Abstract
Interleukin (IL)-6 has been detected in serum and ascites from patients affected by epithelial ovarian cancers, and also in some human ovarian cancer cell lines. To investigate the role of IL-6 in ovarian lesions, we first measured its levels in serum samples of 24 healthy donors and in 17 and 9 patients affected by ovarian carcinomas and ovarian benign cysts respectively. IL-6 levels were significantly higher than healthy donors in serum samples from ovarian cancer patients, but not in benign ovarian cysts. We then investigated the mechanism of IL-6 production in two cell lines obtained from the same patient with high grade serous ovarian carcinoma before (PEA1) and after (PEA2) development of cisplatinum resistance. The levels of intracellular IL-6, analysed by western blotting, did not relevantly differ in the two cell lines, and they did not change after the cell treatment with an AKT inhibitor. Although the interleukin was present in supernatants from both cell lines, its concentration in the supernatant of chemoresistant cells was significantly higher than chemosensitive cells. Interestingly, exposure to the AKT inhibitor resulted in a reduced IL-6 release in PEA1, but not in PEA2 cells. These results let infer different mechanisms of IL-6 release in chemoresistant and chemosensitive cell lines, and contribute new insights in ovarian cancer biology that suggest more in depth studies about the role of AKT in IL-6 release and in development of chemoresistance.
Keywords: Interleukin-6, ovarian cancer, ovarian benign cysts, chemoresistance, AKT inhibitor
Introduction
Interleukin (IL)-6 is a 26 kDa single chain phosphorylated glycoprotein involved in several physiological and pathological settings, where it regulates cell survival, proliferation, release of specific factors and other functional aspects [1]. IL-6 transmits its signals through a cell-surface receptor complex, and that consists of a ligand-binding glycoprotein termed as IL-6 receptor (IL-6R) and a signal-transducing component, gp130. The IL-6/IL-6R complex activates Janus kinase (JAK), which in turn, can trigger three possible signalling routes: dimerization of signal transducer and transcription-3 (STAT3), activation of Ras/Raf pathway, and activation of phosphoinositol-3 kinase (PI3K) pathway [2]. The altered production of IL-6 by in different cell types has implications in several diseases, from rheumatic to oncologic and psychiatric pathologies, and IL-6-inhibition constitutes a therapeutic strategy [3,4]. There is thus a great deal of interest in understanding mechanisms of production and release of the cytokine in different cells and contexts.
Several pieces of evidence indicate that IL-6 plays a critical role in the biology of epithelial ovarian cancer. Indeed, the cytokine has been detected in serum and ascites from patients, and its levels correlate with prognosis [5,6]. IL-6, produced by cells of the ovarian tumour microenvironment, sustains cancer cell survival and growth through the activation of JAK3/STAT3 signalling pathway. The cytokine can also be involved in auto- and paracrine circuits originated from tumour cells: indeed, some ovarian cancer cell lines have been found to secrete IL-6 [7-9].
Our aim was to confirm IL-6 presence in serum samples from patients affected by ovarian cancer and not by benign cysts, and to analyse the mechanism of IL-6 release in chemosensitive and chemoresistant ovarian cancer cells.
Material and methods
Serum samples
Aliquots of serum samples were purchased at BIOIVT (West Sussex, United Kingdom) and stored at -80°C.
In vitro experiments
The human ovarian adenocarcinoma cell lines PEA1 and PEA2 were obtained from European Collection of Authenticated Cell Cultures (ECACC). PEA1 and PEA2 are two adherent cell lines derived from a malignant effusion from the peritoneal ascites of the same patient with a poorly differentiated adenocarcinoma. PEA1 was collected prior to treatment with cisplatin and prednimustine; PEA2 was collected on relapse after the treatment. Both cell lines were cultured in RPMI 1640 (Lonza) medium supplemented with 10% FBS/1% Pen-Strep and grown at 37°C in a 5% CO2 atmosphere. Both cell lines were incubated in 96 well-plates (1×105/mL) in RPMI 1640 medium, with or without an AKT inhibitor (AKT Inhibitor IV and AKT Inhibitor X, Calbiochem) or a JNK inhibitor (SP600125, Sigma-Aldrich), or the sole solvent (0.02% DMSO), at 37°C in 5% CO2 atmosphere for 6 hrs. Supernatants were then collected and IL-6 content was quantified by ELISA (IL-6 Human Uncoated ELISA Kit, Invitrogen) following the manifacturer’s instructions. Cell extracts for western blotting analyses were prepared by harvesting and resuspending the cells in Laemmli buffer 1× (125 mM Tris-HCl at pH 6.8, 2.3% Sodium Dodecyl Sulfate, 10% Glycerol and 2% Bromophenol blue, 5% β-mercaptoethanol). The extracts were boiled and clarified by centrifugation before electroforetic separation and Western blot analysis.
siRNAs and transfections
Specific akt siRNAs (akt1 siRNA: 5’-CAACAUUCAACUUUAGUAUUUUUAC-3’; akt2 siRNA: 5’-AGGUACUUCGAUGAUGAAUUUACCG-3’; akt3 siRNA: 5’-UCCAACUUCACAAAUUGAUAAUATA-3’) and a non-targeted (NT) siRNA (5’-CAGUCGCGUUUGCGACUGG-3’) were purchased from Integrated DNA Technologies, Inc. (Leuven, Belgium). PEA1 and PEA2 cells were transfected with siRNAs at a final concentration of 50 nmol/L using TransIT-X2® (Mirus Bio, Medison, USA), for 48 hours.
Western blot
Total proteins were separated on 10% SDS polyacrilammide gels and electrophoretically transferred to nitrocellulose membrane. Nitrocellulose blots were blocked with 10% bovine serum albumin (BSA) in TBS-T buffer (20 mM Tris-HCl at pH 7.4, 500 mM NaCl and 0.01% Tween), and incubated with primary antibodies: anti-IL-6 antibody (ab6672, Abcam); anti-pAKT (Ser473) antibody (#9271, Cell signaling); anti-AKT antibody (#9272, Cell signaling); anti-GAPDH antibody (sc-32233, Santa Cruz Biotechnology). All antibodies were used at a 1:1000 dilution in TBST containing 5% BSA overnight at 4°C. Immunoreactivity was detected by sequential incubation with horseradish peroxidase-conjugated secondary antibodies (dilution 1:5000) and ECL detection reagents (Pierce™ ECL Western Blotting Substrate, Thermo Scientific). Autoradiographic images were obtained by scanning the autoradiographic film (Santa Cruz Biotechnology). Densitometric analyses were performed with ImageJ software (NHI, USA). The values corresponding to the band intensities were obtained by integration of peak areas of the optical density plot after subtraction of the background signal.
Statistical analysis
Results are expressed as means ± s.d. Data were analysed by Student’s t-test using MedCalc statistical software version 13.3.3 (Ostend, Belgium). p-values from 0.01 to 0.05, from 0.001 to 0.01, or < 0.001 were respectively considered significant (*), very significant (**) or highly significant (***).
Results
17 serum samples of epithelial ovarian cancer (EOC) patients were analyzed for their IL-6 contents. 8 out 17 samples showed high levels of the cytokine if compared to the corresponding levels of 24 healthy donors used as a control (P=0.003). On the other hand, among the 9 serum samples from by benign ovarian cysts no samples with high levels of IL-6 (P=0.04 vs EOC) was detected (Figure 1). These results were in good agreement with the data present in the literature reporting high levels of the cytokine in sera of ovarian cancer patients [5,11,12]. It is worth noting that IL-6 presence in serum was selectively associated with malignant, and not with benign, ovarian lesions, and any correlation with other available patient metadata could be found (Table 1). This evidence prompted us to further investigate the mechanisms of IL-6 production and release in ovarian cancer cells.
Figure 1.
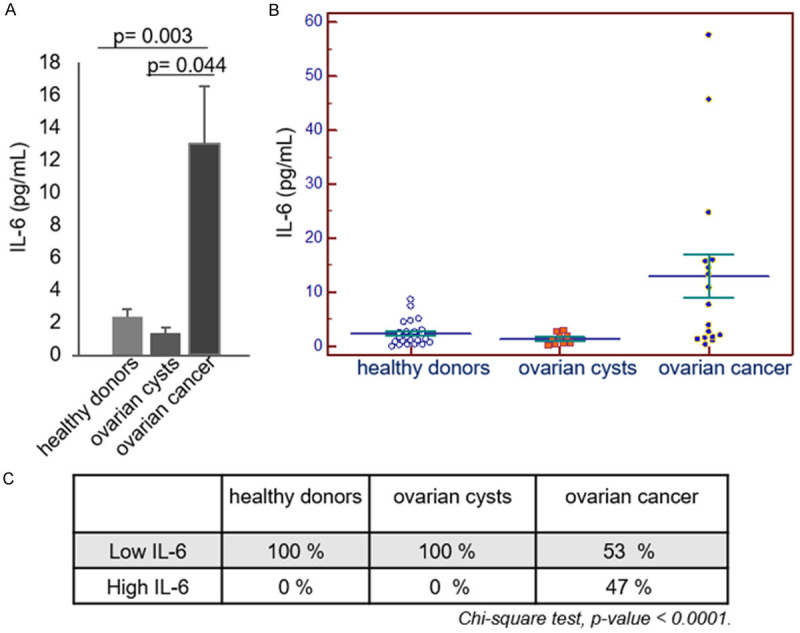
IL-6 content in serum samples from patients affected by ovarian cancers or benign cysts. A, B. Serum samples of 24 healthy donors, and 17 and 9 patients respectively affected by ovarian cancers and ovarian benign cysts were analysed by IL-6 ELISA. C. Contingency table of percentages of samples with low or high IL-6 levels in the three different groups. (p values are calculated by Chi-square test).
Table 1.
EOC patients metadata
| IL-6 | Number of cases | Age (mean ± st. dev.) | BMI (mean ± st. dev.) | Menopausal status | Smoking Status | Alcohol Status | FIGO Staging System |
|---|---|---|---|---|---|---|---|
| Low levels | 9 | 54.4 ± 13.1 | 22.52 ± 2.73 | pre-menopausal n=2 | no n=6 | Never Used n=6 | I n=2 |
| post-menopausal n=5 | yes n=1 | Occasional Use n=1 | II n=0 | ||||
| III n=4 | |||||||
| IV n=2 | |||||||
| High levels | 8 | 55.7 ± 16.7 | 26.73 ± 7.38 | pre-menopausal n=1 | no n=6 | Never Used n=5 | I n=2 |
| post-menopausal n=6 | yes n=0 | Occasional Use n=1 | II n=0 | ||||
| III n=2 | |||||||
| IV n=1 | |||||||
| p value | 0.8592 | 0.1955 | 0.5302 | 0.3545 | 0.9093 | 0.8500 |
Two matched cell lines, PEA1 and PEA2, obtained from the same patient with high grade serous ovarian carcinoma before (PEA1) and after (PEA2) development of clinical platinum resistance [13-15] were utilized to study production and release of IL6. The cytokine was present in both cell lines’ supernatants, but its concentration was significantly higher in the supernatant of chemoresistant compared to chemosensitive cells (Figure 2A). Since IL-6 production can be regulated in some contexts by AKT kinase [16,17], that also plays an important role in ovarian cancer biology [18-20], we verified the effect of an AKT inhibitor on IL-6 production and release in PEA1 and PEA2 cells. The results showed a significant (P=0.02) decrease of IL6 release (> 40%) in cultures of PEA1 cells treated with the AKT inhibitor IV. The inhibition specificity was verified by comparison with cell treated with a JNK inhibitor, used as a control. Interestingly, the AKT inhibitor IV did not significantly affect the amount of IL-6 released by PEA2 cells (Figure 2C). On the other hand, the inhibition of AKT did not affect the intracellular levels of IL-6 in both cell lines (Figure 2B). The specific involvement of AKT in determining the IL-6 levels in PEA1 cells was further confirmed in experiments carried out with a different inhibitor (AKT Inhibitor X), and the results obtained with both inhibitors were also in good agreement with the results obtained down-modulating AKT expression with akt specific siRNA (Figure 3).
Figure 2.
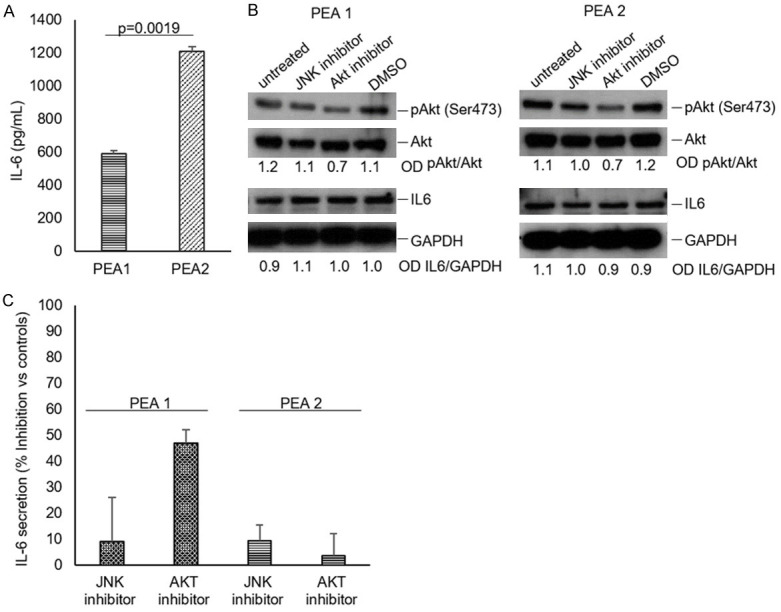
IL-6 intracellular levels in PEA1 and PEA2 cells. Cells (1×105/mL) were incubated in RPMI 1640 medium at 37°C in a 5% CO2 atmosphere for 6 hrs. (A) Supernatants were collected and analysed for IL-6 content by ELISA. (B) Effect of AKT or JNK inhibitors on IL-6 release by PEA1 and PEA2 cells. Cells (1×105/mL) were incubated in RPMI 1640 medium, in the presence or absence of 4 μM AKT inhibitor (AKT Inhibitor IV, Calbiochem) or 10 μM JNK inhibitor (SP600125, Sigma-Aldrich) at 37°C in a 5% CO2 atmosphere for 6 hrs. Cell lysates were analysed by western blot with an anti-IL-6 (ab6672, Abcam), an anti-pAKT (Ser473) (#9271, Cell signaling), an anti-AKT (#9272, Cell signaling) or an anti-GAPDH (sc-32233, Santa Cruz Biotechnology) antibody. (C) Supernatants from (B) were collected and IL-6 content was analysed by ELISA. All data were obtained from triplicate samples and confirmed in three separate experiments.
Figure 3.
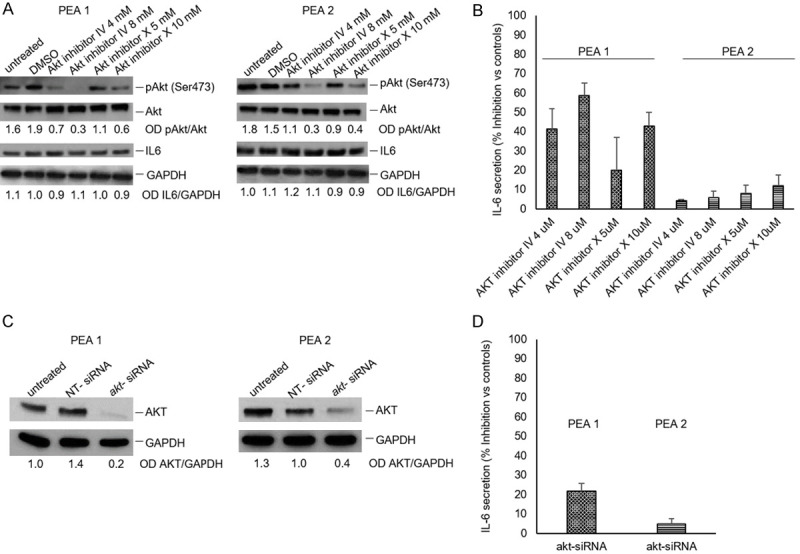
AKT affect IL-6 secretion in PEA1 cells. Cells (1×105/mL) were incubated in RPMI 1640 medium, in the presence or absence of AKT inhibitors at different concentrations for 6 hrs. (A) Cell lysates were analysed by western blot as described in Figure 2. (B) Supernatants were collected and IL-6 content was analysed by ELISA. Data are from triplicate samples and confirmed in two separate experiments. (C) PEA1 and PEA2 cells were transfected with akt siRNAs or a non-targeted (NT) siRNA at a final concentration of 50 nmol/L for 48 hours. Next, the cells were harvested, lysed and analysed by Western blotting with the indicated antibodies. (D) Supernatants from (C) were collected and IL-6 content was analysed by ELISA. Data were obtained from triplicate samples and confirmed in two separate experiments.
Discussion
The poor prognosis for ovarian cancer, fifth in the rankings of cancer deaths among women, is closely related to resistance to conventional platinum-based chemotherapy. It has been shown that IL-6 plays a role in chemoresistance: indeed, production of IL-6 by ovarian cancer cells can induce resistance to cisplatin and paclitaxel [21,22]. Mechanisms of IL-6 production and release in ovarian cancer cells are therefore relevant topics to better understand ovarian cancer cell biology and the onset of cancer chemoresistance.
IL-6 production can be regulated by at transcriptional and posttranscriptional levels with complex mechanisms. In fact, functional cis-regulatory elements in the human IL-6 gene include binding sites for nuclear factor kappa B (NF-κB), nuclear factor IL6 (NF-IL6), specificity protein 1 (SP1), cyclic AMP response element binding protein (CREB), interferon regulatory factor 1 (IRF-1) and activator protein 1 (AP-1) [23]. An RNA-binding protein, the AT-rich interactive domain-containing protein 5a (Arid5a), exports IL-6 mRNA to the cytoplasm bound to the up-frameshift protein 1 (UPF1) through the chromosomal region maintenance 1 (CRM1) pathway [24]. IL-6 mRNA is regulated by adenylate-uridylate-rich elements (AREs) located in the 3’UTR region of IL-6 mRNA; a regulatory RNase-1 (Regnase-1) degrades transcriptionally active IL-6 mRNA by binding to IL-6 3’UTR in the cytoplasm, endoplasmic reticulum and ribosomes [25]. Cells can also increase or decrease the rate of post-Golgi trafficking to modulate IL-6 release [26]. Dysregulation of any of these pathways may cause an abnormal increase of secreted IL-6.
It has been shown that AKT play an important role in ovarian cancer biology, and that can be hyper-activated in a majority of ovarian cancers and stimulates the proliferation and invasion of ovarian cancer cells [18,19]. The results obtained with chemosensitive and chemoresistant cell lines show that the release of IL-6 is AKT-dependent only in chemosensitive cells PEA1 and, on the other hand, it seems that a modification in the secretory pathway occurred in the cells during the acquisition of chemoresistance. Indeed, PEA2 cells, derived from the same patient after the development of chemoresistance, produce the same amount of IL-6 protein but release higher amounts of cytokine although the secretion pathway does not depend on AKT activity. Further studies are necessary to better characterise this AKT-independent pathway of IL-6 secretion associated with chemoresistance, and the possible correlation with the expression of ATP-binding cassette (ABC) transporters. In fact, these transporters lower the intracellular concentration of chemotherapeutic drugs [27,28] and have also been involved, in other contexts, in the efflux of IL-6 and other cytokines from the intracellular compartment to the extracellular space [29,30].
Standard of care therapy in ovarian cancer did not change through the last three decades [31]. In-depth study of pathways involving molecules, such as IL-6 and AKT, with a recognized role in the biology of this tumour [8,19,32,33] can contribute to a critical review of the failures in therapy innovation attempts and to the improvement of clinical experimentation.
Our findings indicate that a different mechanism of secretion can account for higher IL-6 release from chemoresistant compared to chemosensitive ovarian cancer cells. Such mechanism operating in chemoresistant cells does not appear to involve the activity of AKT kinase, which instead can regulate IL-6 release from chemosensitive cells. We believe that this AKT-independent pathway of IL-6 release deserves deeper investigation, in order to verify its role in ovarian cancer progression and in the development of chemoresistance.
Acknowledgements
This work was supported by University of Salerno (FARB 2018) grants to LM and Progetto (ex) PON03PE_00060_4 “Nuove strategie per la diagnostica medica e molecolare e per la tracciabilità ed il monitoraggio dei prodotti alimentari” to AR, RM and LM.
Disclosure of conflict of interest
None.
References
- 1.Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol. 2018;10:a028415. doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Sanguinete MMM, Oliveira PH, Martins-Filho A, Micheli DC, Tavares-Murta BM, Murta EFC, Nomelini RS. Serum IL-6 and IL-8 correlate with prognostic factors in ovarian cancer. Immunol Invest. 2017;46:677–688. doi: 10.1080/08820139.2017.1360342. [DOI] [PubMed] [Google Scholar]
- 6.Dalal V, Kumar R, Kumar S, Sharma A, Kumar L, Sharma JB, Roy KK, Singh N, Vanamail P. Biomarker potential of IL-6 and VEGF-A in ascetic fluid of epithelial ovarian cancer patients. Clin Chim Acta. 2018;482:27–32. doi: 10.1016/j.cca.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Jiang B, Zhu SJ, Xiao SS, Xue M. MiR-217 inhibits M2-like macrophage polarization by suppressing secretion of interleukin-6 in ovarian cancer. Inflammation. 2019;42:1517–1529. doi: 10.1007/s10753-019-01004-2. [DOI] [PubMed] [Google Scholar]
- 8.McLean K, Tan L, Bolland DE, Coffman LG, Peterson LF, Talpaz M, Neamati N, Buckanovich RJ. Leukemia inhibitory factor functions in parallel with interleukin-6 to promote ovarian cancer growth. Oncogene. 2019;38:1576–1584. doi: 10.1038/s41388-018-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh YA, Jo SY, Lee HY, Lee C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int J Oncol. 2015;46:1405–1411. doi: 10.3892/ijo.2014.2808. [DOI] [PubMed] [Google Scholar]
- 10.Rosati A, Basile A, D’Auria R, d’Avenia M, De Marco M, Falco A, Festa M, Guerriero L, Iorio V, Parente R, Pascale M, Marzullo L, Franco R, Arra C, Barbieri A, Rea D, Menichini G, Hahne M, Bijlsma M, Barcaroli D, Sala G, di Mola FF, di Sebastiano P, Todoric J, Antonucci L, Corvest V, Jawhari A, Firpo MA, Tuveson DA, Capunzo M, Karin M, De Laurenzi V, Turco MC. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat Commun. 2015;6:8695. doi: 10.1038/ncomms9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Yi Y, Zhao M. Effect of dexmedetomidine anesthesia on perioperative levels of TNF-α and IL-6 in patients with ovarian cancer. Oncol Lett. 2019;17:5517–5522. doi: 10.3892/ol.2019.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM, Kulesza-Bronczyk B, Kinalski M, Terlikowski SJ. Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. Eur Cytokine Netw. 2013;24:106–113. doi: 10.1684/ecn.2013.0340. [DOI] [PubMed] [Google Scholar]
- 13.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, Schol DJ, Hilgers J, Leonard RC, Smyth JF. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–6172. [PubMed] [Google Scholar]
- 14.Stronach EA, Alfraidi A, Rama N, Datler C, Studd JB, Agarwal R, Guney TG, Gourley C, Hennessy BT, Mills GB, Mai A, Brown R, Dina R, Gabra H. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 2011;71:4412–4422. doi: 10.1158/0008-5472.CAN-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matassa DS, Amoroso MR, Lu H, Avolio R, Arzeni D, Procaccini C, Faicchia D, Maddalena F, Simeon V, Agliarulo I, Zanini E, Mazzoccoli C, Recchi C, Stronach E, Marone G, Gabra H, Matarese G, Landriscina M, Esposito F. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016;23:1542–1554. doi: 10.1038/cdd.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan TK, Lay FT, Hulett MD. Importance of phosphoinositide binding by human beta-defensin 3 for Akt-dependent cytokine induction. Immunol Cell Biol. 2018;96:54–67. doi: 10.1111/imcb.1017. [DOI] [PubMed] [Google Scholar]
- 17.Hamel-Côté G, Lapointe F, Véronneau S, Mayhue M, Rola-Pleszczynski M, Stankova J. Regulation of platelet-activating factor-mediated interleukin-6 promoter activation by the 48 kDa but not the 45 kDa isoform of protein tyrosine phosphatase non-receptor type 2. Cell Biosci. 2019;9:51. doi: 10.1186/s13578-019-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoneum A, Said N. PI3K-AKT-mTOR and NF-B pathways in ovarian cancer: implications for targeted therapeutics. Cancers (Basel) 2019;11:949. doi: 10.3390/cancers11070949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–160. doi: 10.1016/j.semcancer.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Savant SS, Sriramkumar S, O’Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancer (Basel) 2018;10:251. doi: 10.3390/cancers10080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, Li LZ. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110–123. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Meng J, Liu K, Shao Y, Feng X, Ji Z, Chang B, Wang Y, Xu L, Yang G. ID1 confers cancer cell chemoresistance through STAT3/ATF6-mediated induction of autophagy. Cell Death Dis. 2020;11:137. doi: 10.1038/s41419-020-2327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, Zheng SG. Hall of fame among pro-inflammatory cytokines: interleukin-6 gene and its transcriptional regulation mechanisms. Front Immunol. 2016;7:604. doi: 10.3389/fimmu.2016.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda K, Ripley B, Nishimura R, Mino T, Takeuchi O, Shioi G, Kiyonari H, Kishimoto T. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci U S A. 2013;110:9409–9414. doi: 10.1073/pnas.1307419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao R, Yang R, Chen X, Harhaj EW, Wang X, Fan Y. Regnase-1, a rapid response ribonuclease regulating inflammation and stress responses. Cell Mol Immunol. 2017;14:412–422. doi: 10.1038/cmi.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revelo NH, Ter Beest M, van den Bogaart G. Membrane trafficking as an active regulator of constitutively secreted cytokines. J Cell Sci. 2020;133:jcs234781. doi: 10.1242/jcs.234781. [DOI] [PubMed] [Google Scholar]
- 27.Briz O, Perez-Silva L, Al-Abdulla R, Abete L, Reviejo M, Romero MR, Marin JJG. What “The Cancer Genome Atlas” database tells us about the role of ATP-binding cassette (ABC) proteins in chemoresistance to anticancer drugs. Expert Opin Drug Metab Toxicol. 2019;15:577–593. doi: 10.1080/17425255.2019.1631285. [DOI] [PubMed] [Google Scholar]
- 28.Domenichini A, Adamska A, Falasca M. ABC transporters as cancer drivers: potential functions in cancer development. Biochim Biophys Acta Gen Subj. 2019;1863:52–60. doi: 10.1016/j.bbagen.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118:9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 30.Bleier BS, Nocera AL, Iqbal H, Hoang JD, Alvarez U, Feldman RE, Han X. P-glycoprotein promotes epithelial T helper 2-associated cytokine secretion in chronic sinusitis with nasal polyps. Int Forum Allergy Rhinol. 2014;4:488–494. doi: 10.1002/alr.21316. [DOI] [PubMed] [Google Scholar]
- 31.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 32.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 33.Maniati E, Berlato C, Gopinathan G, Heath O, Kotantaki P, Lakhani A, McDermott J, Pegrum C, Delaine-Smith RM, Pearce OMT, Hirani P, Joy JD, Szabova L, Perets R, Sansom OJ, Drapkin R, Bailey P, Balkwill FR. Mouse ovarian cancer models recapitulate the human tumor microenvironment and patient response to treatment. Cell Rep. 2020;30:525–540. e7. doi: 10.1016/j.celrep.2019.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


