom duration of ARI. The results of this feasibility trial suggest that a full-scale RCT with a sufficient number of participants to achieve statistical power is warranted to demonstrate the protective effects of GS-3K8 and GINST against ARI.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by the Export Promotion Technology Development Program of the Ministry of Agriculture, Food and Rural Affairs (312064-03) of South Korea.
References
- 1.Allan G.M., Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190–199. doi: 10.1503/cmaj.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A., Thomas M., Whitney H. Effects of upper respiratory tract illnesses on mood and performance over the working day. Ergonomics. 2000;43:752–763. doi: 10.1080/001401300404724. [DOI] [PubMed] [Google Scholar]
- 3.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 4.Health Insurance Review and Assessment Service (HIRA) HIRA; Seoul: 2013. The common cold in spring during the change of seasons, more attention in young people age group [Internet]http://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=8470 Mar [cited 2018 Feb 12]. Available from: [Google Scholar]
- 5.McElhaney J.E., Simor A.E., McNeil S., Predy G.N. Efficacy and safety of CVT-E002, a proprietary extract of Panax quinquefolius in the prevention of respiratory infections in influenza-vaccinated community-dwelling adults: a multicenter, randomized, double-blind, and placebo-controlled trial. Influenza Res Treat. 2011;2011:759051. doi: 10.1155/2011/759051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jefferson T., Rivetti D., Rivetti A., Rudin M., Di Pietrantonj C., Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 7.Michiels B., Van Puyenbroeck K., Verhoeven V., Vermeire E., Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., Kwon D.Y., Ha K.C., Park E.O., Lee N. Preventive effect of Korean red ginseng for acute respiratory illness: a randomized and double-blind clinical trial. J Korean Med Sci. 2012;27:1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElhaney J.E., Gravenstein S., Cole S.K., Davidson E., O'neill D., Petitjean S., Rumble B., Shan J.J. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52:13–19. doi: 10.1111/j.1532-5415.2004.52004.x. [DOI] [PubMed] [Google Scholar]
- 10.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M.H., Lee B.H., Lee S., Choi C. Reduction of hepatitis A virus on FRhK-4 cells treated with Korean red ginseng extract and ginsenosides. J Food Sci. 2013;78:M1412–M1415. doi: 10.1111/1750-3841.12205. [DOI] [PubMed] [Google Scholar]
- 12.Lee M.H., Seo D.J., Kang J.H., Oh S.H., Choi C. Expression of antiviral cytokines in Crandell-Reese feline kidney cells pretreated with Korean red ginseng extract or ginsenosides. Food Chem Toxicol. 2014;70:19–25. doi: 10.1016/j.fct.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y.Y., Wang B., Qian D.M., Li L., Wang Z.H., Hu M., Song X.X. Inhibitory effects of Ginsenoside Rb1 on apoptosis caused by HSV-1 in human glioma cells. Virol Sin. 2012;27:19–25. doi: 10.1007/s12250-012-3220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae E.A., Kim N.Y., Han M.J., Choo M.K., Kim D.H. Transformation of ginsenosides to compound K(IH-901) by lactic acid bacteria of human intestine. J Microbiol Biotechnol. 2003;13:9–14. [Google Scholar]
- 15.Jin H., Seo J.H., Uhm Y.K., Jung C.Y., Lee S.K., Yim S.V. Pharmacokinetic comparison of ginsenoside metabolite IH-901 from fermented and non-fermented ginseng in healthy Korean volunteers. J Ethnopharmacol. 2012;139:664–667. doi: 10.1016/j.jep.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Seol S.Y., Kim B.R., Hong S.C., Yoo J.H., Lee K.H., Lee H.J., Park J.D., Pyo M.K. The effective preparation of protopanaxadiol saponin enriched fraction from ginseng using the ultrafiltration. Nat Prod Sci. 2014;20:58–64. [Google Scholar]
- 17.Park S.H., Oh M.R., Choi E.K., Kim M.G., Ha K.C., Lee S.K., Kim Y.G., Park B.H., Kim D.S., Chae S.W. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J Ginseng Res. 2014;38:239–243. doi: 10.1016/j.jgr.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer J.A., Roy A., Burrell A., Fairchild C.J., Fuldeore M.J., Ollendorf D.A., Wong P.K. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.High K.P., Case D., Hurd D., Powell B., Lesser G., Falsey A.R., Siegel R., Metzner-Sadurski J., Krauss J.C., Chinnasami B. A randomized, controlled trial of Panax quinquefolius extract (CVT-E002) to reduce respiratory infection in patients with chronic lymphocytic leukemia. J Support Oncol. 2012;10:195–201. doi: 10.1016/j.suponc.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im K., Kim J., Min H. Ginseng, the natural effectual antiviral: protective effects of Korean Red Ginseng against viral infection. J Ginseng Res. 2016;40:309–314. doi: 10.1016/j.jgr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Predy G.N., Goel V., Lovlin R., Donner A., Stitt L., Basu T.K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005;173:1043–1048. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seida J.K., Durec T., Kuhle S. North American (Panax quinquefolius) and Asian ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review. Evid Based Complement Alternat Med: eCAM. 2011;2011:282151. doi: 10.1093/ecam/nep068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Jung Y.J., Kim K.H., Kwon Y., Kim Y.J., Zhang Z., Kang H.S., Wang B.Z., Quan F.S., Kang S.M. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses. 2018;10 doi: 10.3390/v10090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyo MK, Seol SY, Yoo JH, Lee KH, Hong SC, Park JD, International Ginseng & Herb Research Institute. Composition of anti-influenza H1N1 virus using ultrafiltration and preparation method thereof. Korean patent KR 101485982B1. 2015 Jan 19. Korean.