ABSTRACT
Objective:
To clarify the effect of early dysphagia rehabilitation, early rehabilitation was started within 2 days of admission by speech-language-hearing therapists in patients with severe aspiration pneumonia.
Methods:
The subjects were inpatients with severe aspiration pneumonia (A-DROP≥3) admitted to our hospital between April 2014 and March 2019. We retrospectively investigated patient age, sex, A-DROP score, community-acquired or nursing- and healthcare-associated pneumonia, invasive and noninvasive ventilation, comorbidities, nutritional risk, admission from nursing home, discharge to nursing home, walking ability before admission and at discharge, Food Intake LEVEL Scale (FILS) score at the start of rehabilitation and at discharge, the achievement of oral intake, alternative nutrition in use at discharge, number of days from admission to the start of rehabilitation, and number of days from admission to oral intake. We compared the patient characteristics and rehabilitation outcomes between 159 patients who underwent early dysphagia rehabilitation and 67 patients who underwent later dysphagia rehabilitation. To assess the association between alternative nutrition at discharge and early dysphagia rehabilitation, binominal logistic regression analysis was performed.
Results:
Early dysphagia rehabilitation was significantly associated with shorter hospital stays, fewer discharges to nursing homes, higher likelihood of oral intake, the removal of alternative nutrition at discharge, fewer days from admission to oral intake, and higher FILS scores at discharge. Early dysphagia rehabilitation was significantly associated with no alternative nutrition at discharge in binominal logistic regression analysis (odds ratio 3.26; P <0.01).
Conclusions:
This study suggested that early dysphagia rehabilitation was effective in improving outcomes of severe aspiration pneumonia including the removal of alternative nutrition at discharge.
Keywords: Dysphagia rehabilitation, Pneumonia, Speech-language-hearing therapist
INTRODUCTION
In hospitalized patients, the incidence of aspiration pneumonia is high in cases of community-acquired pneumonia (CAP), nursing- and healthcare-associated pneumonia (NHCAP), and hospital-acquired pneumonia (HAP).1) Dysphagia rehabilitation is provided to patients with aspiration pneumonia. However, such rehabilitation may carry the risk of re-exacerbation of pneumonia due to aspiration. Consequently, dysphagia rehabilitation requires an interdisciplinary approach by experts, including speech-language-hearing therapists (STs). The calculation of the respiratory rehabilitation fee by STs was accepted in Japan from April 2020. STs are expected to play an important role in dysphagia rehabilitation for aspiration pneumonia.
The Japanese Society of Intensive Care Medicine defined early rehabilitation as a set of measures that assists in the maintenance, improvement, and restoration of function started within 48 h from the onset of disease or the time of surgery or acute exacerbation.2) The functions for restoration include swallowing as well as motor and respiratory functions. Early rehabilitation administered by physical therapists potentially prevents hospitalization-associated disabilities in elderly inpatients3) and reduces the 30-day in-hospital mortality rates in elderly patients with aspiration pneumonia.4) However, there is no accepted standard for such rehabilitation and there is insufficient evidence for the early initiation of dysphagia rehabilitation. It is notable that severe pneumonia carries a high risk of re-exacerbation, and the indications for early dysphagia rehabilitation should be carefully considered. Moreover, the presence of several comorbidities that accompany aspiration pneumonia influences the progress of treatment of pneumonia and dysphagia.
The Japanese Respiratory Society proposes the A-DROP scoring system for assessing pneumonia severity in patients with CAP. This system is a modified version of the British Thoracic Society’s CURB-65 criteria and consists of the following factors: age (men, ≥70 years; women ≥75 years), dehydration (blood urea nitrogen ≥21 mg/ml), respiratory failure (pulse oximetry saturation, SpO2 ≤90%); consciousness disturbance, and low blood pressure (systolic blood pressure ≤90 mmHg).5) Patients with A-DROP scores of 3–5 were categorized as having severe pneumonia and those with A-DROP scores of 4 or 5 were categorized as having very severe pneumonia. The Japanese Respiratory Society also proposes use of the I-ROAD scoring system for assessing pneumonia severity in patients with HAP.6) This system includes the presence of a malignant tumor or immunodeficiency as prognostic factors for pneumonia. The Infectious Disease Society of America proposes the Pneumonia Severity Index (PSI).7) This system includes nursing home residency and comorbidities such as neoplasia, liver disease, congestive heart failure, cerebrovascular disease, and renal disease. Most cases of NHCAP have aspiration pneumonia. A-DROP, I-ROAD, and PSI scores help in evaluating the severity of NHCAP. 8,9) In a systematic literature review of the risks of aspiration pneumonia in frail older people, 13 significant risk factors were identified: age, male sex, lung diseases, dysphagia, diabetes mellitus, severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, bad oral health, malnutrition, Parkinson’s disease, the use of antipsychotic drugs, the use of proton pump inhibitors, and the use of angiotensin-converting enzyme inhibitors.10)
The purpose of this study was to retrospectively investigate the comorbidities and training progress of inpatients with severe aspiration pneumonia and to clarify the effect of early dysphagia rehabilitation carried out by STs.
MATERIALS AND METHODS
Subjects
We retrospectively reviewed the medical records of inpatients with severe pneumonia (A-DROP ≥3) and dysphagia as evaluated by STs. The patients were admitted to our hospital between April 2014 and March 2019. The attending physicians diagnosed pneumonia according to the presence of two or more of four findings (white blood cell count >9000/dL, body temperature >37.5°C, purulent sputum, and high levels of plasma C-reactive protein) along with a new abnormal shadow on chest X-ray or computed tomography.11) We excluded the following: (i) patients who were on alternative nutrition before admission; (ii) patients who were evaluated with no dysphagia by ST; (iii) patients who were moved from other hospitals because of HAP; (iv) patients who had acute cerebrovascular disease or other acute brain disease; (v) patients who had mild and moderate pneumonia (A-DROP ≤2); (vi) patients who died in the hospital; and (vii) patients who started rehabilitation after 8 or more days from admission because tube feeding within 7 days of admission was recommended.12)
This study was approved by the Ethics Committee of our institute (approval number. 2020–11) and was carried out by the opt-out method of our hospital website. This study was conducted in accordance with the Declaration of Helsinki.
Patient Characteristics and Condition Before Undergoing Rehabilitation
We investigated the age, sex, A-DROP score, CAP or NHCAP, invasive and noninvasive ventilation, comorbidities, nutritional risk, admission from nursing home, walking ability before admission, and the severity of dysphagia at start of rehabilitation as the characteristics of patients and their condition before undergoing rehabilitation. The comorbidities included lung diseases other than pneumonia, malignant tumor, immunodeficiency, liver disease, congestive heart failure, chronic cerebrovascular disease, chronic brain diseases other than cerebrovascular disease, renal disease, diabetes mellitus, and dementia. Dementia was defined by past diagnoses or a Hasegawa dementia rating scale-revised score of ≤20. The nutritional risk was evaluated by the Controlling Nutritional Status (CONUT) score on admission. The CONUT score is generated from the levels of serum albumin and serum total cholesterol and the total lymphocyte count. CONUT scores divide a patient’s nutritional status into four phases; moderate and severe nutritional risk was defined by CONUT scores of 5 or more.13) The severity of dysphagia was evaluated by the Food Intake LEVEL Scale (FILS). FILS is a 10-point observer-rating scale, and no alternative nutrition is defined as FILS level 7 or more.14)
Dysphagia Rehabilitation
The attending physicians consulted with rehabilitation doctors and STs to evaluate dysphagia at admission and when patients had recovered from pneumonia. The ST evaluated the severity of dysphagia and started training based on the training method summary of the Japanese Society of Dysphagia Rehabilitation15) the next day after the consultation or after removal of invasive or noninvasive ventilation. Videofluoroscopy16) and endoscopic evaluation17) were performed by rehabilitation doctors or dentists if further evaluation was necessary.
Patients with a consciousness disturbance worse than Japan Coma Scale (JCS) II-10 underwent indirect swallowing exercises such as respiratory exercises, range-of-motion exercises for organs involved with swallowing, and muscle-strengthening exercises for the muscles involved in swallowing and the facilitation of dry swallowing. Patients with improved consciousness (better than JCS I-3) underwent direct swallowing exercises with a small quantity of easy-to-swallow food after a modified water swallowing test, a food test, video fluoroscopy, or endoscopic evaluation. After patients could eat a large quantity of food as a result of direct swallowing exercises, meals were provided using safe positioning for feeding and dietary modification. Patients with poor dental prostheses or poor oral hygiene were treated by dentists and dental hygienists. Patients with voice disorders, dysarthria, or cognitive impairments underwent voice training, dysarthria training, or cognitive training in addition to dysphagia rehabilitation by STs. Patients at nutritional risk underwent nutrition care provided by the nutrition support team. All patients underwent nursing care and physical therapy.
Early dysphagia rehabilitation was defined as dysphagia rehabilitation started within 2 days of admission. The subjects were divided into two groups: the early group, which underwent early dysphagia rehabilitation, and the control group, for which dysphagia rehabilitation started between 3 and 7 days after admission. In the majority of patients in the early group, the attending physician consulted the ST at admission. Additionally, most patients in the control group consulted with STs after recovery from pneumonia.
Outcomes
We investigated the dysphagia training time per day, the length of hospital stay, discharge to a nursing home, walking ability at discharge, FILS score at discharge, the achievement of oral intake, alternative nutrition at discharge, number of days from admission to start of rehabilitation, and number of days from admission to oral intake as the outcomes of rehabilitation.
Statistical Analysis
We compared the patient characteristics and the outcomes of rehabilitation between the early and control groups. In univariate comparisons, the age, training duration per day, length of hospital stay, the number of days from admission to the start of rehabilitation, and the number of days from admission to oral intake were compared using unpaired Student’s t-tests. The sex, A-DROP score, CAP or NHCAP, invasive and noninvasive ventilation, comorbidities, admission from a nursing home, discharge to a nursing home, walking ability before admission and at discharge, nutritional risk, achievement of oral intake, and alternative nutrition at discharge were compared using Fisher’s exact test. The FILS scores at the start of rehabilitation and discharge were compared using the Mann-Whitney U test.
To assess the association between alternative nutrition at discharge and early dysphagia rehabilitation, binominal logistic regression analysis was performed. Alternative nutrition at discharge was included in the binominal logistic regression as the objective variable, and the FILS score before undergoing rehabilitation and early dysphagia rehabilitation (the only parameters with a P-value less than 0.05 in the univariate analyses) were included as explanatory variables. Subsequently, we performed subanalysis for very severe pneumonia (A-DROP ≥4). As with the analysis for severe pneumonia, we compared each group and performed binominal logistic regression.
The threshold for significance was a value of P<0.05. All statistical analyses were performed using IBM SPSS version 23.0 (IBM SPSS, Armonk, NY, US).
RESULTS
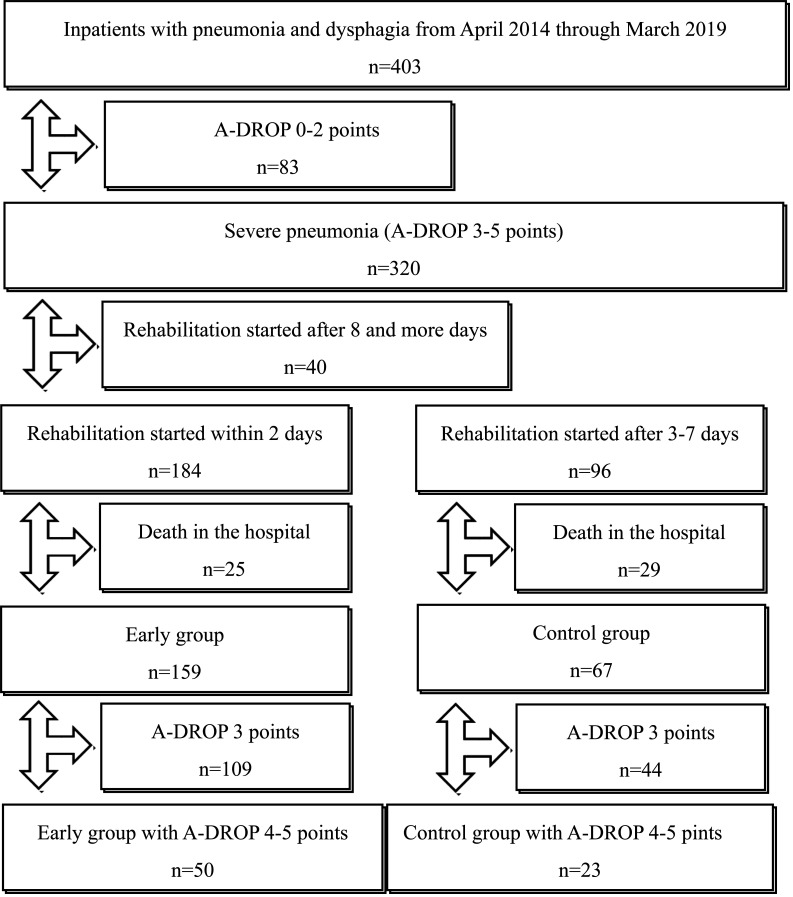
Figure 1 shows the selection of subjects for this study. A total of 403 inpatients with pneumonia and dysphagia were admitted to our hospital between April 2014 and March 2019. Of these, 83 patients with mild or moderate pneumonia (A-DROP≤2) and 40 patients who started rehabilitation after 8 or more days from admission were excluded. A total of 184 patients started rehabilitation within 2 days, and 25 (13.6%) of these died in the hospital. As a result, 159 patients were included in the early group and 50 of these experienced very severe pneumonia (A-DROP 4 or 5 points). A total of 96 patients started rehabilitation between 3 and 7 days after admission, and 29 (30.2%) of these died in the hospital. Consequently, 67 patients were included in the control group, and 23 of these had very severe pneumonia.
Figure 1.
Flowchart showing the selection process of subjects in this study. We excluded patients with mild or moderate pneumonia (A-DROP ≤2), patients who started rehabilitation 8 or more days after admission, and patients who died in the hospital. The remaining patients were divided into the early dysphagia rehabilitation group and the control group (i.e., dysphagia rehabilitation started between 3 and 7 days after admission).
Table 1 shows the patient characteristics and condition for the early and control groups before undergoing rehabilitation. There were no significant differences between the groups for any characteristic or condition before rehabilitation was started. In both groups, the average age was more than 80 years. More than 90% of the patients had comorbidities. Notably, approximately 80% of the patients had respiratory failure, consciousness disturbance, and NHCAP; more than 60% of patients had dementia and nutritional risk; and more than 50% of the patients were not ambulatory before admission. A few patients were treated by invasive or noninvasive ventilation. The two groups had almost the same FILS scores at the start of the rehabilitation.
Table 1. Characteristics and condition of patients with severe pneumonia before undergoing general rehabilitation and dysphagia rehabilitation.
| Characteristic | Early groupa
(n=159) |
Control groupb
(n=67) |
Pc |
| Age (years) | 84.6 ± 7.2 | 83.3 ± 7.7 | 0.25 |
| Sex: female | 63 (39.6) | 25 (37.3) | 0.77 |
| A-DROP score | |||
| 3 points | 109 (68.6) | 44 (65.7) | |
| 4 points | 43 (27.0) | 22 (32.8) | |
| 5 points | 7 (4.4) | 1 (1.5) | 0.47 |
| Age (years): men ≥70, women ≥75 | 154 (96.9) | 62 (92.5) | 0.17 |
| BUN ≥21 mg/dl | 102 (64.2) | 50 (74.6) | 0.16 |
| SpO2 ≤90% | 136 (85.5) | 56 (83.6) | 0.84 |
| Consciousness disturbance | 124 (78.0) | 52 (77.6) | 1.00 |
| Systolic BP ≤90 mmHg | 17 (10.7) | 5 (7.5) | 0.48 |
| NHCAP | 131 (82.4) | 55 (82.1) | 1.00 |
| Invasive and noninvasive ventilation | 2 (1.3) | 4 (6.0) | 0.07 |
| Comorbidities | 149 (93.7) | 63 (94.0) | 1.00 |
| Lung disease | 36 (22.6) | 19 (28.4) | 0.40 |
| Malignant tumor | 25 (15.7) | 13 (19.4) | 0.56 |
| Immunodeficiency | 12 (7.5) | 6 (9.0) | 0.79 |
| Liver disease | 2 (1.3) | 2 (3.0) | 0.58 |
| Congestive heart failure | 22 (13.8) | 10 (14.9) | 0.84 |
| Cerebrovascular disease | 45 (28.3) | 21 (31.3) | 0.75 |
| Brain disease | 22 (16.1) | 10 (14.9) | 0.84 |
| Renal disease | 16 (10.1) | 8 (11.9) | 0.81 |
| Diabetes mellitus | 26 (16.4) | 14 (20.9) | 0.45 |
| Dementia | 108 (67.9) | 48 (71.6) | 0.64 |
| Moderate or severe nutritional risk | 105 (66.1) | 49 (73.1) | 0.35 |
| Admission from nursing home | 51 (32.1) | 24 (35.8) | 0.64 |
| Ambulatory before admission | 66 (41.5) | 34 (50.7) | 0.24 |
| FILS score at the start of the rehabilitation | 5 (4–7) | 5 (3–7) | 0.34 |
aDysphagia rehabilitation was started within 2 days after admission.
bDysphagia rehabilitation was started between 3 and 7 days after admission.
cUnpaired Student's t-test, Fisher's exact test, or the Mann-Whitney U test was used.
Data are shown as mean±SD, n (%), or median (first quartile–third quartile).
BUN, blood urea nitrogen; BP, blood pressure.
Table 2 shows the outcomes of dysphagia rehabilitation for patients with severe pneumonia in the early and control groups. Patients in the early group experienced significantly shorter lengths of hospital stay and significantly fewer were discharged to nursing homes than patients in the control group. In the early group, it was observed that a significantly larger proportion of patients achieved oral intake and had no need of alternative nutrition at discharge. The early group had significantly fewer days between admission and oral intake and a significantly higher FILS score at discharge than the control group.
Table 2. Outcomes of dysphagia rehabilitation for patients with severe pneumonia .
| Outcomes | Early groupa (n=159) | Control groupb (n=67) | Pc |
| Training time per day (min) | 9.6 ± 5.4 | 10.8 ± 6.4 | 0.12 |
| Length of hospital stay (days) | 31.1 ± 23.3 | 43.0 ± 29.0 | <0.01 |
| Discharge to nursing home | 82 (51.6) | 45 (67.2) | 0.04 |
| Ambulatory at discharge | 51 (32.1) | 17 (25.4) | 0.34 |
| FILS score at discharge | 7 (7–8) | 7 (4–8) | 0.02 |
| Achievement of oral intake | 150 (94.3) | 56 (83.6) | 0.01 |
| No alternative nutrition at discharge | 132 (83.0) | 41 (61.2) | <0.001 |
| Days from admission to start of rehabilitation | 1.2 ± 0.6 | 4.4 ±1.3 | <0.001 |
| Days from admission to start of oral intake | 3.7 ± 3.9 | 7.7 ± 7.0 | <0.001 |
aDysphagia rehabilitation was started within 2 days after admission.
bDysphagia rehabilitation was started between 3 and 7 days after admission.
cUnpaired Student's t-test, Fisher's exact test, or the Mann-Whitney U test was used.
Data are shown as mean±SD, n (%), or median (first quartile–third quartile).
Table 3 shows the patient characteristics and condition before rehabilitation for severe pneumonia for those who did and did not need alternative nutrition at discharge. Univariate analyses revealed that a significantly higher proportion of patients who needed alternative nutrition at discharge experienced consciousness disturbance and malnutrition than patients who did not need alternative nutrition. Notably, fewer patients needing alternative nutrition were ambulatory before admission and fewer underwent early rehabilitation. Patients needing alternative nutrition at discharge had significantly lower FILS scores at the start of the rehabilitation.
Table 3. Patient characteristics and condition before undergoing rehabilitation for severe pneumonia with respect to the need for alternative nutrition at discharge.
| Characteristics | No alternative nutrition (n=173) | Alternative nutrition (n=53) | Pa |
| Age (years) | 84.3 ± 7.2 | 83.8 ± 7.9 | 0.66 |
| Sex: female | 68 (39.3) | 20 (37.7) | 0.87 |
| A-DROP score | |||
| 3 points | 121 (69.9) | 32 (60.4) | |
| 4 points | 45 (26.0) | 20 (37.7) | |
| 5 points | 7 (4.1) | 1 (1.9) | 0.25 |
| Age (years): men ≥70, women ≥75 | 167 (96.5) | 49 (92.5) | 0.25 |
| BUN ≥21 mg/dl | 119 (68.8) | 33 (62.3) | 0.41 |
| SpO2 ≤90% | 146 (84.4) | 46 (86.8) | 0.83 |
| Consciousness disturbance | 129 (74.6) | 47 (88.7) | 0.04 |
| Systolic BP ≤90 mmHg | 16 (9.2) | 6 (11.3) | 0.61 |
| NHCAP | 138 (79.8) | 48 (90.6) | 0.10 |
| Invasive or noninvasive ventilation | 4 (2.3) | 2 (3.8) | 0.63 |
| Comorbidities | 160 (92.5) | 52 (98.1) | 0.20 |
| Lung disease | 41 (23.7) | 14 (26.4) | 0.72 |
| Malignant tumor | 30 (17.3) | 8 (15.1) | 0.83 |
| Immunodeficiency | 17 (9.8) | 1 (1.9) | 0.08 |
| Liver disease | 4 (2.3) | 0 (0) | 0.58 |
| Congestive heart failure | 25 (14.5) | 7 (13.2) | 1.00 |
| Cerebrovascular disease | 51 (29.5) | 15 (28.3) | 1.00 |
| Brain disease | 24 (13.9) | 8 (15.1) | 0.66 |
| Renal disease | 21 (12.1) | 3 (5.7) | 0.21 |
| Diabetes mellitus | 33 (19.1) | 7 (13.2) | 0.41 |
| Dementia | 114 (65.9) | 42 (79.2) | 0.09 |
| Moderate or severe nutritional risk | 110 (63.6) | 44 (83.0) | <0.01 |
| Admission from nursing home | 53 (30.6) | 22 (41.5) | 0.18 |
| Ambulatory before admission | 85 (49.1) | 15 (28.3) | 0.01 |
| FILS score at the start of the rehabilitation | 5 (5–7) | 3 (2–5) | <0.001 |
| Early rehabilitation | 132 (76.3) | 27 (50.9) | <0.001 |
aUnpaired Student's t-test, Fisher's exact test, or the Mann-Whitney U test was used.
Data are shown as mean±SD, n (%), or median (first quartile–third quartile).
Table 4 shows the results of binominal logistic regression analysis for the prediction of no alternative nutrition needed at discharge in patients with severe pneumonia. Consciousness disturbance, nutritional risk, ambulatory before admission, FILS score at the start of rehabilitation, and early rehabilitation were included as explanatory variables. Early rehabilitation and the FILS score at the start of rehabilitation were significant independent factors for the prediction of no alternative nutrition needed on discharge. The odds ratio of patients who underwent early rehabilitation not needing alternative nutrition at discharge was 3.26 (95% confidence interval 1.52–6.98; P<0.01) compared with patients who did not undergo early rehabilitation, after adjustment for all covariates.
Table 4. Binominal logistic regression analysis for predicting no alternative nutrition at discharge in patients with severe pneumonia.
| Variable | Odds ratio | 95% CI | P |
| Consciousness disturbance | 1.95 | 0.69–5.56 | 0.21 |
| Ambulatory before admission | 1.98 | 0.90–4.35 | 0.09 |
| Moderate or severe nutritional risk | 1.45 | 0.92–2.29 | 0.11 |
| FILS score at the start of the rehabilitation | 0.57 | 0.46–0.72 | <0.001 |
| Early rehabilitation | 3.26 | 1.52–6.98 | <0.01 |
CI, confidence interval.
Tables 5–8 show the results of subanalyses for patients with very severe pneumonia. Table 5 shows the characteristics and condition of patients in the early and control groups before undergoing rehabilitation for extremely severe pneumonia. As with severe pneumonia, there were no significance differences between the two groups before rehabilitation. In both groups, the average age was more than 80 years. It was observed that more than 80% of the patients had respiratory failure, consciousness disturbance, and NHCAP. Dementia and nutritional risk were observed in more than 70% of the patients. The FILS score at the start of rehabilitation in the control group was lower than that in the early group, but the difference was not significant (P=0.07)
Table 5. Characteristics and condition of patients with very severe pneumonia before undergoing general rehabilitation and dysphagia rehabilitation.
| Characteristics | Early groupa (n=50) | Control groupb (n=23) | Pc |
| Age (years) | 85.1±6.0 | 85.3±7.5 | 0.88 |
| Sex: female | 18 (36.0) | 10 (43.5) | 0.61 |
| A-DROP score | |||
| 4 points | 43 (86.0) | 22 (95.7) | |
| 5 points | 7 (14.0) | 1 (4.3) | 0.27 |
| Age (years): men ≥70, women ≥75 | 50 (100.0) | 22 (95.7) | 0.32 |
| BUN ≥21 mg/dl | 48 (96.0) | 22 (95.7) | 1.00 |
| SpO2 ≤90% | 48 (96.0) | 23 (100.0) | 0.56 |
| Consciousness disturbance | 48 (96.0) | 22 (95.7) | 1.00 |
| Systolic BP ≤90 mmHg | 12 (24.0) | 4 (17.4) | 0.56 |
| NHCAP | 43 (86.0) | 19 (82.6) | 0.73 |
| Invasive or noninvasive ventilation | 0 (0.0) | 1 (4.3) | 0.32 |
| Comorbidities | 48 (96.0) | 23 (100.0) | 1.00 |
| Lung disease | 11 (22.0) | 8 (34.8) | 0.26 |
| Malignant tumor | 8 (16.0) | 3 (13.0) | 1.00 |
| Immunodeficiency | 6 (12.0) | 1 (4.3) | 0.42 |
| Liver disease | 1 (2.0) | 1 (4.3) | 1.00 |
| Congestive heart failure | 11 (22.0) | 1 (4.3) | 0.09 |
| Cerebrovascular disease | 13 (26.0) | 10 (43.5) | 0.18 |
| Brain disease | 6 (12.0) | 1 (4.3) | 0.42 |
| Renal disease | 10 (20.0) | 3 (13.0) | 0.53 |
| Diabetes mellitus | 7 (14.0) | 3 (13.0) | 1.00 |
| Dementia | 35 (70.0) | 19 (82.6) | 0.64 |
| Admission from nursing home | 15 (30.0) | 11 (47.8) | 0.19 |
| Ambulatory before admission | 20 (40.0) | 7 (30.4) | 0.45 |
| Moderate or severe nutritional risk | 36 (72.0) | 18 (78.3) | 0.77 |
| FILS score at the start of the rehabilitation | 5 (4–7) | 4 (2.5–5) | 0.07 |
aDysphagia rehabilitation was started within 2 days after admission.
bDysphagia rehabilitation was started between 3 and 7 days after admission.
cUnpaired Student's t-test, Fisher's exact test, or the Mann-Whitney U test was used.
Data are shown as mean±SD, n (%), or median (first quartile–third quartile).
Table 8. Binominal logistic regression analysis for predicting no alternative nutrition at discharge in patients with very severe pneumonia.
| Variable | Odds ratio | 95% CI | P |
| FILS score at the start of the rehabilitation | 1.53 | 1.09–2.15 | 0.01 |
| Early rehabilitation | 0.35 | 0.11–1.08 | 0.07 |
Table 6 shows the outcomes of dysphagia rehabilitation for patients with very severe pneumonia in the early and control groups. Patients in the early group had a significantly shorter length of hospital stay than patients in the control group, and significantly fewer were discharged to a nursing home. At discharge in the early group, significantly more patients were ambulatory and significantly more needed no alternative nutrition. Also, the period between admission and oral intake was significantly shorter in the early group and the FILS score at discharge was higher.
Table 6. Outcomes of dysphagia rehabilitation for patients with very severe pneumonia.
| Outcomes | Early groupa (n=50) | Control groupb (n=23) | Pc |
| Training time per day (min) | 10.0 ± 5.4 | 10.8 ± 8.6 | 0.18 |
| Length of hospital stay (days) | 32.4 ± 22.3 | 51.2 ± 37.5 | <0.01 |
| Discharge to nursing home | 25 (50.0) | 18 (78.3) | 0.04 |
| Ambulatory at discharge | 18 (36.0) | 2 (8.7) | 0.02 |
| FILS score at discharge | 7 (7–8) | 7 (4–8) | 0.04 |
| Achievement of oral intake | 48 (96.0) | 20 (87.0) | 0.32 |
| No alternative nutrition at discharge | 40 (80.0) | 12 (52.2) | 0.02 |
| Days from admission to start of rehabilitation | 1.3 ± 0.6 | 4.5 ± 1.5 | <0.001 |
| Days from admission to start of oral intake | 4.5 ± 4.7 | 8.4 ± 5.6 | <0.01 |
Table 7 shows the characteristics and condition of patients before undergoing rehabilitation for very severe pneumonia for those who did and did not need alternative nutrition at discharge. In the univariate analyses, significantly fewer patients needing alternative nutrition underwent early rehabilitation. Patients who needed alternative nutrition at discharge had a significantly lower FILS score at the start of rehabilitation.
Table 7. Patient characteristics and condition before undergoing rehabilitation for very severe pneumonia analyzed with respect to the need for alternative nutrition at discharge .
| Characteristics | No alternative nutrition (n=52) | Alternative nutrition (n=21) | Pa |
| Age (years) | 85.6 ± 6.2 | 84.2 ± 7.1 | 0.43 |
| Sex: female | 22 (42.3) | 6 (28.6) | 0.30 |
| A-DROP score | |||
| 4 points | 45 (86.5) | 20 (95.2) | |
| 5 points | 7 (13.5) | 1 (4.8) | 0.28 |
| Age (years): men ≥70, women ≥75 | 52 (100.0) | 20 (95.2) | 0.29 |
| BUN ≥21 mg/dl | 50 (96.2) | 20 (95.2) | 1.00 |
| SpO2 ≤90% | 50 (96.2) | 21 (100.0) | 1.00 |
| Consciousness disturbance | 49 (94.2) | 21 (100.0) | 0.55 |
| Systolic BP ≤90 mmHg | 13 (25.0) | 3 (14.3) | 0.37 |
| NHCAP | 43 (82.7) | 19 (90.5) | 0.49 |
| Invasive or noninvasive ventilation | 1 (1.9) | 0 (0.0) | 1.00 |
| Comorbidities | 50 (96.2) | 100 (100.0) | 1.00 |
| Lung disease | 13 (25.0) | 6 (28.6) | 0.77 |
| Malignant tumor | 8 (15.4) | 3 (14.3) | 1.00 |
| Immunodeficiency | 7 (13.5) | 0 (0.0) | 0.18 |
| Liver disease | 7 (13.5) | 0 (0.0) | 0.18 |
| Congestive heart failure | 10 (19.2) | 2 (9.5) | 0.49 |
| Cerebrovascular disease | 15 (28.8) | 8 (38.1) | 0.58 |
| Brain disease | 4 (7.7) | 3 (14.3) | 0.40 |
| Renal disease | 11 (21.2) | 2 (9.5) | 0.32 |
| Diabetes mellitus | 8 (15.4) | 2 (9.5) | 0.71 |
| Dementia | 36 (69.2) | 18 (85.7) | 0.24 |
| Moderate or severe nutritional risk | 38 (73.1) | 16 (76.2) | 1.00 |
| Admission from nursing home | 15 (28.8) | 11 (52.4) | 0.07 |
| Ambulatory before admission | 21 (40.4) | 6 (28.6) | 0.43 |
| FILS score at the start of the rehabilitation | 5 (4–7) | 4 (2–5) | <0.01 |
| Early rehabilitation | 40 (76.9) | 10 (47.6) | 0.03 |
aUnpaired Student's t-test, Fisher's exact test, or the Mann-Whitney U test was used.
Data are shown as mean±SD, n (%), or median (first quartile–third quartile).
Table 8 shows the results of binominal logistic regression analysis for the prediction of no alternative nutrition on discharge in patients with very severe pneumonia. The FILS score at the start of rehabilitation and early rehabilitation were included as explanatory variables. The FILS score at the start of rehabilitation was a significant independent factor for the prediction of no alternative nutrition at discharge; however, the contribution of early rehabilitation was not significant.
DISCUSSION
This study suggested that early dysphagia rehabilitation was effective in the removal of alternative nutrition by the time of discharge. Momosaki et al. noted that dysphagia rehabilitation showed a positive effect on the total oral intake in elderly patients with aspiration pneumonia.18) The current study showed similar results. However, Momosaki et al. defined early dysphagia rehabilitation as rehabilitation started earlier than 5 days from admission; nonetheless, they found that patients undergoing early rehabilitation were more likely to achieve total oral intake at discharge than those undergoing late rehabilitation. The current study suggested that early dysphagia rehabilitation started within 2 days of admission was more effective. Furthermore, Momosaki et al. established that patients with mild pneumonia had a higher odds ratio for total oral intake associated with dysphagia rehabilitation than patients with moderate or severe pneumonia. The current study suggested that earlier dysphagia rehabilitation performed by STs could improve the outcomes of severe aspiration pneumonia.
Koyama et al. noted that the early commencement of both oral intake and physical function was associated with early hospital discharge with oral intake in hospitalized elderly adults with pneumonia.19) In the current study, it was observed that patients undergoing early dysphagia rehabilitation experienced a significantly shorter duration between admission and the first oral intake. Early oral intake may significantly contribute to a shorter length of hospital stay, a lower proportion of discharges to nursing homes, a higher likelihood of removal of alternative nutrition at discharge, and higher FILS scores at discharge. Maeda et al. suggested that nil per os (NPO) with aspiration pneumonia during hospital admission resulted in a prolonged treatment duration and a decline in swallowing ability of patients after exclusions of respiratory insufficiency.20) In the current study, approximately 80% of patients had respiratory failure and consciousness disturbance. Our findings suggested that early dysphagia rehabilitation may prevent declines in swallowing ability, aid in the avoidance of unnecessary NPO after recovery from respiratory failure and consciousness disturbance, and contribute to significantly shorter lengths of hospital stay, improved swallowing ability, and the removal of alternative nutrition at discharge for severe pneumonia.
Stegemann et al. found that patients in long-term care facilities experienced serious difficulties with swallowing solid oral dosage forms.21) Dysphagia has an influence on not only ingestion but also oral drug administration. In the current study, more than 90% of patients had comorbidities, and the majority of patients had to be treated by oral administrations. Early dysphagia rehabilitation might facilitate continuation of the treatment of comorbidities by safe and certain oral administration and thereby lead to better outcomes.
Fujishima et al. noted that aging and secondary sarcopenia after inactivity, malnutrition, and disease are associated with sarcopenic dysphagia.22) In the current study, the average age of patients was more than 80 years; dementia and nutritional risk were observed in more than 60% of patients, and more than 50% of patients were not ambulatory before admission. Consequently, the patients in this study had multiple risk factors of sarcopenia and sarcopenic dysphagia. Yoshimura et al. found that sarcopenia was associated with lower rates of recovery of activities of daily living, dysphagia, and a lower rate of home discharge, and the early detection of sarcopenia and treatment by rehabilitation nutrition was recommended.23) Wakabayashi et al. suggested that a combination of both rehabilitation and nutrition care management may improve the outcomes in disabled elderly patients with malnutrition and sarcopenia.24) The current study suggested that early dysphagia rehabilitation, including speech-language-hearing therapy, physical therapy, and the nutrition support, might prevent, and aid recovery from, sarcopenia and sarcopenic dysphagia.
The results of sub-analyses of patients with very severe pneumonia were mostly similar to the results for severe pneumonia. Our findings suggested that early dysphagia rehabilitation could also improve the outcomes of very severe pneumonia. However, unlike the findings for all severe pneumonia cases, early rehabilitation was not a significant factor for the prediction of no alternative nutrition at discharge in patients with very severe pneumonia, whereas the FILS score at the start of rehabilitation was a significant factor. In very severe pneumonia, it may be difficult to recover impaired swallowing ability, and recovery may be unachievable with or without early dysphagia rehabilitation. For patients with very severe pneumonia, the FILS score at the start of rehabilitation in the control group was lower than that in the early group. A late start of rehabilitation might result in an additional decline in swallowing ability. Consequently, any unnecessary delay of rehabilitation should be avoided.
Our study has some limitations. First, the timing of consultations between rehabilitation doctors and STs depended on the judgment of the attending physician and was not randomized. Second, the program of dysphagia rehabilitation was individualized. For example, all patients underwent dysphagia rehabilitation with the aid of STs, rehabilitation doctors, nurses, and physical therapists. Additionally, patients who did not require attention for dental or nutritional issues did not undergo treatment by dentists, dental hygienists, or the nutrition support team. The effect of early dysphagia rehabilitation should be evaluated by randomized controlled trials following a uniform protocol. However, various comorbidities and social and ethical problems with severe pneumonia make the implementation of such a trial difficult. Third, few patients in the current study were treated with invasive or noninvasive ventilation because this study excluded patients who died in the hospital. Additionally, several patients and their families declined ventilation for ethical reasons. As a result, the influence of ventilation was not evaluated in this study. Other studies should be implemented to evaluate the effect of early dysphagia rehabilitation in patients with ventilation. Fourth, the dysphagia training time per day was short at around 10 min. Consequently, it is unclear whether the training was sufficient to be effective. Whether the effect of early rehabilitation was a result of early evaluation and reduction of the fasting period or whether rehabilitation for functional recovery was effective should be further investigated.
In conclusion, we retrospectively investigated the comorbidities and training progress of inpatients with severe aspiration pneumonia and clarified the effects of early dysphagia rehabilitation by STs. Our results suggested that early dysphagia rehabilitation starting within 2 days of admission was effective with respect to the outcomes of severe aspiration pneumonia including the removal of alternative nutrition at discharge. Dysphagia rehabilitation for severe pneumonia should be started early using an interdisciplinary approach of experts including STs.
ACKNOWLEDGMENTS
We would like to thank the speech-language-hearing therapists, dentists, dental hygienists, and other staff members of the Department of Rehabilitation of our hospital.
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
REFERENCES
- 1.Teramoto S,Fukuchi Y,Sasaki H,Sato K,Sekizawa K,Matsuse T, Japanese Study Group on Aspiration Pulmonary Disease: High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc 2008;56:577–579. 10.1111/j.1532-5415.2008.01597.x [DOI] [PubMed] [Google Scholar]
- 2.Committee for Early Rehabilitation: Evidence-based expert consensus for early rehabilitation in the intensive care unit. Nihon Shuchu Chiryo Igakukai Zasshi 2017;24:255–303. 10.3918/jsicm.24_255 [DOI] [Google Scholar]
- 3.Covinsky KE,Pierluissi E,Johnston CB: Hospitalization-associated disability. JAMA 2011;306:1782–1793. 10.1001/jama.2011.1556 [DOI] [PubMed] [Google Scholar]
- 4.Momosaki R,Yasunaga H,Matsui H,Horiguchi H,Fushimi K,Abo M: Effect of early rehabilitation by physical therapists on in-hospital mortality after aspiration pneumonia in the elderly. Arch Phys Med Rehabil 2015;96:205–209. 10.1016/j.apmr.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 5.Shindo Y,Sato S,Maruyama E,Ohashi T,Ogawa M,Hashimoto N,Imaizumi K,Sato T,Hasegawa Y: Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest 2009;135:633–640. 10.1378/chest.08-1357 [DOI] [PubMed] [Google Scholar]
- 6.Matsunuma R,Asai N,Ohkuni Y,Nakashima K,Iwasaki T,Misawa M,Norihiro K: I-ROAD could be efficient in predicting severity of community-acquired pneumonia or healthcare-associated pneumonia. Singapore Med J 2014;55:318–324. 10.11622/smedj.2014082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine MJ,Hough LJ,Medsger AR,Li YH,Ricci EM,Singer DE,Marrie TJ,Coley CM,Walsh MB,Karpf M,Lahive KC,Kapoor WN: The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med 1997;157:36–44. 10.1001/archinte.1997.00440220040006 [DOI] [PubMed] [Google Scholar]
- 8.Koizumi T,Tsukada H,Ito K,Shibata S,Hokari S,Tetsuka T,Aoki N,Moro H,Tanabe Y,Kikuchi T: A-DROP system for prognostication of NHCAP inpatients. J Infect Chemother 2017;23:523–530. 10.1016/j.jiac.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Ito A,Ishida T,Tokumasu H,Yamazaki A,Washio Y: Evaluation of pneumonia severity scoring systems in nursing and healthcare-associated pneumonia for predicting prognosis: a prospective, cohort study. J Infect Chemother 2020;26:372–378. 10.1016/j.jiac.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 10.van der Maarel-Wierink CD,Vanobbergen JN,Bronkhorst EM,Schols JM,de Baat C: Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc 2011;12:344–354. 10.1016/j.jamda.2010.12.099 [DOI] [PubMed] [Google Scholar]
- 11.Kohno S,Imamura Y,Shindo Y,Seki M,Ishida T,Teramoto S,Kadota J,Tomono K,Watanabe A: Clinical practice guidelines for nursing- and healthcare-associated pneumonia (NHCAP) [complete translation]. Respir Investig 2013;51:103–126. 10.1016/j.resinv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Dennis MS,Lewis SC,Warlow C, FOOD Trial Collaboration: Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 2005;365:764–772. 10.1016/S0140-6736(05)17983-5 [DOI] [PubMed] [Google Scholar]
- 13.Ignacio de Ulíbarri J,González-Madroño A,de Villar NG,González P,González B,Mancha A,Rodríguez F,Fernández G: CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38–45. [PubMed] [Google Scholar]
- 14.Kunieda K,Ohno T,Fujishima I,Hojo K,Morita T: Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J Pain Symptom Manage 2013;46:201–206. 10.1016/j.jpainsymman.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 15.Takehara K,Yamamoto H,Takahashi K,Hironaka S,Katsumata A,Nito T,Koyama T,Fujiwara Y,Fujishima I: The training method summary (Revised edition 2014) [in Japanese]. Jpn J Rehabil Med 2014;18:55–89. [Google Scholar]
- 16.Nito T,Katsumata A,Koyama T,Takahashi K,Takehara K,Hironaka S,Fujiwara Y,Yamamoto H,Fujishima I: The method of videofluoroscopic examination of swallowing (Revised edition 2014) [in Japanese]. Jpn J Rehabil Med 2014;18:166–186. [Google Scholar]
- 17.Takehara K,Ishii M,Katsumata A,Koyama T,Takahashi K,Fujiwara Y,Horiguchi T,Hironaka S,Fujishima I: The method of videoendoscopic examination of swallowing (Revised edition 2012) [in Japanese]. Jpn J Rehabil Med 2013;17:87–99. [Google Scholar]
- 18.Momosaki R,Yasunaga H,Matsui H,Horiguchi H,Fushimi K,Abo M: Effect of dysphagia rehabilitation on oral intake in elderly patients with aspiration pneumonia. Geriatr Gerontol Int 2015;15:694–699. 10.1111/ggi.12333 [DOI] [PubMed] [Google Scholar]
- 19.Koyama T,Maeda K,Anzai H,Koganei Y,Shamoto H,Wakabayashi H: Early commencement of oral intake and physical function are associated with early hospital discharge with oral intake in hospitalized elderly individuals with pneumonia. J Am Geriatr Soc 2015;63:2183–2185. 10.1111/jgs.13679 [DOI] [PubMed] [Google Scholar]
- 20.Maeda K,Koga T,Akagi J: Tentative nil per os leads to poor outcomes in older adults with aspiration pneumonia. Clin Nutr 2016;35:1147–1152. 10.1016/j.clnu.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 21.Stegemann S,Gosch M,Breitkreutz J: Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int J Pharm 2012;430:197–206. 10.1016/j.ijpharm.2012.04.022 [DOI] [PubMed] [Google Scholar]
- 22.Fujishima I,Fujiu-Kurachi M,Arai H,Hyodo M,Kagaya H,Maeda K,Mori T,Nishioka S,Oshima F,Ogawa S,Ueda K,Umezaki T,Wakabayashi H,Yamawaki M,Yoshimura Y: Sarcopenia and dysphagia: position paper by four professional organizations. Geriatr Gerontol Int 2019;19:91–97. 10.1111/ggi.13591 [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura Y,Wakabayashi H,Bise T,Nagano F,Shimazu S,Shiraishi A,Yamaga M,Koga H: Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019;61:111–118. 10.1016/j.nut.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi H,Sakuma K: Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle 2014;5:269–277. 10.1007/s13539-014-0162-x [DOI] [PMC free article] [PubMed] [Google Scholar]