Abstract
The expression of RNA-binding proteins (RBPs) is dysregulated in colorectal cancer (CRC) and in other types of cancer. Among the RBPs, the insulin-like growth factor-2 messenger RNA binding protein (IGF2BP1-3) family is involved in the development of the colon and the progression of CRC. However, the regulation of mRNA fate by IGF2BP3 in CRC remains less well understood. Here, we show that IGF2BP3 interacts with ELAVL1 to coregulate a cohort of genes involved in the cell cycle and cell proliferation. Mechanistically, recognition of these mRNAs by the IGF2BP3/ELAVL1 complex leads to prolonged half-lives of the mRNA molecules and increased expression of the target genes, thereby driving CRC cell proliferation. Interestingly, knockdown of either IGF2BP3 or ELAVL1 impairs the IGF2BP3/ELAVL1 complex-enhanced mRNA stability, suggesting a functional interdependency between IGF2BP3 and ELAVL1 in CRC. Our findings reveal the molecular mechanism by which IGF2BP3 regulates mRNA stability and identify the cooperativity of the IGF2BP3/ELAVL1 complex as a novel therapeutic target in CRC.
Keywords: Colorectal cancer, RBP, IGF2BP3, ELAVL1, mRNA stability
Introduction
Colorectal cancer (CRC) is one of most deadly types of cancer in the digestive tract, and it was estimated that more than 1.8 million new CRC cases and 881,000 CRC-related deaths occurred in 2018 [1]. Globally, CRC remains the third most commonly diagnosed cancer and accounts for 10% of cancer-related deaths [2]. Most CRC cases arise sporadically and are often diagnosed at advanced stages [3]. After curative treatment, CRC patients often develop recurrent disease, which is highly correlated with the unfavorable prognosis of CRC [4].
The development of CRC is well-understood and characterized by distinct genetic and epigenetic events [5]. Traditional adenoma-carcinoma carcinogenesis is first initiated by an APC mutation followed by activation of RAS or functional loss of TP53, which are further accompanied by the accumulation of different molecular alterations [2,5-7]. Importantly, emerging evidence has shown that RNA-binding proteins (RBPs) play critical roles in colorectal cancer progression [8]. RBPs bind target RNAs to form ribonucleoprotein (RNP) complexes in both context-dependent and context-independent manners, and these RNP complexes regulate the fate and functions of RNAs, including RNA biogenesis, stability, transport and cellular localization [9,10].
Multiple RBPs have been implicated in the modulation of gene expression at the posttranscriptional level in CRC [11,12]. The insulin-like growth factor-2 messenger RNA binding protein (IGF2BP) family comprises three RNA-binding proteins (IGF2BP1-3), all of which are highly expressed during both colon development and CRC progression [12,13]. The IGF2BPs exhibit distinct RNA-recognition properties, and numerous oncogenic transcripts have been identified as functional targets of IGF2BP1/2 in CRC [12]. However, it remains largely unknown how IGF2BP3 functions in binding and regulating its target transcripts in CRC progression.
In this study, we first determined the oncogenic roles of IGF2BP3 in CRC in vitro and in vivo. Through an approach of coimmunoprecipitation (Co-IP) combined with LC-MS/MS, we showed that IGF2BP3 physically interacted with the well-defined RNA-binding protein ELAVL1. We further predicted that 3070 target transcripts were directly coregulated by these two RBPs, indicating their collaborative roles in mRNA recognition. Interestingly, loss of either ELAVL1 or IGF2BP3 impaired the IGF2BP3/ELAVL1 complex-mediated target mRNA stability. Taken together, our findings reveal the molecular mechanism by which IGF2BP3 regulates target mRNA transcripts, which could be considered a therapeutic target in CRC treatment.
Material and methods
Cell culture and transfection
The human CRC cell lines SW480, HCT116, and DLD1 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Gibco). Transient transfection of siRNA was performed with the Lipofectamine™ RNAi MAX Transfection Reagent (Invitrogen #13778150) following the standard protocol. The siRNAs used in this study were purchased from RiboBio (stB0004745, MAP2K1; stB0008179, TPR).
RIP-qPCR assay
The RIP assay was performed using the EZ-Magna RIP Kit (Millipore #17-701). Briefly, 1×107 IGF2BP3-overexpressing SW480 cells and control cells were collected. The cell pellets were resuspended in the RIP Lysis Buffer supplemented with the Protease Inhibitor Cocktail and RNase Inhibitor and incubated on ice for 5 min. The magnetic beads were washed with the RIP Wash Buffer, and then, 5 µg of RIPAb+ IGF2BP3-RIP Validated Antibody (Sigma-Aldrich #03-198) or ELAVL1 (Abcam #ab136542) antibody was added to each tube. The magnetic beads and antibodies were incubated with rotation for 30 min at room temperature. Then, the RIP lysates were added to the antibody-coated magnetic beads and incubated in tubes with rotation for 3 h to overnight at 4°C. The beads were collected and washed six times. The immunoprecipitated RNA was eluted with a proteinase K buffer, extracted with a phenol:chloroform:isoamyl alcohol l (125:24:1, pH 4.3) buffer and analyzed with quantitative RT-qPCR.
RNA extraction and quantitative RT-qPCR
Total RNA extraction was performed with the TRIzol reagent (Invitrogen #15596018) as previously described [14]. Briefly, the total RNA samples were reverse transcribed with the PrimeScript™ RT reagent Kit (Takara #RR037A), and RT-qPCR was performed using TB Green® Premix Ex Taq™ II (Takara #RR820A) according to the manufacturer’s instructions. Each experiment was repeated independently at least three times. The primers used are listed in the Table S1.
Measurement of RNA stability
CRC cells were seeded in 6-well plates to obtain 50% confluence after 24 h. The cells were treated with 5 μg/ml actinomycin D (ApexBio #A4448) and were collected at the indicated time points. The total RNA was extracted by TRIzol reagent and analyzed by RT-qPCR. The turnover rates and half-lives of the mRNAs were estimated according to previous studies [15,16].
Plasmid construction and lentivirus packaging
The IGF2BP3 cDNA clone was constructed and subcloned into the pLVX-puro lenti-vector. The ELAVL1 (EX-Q0365-Lv101), MAP2K1 (EX-A0826-Lv101) and TPR (EX-Z8318-Lv101) cDNA clones were purchased from GeneCopoeia. The shRNA oligos targeting IGF2BP3 or ELAVL1 and a nontargeting oligo control were engineered into the pSIH-puro lenti-vector. For pLVX-puro lentivirus production, the packaging plasmids delta 8.9 and pLP2 were used. For pSIH-puro lentivirus production, the packaging plasmids vSVG, pLP1 and pLP2 were used. The packaging plasmids and the indicated lentiviral vectors were cotransfected into HEK293T cells. After transfection for 48 h, the supernatants containing lentivirus particles were collected and stored in aliquots at -80°C. For lentivirus infection, CRC cells were first treated with polybrene (5 µg/ml) (Santa Cruz #Sc-134220) and then infected with the indicated lentivirus. Stable cell populations were established by selecting with puromycin (1 μg/ml) (Gibco #A11138-03) or neomycin (400 μg/ml) (Gibco #10131027) for 2 weeks.
Western blotting assays
Western blotting was performed as previously described [14]. Briefly, the cells were collected and lysed in lysis buffer (1% NP-40, 0.1% sodium dodecyl sulfate, 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA, and 1× Proteinase Inhibitor Cocktail) for 30 min on ice. Equal amounts of proteins were loaded and resolved by 10% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked with 5% BSA solution and then incubated with specific antibodies at 4°C overnight. Following incubation with secondary antibodies, the immunoblots were detected using Western Lightning Plus ECL (PerkinElmer #203-17071). The antibodies used for western blotting were as follows: anti-IGF2BP3 (Proteintech #14642-1-AP), anti-TPR (Abcam #ab170940), and anti-MAP2K1 (Abcam #ab32576), anti-ELAVL1 (Abcam #ab136542).
Cell proliferation assays
Cell proliferation was quantified by CCK-8 assays. CRC cells were seeded into 96-well plates (2×104 cells/ml; 100 µl/well). The Cell Proliferation Reagent CCK-8 (Dojindo Molecular Technologies #CK04) was used to measure cell proliferation. After 2 h of incubation, the absorbance was measured at 450 nm using a microplate reader.
Colony formation assays
HCT116/DLD1 cells (2×103) or SW480 cells (1×104) with indicated treatment were seeded in 6-well plates. After 5-7 days, the cells were fixed with methanol followed by crystal violet staining. The colonies were counted in three random fields under a microscope. All the experiments were performed at least three times in triplicate.
Immunoprecipitation and mass spectrometry
For immunoprecipitation, 900 µg of protein was incubated with specific antibodies (2-3 µg) overnight at 4°C with rotation. Then, 30 µL anti-FLAG M2 affinity gel (Sigma) was added, and the incubation was continued for an additional 2 h. The beads were then washed 5 times using Buffer CF 0.15 (1 M HEPES, pH 7.9, glycerol, 5 M NaCl, 2 M MgCl2, 0.5 M EDTA, 10% NP 40, 1 M DTT, and 250 mM PMSF). The precipitated proteins were eluted from the beads by resuspending the beads in 2× SDS-PAGE loading buffer and boiling for 10 min. The eluted proteins were then analyzed by western blotting. For mass spectrometry, the proteins harvested from the immunoprecipitation were separated with 10% SDS-PAGE gels followed by silver staining according to the manufacturer’s protocol (Beyotime #P0017S). The desired bands were excised and subjected to LC-MS/MS sequencing and analysis. The result of mass spectrometry is listed in the Table S2.
Mouse xenograft studies
Six-week-old male BALB/c nude mice were purchased from Vital River (Beijing, China). Stable IGF2BP3-deficient HCT116 and DLD1 cells (1×106) were subcutaneously injected into the BALB/c nude mice. The tumor volumes were measured every four days, and the tumor masses were harvested and weighed 2-3 weeks after the initial cell implantation. All the procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Medical Sciences Cancer Hospital.
Immunohistochemistry analysis
Human CRC tissue microarrays were purchased from Servicebio Company (G6037-3). This study was approved by the ethical committee of the hospital, and informed consent was obtained from each enrolled patient. The tissue microarrays were stained with the anti-IGF2BP3 antibody (Proteintech #14642-1-AP) following the IHC procedure that was previously described [17]. The images were captured by an Aperio ScanScope (Leica, Nussloch, Germany).
Statistical analysis
Data analysis was conducted using GraphPad Prism (Version 6). The data was reported as the mean ± S.D. Statistical significance was calculated using two-sided Student’s t-tests or Wilcoxon matched-pairs signed rank test. ANOVA or the Friedman test was used for statistical analysis in multiple experimental groups. A P-value <0.05 was considered statistically significant.
Results
IGF2BP3 is overexpressed in CRC
To investigate the roles of IGF2BP3 in CRC, we first examined the expression of IGF2BP3 and its correlation with the clinical outcomes of colorectal cancer patients. In a cohort of 269 CRC patients from TCGA database [18], we found that high expression of IGF2BP3 was associated with worse overall survival of CRC patients (Figure 1A). Next, we measured the expression of IGF2BP3 in 16 paired patient samples of CRC tissue and adjacent normal tissue with immunohistochemistry (IHC). Consistent with previous findings that the expression of IGF2BP3 was significantly increased in CRC [19,20], higher expression of IGF2BP3 was observed in the cytoplasm and membrane of CRC cells. The H-scores of IGF2BP3 were markedly increased in the CRC tissues compared to the paired normal tissues (N=16) (Figure 1B). These findings were further validated in the two cohorts of CRC patients from the GEO databases (Figure 1C). Importantly, the IGF2BP3 levels significantly increased with CRC progression from early to late stages (Figure 1D). Taken together, these data suggest that IGF2BP3 expression is aberrantly increased in CRC tissues and strongly correlated with CRC malignancy.
Figure 1.
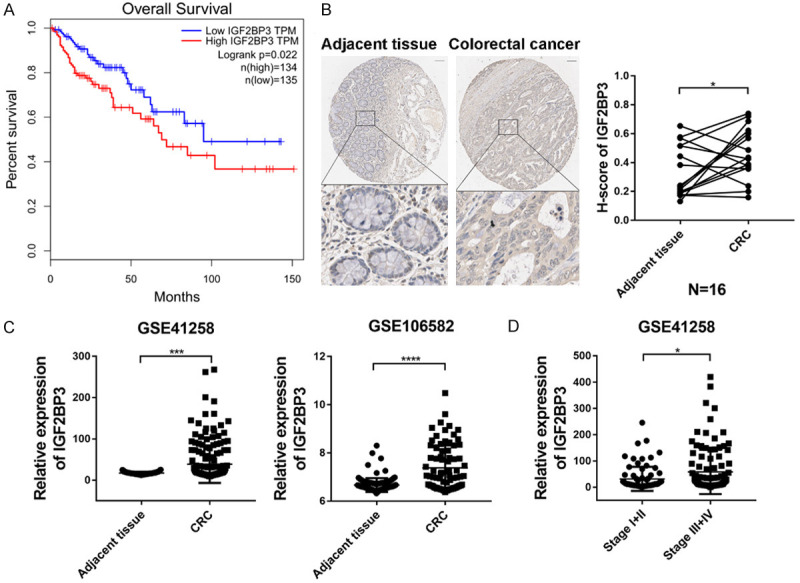
Analysis of clinical significance of IGF2BP3 in CRC. (A) Kaplan-Meier analysis of CRC patient survival with the GEPIA database [18] in strata defined according to high or low expression of IGF2BP3. (B) Representative images of IHC staining for IGF2BP3 in colorectal normal tissues and colorectal cancer tissues. Scale bar, 100 µm (left panel). The IHC score of IGF2BP3 was markedly higher in the CRC tissues than in the adjacent normal tissues (N=16) (right panel). (C) The mRNA level of IGF2BP3 was analyzed in two independent CRC patient cohorts, GSE41258 (left panel) and GSE106582 (right panel), showing that the IGF2BP3 mRNA levels were increased in the CRC tissues compared to the paired adjacent tissues. (D) The mRNA level of IGF2BP3 was analyzed in CRC patients (GSE41258) with higher or lower stage. *P<0.05, ***P<0.001, ****P<0.0001.
IGF2BP3 promotes CRC cell proliferation in vitro and in vivo
To further delineate the functional roles of IGF2BP3 in CRC, we first analyzed the expression of IGF2BP3 in CRC cells. As shown in Figure S1A, the mRNA expression of IGF2BP3 was much higher in the four CRC cell lines than in the normal colon FHC cell line, which was supportive of the oncogenic role of IGF2BP3 in the CRC cell model. We further analyzed the protein level of the four CRC cell lines (Figure S1B) and found that the HCT116 and DLD1 cells harbored relatively high expression of IGF2BP3 compared to the SW480 and HT29 cells. To investigate the functions of IGF2BP3 in CRC, we first infected SW480 cells with lentivirus encoding IGF2BP3 and then examined the cell viability of both the IGF2BP3-overexpressing cells and control cells. We found that the ectopic overexpression of IGF2BP3 dramatically promoted the proliferation and survival of the SW480 cells (Figures 2A-C, S1C). We also generated stable IGF2BP3-deficient HCT116 and DLD1 cell lines with lentivirus carrying shRNA targeting IGF2BP3 (shIGF2BP3 #1-#4) or nontargeting shCtrl as a control (Figure S1D-G). Consistently, stable knockdown of IGF2BP3 significantly suppressed the proliferation and survival of both cell lines (Figure 2D-G). To further demonstrate the growth-promoting effect of IGF2BP3 in vivo, we subcutaneously injected the stable IGF2BP3-deficient HCT116 and DLD1 cells and control cells into BALB/c nude mice. Compared to the control cells, knockdown of IGF2BP3 in both cell lines significantly inhibited the tumor growth and decreased the tumor weights (Figure 2H-K). Together, these results clearly reveal that IGF2BP3 drives CRC cell proliferation in vitro and outgrowth in vivo.
Figure 2.
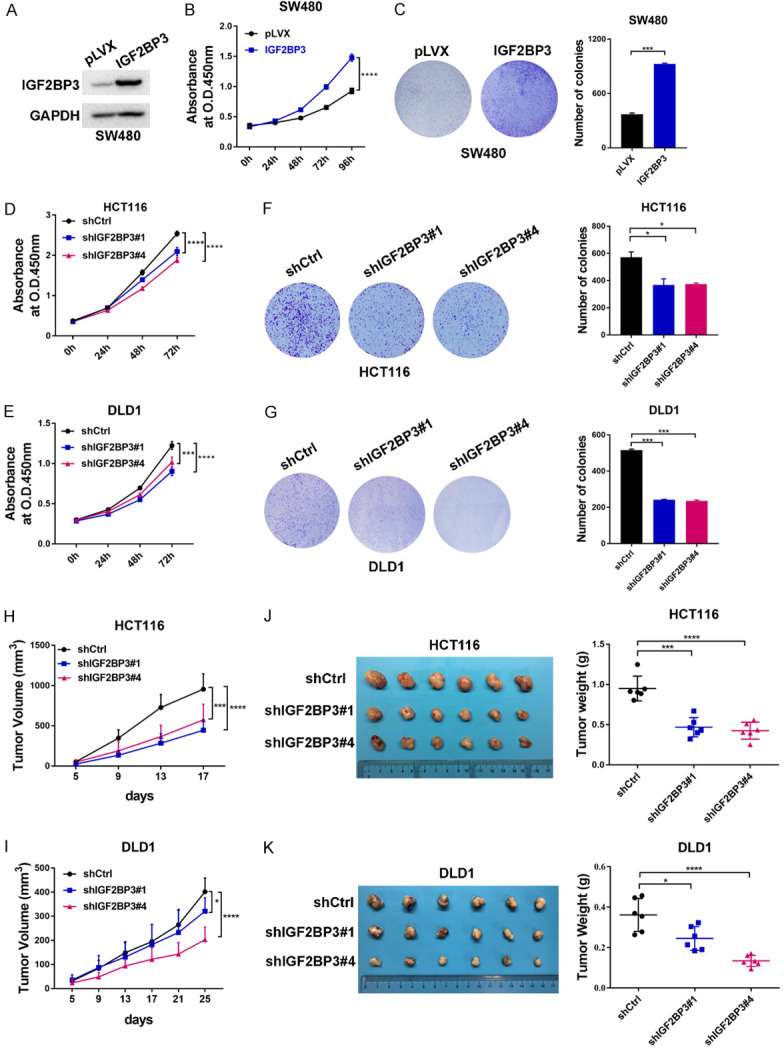
Oncogenic roles of IGF2BP3 in CRC. (A) Establishment of IGF2BP3-overexpressing SW480 cells. The expression of IGF2BP3 was analyzed with western blotting analysis. (B) Cell proliferation assays were performed in the IGF2BP3-overexpressing SW480 cells and control cells. (C) A total of 10,000 IGF2BP3-overexpressing SW480 cells and control cells were seeded into 6-well plates, and the colony formation ability of each group was assessed. Representative images are shown in (C) (left panel). The results were quantitatively analyzed (right panel). (D, E) Cell proliferation assays were performed in the stable IGF2BP3-deficient HCT116 (D) and DLD1 (E) cells and control cells. (F, G) A total of 2,000 stable IGF2BP3- deficient HCT116 (F) and DLD1 (G) cells and control cells were seeded into 6-well plates, and the colony formation ability of each group was assessed. Representative images are shown in (F and G) (left panel). The results were quantitatively analyzed (right panel). (H-K) Stable IGF2BP3-depleted HCT116 (H) or DLD1 (I) cells and control cells were subcutaneously injected into male BALB/c nude mice. The tumor growth was measured (H, I). Representative images are shown (J, K left panel), and the tumor weights from each group were statistically analyzed (J, K right panel). *P<0.05, ***P<0.001, ****P<0.0001.
IGF2BP3 physically interacts with the RNA binding protein ELAVL1 to recognize common mRNA transcripts in CRC
Given that RNA binding proteins are able to interact with multiple protein partners to regulate downstream targets [21-23], we proposed that IGF2BP3 recognized and regulated mRNA transcripts through potential collaborative interactions with other proteins. To validate this hypothesis, we first employed affinity purification and mass spectrometry to determine the IGF2BP3 interactome in vivo. In detail, FLAG-tagged IGF2BP3 (FLAG-IGF2BP3) was stably expressed in SW480 cells (Figure S2A). Whole-cell extracts were then collected and subjected to affinity purification using an anti-FLAG affinity gel. The purified protein complex was analyzed by western blotting followed by silver staining (Figures 3A, S2A). Through mass spectrometric analysis, we identified 449 IGF2BP3-interacting proteins in total, 436 of which were IGF2BP3-specific protein targets (Figure S2B). GO analysis of the IGF2BP3-specific binding targets further indicated that most of them were implicated in multiple activities related to RNA regulation (Figure S2C, S2D). Accordingly, IGF2BP3 was copurified with the well-defined RNA-binding protein ELAVL1 (Figure 3A), and this interaction was confirmed by coimmunoprecipitation (Co-IP) in FLAG-IGF2BP3-expressing SW480 cells (Figure 3B). Reciprocally, IP with an antibody against ELAVL1 followed by western blotting analysis also showed that IGF2BP3 was efficiently coimmunoprecipitated by ELAVL1 in HCT116 cells (Figure 3C).
Figure 3.
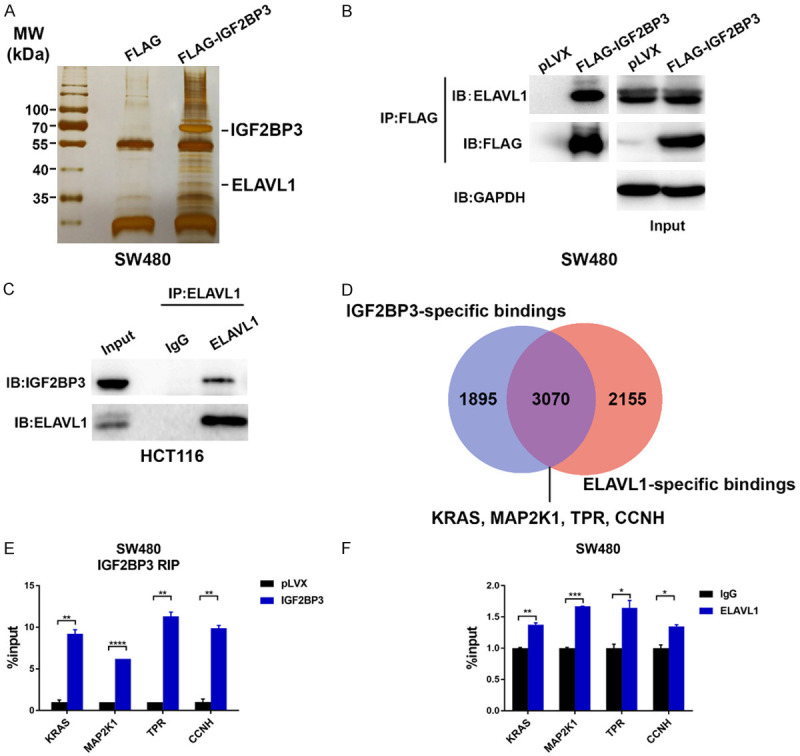
Identification of IGF2BP3-interacted proteins and target mRNAs. (A) Cellular extracts from the SW480 cells stably expressing FLAG (control) or FLAG-IGF2BP3 were immunoprecipitated with an anti-FLAG M2 affinity gel and eluted with 2x loading buffer. The protein eluates were resolved by 10% SDS-PAGE gel electrophoresis and silver-stained. The indicated protein bands were retrieved for mass spectrometry analysis. (B) Whole-cell lysates from the FLAG-IGF2BP3-overexpressing SW480 cells and control cells were prepared, and co-IP was performed with an anti-FLAG M2 affinity gel. The immunoprecipitates were then blotted using antibodies against the indicated proteins. (C) Whole-cell lysates from HCT116 cells were prepared, and co-IP was performed with an antibody against ELAVL1. The immunoprecipitates were then analyzed by using antibodies against the indicated proteins. (D) Analysis of the potential overlapping mRNA targets of IGF2BP3 and ELAVL1 according to the Starbase database (http://starbase.sysu.edu.cn). (E) RIP analysis was performed to validate the binding of IGF2BP3 to the regulatory regions of the KRAS, MAP2K1, TPR, and CCNH mRNA transcripts in the IGF2BP3-overexpressing SW480 cells and control cells. (F) RIP analysis was performed to validate the binding of ELAVL1 to the regulatory regions of the KRAS, MAP2K1, TPR, and CCNH mRNA transcripts in SW480 cells. **P<0.01, ****P<0.0001.
To identify the mRNA transcripts that are coregulated by these two RBPs, we then searched the online RBP database, which provided both IGF2BP3 and ELAVL1 CLIP-seq data [24]. Surprisingly, we found that 3070 mRNA transcripts were coregulated by IGF2BP3 and ELAVL1 (Figure 3D), which implied that IGF2BP3 may cooperate with ELAVL1 to modulate the functions of these mRNAs. GO analysis revealed that these common mRNA targets were involved in the cell cycle transition and cell proliferation (Figure S2E). Next, we performed an RIP assay in IGF2BP3-overexpressing SW480 cells to validate the binding of IGF2BP3 to four randomly selected mRNA transcripts (KRAS, MAP2K1, TPR, and CCNH). As expected, in IGF2BP3-overexpressing SW480 cells, we observed increased IGF2BP3 bindings to these mRNA transcripts (Figure 3E). Finally, we proposed that IGF2BP3 interacting with ELAVL1 recognized and bound to these mRNAs. Based on the RIP assay, we observed increased enrichment of ELAVL1 on these mRNA transcripts, implying collaborative roles of these two RBPs in binding to target mRNAs (Figure 3F). Taken together, these findings reveal that IGF2BP3 physically interacts with ELAVL1 to recognize mRNA transcripts in CRC cells.
IGF2BP3 synergizes with ELAVL1 to regulate mRNA stabilization in CRC
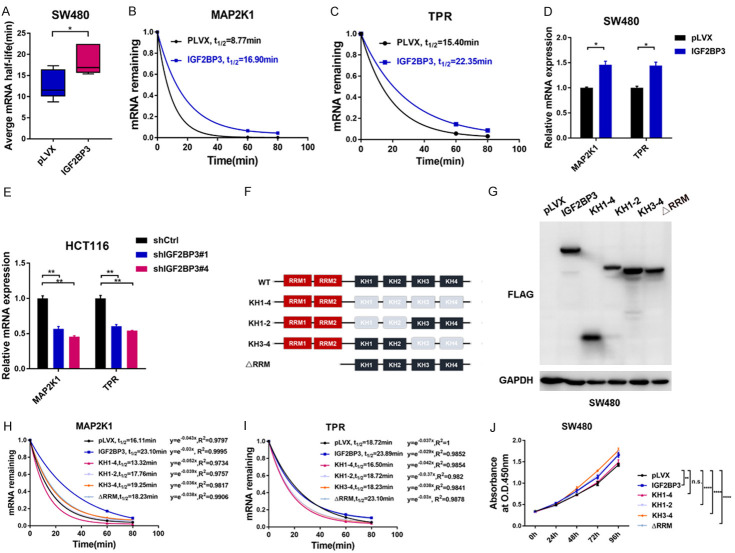
Previous studies showed that RBPs bind to target mRNA transcripts to regulate mRNA stability [10]. We next tested the stability of mRNAs bound by both IGF2BP3 and ELAVL1 by calculating the half-lives of these mRNAs. Both IGF2BP3-overexpressing SW480 cells and control cells were treated with actinomycin D for 60 min and 80 min respectively followed by the quantification of the relative mRNA levels. We found that overexpression of IGF2BP3 significantly increased the half-lives of these four selected mRNA transcripts (KRAS, MAP2K1, TPR and CCNH) (Figure 4A), indicating that IGF2BP3 plays critical roles in regulating mRNA stability in CRC cells. Among these transcripts, the half-lives of MAP2K1 and TPR mRNA almost doubled in the IGF2BP3-expressing SW480 cells (Figures 4B, 4C, S3A, S3B). RT-qPCR analysis further revealed that the mRNA levels of these genes were significantly increased in the IGF2BP3-overexpressing SW480 cells compared to the control cells (Figures 4D, S3C). Conversely, knockdown of IGF2BP3 in HCT116 cells significantly decreased the mRNA levels of these genes (Figures 4E, S3D). IGF2BP3 contains two RRMs and four KH domains, The C-terminal KH domains (KH) of the IGF2BP3 are indispensable for target RNA-binding, whereas the RRM domains mediate protein-RNA complexes stabilization as well as association with other RBPs [25]. We next constructed different IGF2BP3 truncated mutants to examine which domains were essential for target mRNA stability (Figure 4F, 4G). We found that deletion of KH domains (KH1-4), but not the RRM domains, significantly decreased the mRNAs stability of four indicated genes compared to IGF2BP3 wide-type control. Interestingly, deletion of either KH1-2 or KH3-4 domain of IGF2BP3 retained the abilities to stabilize target mRNAs. These data suggested that the KH domains are required for IGF2BP3-mediated mRNA stabilization (Figures 4H, 4I, S3E, S3F). Consistently, overexpression of IGF2BP3 with KH domains deletion (KH1-4) exhibited no effect on SW480 cell proliferation, whereas other deletion mutants remained the growth-promoting effects (Figure 4J). In summary, KH domains of IGF2BP3 are indispensable for target mRNA stability and cell proliferation in CRC.
Figure 4.
Analysis of IGF2BP3-mediated mRNA stability. (A) Analysis of the average mRNA half-lives of KRAS, MAP2K1, TPR and CCNH in the SW480 cells stably overexpressing IGF2BP3 and the control cells. (B, C) Both the IGF2BP3-overexpressing SW480 cells and control cells were treated with actinomycin D for 60 and 80 min respectively. Total RNA was extracted, the relative expression of MAP2K1 (B) and TPR (C) was calculated, and the mRNA half-lives of these genes were analyzed. (D) RT-qPCR analysis was performed to analyze the mRNA expression of MAP2K1 and TPR in the SW480 cells stably overexpressing IGF2BP3 and the control cells. (E) RT-qPCR analysis was performed to analyze the mRNA expression of MAP2K1 and TPR in the IGF2BP3-deficient HCT116 cells and control cells. (F) Schematic graph depicts the RNA-binding domains of IGF2BP3 and indicated deletions mutants constructed for the following study. (G) Western blot analysis showed the molecular weights of indicated IGF2BP3 deletion mutants. (H, I) The indicated IGF2BP3 deletion mutants and IGF2BP3 (WT) were stably overexpressed in SW480 cells. Cells were treated with actinomycin D for 60 and 80 min respectively. Total RNA was extracted, the relative expression of MAP2K1 (H) and TPR (I) was calculated, and the mRNA half-lives of these genes were analyzed. (J) The indicated IGF2BP3 deletion mutants and IGF2BP3 (WT) were stably overexpressed in SW480 cells and a CCK-8 assay was performed to measure the cell proliferation in the indicated groups. *P<0.05, **P<0.01, ****P<0.0001.
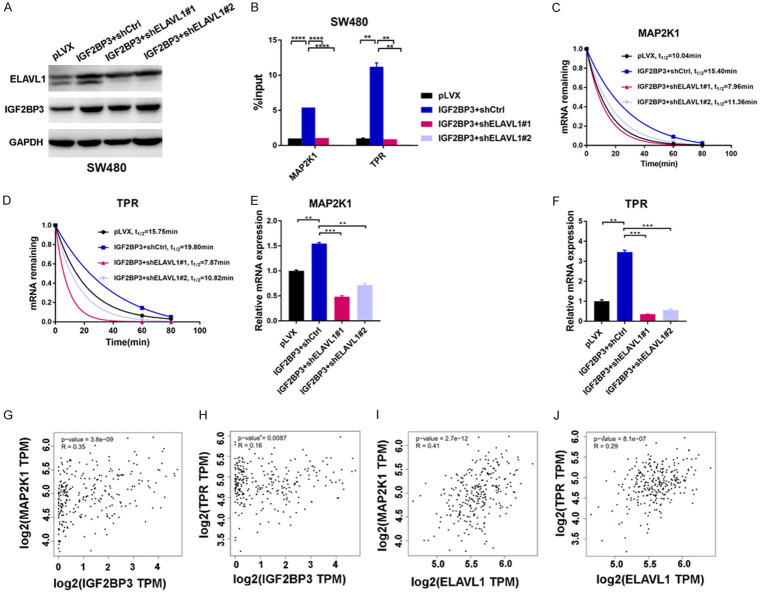
Given that ELAVL1 was colocalized with IGF2BP3 and bound to the same set of mRNA targets (Figure 3), we next investigated if ELAVL1 was able to affect the stability of these mRNAs. We stably knocked down the expression of ELAVL1 in the IGF2BP3-overexpressing SW480 cells (Figures 5A, S4A). Interestingly, in the IGF2BP3-overexpressing SW480 cells, knockdown of ELAVL1 significantly decreased the enhanced bindings of IGF2BP3 to mRNAs, leading to attenuated mRNA stability and decreased mRNA expression (Figures 5B-F, S4B-F). These data suggested that ELAVL1 is indispensable for the regulation of mRNA stability by IGF2BP3. Similarly, when IGF2BP3 was depleted in ELAVL1-overexpressing HCT116 cells (Figure S4G, S4H), ELAVL1-regulated mRNA stability was also significantly reduced (Figure S4I-L). Moreover, the expression of IGF2BP3 was positively correlated with ELAVL1 in CRC patient tissues (Figure S4M). Taken together, these data suggest IGF2BP3 synergizes with ELAVL1 to regulate downstream mRNAs.
Figure 5.
ELAVL1 facilitates IGF2BP3-regulated mRNA stability. (A) Establishment of IGF2BP3-overexpressing SW480 cells with stable ELAVL1 depletion. The expression of ELAVL1 and IGF2BP3 was analyzed by western blotting. (B) RIP analysis was performed to analyze the binding of IGF2BP3 to the regulatory regions of the MAP2K1 and TPR mRNAs in the SW480 cells overexpressing IGF2BP3 and these cells with the additional stable depletion of ELAVL1. (C, D) Analysis of the mRNA half-lives of MAP2K1 (C) and TPR (D) in the SW480 cells overexpressing IGF2BP3 and these cells with the additional stable depletion of ELAVL1. (E, F) The mRNA expression of MAP2K1 (E) and TPR (F) in the IGF2BP3-overexpressing SW480 cells and these cells with the additional stable depletion of ELAVL1 was analyzed with RT-qPCR assay. (G-J) Analysis of the clinical correlations between IGF2BP3/ELAVL1 and MAP2K1/TPR expression with the GEPIA database [18]. **P<0.01, ***P<0.001, ****P<0.0001.
Finally, we sought to determine the clinical correlations between IGF2BP3/ELAVL1 and their regulated targets in CRC. We found that the expression of both MAP2K1 and TPR was positively correlated with IGF2BP3 or ELAVL1 in CRC patient tissues (Figure 5G-J), demonstrating functional dependency and clinical significance between IGF2BP3/ELAVL1 and MAP2K1/TPR in CRC. Taken together, our findings suggest that IGF2BP3 increases mRNA stability through collaborative interaction with ELAVL1 in CRC.
Loss of MAP2K1 or TPR impairs IGF2BP3-driven cell proliferation in CRC
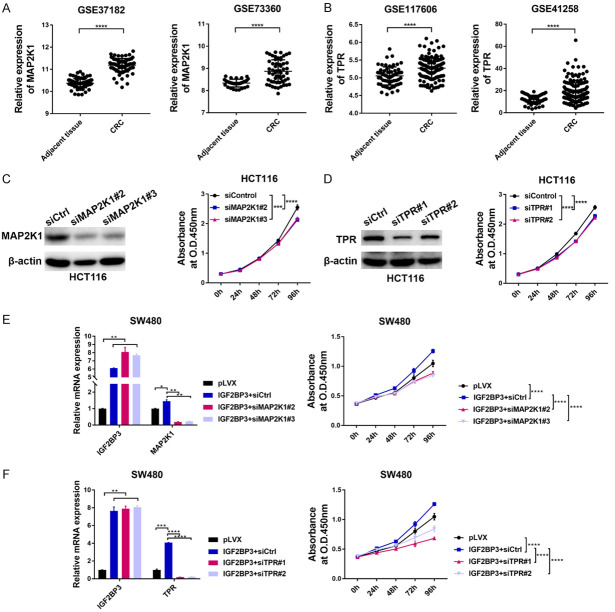
Given that the expression of MAP2K1 and TPR was strongly correlated with IGF2BP3 or ELAVL1 in the CRC specimens, we next examined the functions of these two genes in CRC. By analyzing two CRC patient cohorts from the GEO datasets, we found that the mRNA expression of these two genes was markedly upregulated in CRC patient tissues compared to adjacent tissues (Figure 6A, 6B). Additionally, transient silencing of these two genes in HCT116 and DLD1 cells with small interfering RNA (siRNA) significantly suppressed cell proliferation in vitro, which confirmed the oncogenic roles of these two genes (Figures 6C, 6D, S5A-F). To further verify that IGF2BP3 is an upstream regulator of these two onco-transcripts, we transiently transfected siRNA targeting MAP2K1 or TPR into the IGF2BP3-overexpressing SW480 cells and examined cell proliferation in each group. As ectopic expression of IGF2BP3 in SW480 cells enhanced its binding to these two mRNAs and led to increased mRNA stability (Figures 3E, 4B, 4C), we further determined that the mRNA expression of MAP2K1 and TPR was increased in the IGF2BP3-overexpressing SW480 cells and was decreased after siRNA-mediated gene silencing (Figure 6E, 6F). Consistently, the cell proliferation of the SW480 cells was dramatically increased by IGF2BP3 overexpression, and this increase was counteracted by the silencing of its downstream effector MAP2K1 or TPR (Figure 6E, 6F). Reciprocally, the ectopic expression of MAP2K1 or TPR was sufficient to rescue the proliferative abilities of the IGF2BP3-depleted HCT116 or DLD1 cells (Figure S6A-H). Collectively, these data clearly demonstrate that MAP2K1 and TPR act as direct targets of IGF2BP3 to exert protumorigenic functions in CRC.
Figure 6.
Functional characterizations of two IGF2BP3-regulated targets in CRC. (A, B) The mRNA levels of MAP2K1 (A) and TPR (B) were analyzed in two independent CRC patient cohorts. (C) HCT116 cells were transfected with siRNA targeting MAP2K1 and a nontargeting siRNA control, and the knockdown efficiency of MAP2K1 was confirmed by western blotting analysis (left panel). The effect of MAP2K1 silencing on the proliferation of HCT116 cells was analyzed with a CCK-8 assay (right panel). (D) HCT116 cells were transfected with siRNA targeting TPR and a nontargeting siRNA control, and the knockdown efficiency of TPR was confirmed by western blotting analysis (left panel). The effect of TPR silencing on the proliferation of HCT116 cells was analyzed with a CCK-8 assay (right panel). (E, F) The SW480 cells overexpressing IGF2BP3 were further transfected with siRNA targeting MAP2K1 (E) and TPR (F). The expression of IGF2BP3, MAP2K1 and TPR was analyzed with RT-qPCR analysis (E, F left panel). The proliferation of the indicated cells was examined with a CCK-8 assay (E, F right panel). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Discussion
Sequence-specific RNA-binding proteins (RBPs) recognize and interact with a large number of RNAs to form ribonucleoproteins (RNPs), thereby controlling the fate of these RNAs [9-11]. Increasing evidence shows that RBPs are dysregulated in many human cancers and play crucial roles in cancer development [8,26]. Of note, many RBPs are implicated in the maintenance of intestinal epithelial homeostasis and the progression of colorectal cancer [12]. Among the RBPs that are critically involved in CRC development, our study focused on one of the IGF2BP family members, IGF2BP3, which has been shown to be functional in multiple cancer types [13,25]. However, there is still limited evidence regarding its RNA binding properties. Previous findings showed that the expression of IGF2BP3 is highly correlated with unfavorable prognosis in CRC patients and that IGF2BP3 promotes CRC cells malignant progression [19,27]. However, the mechanism by which IGF2BP3 modulates the fates of its target RNAs in CRC development remains largely unexplored.
Indeed, IGF2BP3 is overexpressed in CRC tissues compared to normal tissues (Figure 1B, 1C). Functional studies also further support the tumorigenic role of IGF2BP3 in both CRC cells and xenograft models (Figure 2). Given that different RBPs are able to bind to the same RNA targets and exert concordant or diverse effects on the target RNAs [21-23], we next performed an IP-based LC-MS/MS assay to characterize the IGF2BP3-interacting proteins in CRC cells. Interestingly, numerous IGF2BP3-bound proteins are involved in RNA binding and processing (Figure S2B-D), suggesting that IGF2BP3 may interact with other RBPs to regulate mRNAs. This notion prompts us to discover ELAVL1, a well-characterized oncogenic RBP in multiple cancer types [11,28]. ELAVL1 is a ubiquitously expressed member of the ELAVL protein family, which contains three RNA-binding domains to selectively bind to cis-acting AU-rich elements (AREs) and stabilize ARE-containing mRNAs to regulate target gene expression [28,29]. Reportedly, IL-18 can induce the interaction between IGF2BP3 and ELAVL1, leading to COX-2 mRNA stabilization in acute myeloid leukemia [30]. Interestingly, all the IGF2BP family members, IGF2BP1-3, were also confirmed to interact and colocalized with ELAVL1 in HeLa cells [16]. These findings may indicated that ELAVL1 binds to specific domains that share structural similarities among the IGF2BP family members and that might be conserved in different cell types. In our study, we predicted that 3070 mRNAs were coregulated by these two RBPs and identified two clinically relevant oncogenes (TPR and MAP2K1) in CRC (Figure 3D-F), suggesting a functional interplay between RBPs to cooperatively regulate the same target mRNAs.
Many cellular factors and regulatory mechanisms contribute to the stability of mRNA [31]. Generally, the mechanism of mRNA decay comprises two types: mRNA surveillance pathways that target aberrant mRNAs and mRNA halflife regulation that changes the abundance of proteins [31,32]. ARE-mediated mRNA decay is one of the most well-defined mechanisms that governs the stability of ARE-containing mRNAs. ARE-bound ELAVL1 potentially antagonizes mRNA degradation by competing with the binding sites of the destabilizing proteins TTP, BRF1, KSRP and AUF1 [29,32-34]. Thus, we proposed that the stability of the mRNAs that were coregulated by IGF2BP3/ELAVL1 was facilitated by ELAVL1. Consistently, loss of ELAVL1 significantly compromised the mRNA binding efficacy of IGF2BP3, leading to shortened mRNA half-lives and decreased mRNA expression levels (Figure 5A-F). Additionally, the mRNA fate was also regulated by posttranscriptional modifications of mRNAs. The N6-methyladenosine (m6A) modification has been identified as most abundant internal modification, which is enriched in 3’ untranslated regions (UTRs) and close to the stop codons of target mRNA [35]. Recent study showed that IGF2BP family members functioned as m6A readers through its KH domains (KH), which recognized and promoted mRNA stability in m6A-dependent manner [16]. Our findings indicated the relationship between IGF2BP3 and ELAVL1 was interdependent and synergistic (Figures 5, S4), and the crosstalk between IGF2BP3 and ELAVL1 mediated mRNA stability remained to be further investigated.
In summary, our study reveals that the IGF2BP3/ELAVL1 complex is essential in regulating oncogenic mRNA stability in CRC. Due to the complexity of gene regulation at the posttranscriptional level, the comprehensive characterization of IGF2BP3/ELAVL1 complex-mediated mRNA stability will help provide novel molecular insights into the functions of RBPs in RNA metabolism.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81872038, 81902503).
Disclosure of conflict of interest
None.
Abbreviations
- CRC
colorectal cancer
- IGF2BP3
the insulin-like growth factor-2 messenger RNA binding protein
- Co-IP
coimmunoprecipitation
- RBPs
RNA-binding proteins
- RNP
ribonucleoprotein
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (London, England) 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 3.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 4.van der Stok EP, Spaander MCW, Grünhagen DJ, Verhoef C, Kuipers EJ. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol. 2017;14:297–315. doi: 10.1038/nrclinonc.2016.199. [DOI] [PubMed] [Google Scholar]
- 5.Grazioso TP, Brandt M, Djouder N. Diet, microbiota, and colorectal cancer. iScience. 2019;21:168–187. doi: 10.1016/j.isci.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. Trends Cancer. 2017;3:506–528. doi: 10.1016/j.trecan.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nature reviews. Mol Cell Biol. 2018;19:327–341. doi: 10.1038/nrm.2017.130. [DOI] [PubMed] [Google Scholar]
- 10.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Cárdenas JM, Guerrero S, López-Cortés A, Armendáriz-Castillo I, Guevara-Ramírez P, Pérez-Villa A, Yumiceba V, Zambrano AK, Leone PE, Paz-Y-Miño C. Post-transcriptional regulation of colorectal cancer: a focus on RNA-binding proteins. Front Mol Biosci. 2019;6:65. doi: 10.3389/fmolb.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterji P, Rustgi AK. RNA binding proteins in intestinal epithelial biology and colorectal cancer. Trends Mol Med. 2018;24:490–506. doi: 10.1016/j.molmed.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell JL, Wächter K, Mühleck B, Pazaitis N, Köhn M, Lederer M, Hüttelmaier S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70:2657–2675. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Li Y, Zhao M, Lin H, Wang W, Li D, Cui W, Zhou C, Zhong J, Huang C. MiR-494 acts as a tumor promoter by targeting CASP2 in non-small cell lung cancer. Sci Rep. 2019;9:3008. doi: 10.1038/s41598-019-39453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CY, Ezzeddine N, Shyu AB. Messenger RNA half-life measurements in mammalian cells. Methods Enzymol. 2008;448:335–357. doi: 10.1016/S0076-6879(08)02617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, Hu YC, Hüttelmaier S, Skibbe JR, Su R, Deng X, Dong L, Sun M, Li C, Nachtergaele S, Wang Y, Hu C, Ferchen K, Greis KD, Jiang X, Wei M, Qu L, Guan JL, He C, Yang J, Chen J. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, Li Y, Yuan X, Zhao M, Wang J, Li Y, Li Y, Lin H, Zhang Q, Wang W, Li D, Dong X, Li L, Liu M, Huang W, Huang C. The UbL-UBA Ubiquilin4 protein functions as a tumor suppressor in gastric cancer by p53-dependent and p53-independent regulation of p21. Cell Death Differ. 2019;26:516–530. doi: 10.1038/s41418-018-0141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Sheng Y, Guo Y, Huang Z, Huang Y, Wen D, Liu CY, Cui L, Yang Y, Du P. Increased IGF2BP3 expression promotes the aggressive phenotypes of colorectal cancer cells in vitro and vivo. J Cell Physiol. 2019;234:18466–18479. doi: 10.1002/jcp.28483. [DOI] [PubMed] [Google Scholar]
- 20.Lochhead P, Imamura Y, Morikawa T, Kuchiba A, Yamauchi M, Liao X, Qian ZR, Nishihara R, Wu K, Meyerhardt JA, Fuchs CS, Ogino S. Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur J Cancer. 2012;48:3405–3413. doi: 10.1016/j.ejca.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengupta TK, Bandyopadhyay S, Fernandes DJ, Spicer EK. Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J Biol Chem. 2004;279:10855–10863. doi: 10.1074/jbc.M309111200. [DOI] [PubMed] [Google Scholar]
- 22.Ishimaru D, Ramalingam S, Sengupta TK, Bandyopadhyay S, Dellis S, Tholanikunnel BG, Fernandes DJ, Spicer EK. Regulation of Bcl-2 expression by HuR in HL60 leukemia cells and A431 carcinoma cells. Mol Cancer Res. 2009;7:1354–1366. doi: 10.1158/1541-7786.MCR-08-0476. [DOI] [PubMed] [Google Scholar]
- 23.Sureban SM, Murmu N, Rodriguez P, May R, Maheshwari R, Dieckgraefe BK, Houchen CW, Anant S. Functional antagonism between RNA binding proteins HuR and CUGBP2 determines the fate of COX-2 mRNA translation. Gastroenterology. 2007;132:1055–1065. doi: 10.1053/j.gastro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lederer M, Bley N, Schleifer C, Hüttelmaier S. The role of the oncofetal IGF2 mRNA-binding protein 3 (IGF2BP3) in cancer. Semin Cancer Biol. 2014;29:3–12. doi: 10.1016/j.semcancer.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZL, Li B, Luo YX, Lin Q, Liu SR, Zhang XQ, Zhou H, Yang JH, Qu LH. Comprehensive genomic characterization of RNA-binding proteins across human cancers. Cell Rep. 2018;22:286–298. doi: 10.1016/j.celrep.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Yan D, Tang H, Zhou C, Fan J, Li S, Wang X, Xia J, Huang F, Qiu G, Peng Z. IMP3 is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Ann Surg Oncol. 2009;16:3499–3506. doi: 10.1245/s10434-009-0648-5. [DOI] [PubMed] [Google Scholar]
- 28.García-Mauriño SM, Rivero-Rodríguez F, Velázquez-Cruz A, Hernández-Vellisca M, Díaz-Quintana A, De la Rosa MA, Díaz-Moreno I. RNA binding protein regulation and cross-talk in the control of AU-rich mRNA fate. Front Mol Biosci. 2017;4:71. doi: 10.3389/fmolb.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko CY, Wang WL, Li CF, Jeng YM, Chu YY, Wang HY, Tseng JT, Wang JM. IL-18-induced interaction between IMP3 and HuR contributes to COX-2 mRNA stabilization in acute myeloid leukemia. J Leukoc Biol. 2016;99:131–141. doi: 10.1189/jlb.2A0414-228RR. [DOI] [PubMed] [Google Scholar]
- 31.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nature reviews. Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 32.Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nat Rev Genet. 2012;13:246–259. doi: 10.1038/nrg3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813–4827. doi: 10.1093/nar/gki797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.