Abstract
For pancreatic cancer, the probability of distant metastasis can help choose the best course of treatment. The aim of this study is to establish the efficacy of hydroxyproline as a biomarker for distant metastasis for pancreatic cancer and to clarify the mechanism of EGLN/HIF1A axis that controls the invasion and metastasis. Metabolites (hydroxyproline) and genes (EGLN2 and EGLN3) were identified by metabolome analysis of the serum with pancreatic cancers with and without distant metastasis. The mechanism of EGLN/HIF1A axis including angiogenesis was examined in pancreatic cancer cells. Hydroxyproline associated with these mechanisms was evaluated to suggest the association with overall survival in pancreatic cancer. Decreased expression of EGLN2 and EGLN3 in pancreatic cancer, via the HIF1A and TGF ß1 pathway, was associated with the induction of angiogenic factors, increased vascular invasion, and poor overall patient survival. Hydroxyproline concentrations were regulated via the HIF1A pathway by EGLN2 and EGLN3, and that increased concentrations of hydroxyproline promote the invasion and metastasis of pancreatic cancer cells. These results suggested that the expression of hydroxyproline through the HIF1A pathway induced by EGLN2 and EGLN3 could be a surrogate marker for treatment and might predict distant metastasis in pancreatic cancer.
Keywords: EGLN, HIF1A, hydroxyproline, pancreatic cancer, distant metastasis
Introduction
The overall survival of pancreatic cancer is approximately 10-20% [1], which is one of the worst survival rates of digestive cancers. Curative resection is the only radical treatment; however, pancreatic cancer is often locally advanced at the time of diagnosis and patients may have distant metastases after surgery [2]. The indication for resection is quite important, since several chemotherapy regimens can extend survival [3,4], and combined vascular resection is necessary for surgery to be curative [5,6]. Knowing the probability of distant metastasis can help choose the best course of treatment.
This study aimed to establish individual biomarkers for pancreatic cancer treatment and to clarify the mechanisms that control the invasion and metastasis of pancreatic cancer using a genetic approach. We did metabolome analysis of the blood of patients with pancreatic cancer, we identified metabolites that were differentially expressed in patients with pancreatic cancer and metastases vs. that without metastases, and identified additional genes that were associated with these metabolic pathways.
In our study, we demonstrated that decreased expression of EGLN2 and EGLN3 in pancreatic cancer, via the HIF1A pathway, was associated with the induction of angiogenic factors, increased vascular invasion, and poor overall patient survival. Increased expression of hydroxyproline in the patients’ serum was associated with reduced EGLN2 and EGLN3 expression. Taken together, these results suggest that the expression of hydroxyproline through the HIF1A pathway induced by EGLN2 and EGLN3 could be a surrogate marker for treatment and might predict distant metastasis in pancreatic cancer.
Materials and methods
Identification of candidate metabolites and genes associated with pancreatic cancer metastasis
Serum samples were collected from patients with histologically-diagnosed pancreatic cancer. No patients had received any prior treatment in the form of chemotherapy, radiotherapy, surgery, or alternative therapy. The serum samples were retrieved as previously described [7]. The metabolite standards, capillary electrophoresis-time-of-flight-mass spectrometry (CE-TOFMS) instrumentation, and measurement conditions for cationic and anionic metabolites were described previously [7]. Comparison of the difference between two groups, e.g., with and without distant metastasis, was assessed. Relevant genes were identified using the Human Metabolome Database (http://www.hmdb.ca/).
Cell lines and cell culture
The human pancreatic cancer cell lines, Panc1, MiaPaCa2, and BxPc3 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The identity of all cells was independently authenticated by short tandem repeat genotyping on May 2016. Cell lines were grown in RPMI 1640 medium (Sigma Chemical, St Louis, MO) supplemented with 10% fetal bovine serum at 37°C in a humidified 5% CO2 normoxic atmosphere. Cell lines were confirmed pathogen-free. We used the BIONIX-3 hypoxic culture kit (Sugiyama-Gen, Tokyo, Japan) to produce hypoxic culture conditions.
siRNA-mediated gene knock-down
Stealth™ RNAI siRNAs from Invitrogen were used to knock down endogenous gene expression. Two siRNAs (with different sequences) per target were individually transfected into cells at a final concentration of 100 nM using Lipofectamine RNAiMAX (Invitrogen). siGFP was used as a negative control. These knock-down system was verified by qRT-PCR.
Chemomigration assay
Chemomigration was assessed as previously described [8], using 24-well transwell plates with polyethylene terephthalate membranes (8 μm pore size) (BD Falcon). The undersides were coated with 10 μL of 500 μg/mL chemoattractant (type I collagen) and air-dried. The membranes were rehydrated for 3 h with DMEM at 37°C before use. Cells (5×104) were resuspended in 500 μL DMEM (0.1% FBS), and added to the upper chambers. We added 600 μL DMEM (0.1% FBS) to the lower chambers. After incubation for 24 h at 37°C, cells remaining on the upper surface of the membranes were mechanically removed. Cells that migrated to the underside were stained with crystal violet and counted under a microscope. Nine independent fields were counted per experiment. The control were treated with siGFP under the same conditions.
Tissue samples
We obtained frozen tissue specimens containing cancerous and matched normal pancreas regions from 102 patients who had surgical resection for pancreatic cancer between September 2008 and December 2013 at Tokyo Medical University Hachioji Medical Center. All protocols were performed in accordance with the ethics committee of Tokyo Medical University.
RNA isolation and cDNA synthesis
RNA was extracted from using the RNeasy kit (QIAGEN, Valencia, CA) following manufacturer’s instructions. Conditions for semi-quantitative amplification of cDNA were 95°C for 2 min, followed by 25 cycles at 95°C for 30 s, 56°C for 30 s, and 72°C for 60 s, with a final extension cycle at 72°C for 10 min.
Assessment of EGLN2 and EGLN3 levels in pancreatic tissue by qRT-PCR
qRT-PCR of EGLN2 and EGLN3 mRNA expression was examined in cancerous regions and in adjacent normal pancreatic tissue regions of samples acquired during pancreatic cancer resection surgery at our institute from 2008 to 2013. The relative expression levels (cancer vs. normal pancreatic tissue) were examined using qRT-PCR (quantitative reverse transcription polymerase chain reaction). RT-PCR analysis was run in triplicate for each sample on a Light Cycler 480 Real-Time PCR System using SYBR Green 1 Master Mix (Roche). The following program was run: pre-incubation for 5 min at 95°C, amplification for 45 cycles (10 s of denaturation at 95°C, 10 s of annealing at 57°C, and 10 s of extension at 72°C), with melt-curve analysis. mRNA levels of the target genes were normalized using GAPDH as the internal control. The sequences of all the primers used are listed as follows:
EGLN2: Forward: 5’ TGGCCCTGGACTATATCGTG 3’; Backward: 5’ GGCACCAATGCTTCGACAG 3’. EGLN3: Forward: 5’ CTGGGCAAATACTACGTCAAGG 3’; Backward: 5’ GACCATCACCGTTGGGGTT 3’. GAPDH: Forward: 5’ ATCATCCCTGCCTCTACTGG 3’; Backward: 5’ TTTCTAGACCGGCAGGTCAGGT 3’. ANGPTL-2: Forward: 5’ CACCGACCTCCCGTTAGC 3’; Backward: 5’ GGCCACCTTGTGGAAGAGT 3’. b-FGF: Forward: 5’ TTCTTCCTGCGCATCCAC 3’; Backward: 5’ CGGTTAGCACACACTCCTTTGAT 3’. IL-8: Forward: 5’ TTGGCAGCCTTCCTGATTT 3’; Backward: 5’ GGGTGGAAAGGTTTGGAGTAT 3’. TGF-ß1: Forward: 5’ CAGCAACAATTCCTGGCGATA 3’; Backward: 5’ AAGGCGAAAGCCCTCAATTT 3’. TGF-ß2: Forward: 5’ CTGATCCTGCATCTGGTCACG 3’; Backward: 5’ TGGGGGACTGGTGAGCTTC 3’. VEGF: Forward: 5’ AGAAGGAGGAGGGCAGAATC 3’; Backward: 5’ TGGCTTGAAGATGTACTCGATCTC 3’. CTGF: Forward: 5’ CAGCATGGACGTTCGTCTG 3’; Backward: 5’ AACCACGGTTTGGTCCTTGG 3’. SERPINE-1: Forward: 5’ ACCGCAACGTGGTTTTCTCA 3’; Backward: 5’ TTGAATCCCATAGCTGCTTGAAT 3’. SPARC: Forward: 5’ TGAGGTATCTGTGGGAGCTAATC 3’; Backward: 5’ CCTTGCCGTGTTTGCAGTG 3’.THBS-1: Forward: 5’ AGACTCCGCATCGCAAAGG 3’; Backward: 5’ TCACCACGTTGTTGTCAAGGG 3’.
Western blot analysis
Protein expression was assessed by western blot analysis using antibodies against HIF1A (Cell Signaling Technology), EGLN2 (Cell Signaling Technology), EGLN3 (Cell Signaling Technology) and actin (ABCAM). The western blot protocols used have been described previously [9].
HIF1A antibody and TGF-ß1 inhibitor treatment
Cells were plated into 12- to 24-well tissue culture plates or 10 cm2 dishes and cultured overnight. HIF1A antibody (ab51608; Abcam plc, Cambridge, UK) or LY364947 (Sigma-Aldrich, Germany) was added to the cells 48 h before mRNA, protein, and supernatant extraction. The final dimethyl sulfoxide (DMSO) concentration in all experiments was <0.05%.
Hydroxyproline measurement
The supernatants from pancreatic cancer cells or the serum from patients with pancreatic cancer were assessed for hydroxyproline levels (A measurement kit by Cell Biolabs, Inc., CA). In vitro, cells were plated in 12- to 24-well tissue culture plates or 10 cm2 dishes and cultured overnight. siRNA reagents with and without HIF1A antibody or TGF-ß1 antibody were added to the cells 48 h before supernatant extraction. Serum from patients was collected before resection surgery.
Statistical analyses
Data represent the mean ± standard error of the mean (SEM). Comparisons between groups were made using 2-tailed Mann-Whitney U-test for continuous variables, and Fisher exact test for comparison of proportions. Correlations were calculated with the nonparametric Spearman’s coefficient. All calculations were made using the SPSS package (SPSS 18.0). Overall survival (OS) curves were represented according to Kaplan-Meier estimates and compared using log-rank tests. P<0.05 was considered statistically significant.
Results
Identification of candidate genes by metabolome assay
Metabolome analysis was performed using the serum of 26 patients diagnosed with pancreatic cancer. The clinicopathological characteristics of all 26 patients are summarized in Table 1. Concentrations of three serum metabolites, glucose-6-phosphate (G6P), malonate, and hydroxyproline, were significantly higher in patients with distant metastases compared to those without distant metastases. Ten genes shown in Figure 1B were identified, after some genes involved in the above three serum metabolites were searched by Human Metabolome Database. Among these, levels of EGLN2 and EGLN3 genes were significantly upregulated compared with the control (Figure 1A). Therefore, we chose to evaluate the expression of EGLN2 and EGLN3 in pancreatic cancer cell lines and their potential role in the invasion and metastasis of pancreatic cancer cells.
Table 1.
Clinicopathological characteristics of 26 patients performed metabolome analysis
| Distant meta (-) (n=10) | Distant meta (+) (n=16) | p-value | |
|---|---|---|---|
| Age | 70 | 69 | 0.740 |
| Gender | 0.813 | ||
| Male | 6 | 7 | |
| Female | 4 | 9 | |
| UICC Stage | |||
| II | 3 | 0 | |
| III | 7 | 0 | |
| IV | 0 | 16 | |
| Tumor marker | |||
| CEA (ng/ml) | 10.1 | 71.6 | 0.273 |
| CA19-9 (U/ml) | 1899.6 | 4628.7 | 0.443 |
| Dupan2 (U/ml) | 525.4 | 438.4 | 0.699 |
| Span1 (U/ml) | 810.6 | 703.1 | 0.822 |
| Serum metabolite with significant difference (umol/L) | |||
| Glucose 6 phosphate | 0.17 | 0.222 | 0.007 |
| Malonate | 0.564 | 0.675 | 0.023 |
| Hydroxyproline | 10.73 | 19.17 | 0.010 |
Values are mean. CEA, Carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; Dupan2, pancreatic cancer-associated antigen-2; Span1, s-pancreas-1 antigen.
Figure 1.
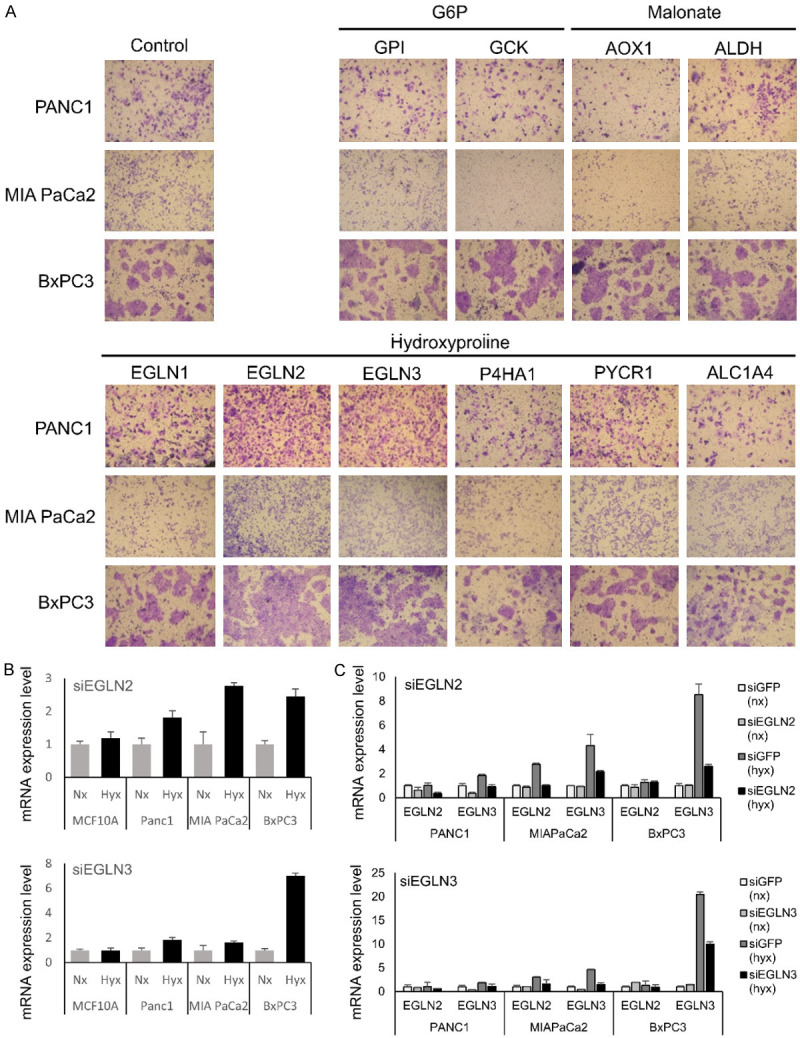
Identification of candidate genes by metabolome assay and confirmation of EGLN2 and EGLN3 expression levels and knock-down by siRNA system. (A) Levels of three serum metabolites, glucose-6-phosphate (G6P), malonate, and hydroxyproline, were significantly higher in patients with distant metastases compared to those without distant metastases. Ten genes shown in figure were identified, after some genes involved in the above three serum metabolites were searched by Human Metabolome Database. Among these, levels of EGLN2 and EGLN3 genes were significantly upregulated compared with the control. EGLN2 and EGLN3 levels were relatively lower in the pancreatic cancer cell lines compared to MCF10A in (B). And EGLN2 and EGLN3 mRNA levels were higher in hypoxic conditions compared to normoxia in (B). The siRNAs decreased EGLN2 or EGLN3 expression in pancreatic cancer cells, especially under hypoxic conditions in (C).
Confirmation of EGLN2 and EGLN3 expression levels in pancreatic cancer cell lines
We evaluated mRNA expression of EGLN2 and EGLN3 in pancreatic cancer cell lines, Panc1, MIAPaCa2, and BxPC3; and in the human mammary epithelial cell line MCF10A (as normal cell line). EGLN2 and EGLN3 levels were relatively lower in the pancreatic cancer cell lines compared to MCF10A (data not shown). We then compared their expression levels in normoxia and hypoxia conditions. EGLN2 and EGLN3 mRNA levels were higher in hypoxic conditions compared to normoxia (Figure 1B).
EGLN2 and EGLN3 regulate the expression of angiogenic factors and via TGF-ß1 and HIF1A signaling, as a result expression of hydroxyproline
To ascertain the potential roles of EGLN2 and EGLN3 in pancreatic cancer cells, we depleted EGLN2 or EGLN3 mRNA using siRNAs in normoxia and hypoxia. The siRNAs decreased EGLN2 or/and EGLN3 expression in pancreatic cancer cells, especially under hypoxic conditions (Figure 1C). Reducing EGLN2 or EGLN3 mRNA levels increased the expression of several angiogenic factors (Figure 2A) and TGF-ß signatures (Figure 2B). Moreover, reduction of EGLN2 or EGLN3 in pancreatic cancer cell lines also mitigated HIF1A signaling (Figure 2C). Knockdown of EGLN2 or EGLN3 led to upregulation of HIF1A (Figure 2D). Supernatant concentrations of hydroxyproline increased when EGLN2 or EGLN3 were knocked down (Figure 2E). These in vitro findings were more prominent in hypoxic vs. normoxic conditions.
Figure 2.
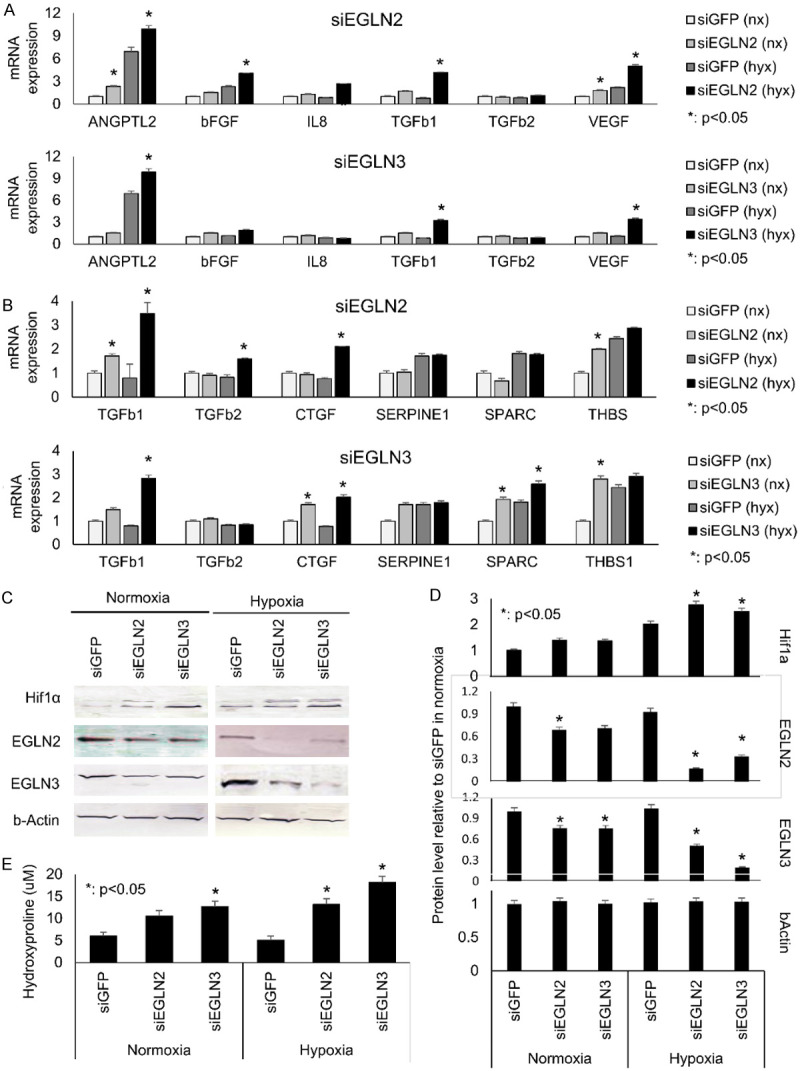
EGLN2 and EGLN3 regulate the expression of angiogenic factors and via TGF-ß1 and HIF1A signaling, as a result expression of hydroxyproline. Reducing EGLN2 or EGLN3 mRNA levels decreased the expression of several angiogenic factors in (A) and TGF-ß signatures in (B). Moreover, reduction of EGLN2 or EGLN3 in pancreatic cancer cell lines also mitigated HIF1A signaling (C). Knockdown of EGLN2 or EGLN3 led to upregulation of HIF1A (D). Supernatant concentrations of hydroxyproline increased when EGLN2 or EGLN3 were knocked down in (E). These in vitro findings were more prominent in hypoxic vs. normoxic conditions.
TGF-ß1 inhibition or HIF1A antibody treatment induces the expression of angiogenic factors and hydroxyproline
To determine the mechanism by which angiogenic factors and hydroxyproline regulate EGLN2 and EGLN3 expression, we treated cells with reduced EGLN2 and EGLN3 expression with LY364947 (a TGF-ß1 receptor inhibitor) or an HIF1A antibody. Angiogenic factors amplified by EGLN2/3 and TGF-ß signaling were suppressed by incubation with the HIF1A antibody (Figure 3A and 3B). On the other hand, the effects of TGF-ß were suppressed by LY364947 (Figure 3A and 3B). These results suggest that EGLN2 or EGLN3 directly regulate HIF1A and TGF-ß1 signaling; however, angiogenic factors are independently regulated.
Figure 3.
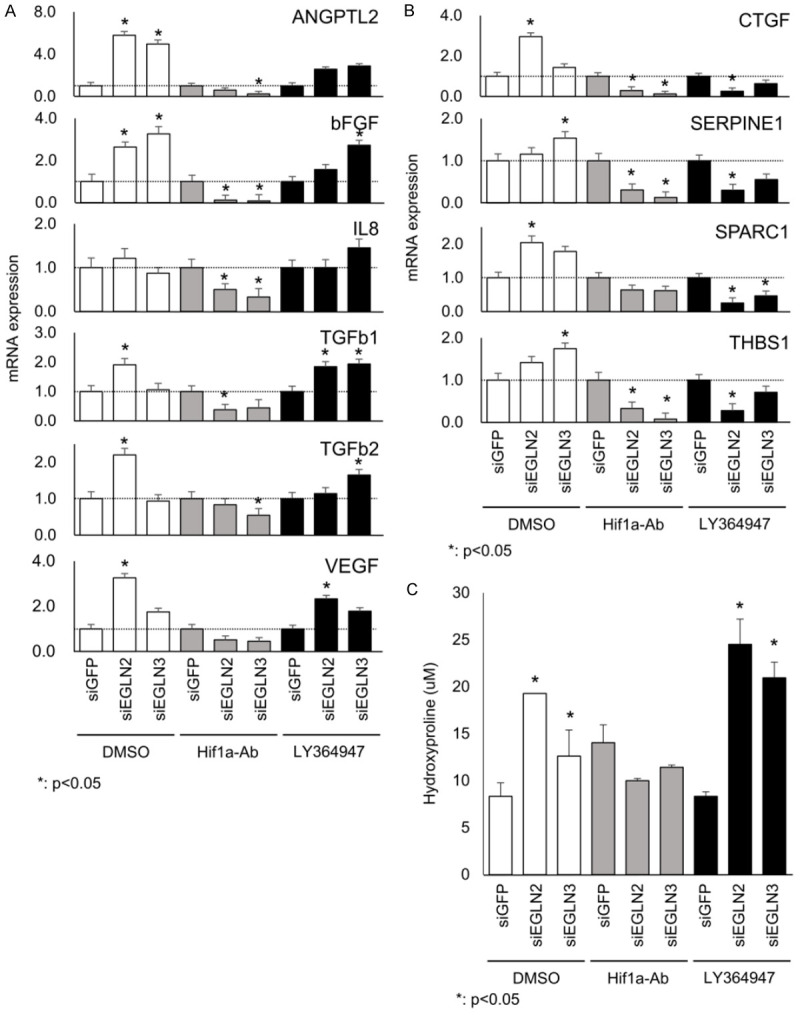
TGF-ß1 inhibition or HIF1A antibody treatment induces the expression of angiogenic factors and hydroxyproline. Several angiogenic factors amplified by EGLN2/3 and TGF-ß signaling were suppressed by incubation with the HIF1A antibody (A and B). On the other hand, the effects of TGF-ß were suppressed by LY364947 (A and B). Moreover, hydroxyproline concentrations in the culture supernatants were not suppressed by LY364947; however, they were suppressed by the HIF1A antibody (C).
To confirm HIF1A protein level by siEGLN2 or siEGLN3 treated with LY364947 and HIF1A antibody. We found no significant change in HIF1A protein expression, because HIF1A pathway downstream (data not shown). Moreover, hydroxyproline concentrations in the culture supernatants were not suppressed by LY364947; however, they were suppressed by the HIF1A antibody (Figure 3C). These results suggest that hydroxyproline is a downstream target of the HIF1A pathway.
Significance of EGLN2, EGLN3, and hydroxyproline expression in pancreatic cancer
To investigate EGLN2 and EGLN3 expression in pancreatic cancer, we examined samples from 102 pancreatic cancer patients who received curative resection at our institute. Their clinicopathological characteristics are summarized in Table 2. Low levels of EGLN2 or EGLN3 mRNA expression were found in 49% and 64% of the patients, respectively (Figure 4A). There was a significant positive correlation between EGLN2 mRNA expression and EGLN3 mRNA expression (Figure 4B). Decreased EGLN2 expression was significantly associated with reduced disease-free survival (P=0.001) and overall survival (P=0.004) (Figure 4C). Decreased EGLN3 expression was significantly correlated with decreased disease-free survival (P=0.006) and overall survival (P=0.049) (Figure 4D).
Table 2.
Clinicopathological charcteristics of 102 patients performed curative resection
| N=102 | |
|---|---|
| Age | |
| Median | 70 |
| Range | 27-84 |
| Gender | |
| Male | 55 (54%) |
| Female | 47 (46%) |
| UICC Stage | |
| I | 22 (22%) |
| II | 56 (55%) |
| III | 24 (23%) |
| EGLN2 | Median; 1.77 |
| High EGLN2 | 52 (51%) |
| Low EGLN2 | 50 (49%) |
| EGLN3 | Median; 0.10 |
| High EGLN3 | 37 (36%) |
| Low EGLN3 | 65 (64%) |
Values in parentheses are percentages unless indicated otherwise; values are median.
Figure 4.
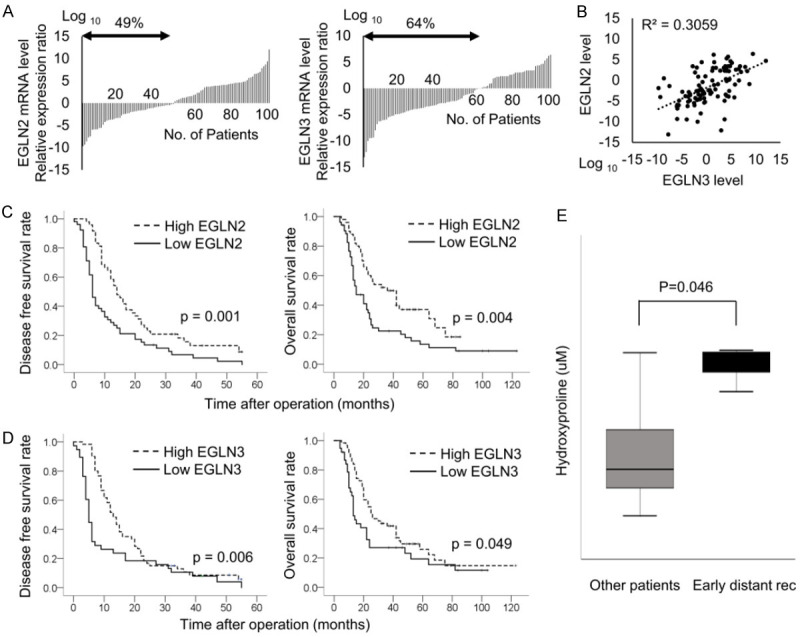
Significance of EGLN2, EGLN3, and hydroxyproline expression in pancreatic cancer. Low levels of EGLN2 or EGLN3 mRNA expression were found in 49% and 64% of the patients, respectively (A). There was a significant positive correlation between EGLN2 mRNA expression and EGLN3 mRNA expression (B). Decreased EGLN2 expression was significantly associated with reduced disease-free survival and overall survival in (C). Decreased EGLN3 expression was significantly correlated with decreased disease-free survival and overall survival in (D). High expression of hydroxyproline was associated with early emergence of early distant recurrence (E).
To clarify the clinical significance of hydroxyproline expression, we conducted a prospective study in pancreatic cancer patients before curative resection at our institute between 2016 and 2018 (n=25), and compared its expression among patients with recurrence within 6 months of surgery and patients without recurrence within 6 months of surgery. The demographic and clinical data showed no significant differences with regard to age, sex, UICC stage or other values between the two groups without hydroxyproline concentration (Table 3). High concentration of hydroxyproline was associated with early emergence of distant metastasis (P=0.046) (Figure 4E).
Table 3.
Clinicopathological characteristics of 25 patients performed analysis of hydroxyproline concentration
| Early distant rec (n=6) | Other patients (n=19) | P value | |
|---|---|---|---|
| Age | |||
| Median | 76 | 69 | 0.086 |
| Range | 65-84 | 48-81 | |
| Gender | |||
| Male | 3 (50%) | 11 (58%) | 0.735 |
| Female | 3 (50%) | 8 (42%) | |
| UICC Stage | |||
| I | 0 | 5 (26%) | 0.100 |
| II | 4 (67%) | 12 (63%) | |
| III | 2 (33%) | 2 (11%) | |
| Neoadjuvant chemotherapy | 1 (17%) | 3 (16%) | 0.959 |
| Surgical procedure | |||
| PPPD or SSPPD | 4 (67%) | 14 (74%) | 0.985 |
| DP | 2 (33%) | 5 (26%) | |
| Vascular reconstruction | 1 (17%) | 6 (32%) | 0.235 |
| Blood loss (g) | |||
| Median | 280 | 390 | 0.725 |
| Range | 155-685 | 20-960 | |
| Operative time (min) | |||
| Median | 310 | 372 | 0.242 |
| Range | 104-416 | 277-574 | |
| Hydroxyproline (uM) | |||
| Median | 21.28 | 9.24 | 0.046 |
| Range | 17.24-25.35 | 4.47-21.24 |
Values in parentheses are percentages unless indicated otherwise; values are median.
Discussion
In our study, we identified EGLN2 and EGLN3 as predictive markers for disease-free survival and overall survival of patients with pancreatic cancer, and hydroxyproline as a predictive marker for distant metastasis. Moreover, we identified mechanisms for their metabolism effects, indicating a potential role for hydroxyproline in aerobic glycolysis regulation by targeting the EGLN2 and EGLN3/HIF1A axis.
EGLN is an oxygen-sensing enzyme, which can hydroxylate proteins to modulate diverse physiopathological signals. Aberrant regulation of EGLN results in many human diseases, including cancer. It is well known that EGLN function largely depends on the role of HIF1A in tumors, but the detailed regulatory mechanisms of EGLN, especially in pancreatic cancer settings, remain unclear [10]. In lung carcinoma cells, overexpression of EGLN2 induces cell cycle arrest and suppresses proliferation by inhibiting nuclear factor-кB (NF-кB) activity [11]. In human colon and colorectal cancer, overexpression of EGLN2 inhibits tumor growth [12]. In pancreatic cancer, TCF7L2 and EGLN2 were first reported as novel prognostic markers and provided a possible regulatory mechanism [13], and EGLN3 was reported to be associated with tumor growth, apoptosis, and angiogenesis, through HIF1A-dependent pathways [14]. However, the role of EGLN and the correlation of EGLN and hydroxyproline in pancreatic cancer has seldom been discussed. Our observations suggest that loss of EGLN2 and EGLN3 upregulates HIF1A pathway signaling, and these genes are prognostic factors for pancreatic cancers. Moreover, we found that the HIF1A pathway induced by EGLN independently upregulated the TGF-ß pathway and angiogenesis.
Overexpression of HIF1A is a characteristic of several human cancers and their metastases [15]. HIF1A protein is the major regulator of oxygen homoeostasis, and tumor hypoxia activates HIF1A to induce the transcription of vascular endothelial growth factor (VEGF) and neovascularization to support cell survival and invasion [16,17]. Three known EGLN proteins have been identified as cellular oxygen sensors in the mediation of HIF1A degradation. As mentioned above, EGLN has been reported in various tumors; however, its role is carcinogenesis varies by cancer type. We found that EGLN2 was more strongly associated with angiogenesis and the TGF-ß pathway compared to EGLN3, although EGLN2 and EGLN3 expression were positively correlated in clinical specimens. In this study, the effects of siEGLN and TGF-ß signatures on angiogenic factors tended to be stronger in EGLN2 than in EGLN3. In pancreatic cancer, EGLN, which is involved in invasion and metastasis via the HIF1A pathway, may be modulated primarily through EGLN2, but the specific mechanism has not been characterized.
Hydroxyproline, an abundant amino acid in collagen, is a characteristic biochemical marker that can simplify the chemical analysis of connective tissues [18]. Prior reports have demonstrated the relationship between hydroxyproline concentration in liver biopsy tissue and fibrosis [19] and the invasion and metastasis of cancer cells [20]. And hypoxia activates glutamine, proline, and hydroxyproline metabolism in HCC. Aberrant accumulation of hydroxyproline by attenuated PRODH2 activity promotes HCC pathogenesis through stabilizing HIF1a [21]. However, no report has characterized a detailed mechanism for these activities in pancreatic cancer. One of the limitations of this study is that we did not evaluate the mechanism whereby HIF1A signaling affects hydroxyproline expression.
In conclusions, we found that hydroxyproline levels are regulated via the HIF1A pathway by EGLN2 and EGLN3, and that increased concentrations of hydroxyproline promote the invasion and metastasis of pancreatic cancer cells. Based on these data, we propose that hydroxyproline concentration may be predictive of distant metastasis.
Acknowledgements
This research was funded by the Medical Research Encouragement Prize of the Japan Medical Association, the Tokyo Medical University Cancer Research Foundation, and the Tokyo Medical University School of Medicine Alumni Association.
The study was approved by the ethics committee of Tokyo Medical University. All study participants provided informed consent.
Disclosure of conflict of interest
None.
Abbreviations
- EGLN
Egl nine homolog
- HIF1A
hypoxia-inducible factor 1-alpha
- TGF-ß
transforming growth factor beta
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Postlewait LM, Ethun CG, Kooby DA, Sarmiento JM, Chen Z, Staley CA 3rd, Brutcher E, Adsay V, El-Rayes B, Maithel SK. Combination gemcitabine/cisplatin therapy and ERCC1 expression for resected pancreatic adenocarcinoma: results of a Phase II prospective trial. J Surg Oncol. 2016;114:336–341. doi: 10.1002/jso.24317. [DOI] [PubMed] [Google Scholar]
- 4.Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, Staugaard C, Indukala D, Boustani AM, Patel V, Cha CH, Salem RR, Chang B, Hochster HS, Lacy J. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737–743. doi: 10.1038/bjc.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravikumar R, Sabin C, Abu Hilal M, Bramhall S, White S, Wigmore S, Imber CJ, Fusai G UK Vascular Resection in Pancreatic Cancer Study Group. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg. 2014;218:401–411. doi: 10.1016/j.jamcollsurg.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Kulemann B, Hoeppner J, Wittel U, Glatz T, Keck T, Wellner UF, Bronsert P, Sick O, Hopt UT, Makowiec F, Riediger H. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg. 2015;19:438–444. doi: 10.1007/s11605-014-2725-8. [DOI] [PubMed] [Google Scholar]
- 7.Itoi T, Sugimoto M, Umeda J, Sofuni A, Tsuchiya T, Tsuji S, Tanaka R, Tonozuka R, Honjo M, Moriyasu F, Kasuya K, Nagakawa Y, Abe Y, Takano K, Kawachi S, Shimazu M, Soga T, Tomita M, Sunamura M. Serum metabolomic profiles for human pancreatic cancer discrimination. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashida T, Takahashi F, Chiba N, Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco Md, Wijendran V, Shioda T, Sgroi D, Donahoe PK, Maheswaran S. HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci U S A. 2010;107:1100–5. doi: 10.1073/pnas.0912710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba N, Comaills V, Shiotani B, Takahashi F, Shimada T, Tajima K, Winokur D, Hayashida T, Willers H, Brachtel E, Vivanco MD, Haber DA, Zou L, Maheswaran S. Homeobox B9 induces epithelial-to-mesenchymal transition-associated radioresistance by accelerating DNA damage responses. Proc Natl Acad Sci U S A. 2012;109:2760–5. doi: 10.1073/pnas.1018867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Xie X, Xiao H, Ding F, Zhong H, Zhu J, Ma N, Mei J. Over-expression of prolyl hydroxylase-1 blocks NF-kappaB-mediated cyclin D1 expression and proliferation in lung carcinoma cells. Cancer Genet. 2014;207:188–194. doi: 10.1016/j.cancergen.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Erez N, Milyavsky M, Eilam R, Shats I, Goldfinger N, Rotter V. Expression of prolyl-hydroxylase-1 (PHD1/EGLN2) suppresses hypoxia inducible factor-1alpha activation and inhibits tumor growth. Cancer Res. 2003;63:8777–8783. [PubMed] [Google Scholar]
- 13.Xiang J, Hu Q, Qin Y, Ji S, Xu W, Liu W, Shi S, Liang C, Liu J, Meng Q, Liang D, Ni Q, Xu J, Zhang B, Yu X. TCF7L2 positively regulates aerobic glycolysis via the EGLN2/HIF-1α axis and indicates prognosis in pancreatic cancer. Cell Death Dis. 2018;9:321. doi: 10.1038/s41419-018-0367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su Y, Loos M, Giese N, Hines OJ, Diebold I, Görlach A, Metzen E, Pastorekova S, Friess H, Büchler P. PHD3 regulates differentiation, tumour growth and angiogenesis in pancreatic cancer. Br J Cancer. 2010;103:1571–1579. doi: 10.1038/sj.bjc.6605936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh MY, Lemos R Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1a- to HIF-2a-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–27. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams E, Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–61. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, Shun CT, Chiou LL, Chen CH, Huang GT, Sheu JC. Hydroxyproline content of needle biopsies as an objective measure of liver fibrosis: emphasis on sampling variability. J Gastroenterol Hepatol. 2005;20:1109–14. doi: 10.1111/j.1440-1746.2005.03901.x. [DOI] [PubMed] [Google Scholar]
- 20.Saito J, Imamura Y, Itoh J, Matsuyama S, Maruta A, Hayashi T, Sato A, Wada N, Kashiwazaki K, Inagaki Y, Watanabe T, Kitagawa Y, Okazaki I. ELISA measurement for urinary 3-hydroxyproline-containing peptides and its preliminary application to healthy persons and cancer patients. Anticancer Res. 2010;30:1007–14. [PubMed] [Google Scholar]
- 21.Tang L, Zeng J, Geng P, Fang C, Wang Y, Sun M, Wang C, Wang J, Yin P, Hu C, Guo L, Yu J, Gao P, Li E, Zhuang Z, Xu G, Liu Y. Global metabolic profiling identifies a pivotal role of proline and hydroxyproline metabolism in supporting hypoxic response in hepatocellular carcinoma. Clin Cancer Res. 2018;24:474–485. doi: 10.1158/1078-0432.CCR-17-1707. [DOI] [PubMed] [Google Scholar]


