Abstract
Background
Skin cancer is the most common cancer in the USA. Therefore, it is important to review the contribution of ultraviolet radiation (UVR) exposure to skin cancer in individuals with the highest risk. Documenting the relationship between outdoor sports solar ultraviolet exposure and their risk of skin cancer along with appropriate risk mitigation strategies can help inform clinicians of practical information for counseling sun protective behaviors in this population.
Methods
We conducted a review of the current evidence using PubMed to answer the following research questions: (1) How is ultraviolet radiation measured? (2) What is the modern utility of the ultraviolet index in modifying recreational sun protection behaviors? (3) What is the risk of developing skin cancer for outdoor sport participants? (4) What is the prevalence of skin cancer in sport participants? and (5) Is the number of nevi and solar lentigines elevated in outdoor sport participants?
Results
Based on the literature, individuals who practice outdoor sport-related activities receive high ultraviolet radiation exposure, have a high risk for skin cancer, have a high prevalence for pigmented lesions, and may benefit from electronic sun protection educational interventions.
Conclusions
Individuals who practice outdoor sports experience substantially higher ultraviolet radiation exposure, routinely exceed the recommended exposure limits, and are at a higher risk of developing skin cancer. Therefore, those who are frequently engaged in outdoor leisure activities should be coached about efficient sun protective practices and relevant mobile technologies that may facilitate adherence.
Keywords: Sun exposure, Skin cancer, Ultraviolet index, Dosimeter, Outdoor sports, Patient education, Athletes, Mobile technology
Key Points
Individuals performing outdoor sports experience increased risk of skin cancer, increased prevalence of pigmented lesions in sun-exposed areas, and experience greater overall sun exposure.
Modern technology utilizing the ultraviolet index as tool for modifying sun-protective behavior confers a mildly positive benefit.
This evidence-based assessment supports the assumption of outdoor sportsmen and women being in greater need of sun-protective behavior counseling by their healthcare provider.
Background
Skin cancer is the most common cancer in the USA. It is five times more common than breast or prostate cancer [1]. Moreover, skin cancer incidence is increasing. According to 2012 estimations, the number of patients diagnosed annually with non-melanoma skin cancer (NMSC) approaches 3.3 million, representing a 50% increase from 2006 [2, 3]. The 2020 Annual Report to the Nation on the Status of Cancer reveals an annual incidence of melanoma of 28.5 per 100,000 persons for men and 17.6 per 100,000 for women, which translates to a respective 5-year average annual percent change of 2.2% and 1.9% [4]. The WHO’s International Agency for Research on Cancer reports an age-standardized incidence rate (ASR) of all skin cancers at 68.1 per 100,000 persons in the USA. Elsewhere, such as Australia and New Zealand, the ASR is even higher at 181.1 and 176.1 skin cancers, respectively [5].
Outdoor sports athletes have high rates of sunburn [6–13] and low rates of skin cancer literacy [7, 14], thereby increasing their risk for cutaneous malignancy. A person’s orientation to the sun [15], the amount of sun exposure [16], and population behaviors [17, 18] toward sun exposure and protection may be determinant factors that explain the increasing frequency of skin cancer, but genetic, demographical, geographical, and meteorological differences make it difficult to predict an individual’s risk [18]. However, clinical recognition of high-risk behaviors can help identify those who need and will benefit most from individualized counseling on sun protective behaviors [18, 19].
It has been established that the number and severity of sunburns correlate with increased rates of melanoma later in life, with up to 90% attributed to UV exposure [18, 20]. While both UV-A (320–400 nm) and UV-B (290–320 nm) impact cutaneous health, UV-B is assumed to be the main culprit for inducing carcinogenic sequelae [16]. Equally as important to the risk of cancer is the health benefit of increased vitamin D levels associated with intermittent UV-B exposure [21, 22]. The relationship between systemic vitamin D levels and all-cause and specific-cause mortality has been documented in many studies [23–27]. The recommended exposures required to achieve the desired 25(OH)D levels are minimal in the summer months, although varying latitudes and weather conditions influence the time needed for adequate UV radiation exposure [28–30].
Despite the wealth of knowledge available to the public on sun care etiquette and skin cancer prevention, individuals continue to fail to engage in protective behaviors while outdoors [13, 31–33], and the melanoma incidence continues to rise [19]. Even in locations where UV-intensity remains elevated year-round, sun-protection programs lack widespread institutional adoption [17, 34]. These concerns emphasize the need for a greater understanding of how outdoor behavior impacts cutaneous health. Herein, we will review the strategies for determining personal UV exposure and evaluate the frequency of skin cancer and pigmented lesions in those who perform outdoor sport activities. In addition, we conducted a systematic review of current literature to evaluate the risk of developing skin cancer guided by the following research questions:
How is personal UVR measured?
What is the modern utility of the ultraviolet index in modifying recreational sun protection behaviors?
What is the risk of developing skin cancer for outdoor sport participants?
What is the prevalence of skin cancer in sport participants?
Is the number of nevi and solar lentigines elevated in sport participants?
Methods
Query criteria and search terms are listed in Table 1. Our approach for each question is detailed as follows:
Table 1.
Search terms
| Search terms | |
|---|---|
| Database | Medline |
| Free text words |
“ultraviolet index”a “electronic dosimeters”b “electronic sun journal”c “Ultraviolet Index” OR “UV Index” OR “UVI” AND “Behavior”; “Mobile”; “Email”; “App”d ((“skin cancer”/sports) OR “skin cancer” prevalence AND “sports”)e “skin cancer” risk AND sportsf “Nevi Count AND Sports”g |
| Field | All fieldsa–f |
| Limits |
Language: Englisha–f Species: Humana–f Time: Nonea–d, g 01/1990–12/2018e 03/1986–03/2019f |
| MeSH | Usede |
Superscripts a–f denote the free text word search terms and their corresponding modifiers utilized in our Medline query
For question 1, we leveraged the International Commission on Non-Ionizing Radiation Protection Statement against UVR [35] and Schmalweiser and Siani’s Review on Nonoccupational Personal Solar UV Exposure Measurements [36]. We also performed focused searches using the terms “ultraviolet index” [37]. Finally, we explored approaches used to measure erythemally weighted UV irradiances accumulated over time on research participants based on references from Moehrle’s work [38] and additional focused Medline searches utilizing the terms “electronic dosimeters” and “electronic sun journal” [39–42]. A broader literature and citation search of all UVI measuring techniques yielded further sources [30, 35, 43–50]. For all other questions, we used Boolean text query strategies “AND”, “OR” on Medline. For question 2, we aimed to expand upon Italia et al.’s 2011 systematic review [51] on UVI interventions using technology by searching “Ultraviolet Index” OR “UV Index” OR “UVI” AND “Behavior” in the past 10 years, which resulted in 112 articles. Three relevant articles were found [52, 53] including the systematic review performed by Heckman et al. [54]. Citation searches yielded another publication [55]. Since question 2 is oriented to target electronic means of UVI communication for mobile athletes, we added various restrictions in place of “Behavior” including “Mobile”, “Email”, and “App”, yielding one relevant citation [56]. Additional focused searches discovered 3 more articles [57–59]. For questions 3–5, we evaluated studies from January 1990 through December 2018 utilizing the following search criteria in PubMed: ((“skin cancer”/sports) OR “skin cancer” prevalence AND “sports” [MeSH]). We identified 104 English publications related to humans, and 6 relevant records [60–65] were included for further review. Additional focused search yielded 6 articles [30, 66–71]. We expanded our search for question 3 by evaluating studies from March 1986 through March 2019 utilizing the following criteria in PubMed: “skin cancer” risk AND sports. Using this query, we found 67 publications related to humans written in English, of which we included 2 additional relevant records [72, 73]. For question 5, we specifically leveraged the work done by Richtig et al. [64], Ambros-Rudolph et al. [65], and Mahe et al. [69]. Of note, question 5 was added post hoc given that high densities of nevi have been associated with an increased risk of developing melanoma [69, 74, 75]. No additional records were found utilizing the following search criteria in PubMed: “nevi count AND sports.” The manuscript quality rating used in this review was based on the type of study, study sample size, and the relative strengths of outcomes measured.
Results
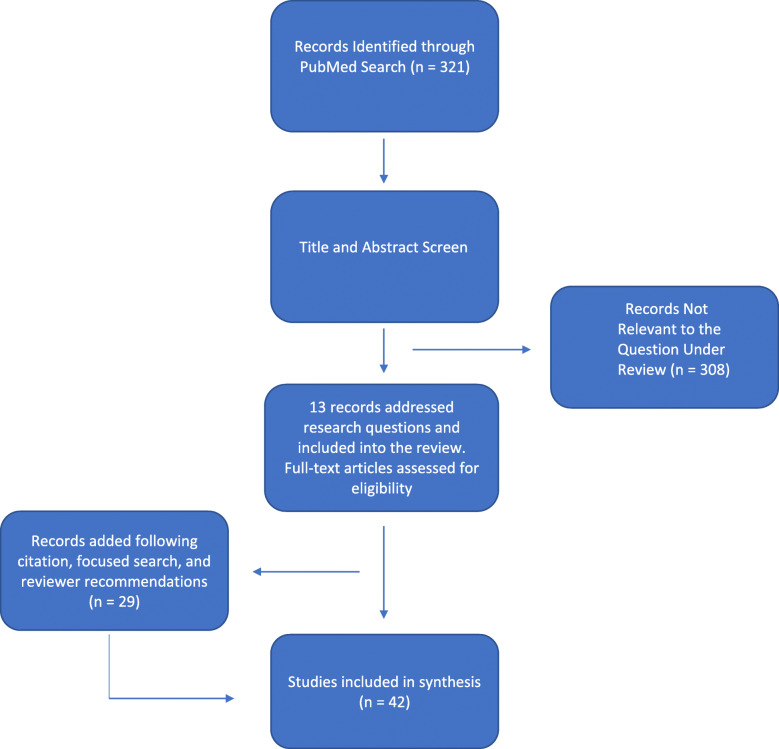
Our search identified 321 records. After scanning titles and abstracts as described above, 13 studies were included for this systematic review (Fig. 1). A total of 29 records were added following citation search, focused search, and reviewer recommendations. A review of the included studies is listed below:
Fig. 1.
PRISMA flow chart of search strategy
Question 1: How is personal UVR measured?
The ambient erythemal dose, or ambient exposure, is defined as the incident erythemally weighted irradiance on a horizontal surface (W/m2) over a specified period of time (J/m2). Accurate measurements of ambient doses can be performed by calibrated broad band radiometers or spectroradiometers [76]. For clinical studies, minimal erythema dose (MED) and standard erythema dose (SED) are the most common radiometric parameters. MED is the lowest UVR exposure sufficient to produce erythema within 8–24 h [35] and varies depending on the tanning and susceptibility to sunburn of each individual [43] (Supplementary Table 1). One SED has a set equivalent to an erythemal effective radiant exposure of 100 J/m2 using the C.I.E. action erythemal spectrum normalized to 298 nm [44]. In contrast to the MED, the SED measure is independent of skin type and is a more objective unit for the measurement of personal UV exposure (PE) via dosimetry [44, 45]. To put this in perspective, the International Commission on Non-Ionizing Radiation Protection (ICNIRP) recommends a daily occupational exposure limit (EL), defined as a maximum PE of 30 J m−2 (0.3 SED) within an 8-h time-frame for sensitive, unprotected skin [35].
Measuring UV exposure in the sports setting is complex; however, different dosimeters have been utilized to measure PE on a variety of platforms [45, 46]. Polysulfone plastic films [77, 78] and Bacillus subtilis spore films [79] are used as chemical and biological dosimeters, respectively. In addition, electronic UV dosimeters have been utilized [39–42], and electronic sun journals (ESJ) are available to track cumulative sun exposure [42]. PE quantified by SED can be utilized by researchers to track UV radiation over time, but individual measurements lack external validity due to different dosimeter orientations secondary to posture and varying environmental conditions [47, 48]. A more pragmatic calculation for comparing PE between sports is the exposure ratio to ambient (ERTA or ER). The ERTA is a ratio of PE relative to ambient UV radiation, which allows researchers to compare accurate dosimetry measurements across different settings while accounting for personal orientation, solar elevation, and other idiosyncratic confounders of precise UV exposure [36]. Since ratio changes can be relatively interpreted over time and across settings, it is a valuable method researchers can use to contrast UV exposure across sporting events in particular. The evolution of these UV radiation measurement technologies and inter-sport ERTA values is comprehensively described in the reviews of nonoccupational UV exposure by Schmalwieser and Siani [36] and Downs et al. [48].
While PE and ERTA are important quantitative measures for industries and researchers, their practical and primary preventative, non-research value to the public may be inhibited by the lack of dosimeter ubiquity, complex calibration requirements [46], and poor inter-reliability in comparison to meteorological grade instruments [45, 49]. The Global Solar Ultraviolet Index (UV Index, UVI, World Health Organization) [37] does not track cumulative PE; however, it is useful for predicting PE and erythemal skin damage risk. The UVI is calculated from the erythemally weighted UV irradiance by convolving the spectral irradiances (280–400 nm) with the spectral weighting function for erythema. It is individually interpreted by skin type and predisposition to sunburn [80] (Supplementary Table 2). Its unitless value can be quantified by the below equation:
where Eλ is the solar spectral irradiance expressed in W/(m2·nm1) at wavelength λ and dλ is the wavelength interval used in the summation. ser is the erythema reference action spectrum, and ker is a constant equal to 40 m2/W [37]. The integer output of the equation ranges from 1 to 11+, which provides individuals with a reference action spectrum for ultraviolet-induced erythema on human skin. Its primary role to the user is to serve as a numerical predictor of cutaneous damage from sun exposure. Moreover, the WHO has put forth scaled sun protection recommendations that complement the increasing risk of erythema or sunburn: 1–2, no protection required; 3–7, protection required; 8–11+, extra protection required [37]. By following these suggestions, individuals can reduce their risk of sunburn and thereby reducing their later risk of skin cancer.
The UVI is not an exact measure of ultraviolet exposure intensity, which varies with geographic location, solar altitude and angle, cloudiness, ozone thickness, aerosols, altitude, and surface albedo from adjacent surfaces such as water or snow [30, 50, 81]. It is measured or predicted by models using satellite-based instruments or from ground-level commercial radiation detectors, with the latter being more precise by being able to account for the aforementioned variables per location in real-time [46]. Geospecific UVIs are included in weather forecasts across many countries and integrated in smartphone applications [37, 82], making sun exposure prevention guidelines widely accessible to patients. During summer in the USA, the UV index can be either very high (8–10) or extreme (11+) at midday. The average UVI in July ranges from 6.5 at the continental US northernmost border to 11.5 in southern Texas [81]. For these conditions, the time needed to achieve erythema ranges from 12–15 min, based on conversions provided by the ICNIRP [35]. In the southern hemisphere, dangerously elevated UVIs are even more prevalent; extreme values of 20+ have been reported in the mountains of Hawaii, the Andes, and the Himalayas [81, 83]. Prior studies have noted increased skin cancer rates along the US latitudinal gradient [84]. Therefore, the UV index can be a powerful educational tool used to alert individuals about weather conditions permitting potentially damaging sun exposure while outdoors.
Question 2: What is the modern utility of the UVI in modifying recreational sun protection behaviors? (Supplementary Table 3)
Despite overwhelming scrutiny, the UVI has been adopted by many governments as the standard platform for public communication of UV exposure risk since its inception by the WHO 1994 [37]. Many of the research efforts detailing UVI knowledge have shown there is a minimal understanding of the UVI by the public [51, 54, 85–90] and that lower UVI value recommendations underpredict erythemal risk [91–93]. Medical professionals have similarly displayed limited knowledge or use of the UVI [94–96]. However, personal knowledge of the UVI may not be necessary to improve sun protection behaviors if mobile health technology can provide tailored recommendations on behalf of the individual. Thus, research examining the efficacy of technology-based interventions elicits the contemporary primary preventative value of the UVI in a different light.
Italia et al. [51] performed a thorough systematic review of the literature addressing this topic prior to 2011, approximately half a year before the iPhone 4S had been revealed [97]. They reviewed 25 studies that investigated the knowledge of, familiarity with, attitude towards, and behavioral impacts of the UVI in the public domain. In regard to familiarity, they reported that awareness of the UVI varied significantly across countries and that understanding of the index was minimal. They also found that the UVI had no impact on knowledge or attitude about UVR or skin cancer. Moreover, behavioral changes in response to UVI interventions were limited or nonexistent in the studies they reviewed. Heckman et al. [54] performed a recent systematic review (2019) of UVI-impact literature (n = 31) in which they compared research between countries. They also found sharp contrasts in UVI awareness between nations, with poor overall comprehension. Unlike Italia et al.’s review [51], Heckman et al. [54] found mixed results on UVI studies utilizing interventions, but stratification based on means of intervention was not addressed. One possible explanation for this change is the advancement and personalization of health technology over the past decade.
The proliferation of mobile and wearable technology and increased demand for electronic health information will continue to alter the UVI research landscape [98]. The number of connected devices worldwide has doubled since 2015 [99], and emerging generations have increasing levels of internet literacy [82]. From 2012 to 2015, the number of individuals who use portable electronic devices for accessing health information increased from 38 to 86% [100]. Moreover, there is public interest in receiving sun protection advice electronically [100, 101]. Since the studies covering this topic prior to 2011 have been extensively described, the following descriptions relate only to technology-based UVI interventions outside of the workplace published since 2011.
In 2015, Buller et al. [53] proposed the use of a mobile smartphone application, “Solar Cell,” to provide individuals with tailored data on UV exposure risk including the current and forecasted UVI. Participants in the intervention group increased use of shade when outdoors (41.0% vs 33.7%, p = 0.03) but reduced use of sunscreen (28.6% vs 34.5%, p = 0.48). People who used the app also reported a decreased average number of days in the sun (60.4% vs 49.3%, p = 0.04) and were more likely to use all sun protection behaviors combined (39.4% vs 33.8%, p = 0.04). No other significant associations between use of the app and sun protection habits were detected. Individuals in lower income brackets who used the app also displayed a greater confidence in sun protection strategies (F = 3.53, p = 0.01).
Their team performed an additional study [52] with a pretest-posttest design to investigate the effectiveness of SolarCell in altering sun protection habits. At the 7-week interim analysis, there was an increase in use of wide-brimmed hats among younger app users (23.8% vs 17.4%, p = 0.045), but the trend did not remain significant by the 12-week posttest analysis. No other associations with positive behavioral changes were found.
Buller et al. [58] performed a pair-matched pretest-posttest quasi-experimental study on the effect of the multi-component “Go Smart Sun” (GSS) educational intervention versus no intervention across 41 US resorts over 2 years. One component of the GSS educational campaign includes sharing the UV index to alert individuals to sun safety. The UVI was at least high (UVI > 5) in 55.5% of 3531 of the interviewed participants and 42.4% of the 4357 prospectively observed participants. In addition to printed materials, they shared sun protection education information via pre-arrival emails, social media messages, and videos that covered a wide range of sun safety techniques beyond UVI awareness. No differences were detected between arms. When stratified by venue type, waterside venues displayed improvements in sun protection behaviors per composite z score (p < 0.01).
Of note, Anderson et al. [59] analyzed the trends of sun protection behaviors in the baseline, pretest cohort. Although sun protection behaviors correlated most highly with increasing temperatures, they found that the UVI was significantly positively associated with sunscreen use and sunscreen reapplication in the retrospective sample (OR = 1.07, 1.19, p < 0.001). The relationship between UVI and shade use was positively significant in the observational sample (β = 0.01, p < 0.001), but UVI was negatively associated with clothing coverage (β = − 0.003, p = 0.004).
In 2016, a study on sun protection training of 26 adolescent organ transplant recipients via text messages was performed by Sachse et al. [55]. Initial in-person training of sun protection strategies that emphasized the utility of the UVI as a “sun protection traffic light” preceded 4 weeks of daily text reminders of the UVI traffic light forecast and behavioral recommendations. The pretest-posttest survey revealed an increased comprehension of the UVI (16% vs 74%), ABCDE mnemonic understanding (0% vs 37%), and recognition of sunburns being delayed from UV exposure onset (26% vs 47%). Sun avoidance behaviors related to redness (16% vs 5%) or warmth of skin (31% vs 31%) did not improve. At 8 weeks, 95% of patients read the messages daily and described the intervention as “very helpful.” Fifty-eight percent of participants reported changing their sun protection behaviors when the UVI was high, 53% increased sunscreen use, and 21% described protective clothing as more important relative to baseline.
Hacker et al. [57] performed a prospective study in 2018 comparing the effectiveness of a personal UVR dosimeter and a “SunSmart” mobile application in altering sun protection habits over 3 months. The app displayed the daily UVI, information on interpreting the UVI, the weather forecast, and a vitamin D tracker tool. Outcomes were measured on the validated Sun Protection Habits (SPH) scale. The SPH index increase was marginally higher in the app group than the UVR monitor group at the 3-month follow-up at + .14 and + .13, respectively, but differences between the two and the control group were nonsignificant. While the dosimeter arm was the only one to have a significant association between use and UV exposure (1-week reduced unprotected UVR exposure OR = 2.706, p = 0.04, 3-month 3.130, p = 0.02), the participants reported they found the app to be more encouraging and engaging (63% vs. 47%) and were more likely to download the app than to buy the dosimeter (40% vs. 19%).
A qualitative study comparing experiences of new (n = 45) and existing users (n = 15) of the SunSmart app was performed by Nicholson et al. [56] in 2019. They found that across groups there was a lack of comprehension of the UVI and that new users described the app’s recommendations as too prudent in comparison to their personal interpretations of daily risk. Importantly, they found that some existing users recognized their inability to gauge the daily UVI, and therefore relied on the SunSmart app to guide daily sun protection behaviors even though they also lacked comprehension of the UVI scale. The benefit was found mostly in individuals who adopted use of the app as part of their daily hygiene regimen.
Although the UVI was not a major component of their interventions, the Healthy Texts [102, 103], UV4.me [104], and Ho et al. [105] studies employed a similar method of technology-based educational interventions to improve sun protection behaviors. All displayed significant improvements in positive sun protection behaviors in the experimental populations (Supplementary Table 3). This underscores that behavior modification cannot be expected by sharing the UVI alone, but rather as an adjunct to other tailored information.
In summary, modern technology has enabled researchers to elicit mild to, at most, moderate sun-protective behavioral changes through electronic multimedia platform interventions. The UVI often serves as a referential crux for which personalized recommendations can be made rather than the impetus for change itself. Increased adherence to these sun protection strategies, as guided by the UVI scale, can decrease the risk of UV-induced erythemal damage to human skin and ultimately reduce the insidious risk of melanoma and other skin cancers attributed to excessive UV exposure. In future research, the utility of real-time UVR detection and UVI feedback in mobile and wearable devices has promising potential to guide patient heliotherapy and vitamin D exposure [82], predict and track UVR exposure at major sporting events [106], and enhance the sun-safety of individuals through extensive user-personalization [107].
Question 3: What is the risk of developing skin cancer in outdoor sports participants? (Table 2)
Table 2.
Risk for outdoor sporting participants to develop skin cancer
| Study | Type of study | Quality rating | Region | N | Outcomes | Hours of exposure |
|---|---|---|---|---|---|---|
| Rosso et al. “Helios”. II [66] | Case control | 3 | Southern Europe | 1549 BCC, 228 SCC, 1795 controls |
Significant association between BCC and water sports (swimming, surfing, boating, and sailing). Non-significant association between BCC and mountain sports (skiing, climbing, hiking) and air sports (flying, hang-gliding and parachuting). Non-significant association between SCC and water sports stronger with > 2112 h of exposure. Holidays at the beach OR 1.5 > 2464 cumulated hours Water sports: OR 1.5 > 771–2112 h |
Risk of SCC = significantly increased at > 70,000 h of lifetime sun exposure Risk of BCC = 2-fold increase risk at 8000–10,000 cumulated hours in a lifetime |
| Rosso et al. [72] | Case control | 3 | Sion Switzerland | n = 146, controls = 144 | Outdoor sports conveyed an increased risk for basal cell carcinoma: average OR 2.2 p = 0.05 | 288– > 3420 h of cumulated exposure |
| Schnohr et al. [67] | Cohort | 4 | Denmark | 28,259 persons | Rate ratio 1.72 (95% CI 1.23–2.40; p = 0.001) for vigorous physical activity compared with low activity and non-melanoma skin cancer in men but not in women. | |
| Holman et al. [68] | Case control | 3 | Australia | 507 melanoma patients, 507 age-, gender-, and location-matched controls |
Boating increased risk for melanoma (OR = 2.43 p = 0.04) Fishing increased risk for melanoma OR = 2.72, p = 0.07 Whenever these sports were practiced one or more times a week |
Quality rating is based on the robustness of the type of study performed, sample size, and strength of the measured outcomes
BCC basal cell carcinoma, SCC squamous cell carcinoma, OR odds ratio, CI confidence interval
The multicenter south European study Helios II indicated that athletes participating in intense UVR exposure water sports such as swimming, surfing, boating, and sailing are at increased risk for development of BCC (odds ratio 1.6 for more than 2600 accumulated hours of exposure in a lifetime). Sports practiced in the mountains such as skiing, climbing, and hiking or in the air such as flying, hang-gliding, and parachuting had weaker or non-significant BCC association [66]. Zanetti et al.’s subanalyses of the Helios II data corroborated these trends by comparing the number of lifetime weighted hours against development of skin cancers. Although the results were not significant at p = 0.05, adjusted odds ratios for CMM, SCC, and BCC in outdoor sports and beach sports were still elevated at 1.5, 1.3, 0.9, and 1.2, 1.2, and 1.0 respectively [70]. Rosso et al. also found that outdoor sports participants had a twofold increase in risk of BCC with a borderline independent significance (p = 0.05) when evaluating a case control population from Switzerland [72].
Analyses performed by a Danish group revealed there was a significantly increased risk of non-melanoma skin cancer for men who participated in vigorous outdoor physical activities compared with those performing low-level physical activities (p = 0.001). No association was found in women [67]. With respect to melanoma, Moore et al. utilized data from twelve prospective studies and found that leisure time physical activity was associated with a higher risk of malignant melanoma. This association was found to be stronger in areas with high UV exposure [71]. Holman et al. found that participation in water sports such as boating had an increased risk of developing melanoma with an odds ratio (OR) of 2.43 (p = 0.04). Similarly, fishing had an increased risk with an OR of 2.72 (p = 0.07). These results applied whenever sports were practiced once or more per week [68].
Question 4: What is the prevalence of skin cancer in sport participants? (Table 3)
Table 3.
Prevalence of pigmented lesions and skin cancer in participants in golf, cricket and surfing
| Study | Quality rating | Sport | Region | N | Measures | Outcomes |
|---|---|---|---|---|---|---|
| del Boz et al. [73] | 4 | Golf | Spain | 195 | Physical examinations |
Actinic keratosis was found in 40%; clinical suspicion of BCC in 7.7% Atypical nevi in 7.7%, SCC in 2.1%, melanoma in 1.5% |
| Noble-Jerks et al. [62] | 4 | Cricket | Australia | 164 | Questionnaires about lifetime diagnosis of skin cancer | 38.4% (63) had at least one skin cancer |
| Dozier et al. [63] | 3 | Surf | Texas, USA | 49 surfers, 60 controls | Physical examinations |
AK 20 surfers; 8 controls (not significant) Atypical nevi 18 surfers; 6 controls (not significant) BCC 8 surfers; 1 control p < 0.047 |
| Climstein et al. [60] | 4 | Surf | Australia | 1348 | Questionnaires about lifetime diagnosis of skin cancer | 184 (13.6%) participants reported skin cancer. Higher relative risk (p < 0.001) in competitive vs recreational surfers (odds ratio 1.74 (CI 1.28–2.31)). BCC was the most frequent skin cancer reported (6.8%), followed by melanoma (1.4%) and SCC (0.6%) |
| Zink et al. [61] | 4 | Ski guides | Switzerland | 62 | Physical examinations | 22 (35.4%) AK, 4 BCC (6.4%), 1 SCC (1.6%) |
Quality rating is based on the robustness of the type of study performed, sample size, and strength of the measured outcomes
BCC basal cell carcinoma, SCC squamous cell carcinoma, AK actinic keratosis
In a study by del Boz, physical examinations were conducted in 195 golfers: actinic keratosis was found in 40%, atypical nevi in 7.7%, clinical suspicion of melanoma in 1.5%, suspicion of SCC in 2.1%, and suspicion of BCC in 7.7% [73]. When former Australian male cricket players (N = 164) responded to questionnaires about lifetime diagnosis of skin cancer, 38.4% (n = 63) of respondents had been diagnosed with at least one skin cancer. Twenty-three responders with histories of skin cancer indicated that they either occasionally, very rarely, or never used at least 2 of the 3 recommended skin protection strategies (wearing a wide-brimmed hat, long-sleeved shirt, and the use of sunscreen) [62]. Zink et al. performed a cross-sectional analysis of skin cancer in 62 mountain and ski guides in Switzerland via physical examination. 43.5% (n = 27) was diagnosed with malignant lesions including AK (n = 22, 35.4%), BCC (n = 4, 6.4%), and SCC (n = 1, 1.6%) [61].
Dozier et al. performed a skin cancer screen of 49 surfers during a competition in Texas via physical examination. Investigators found 8 BCCs on surfers whereas only 1 BCC was found in the control group (p < 0.047) [63]. Australian investigators performed surveys on 1348 recreational and competitive surfers for lifetime prevalence of skin cancer, of which 184 (13.6%) participants reported skin cancer. The relative risk of developing skin cancer was significantly higher (p < 0.001) in competitive vs recreational surfers (odds ratio 1.74 (CI 1.28–2.31). BCC was the most frequent skin cancer reported (6.8%), followed by melanoma (1.4%) and SCC (0.6%) [60].
Question 5. Is the number of nevi and solar lentigines elevated in sport-participants? (Supplementary Table 4)
Richtig et al. [64] and Ambros-Rudolph et al. [65] performed assessments on 150 and 210 marathon runners, respectively. Both studies revealed a significantly elevated number of nevi, atypical nevi, and solar lentigines in marathon runners. Richtig et al. found 19.6 ± 18.2 lentigines on the shoulder when compared to 0 lentigines on the buttocks in the same group. Runners reporting more than 10 lifetime sunburns had more lentigines on their shoulder (p = 0.032). The mean number of counted nevi on the left shoulder was 1.3 ± 2.1 compared to 0.5 ± 1.0 on the left buttocks (p = 0.000). Ambros-Rudolph found 99 runners with more than 1 atypical nevi compared with 66 in the control group (p = 0.001). An increased number of solar lentigines was found in 64 marathon runners when compared to the 42 participants in the control group (p = 0.01). Another study [69] assessed melanocytic nevi count on children who practiced outdoor sports compared to those who did not. Investigators found a mean of 17.2 nevi on those who practiced outdoor sports and a mean of 15 nevi on those who did not (p < 0.001). When gender differences were assessed, boys were found to have significantly increased nevi count on the back area whereas girls did not show an increased number of nevi on the back (Supplementary Table 4).
Discussion
In this review, we identified a number of methods used to measure solar UVR and discussed the applicability of these measurement tools in the personal and research settings. We also found that, while awareness of the UVI varies significantly and comprehension is low even after intervention, access to an electronic tool that provides preventative sun protection behavior recommendations may be successful in altering habits, possibly by means of instruction rather than teaching. Lastly, we addressed that individuals who practice sport-related activities have a higher risk for skin cancer and higher prevalence for pigmented lesions in sun-exposed areas.
Strengths of this study include a longitudinal and focused review of UVR risk assessment tools and outcomes in a defined population, a comprehensive literature search, and the identification of contemporary interventions to improve upon current sun-safety recommendations. Limitations are narrow inclusion criteria and consequent requirement for extensive citation search, the lack of a validated manuscript appraisal scale, and the inherent biases of data included from observational behavioral studies incorporated in the review.
This evidence-based assessment supports the assumption of outdoor sportsmen and women being in greater need of sun-protective behavior counseling by their healthcare providers. Therefore, we highlight here the national guidelines outlined by the Surgeon General in an effort to prevent skin cancer [6]. Emphasis is placed on the individual to adopt protective behavioral strategies such as wearing tightly woven long clothing, hats, and sunglasses; using at least 15 SPF sunscreen before outdoor activity; seeking shade; and avoiding being outside during hours of peak UV intensity. In particular, adolescents and young adults are considered vulnerable but impressionable. Clinicians are recommended to perform tailored, brief interventions in this demographic. Lastly, the guidelines call for legislative involvement at the local, state, and federal level to expand educational programs as well as enable access to proper protective clothing and shade in the workplace and on campuses nationwide [6].
Current literature corroborates these prudent recommendations, especially for athletes [7, 11, 13]. Recent cross-sectional studies [108–110] provide evidence to discourage the use of sunscreen as the only sun protection strategy [111]. Athlete-specific educational interventions have been shown to be effective, such as the SUNSPORT with NCAA student-athletes [112]. Sharing these strategies seems to be most effective when incorporated as part of a multi-component intervention rather than mass media interventions alone [113]. Since the 1980s, educational initiatives such as “slip, slop, slap, seek, slide” have been implemented in other parts of the world [51] to educate about the use of protective clothing, sunscreen, and broad brimmed hats and the importance of seeking shade and wearing sunglasses. However, in order to maintain generational relevance, it should be stressed that the modern ubiquity of mobile technology can complement this multidecade-old adage. The fact that the SunSmart App has been downloaded 300,000 times is a testament to the success of the modernization of Australia’s famously successful public health effort [56]. Outside of the research setting, the UVI remains a core component of personalizing sun-safety communication in mobile apps designed for commercial use in the USA [114]. Patient counseling on the availability and utility of these resources may help individuals adopt sun-safe hygienic routines before or during outdoor sports, regardless of whether their understanding of the UVI is improved or not.
Lastly, Bloom et al. [115] demonstrated increased interest in skin cancer in the population during summer months; as such, educational campaigns may be most effective when the population is more receptive and actively seeking information during the summertime. In summary, a timely educational program that optimizes the core principles of historically successful programs with avant-garde technology may elicit the greatest results in coaching sun protective habits.
Conclusion
Individuals involved in outdoor daytime activities experience substantially high UVR exposure but continue to misunderstand the public utility of the UVI. In addition, they are at high risk of developing skin cancer. Therefore, clinicians should provide preventative counseling and educational support on sun-protection strategies in this high-risk population. We recommend the use of the following sun protection approaches: seeking shade, wearing protective clothing, and using sunscreen while discouraging the use of sunscreen as the only sun-protection strategy. Smartphones and wearable technology with apps that provide UVR avoidance instructions may help athletes adhere to proper protective behaviors before and during outdoors activities. It remains necessary to investigate UVR exposure with newer technologies to more accurately evaluate the contribution of UVR exposure to skin cancer.
Supplementary information
Additional file 1: Table S1. “Number of SED required to induce erythema according to skin phototype” Adapted from International Commission on Illumination22.
Additional file 2: Table S2. “Comparison of time needed to exceed ICNIRP threshold and to achieve erythema with respective UV index for the different non-adapted skin phototypes”.
Additional file 3: Table S3. Comparison of studies using modern technology to improve sun protection behaviors.
Additional file 4: Table S4. “Prevalence of lentigines and nevi on marathon runners and children who practice outdoor sports”.
Acknowledgements
Not applicable.
Abbreviations
- ICNIRP
International Commission on Non-Ionizing Radiation
- C.I.E.
International Commission on Illumination
- UV
Ultraviolet
- UVR
Ultraviolet radiation
- UVI
Ultraviolet index
- WHO
World Health Organization
- MED
Minimal erythemal dose
- SED
Standard erythemal dose
- PE
Personal exposure
- ERTA
Exposure-to-ambient ratio
- OR
Odds ratio
- CMM
Cutaneous malignant melanoma
- SCC
Squamous cell carcinoma
- BCC
Basal cell carcinoma
Authors’ Contributions
AS, MV, DT, and KA performed the literature review and data analyses as well as drafted the manuscript. KK provided the idea and mentorship and revised the document in preparation for submission. The authors read and approved the final manuscript. All authors have contributed to the manuscript and approved the submission of this manuscript to the Sports Medicine.
Funding
No funding was provided or used in the preparation or submission of this manuscript. No financial assistance was used for the completion of this study.
Availability of Data and Materials
Not applicable.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors, Alan Snyder, Manuel Valdebran, David Terrero, Kyle Amber, and Kristen Kelly, declare that they have no competing interests with the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40798-020-00272-9.
References
- 1.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279–282. doi: 10.1001/archdermatol.2010.4. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 3.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.Henley SJ, Ward EM, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225–2249. doi: 10.1002/cncr.32802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estimated age-standardized incidence rates (World) in 2018, melanoma of skin, non-melanoma skin cancer, both sexes, all ages. World health Organization, International Agency on Research on Cancer. Cancer Today Web site. https://gco.iarc.fr/. Published 2020. Accessed April 18, 2020.
- 6.De Castro-Maqueda G, Gutierrez-Manzanedo JV, Ponce-Gonzalez JG, Fernandez-Santos JR, Linares-Barrios M, De Troya-Martin M. Sun protection habits and sunburn in elite aquatics athletes: surfers, windsurfers and olympic sailors. J Cancer Educ. 2020;35(2):312–320. doi: 10.1007/s13187-018-1466-x. [DOI] [PubMed] [Google Scholar]
- 7.Duarte AF, Nagore E, Silva JNM, Picoto A, Pereira AC, Correia OJC. Sun protection behaviour and skin cancer literacy among outdoor runners. Eur J Dermatol. 2018;28(6):803–808. doi: 10.1684/ejd.2018.3450. [DOI] [PubMed] [Google Scholar]
- 8.Zalaudek I, Conforti C, Corneli P, et al. Sun-protection and sun-exposure habits among sailors: results of the 2018 world’s largest sailing race Barcolana’ skin cancer prevention campaign. J Eur Acad Dermatol Venereol. 2020;34(2):412–418. doi: 10.1111/jdv.15908. [DOI] [PubMed] [Google Scholar]
- 9.Villard M, Bonini J, Criquet-Hayot A, Baubion E, Derancourt C. Kite-surfers’ sun risk in the tropics. J Travel Med. 2017;24(2). [DOI] [PubMed]
- 10.Harrison SC, Bergfeld WF. Ultraviolet light and skin cancer in athletes. Sports Health. 2009;1(4):335–340. doi: 10.1177/1941738109338923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinna S, Adams BB. Ultraviolet radiation and the athlete: risk, sun safety, and barriers to implementation of protective strategies. Sports Med. 2013;43(7):531–537. doi: 10.1007/s40279-013-0021-5. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Cho E, Li WQ, Weinstock MA, Han J, Qureshi AA. History of severe sunburn and risk of skin cancer among women and men in 2 prospective cohort studies. Am J Epidemiol. 2016;183(9):824–833. doi: 10.1093/aje/kwv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamant ES, Adams BB. Sunscreen use among collegiate athletes. J Am Acad Dermatol. 2005;53(2):237–241. doi: 10.1016/j.jaad.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs C, Nahar VK, Ford MA, Bass MA, Brodell RT. Skin cancer knowledge, attitudes, and behaviors in collegiate athletes. J Skin Cancer. 2014;2014:248198. doi: 10.1155/2014/248198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pope SJ, Godar DE. Solar UV geometric conversion factors: horizontal plane to cylinder model. Photochem Photobiol. 2010;86(2):457–466. doi: 10.1111/j.1751-1097.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 16.Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5 Suppl 2):S129–S132. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Eakin P, Maddock J, Techur-Pedro A, Kaliko R, Derauf DC. Sun protection policy in elementary schools in Hawaii. Prev Chronic Dis. 2004;1(3):A05. [PMC free article] [PubMed] [Google Scholar]
- 18.Health USDo, Human S . The surgeon general’s call to action to prevent skin cancer. Washington (DC): Office of the Surgeon General (US); 2014. Reports of the surgeon general. [PubMed] [Google Scholar]
- 19.Sunburn and sun protective behaviors among adults aged 18-29 years--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2012;61(18):317–22. [PubMed]
- 20.Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Ann Epidemiol. 2008;18(8):614–627. doi: 10.1016/j.annepidem.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant WB. Vitamin D: evidence and controversies: comment on the article by Gilaberte et al. Actas Dermosifiliogr. 2012;103(7):591–594. doi: 10.1016/j.ad.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Willis KS, Peterson NJ, Larson-Meyer DE. Should we be concerned about the vitamin D status of athletes? Int J Sport Nutr Exerc Metab. 2008;18(2):204–224. doi: 10.1123/ijsnem.18.2.204. [DOI] [PubMed] [Google Scholar]
- 23.Schottker B, Jorde R, Peasey A, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. Bmj. 2014;348:g3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12(2):e0170791. doi: 10.1371/journal.pone.0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Li B, Gao X, et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(4):810–819. doi: 10.3945/ajcn.116.140392. [DOI] [PubMed] [Google Scholar]
- 26.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 28.Seckmeyer G. Why is it so hard to gain enough Vitamin D by solar exposure in the European winter? Meteorol Z. 2018;27(3):223–233. [Google Scholar]
- 29.McKenzie RL, Lucas RM. Reassessing impacts of extended daily exposure to low level solar UV radiation. Sci Rep. 2018;8(1):13805. doi: 10.1038/s41598-018-32056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diffey BL. Time and place as modifiers of personal UV exposure. Int J Environ Res Public Health. 2018;15(6). [DOI] [PMC free article] [PubMed]
- 31.Gage R, Barr M, Stanley J, et al. Sun protection and shade availability in New Zealand’s outdoor recreation spaces. N Z Med J. 2018;131(1484):30–37. [PubMed] [Google Scholar]
- 32.Julian A, Thorburn S, Geldhof GJ. Health beliefs about UV and skin cancer risk behaviors. Cancer Control. 2020;27(4):1073274819894008. doi: 10.1177/1073274819894008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seite S, Del Marmol V, Moyal D, Friedman AJ. Public primary and secondary skin cancer prevention, perceptions and knowledge: an international cross-sectional survey. J Eur Acad Dermatol Venereol. 2017;31(5):815–820. doi: 10.1111/jdv.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezzedine K, Guinot C, Mauger E, et al. Expatriates in high-UV index and tropical countries: sun exposure and protection behavior in 9,416 French adults. J Travel Med. 2007;14(2):85–91. doi: 10.1111/j.1708-8305.2007.00108.x. [DOI] [PubMed] [Google Scholar]
- 35.ICNIRP Statement--protection of workers against ultraviolet radiation. Health Phys. 2010;99(1):66–87. doi: 10.1097/HP.0b013e3181d85908. [DOI] [PubMed] [Google Scholar]
- 36.Schmalwieser AW, Siani AM. Review on nonoccupational personal solar UV exposure measurements. Photochem Photobiol. 2018;94(5):900–915. doi: 10.1111/php.12946. [DOI] [PubMed] [Google Scholar]
- 37.Protection ICoN-IR . In: Global solar UV index: a practical guide. Organization WM, editor. Geneva: World Health Organization; 2002. [Google Scholar]
- 38.Moehrle M. Outdoor sports and skin cancer. Clin Dermatol. 2008;26(1):12–15. doi: 10.1016/j.clindermatol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Diffey BL, Saunders PJ. Behavior outdoors and its effects on personal ultraviolet exposure rate measured using an ambulatory datalogging dosimeter. Photochem Photobiol. 1995;61(6):615–618. doi: 10.1111/j.1751-1097.1995.tb09877.x. [DOI] [PubMed] [Google Scholar]
- 40.Idorn LW, Datta P, Heydenreich J, Philipsen PA, Wulf HC. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data. Br J Dermatol. 2013;168(2):367–373. doi: 10.1111/bjd.12066. [DOI] [PubMed] [Google Scholar]
- 41.Dobbinson S, Niven P, Buller D, Allen M, Gies P, Warne C. Comparing handheld meters and electronic dosimeters for measuring ultraviolet levels under shade and in the sun. Photochem Photobiol. 2016;92(1):208–214. doi: 10.1111/php.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downs NJ, Parisi AV, Butler H, Rawlings A, Elrahoumi RS. An inexpensive high-temporal resolution electronic sun journal for monitoring personal day to day sun exposure patterns. Front Public Health. 2017;5:310. doi: 10.3389/fpubh.2017.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bech-Thomsen N, Wulf HC. Photoprotection due to pigmentation and epidermal thickness after repeated exposure to ultraviolet light and psoralen plus ultraviolet a therapy. Photodermatol Photoimmunol Photomed. 1996;11(5-6):213–218. doi: 10.1111/j.1600-0781.1995.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 44.Diffey BL, Jansen CT, Urbach F, Wulf HC. The standard erythema dose: a new photobiological concept. Photodermatol Photoimmunol Photomed. 1997;13(1-2):64–66. doi: 10.1111/j.1600-0781.1997.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 45.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28(1):4–13. doi: 10.1016/s1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 46.Kanellis VG. Ultraviolet radiation sensors: a review. Biophys Rev. 2019:895–9. [DOI] [PMC free article] [PubMed]
- 47.Verdebout J. Estimating natural UV personal exposure with radiative transfer calculations. Radiat Prot Dosim. 2010;141(3):275–282. doi: 10.1093/rpd/ncq186. [DOI] [PubMed] [Google Scholar]
- 48.Downs NJ, Parisi AV, Schouten PW, Igoe DP, De Castro-Maqueda G. The simulated ocular and whole-body distribution of natural sunlight to kiteboarders: a high-risk case of UVR exposure for athletes utilizing water surfaces in sport. Photochem Photobiol. 2019. [DOI] [PubMed]
- 49.Seckmeyer G, Klingebiel M, Riechelmann S, et al. A critical assessment of two types of personal UV dosimeters. Photochem Photobiol. 2012;88(1):215–222. doi: 10.1111/j.1751-1097.2011.01018.x. [DOI] [PubMed] [Google Scholar]
- 50.Schmalwieser AW, Enzi C, Wallisch S, Holawe F, Maier B, Weihs P. UV exposition during typical lifestyle behavior in an urban environment. Photochem Photobiol. 2010;86(3):711–715. doi: 10.1111/j.1751-1097.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 51.Italia N, Rehfuess EA. Is the Global Solar UV Index an effective instrument for promoting sun protection? A systematic review. Health Educ Res. 2012;27(2):200–213. doi: 10.1093/her/cyr050. [DOI] [PubMed] [Google Scholar]
- 52.Buller DB, Berwick M, Lantz K, et al. Smartphone mobile application delivering personalized, real-time sun protection advice: a randomized clinical trial. JAMA Dermatol. 2015;151(5):497–504. doi: 10.1001/jamadermatol.2014.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buller DB, Berwick M, Lantz K, et al. Evaluation of immediate and 12-week effects of a smartphone sun-safety mobile application: a randomized clinical trial. JAMA Dermatol. 2015;151(5):505–512. doi: 10.1001/jamadermatol.2014.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heckman CJ, Liang K, Riley M. Awareness, understanding, use, and impact of the UV index: a systematic review of over two decades of international research. Prev Med. 2019;123:71–83. doi: 10.1016/j.ypmed.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sachse MM, Bottcher S, Pape L, et al. Face-to-face sun protection training and text messages improve sun protection behaviour in adolescent organ transplant recipients: HIPPOlino feasibility study. Acta Derm Venereol. 2016;96(3):341–345. doi: 10.2340/00015555-2234. [DOI] [PubMed] [Google Scholar]
- 56.Nicholson A, Murphy M, Walker H, Tinker R, Dobbinson S. Not part of my routine: a qualitative study of use and understanding of UV forecast information and the SunSmart app. BMC Public Health. 2019;19(1):1127. doi: 10.1186/s12889-019-7421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hacker E, Horsham C, Vagenas D, Jones L, Lowe J, Janda M. A mobile technology intervention with ultraviolet radiation dosimeters and smartphone apps for skin cancer prevention in young adults: randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(11):e199. doi: 10.2196/mhealth.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buller DB, Andersen PA, Walkosz BJ, Scott MD, Beck L, Cutter GR. Effect of an intervention on observed sun protection by vacationers in a randomized controlled trial at North American resorts. Prev Med. 2017;99:29–36. doi: 10.1016/j.ypmed.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen PA, Buller DB, Walkosz BJ, et al. Environmental variables associated with vacationers’ sun protection at warm weather resorts in North America. Environ Res. 2016;146:200–206. doi: 10.1016/j.envres.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 60.Climstein M, Furness J, Hing W, Walsh J. Lifetime prevalence of non-melanoma and melanoma skin cancer in Australian recreational and competitive surfers. Photodermatol Photoimmunol Photomed. 2016;32(4):207–213. doi: 10.1111/phpp.12247. [DOI] [PubMed] [Google Scholar]
- 61.Zink A, Koch E, Seifert F, Rotter M, Spinner CD, Biedermann T. Nonmelanoma skin cancer in mountain guides: high prevalence and lack of awareness warrant development of evidence-based prevention tools. Swiss Med Wkly. 2016;146:w14380. doi: 10.4414/smw.2016.14380. [DOI] [PubMed] [Google Scholar]
- 62.Noble-Jerks J, Weatherby RP, Meir R. Self-reported skin cancer protection strategies and location of skin cancer in retired cricketers: a case study from membership of the Emu Cricket Club. J Sci Med Sport. 2006;9(6):441–445. doi: 10.1016/j.jsams.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 63.Dozier S, Wagner RF, Jr, Black SA, Terracina J. Beachfront screening for skin cancer in Texas Gulf coast surfers. South Med J. 1997;90(1):55–58. doi: 10.1097/00007611-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Richtig E, Ambros-Rudolph CM, Trapp M, et al. Melanoma markers in marathon runners: increase with sun exposure and physical strain. Dermatology. 2008;217(1):38–44. doi: 10.1159/000121473. [DOI] [PubMed] [Google Scholar]
- 65.Ambros-Rudolph CM, Hofmann-Wellenhof R, Richtig E, Muller-Furstner M, Soyer HP, Kerl H. Malignant melanoma in marathon runners. Arch Dermatol. 2006;142(11):1471–1474. doi: 10.1001/archderm.142.11.1471. [DOI] [PubMed] [Google Scholar]
- 66.Rosso S, Zanetti R, Martinez C, et al. The multicentre south European study ‘Helios’. II: different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73(11):1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnohr P, Gronbaek M, Petersen L, Hein HO, Sorensen TI. Physical activity in leisure-time and risk of cancer: 14-year follow-up of 28,000 Danish men and women. Scand J Public Health. 2005;33(4):244–249. doi: 10.1080/14034940510005752. [DOI] [PubMed] [Google Scholar]
- 68.Holman CD, Armstrong BK, Heenan PJ. Relationship of cutaneous malignant melanoma to individual sunlight-exposure habits. J Natl Cancer Inst. 1986;76(3):403–414. [PubMed] [Google Scholar]
- 69.Mahe E, Beauchet A, de Paula CM, et al. Outdoor sports and risk of ultraviolet radiation-related skin lesions in children: evaluation of risks and prevention. Br J Dermatol. 2011;165(2):360–367. doi: 10.1111/j.1365-2133.2011.10415.x. [DOI] [PubMed] [Google Scholar]
- 70.Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer. 2006;94(5):743–751. doi: 10.1038/sj.bjc.6602982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosso S, Joris F, Zanetti R. Risk of basal and squamous cell carcinomas of the skin in Sion, Switzerland: a case-control study. Tumori. 1999;85(6):435–442. doi: 10.1177/030089169908500603. [DOI] [PubMed] [Google Scholar]
- 73.del Boz J, Fernandez-Morano T, Padilla-Espana L, Aguilar-Bernier M, Rivas-Ruiz F, de Troya-Martin M. Skin cancer prevention and detection campaign at golf courses on Spain’s Costa del Sol. Actas Dermosifiliogr. 2015;106(1):51–60. doi: 10.1016/j.ad.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Li WQ, Cho E, Weinstock MA, Li S, Stampfer MJ, Qureshi AA. Cutaneous nevi and risk of melanoma death in women and men: a prospective study. J Am Acad Dermatol. 2019;80(5):1284–1291. doi: 10.1016/j.jaad.2018.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin-Gorgojo A, Llinares M, Viros A, et al. Cutaneous melanoma primary site is linked to nevus density. Oncotarget. 2017;8(58):98876–98886. doi: 10.18632/oncotarget.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diemoz H. First national intercomparison of solar ultraviolet radiometers in Italy. In. Vol 4. Atmospheric Measurement Techniques:1689-1703.
- 77.Diffey B. Personal ultraviolet radiation dosimetry with polysulphone film badges. Photodermatol. 1984;1(3):151–157. [PubMed] [Google Scholar]
- 78.Siani AMea . Vol 13. Photochem. Photobiol. 2014. Investigation on the capability of polysulphone for measuring biologically effective solar UV exposures; p. 521. [DOI] [PubMed] [Google Scholar]
- 79.Moehrle M, Garbe C. Personal UV dosimetry by Bacillus subtilis spore films. Dermatology. 2000;200(1):1–5. doi: 10.1159/000018306. [DOI] [PubMed] [Google Scholar]
- 80.C.I.E. Commission Internationale de l’Eclairage . Erythema reference action spectrum and standard erythema dose. CIE S007E-1998. Austria: CIE Central Bureau V; 1998. [Google Scholar]
- 81.Fioletov V, Kerr JB, Fergusson A. The UV index: definition, distribution and factors affecting it. Can J Public Health. 2010;101(4):I5–I9. doi: 10.1007/BF03405303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krzyscin JW, Lesiak A, Narbutt J, Sobolewski P, Guzikowski J. Perspectives of UV nowcasting to monitor personal pro-health outdoor activities. J Photochem Photobiol B. 2018;184:27–33. doi: 10.1016/j.jphotobiol.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Cordero RR, Seckmeyer G, Damiani A, et al. The world’s highest levels of surface UV. Photochem Photobiol Sci. 2014;13(1):70–81. doi: 10.1039/c3pp50221j. [DOI] [PubMed] [Google Scholar]
- 84.Qureshi AA, Laden F, Colditz GA, Hunter DJ. Geographic variation and risk of skin cancer in US women. Differences between melanoma, squamous cell carcinoma, and basal cell carcinoma. Arch Intern Med. 2008;168(5):501–507. doi: 10.1001/archinte.168.5.501. [DOI] [PubMed] [Google Scholar]
- 85.Ziehfreund S, Schuster B, Zink A. Primary prevention of keratinocyte carcinoma among outdoor workers, the general population and medical professionals: a systematic review updated for 2019. J Eur Acad Dermatol Venereol. 2019;33(8):1477–1495. doi: 10.1111/jdv.15525. [DOI] [PubMed] [Google Scholar]
- 86.Alberink AM, Valery PC, Russell A, Green A. Do forecasts of UV indexes influence people’s outdoor behaviour? Aust N Z J Public Health. 2000;24(5):488–491. doi: 10.1111/j.1467-842x.2000.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 87.Blunden A, Lower T, Slevin T. Knowledge, awareness, and use of the UV index amongst the West Australian public. J Health Commun. 2004;9(3):207–221. doi: 10.1080/10810730490447057. [DOI] [PubMed] [Google Scholar]
- 88.Wong CC, Liu W, Gies P, Nixon R. Think UV, not heat! Australas J Dermatol. 2015;56(4):275–278. doi: 10.1111/ajd.12272. [DOI] [PubMed] [Google Scholar]
- 89.Borner FU, Schutz H, Wiedemann P. The influence of the UV-index on attitudes toward sun exposure in the German population. J Cancer Educ. 2010;25(4):643–649. doi: 10.1007/s13187-010-0108-8. [DOI] [PubMed] [Google Scholar]
- 90.Perez M, Donaldson M, Jain N, Robinson JK. Sun protection behaviors in head start and other early childhood education programs in Illinois. JAMA Dermatol. 2018;154(3):336–340. doi: 10.1001/jamadermatol.2017.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lehmann M, Pfahlberg AB, Sandmann H, Uter W, Gefeller O. Public health messages associated with low UV index values need reconsideration. Int J Environ Res Public Health. 2019;16(12). [DOI] [PMC free article] [PubMed]
- 92.Lehmann M, Pfahlberg AB, Sandmann H, Uter W, Gefeller O. Implications of low levels of the UV index for sun protection. Stud Health Technol Inform. 2017;243:25–29. [PubMed] [Google Scholar]
- 93.Lehmann M, Sandmann H, Pfahlberg AB, Uter W, Gefeller O. Erythemal UV radiation on days with low UV index values-an analysis of data from the German solar UV monitoring network over a ten-year period. Photochem Photobiol. 2019;95(4):1076–1082. doi: 10.1111/php.13092. [DOI] [PubMed] [Google Scholar]
- 94.Sin C, Beauchet A, Marchal A, Sigal ML, Mahe E. Understanding and use of the global solar UV index (“UV index”) by French dermatologists. Ann Dermatol Venereol. 2013;140(1):15–20. doi: 10.1016/j.annder.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 95.Holman DM, Qin J, Gottschlich EA, Balk SJ. Clinical counseling on sun protection and indoor tanning avoidance: a survey of current practices among U.S. health care providers. Prev Med. 2019;126:105783. doi: 10.1016/j.ypmed.2019.105783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Snyder AN, Litchman GH, Plante JG, Valdebran MA, Rigel DS. Ultraviolet index counseling as a primary prevention strategy by US dermatologists. JAAD Int. 2020;1(1):48–49. doi: 10.1016/j.jdin.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ziegler C. iPhone 4S announced, available October 14th starting at $199. 2011. Published October 4. Accessed April 22, 2020.
- 98.Nebeker C, Harlow J, Espinoza Giacinto R, Orozco-Linares R, Bloss CS, Weibel N. Ethical and regulatory challenges of research using pervasive sensing and other emerging technologies: IRB perspectives. AJOB Empir Bioeth. 2017;8(4):266–276. doi: 10.1080/23294515.2017.1403980. [DOI] [PubMed] [Google Scholar]
- 99.Association. CTW. Connected devices worldwide 47B in 2021. https://www.ctia.org/docs/default-source/default-document-library/ctia-wireless-snapshot.pdf 2016.
- 100.Cybercitizen Health U.S. https://decisionresourcesgroup.com/report/141914-digital-cybercitizen-health-u-s-2015/: Manhattan Research;2013, 2015.
- 101.Turner J, Igoe D, Parisi AV, McGonigle AJ, Amar A, Wainwright L. A review on the ability of smartphones to detect ultraviolet (UV) radiation and their potential to be used in UV research and for public education purposes. Sci Total Environ. 2020;706:135873. doi: 10.1016/j.scitotenv.2019.135873. [DOI] [PubMed] [Google Scholar]
- 102.Janda M, Youl P, Marshall AL, Soyer HP, Baade P. The HealthyTexts study: a randomized controlled trial to improve skin cancer prevention behaviors among young people. Contemp Clin Trials. 2013;35(1):159–167. doi: 10.1016/j.cct.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 103.Youl PH, Soyer HP, Baade PD, Marshall AL, Finch L, Janda M. Can skin cancer prevention and early detection be improved via mobile phone text messaging? A randomised, attention control trial. Prev Med. 2015;71:50–56. doi: 10.1016/j.ypmed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 104.Heckman CJ, Darlow SD, Ritterband LM, Handorf EA, Manne SL. Efficacy of an intervention to alter skin cancer risk behaviors in young adults. Am J Prev Med. 2016;51(1):1–11. doi: 10.1016/j.amepre.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ho BK, Reidy K, Huerta I, et al. Effectiveness of a multicomponent sun protection program for young children: a randomized clinical trial. JAMA Pediatr. 2016;170(4):334–342. doi: 10.1001/jamapediatrics.2015.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Downs NJ, Axelsen T, Schouten P, Igoe DP, Parisi AV, Vanos J. Biologically effective solar ultraviolet exposures and the potential skin cancer risk for individual gold medalists of the 2020 Tokyo Summer Olympic Games. Temperature (Austin) 2020;7(1):89–108. doi: 10.1080/23328940.2019.1581427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nittas V, Mutsch M, Puhan MA. Preferences for sun protection with a self-monitoring App: protocol of a discrete choice experiment study. JMIR Res Protoc. 2020;9(2):e16087. doi: 10.2196/16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morris KL, Perna FM. Decision tree model vs traditional measures to identify patterns of sun-protective behaviors and sun sensitivity associated with sunburn. JAMA Dermatol. 2018;154(8):897–902. doi: 10.1001/jamadermatol.2018.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holman DM, Ding H, Guy GP, Jr, Watson M, Hartman AM, Perna FM. Prevalence of sun protection use and sunburn and association of demographic and behaviorial characteristics with sunburn among US adults. JAMA Dermatol. 2018;154(5):561–568. doi: 10.1001/jamadermatol.2018.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pettigrew S, Jongenelis M, Strickland M, et al. Predictors of sun protection behaviours and sunburn among Australian adolescents. BMC Public Health. 2016;16:565. doi: 10.1186/s12889-016-3197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinson JK, Dellavalle RP, Bigby M, Callen JP. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol. 2008;144(1):97–99. doi: 10.1001/archdermatol.2007.28. [DOI] [PubMed] [Google Scholar]
- 112.Ally MS, Swetter SM, Hirotsu KE, et al. Promoting sunscreen use and sun-protective practices in NCAA athletes: impact of SUNSPORT educational intervention for student-athletes, athletic trainers, and coaches. J Am Acad Dermatol. 2018;78(2):289–292. doi: 10.1016/j.jaad.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 113.Sandhu PK, Elder R, Patel M, et al. Community-wide interventions to prevent skin cancer: two community guide systematic reviews. Am J Prev Med. 2016;51(4):531–539. doi: 10.1016/j.amepre.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moran C, Zetler E. A review of smartphone applications for promoting sun protection practices. J Am Acad Dermatol. 2019;81(2):613–615. doi: 10.1016/j.jaad.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 115.Bloom R, Amber KT, Hu S, Kirsner R. Google search trends and skin cancer: evaluating the US population’s interest in skin cancer and its association with melanoma outcomes. JAMA Dermatol. 2015;151(8):903–905. doi: 10.1001/jamadermatol.2015.1216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. “Number of SED required to induce erythema according to skin phototype” Adapted from International Commission on Illumination22.
Additional file 2: Table S2. “Comparison of time needed to exceed ICNIRP threshold and to achieve erythema with respective UV index for the different non-adapted skin phototypes”.
Additional file 3: Table S3. Comparison of studies using modern technology to improve sun protection behaviors.
Additional file 4: Table S4. “Prevalence of lentigines and nevi on marathon runners and children who practice outdoor sports”.
Data Availability Statement
Not applicable.