Abstract
Cadmium (Cd) is a nonessential heavy metal and a prevalent environmental toxin that has been shown to induce significant cardiomyocyte apoptosis in neonatal murine engineered cardiac tissues (ECTs). In contrast, zinc (Zn) is a potent metallothionein (MT) inducer, which plays an important role in protection against Cd toxicity. In this study, we investigated the protective effects of Zn against Cd toxicity in ECTs and explore the underlying mechanisms. ECTs were constructed from neonatal ventricular cells of wild-type (WT) mice and mice with global MT gene deletion (MT-KO). In WT-ECTs, Cd (5−20 μM) caused a dose-dependent toxicity that was detected within 8 h evidenced by suppressed beating, apoptosis, and LDH release; Zn (50−200 μM) dose-dependently induced MT expression in ECTs without causing ECT toxicity; co-treatment of ECT with Zn (50 µM) prevented Cd-induced toxicity. In MT-KO ECTs, Cd toxicity was enhanced; but unexpectedly, cotreatment with Zn provided partial protection against Cd toxicity. Furthermore, Cd, but not Zn, significantly activated Nrf2 and its downstream targets, including HO-1; inhibition of HO-1 by a specific HO-1 inhibitor, ZnPP (10 µM), significantly increased Cd-induced toxicity, but did not inhibit Zn protection against Cd injury, suggesting that Nrf2-mediated HO-1 activation was not required for Zn protective effect. Finally, the ability of Zn to reduce Cd uptake provided an additional MT-independent mechanism for reducing Cd toxicity. Thus, Zn exerts protective effects against Cd toxicity for murine ECTs that are partially MT-mediated. Further studies are required to translate these findings towards clinical trials.
Keywords: Zinc, cadmium toxicity, engineered cardiac tissue, metallothionein, Nrf2, heme oxygenase-1, ZnPP
Introduction
Cadmium (Cd) is a nonessential toxic heavy metal and a nondegradable environmental toxin. Cd toxicity is associated with various cellular, metabolic, homeostatic, and repair mechanisms, including increased reactive oxygen species (ROS) production and apoptosis [1]. Since Cd mainly accumulates in the liver and kidneys, most research has focused on Cd toxicity in these organs. However, Cd is also toxic to other tissues including the heart, lungs, and adipose tissues [2]. Epidemiologic and preclinical studies show that low to moderate Cd exposure is associated with cardiovascular (CV) dysfunction and disease including arrhythmias [3, 4], hypertension [5, 6], myocardial infarction [7, 8], and heart failure [9–11]. Urine Cd levels are associated with increased CV disease incidence and mortality [12]. Cd toxicity to cardiomyocyte (CM) occurs at 100-fold lower dose than reported for hepatic and renal cell toxicity [13]. Pathological changes in the heart have been reported following Cd exposure, and the underline mechanisms responsible for Cd toxicity in CV disease [3] and potential preventive strategies have not been fully characterized.
By contrast to Cd, zinc (Zn) is an essential metal that supports a wide range of biological processes. The protective effect of Zn on Cd-induced injury has been studied in vitro and in vivo [14, 15], however, the underlying mechanisms remain undefined. Since Zn is a potent MT inducer via the metal-response element-binding transcription factor 1 (MTF1, [16–19]), it is possible that Zn-induced MT overexpression plays an important role in protection against Cd toxicity [20–24]. In contrast to Zn-mediated MT induction, Cd also results in MT induction as part of the cellular response to injury. Attenuation of ROS activation by Zn also plays an important role [25]. Zn has been shown to be protective against Cd toxicity in liver, kidney, and bone [15, 26, 27]. Our current study further explores underlying mechanisms for Zn protection against CM Cd toxicity.
Engineered cardiac tissues (ECTs) are now used globally as a robust platform technology that is well suited for tissue repair paradigms and for scalable in vitro drug screening and disease modeling [28–33]. One of the strengths of the ECT paradigm is that formulations can incorporate multiple cell lineages including cardiomyocytes, fibroblasts, endothelial cells, and mural cells [33]. In general, ECTs cultured in vitro have not been shown to include immunologic cells. However, this ECT paradigm has not been broadly applied to environmental toxicology related studies. While in vivo environmental toxicity models allow the validation of requisite pathways using transgenic over-expression and knockout strategies, these studies can require treatment for weeks to months to identify phenotypes with both on-target and off-target effects of compounds and countermeasures of interest while in vitro ECT platforms can provide reproducible functional, biochemical, and molecular data within 7 days [4]. We have demonstrated that mouse-derived ECTs can serve as a robust model to study in vitro Cd toxicity with respect to both ECT dysfunction and CM injury, and ECTs derived from MT overexpressing mice display increased tolerance to Cd toxicity [4].
Recognizing that Zn has been described to reduce Cd toxicity in other organs, we used our murine ECT platform to address whether Zn can protect ECTs from Cd toxicity, in part via MT dependent pathways, as has been noted in animal models. We found that Zn supplementation protected ECTs from Cd toxicity. To determine the requirement for MT in Zn-mediated protection from Cd toxicity, we found that ECTs derived from MT-KO ventricular cells showed increased Cd toxicity versus WT-ECT and that Zn could partially protect Cd-induced cell death in MT-KO ECTs, likely due to Zn-mediated, MT-independent, reduced Cd uptake. We also found that although an HO-1 inhibitor enhanced Cd toxicity, it had no impact on Zn protection from Cd toxicity. These findings are consistent with our hypothesis that both direct competition between Zn and Cd uptake and Zn-mediated MT induction play important roles in Zn protection from CM Cd toxicity in murine ECTs.
Materials and methods
Animals
All mice were purchased from the Jackson Laboratory (Bar Harbor, ME). MT-KO (MT−/−, both MT1 and MT2 gene knockout) and its wild-type 129S mice, as well as Friend leukemia virus B (FVB) mice were housed in the University of Louisville Research Resources Center at 22 ◦C with a 12-h light/dark cycle and were provided free access to standard rodent chow and tap water. All animal procedures were approved by the Institutional Animal Case and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care. We bred FVB to generate FVB pups for all experiments that did not relate the investigation of the role of MT knockout on the Cd and Zn responses. We bred 129s WT and 129s-MT−/− to generate 129S pups and MT−/− pups, respectively, in order to generate WT- and MT-KO ECTs.
Isolation of neonatal mouse ventricular cardiac cells
Neonatal mouse ventricular cardiac cells were isolated as previous described [4]. Briefly, hearts of 3-day-old Pups were harvested and digested by trypsin and collagen. CM were enriched by preplating for 45 min followed by rotating for 4 h and then heart cells were collected and counted using a hemocytometer.
ECT construction and treatment
ECTs were constructed as previously described [4, 30]. Approximately 0.8 × 106 heart cells derived from four pup ventricles were mixed with collagen and matrigel, poured to form a cylinder construct within Flexcell Tissue TrainTM culture plates (Flexcell International), and incubated for 2 h (37 °C, 5% CO2) to form a cylindrical ECT construct. Following initial ECT gelation, 4 mL of mouse medium was added to each ECT in the six-well culture dish. ECTs were maintained in vitro for 7 days with media changes every other day. Randomly selected ECTs were treated with 20 μM CdCl2 for 24 h starting on day 6, with 50 µM Zn for 24 h starting on day 6, cotreated with Zn and CdCl2, or pretreated with HO-1 inhibitor (ZnPP 10 µM) for 2 h and then treated with 20 μM CdCl2 or/and 50 µM Zn for another 24 h starting on day 6. The concentrations for CdCl2 used in the Zn cotreatment study were based on the dose–response curve shown in Fig. 1 and Table 1. The Zn dose used in the Zn cotreatment study were based on the dose–response curve shown in Fig. 2.
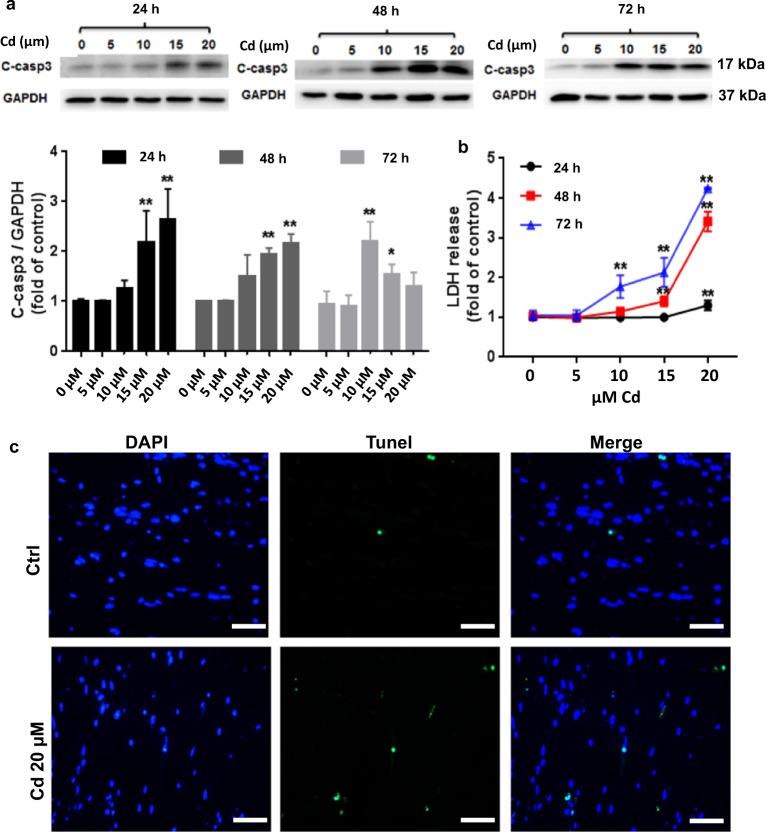
Fig. 1. Dose and time response of toxic Cd effect on murine ECTs.
a Expression of cleaved caspase 3 after increasing Cd concentration (0, 5, 10, 15, 20 µM) for increasing duration (24, 48, 72 h). Group sizes ranged from n = 3–8 per time point. b LDH release after increasing Cd concentration (0, 5,10, 15, 20 µM) for increasing duration (24, 48, 72 h). Group sizes ranged from n = 3–8 per time point. c Representative ECT immunofluorescent staining for terminal transferase dUTP nick end labeling assay (Tunel, green) and nuclei (DAPI, blue) following 24 h 20 μM Cd exposure (×40 magnification, scale bar = 50 μm). *P < 0.05 and **P < 0.01 vs. corresponding Ctrl ECT for the corresponding treatment duration.
Table 1.
Dose- and time-dependent Cd effect on inhibition of ECT beating
| Cd dose | 6 h | 12 h | 24 h | 30 h | 36 h | 48 h | 54 h | 60 h | 72 h |
|---|---|---|---|---|---|---|---|---|---|
| 0 μM (n = 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 μM (n = 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 μM (n = 6) | 0 | 0 | 0 | 0 | 0 | 0 | 13% | 25% | 57% |
| 15 μM (n = 6) | 0 | 0 | 0 | 0 | 0 | 17% | 17% | 50% | 50% |
| 20 μM (n = 10) | 0 | 0 | 0 | 0 | 10% | 80% | 80% | 90% | 100% |
ECT beating following Cd exposure was digitally recorded for 15 s at 6, 12, 24, 30, 36, 48, 54, 60, and 72 h starting at the time of Cd treatment on day 6. Percentages of ECTs with absent beating are displayed, n = 3–10 ECTs per group.
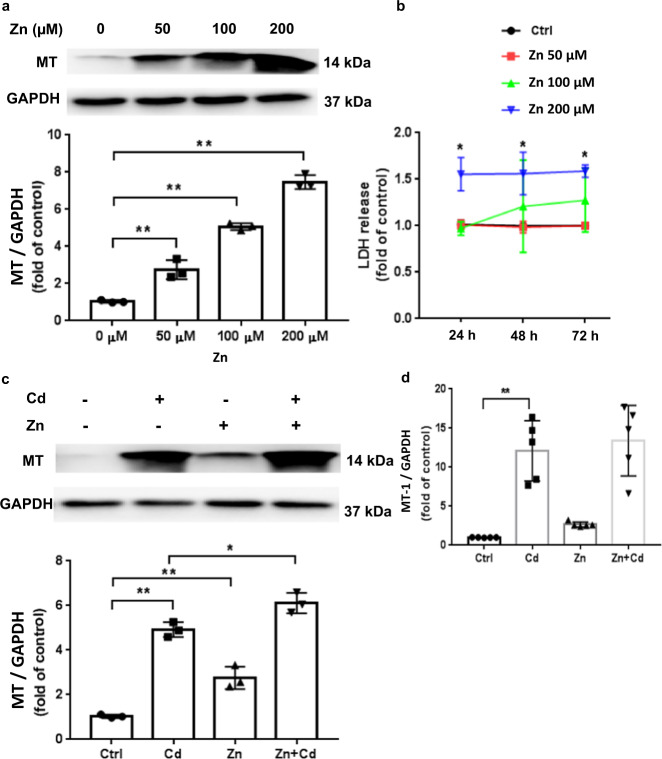
Fig. 2. Zn and Cd effects on ECT MT expression.
a MT expression after increasing Zn dose (50, 100, 200 µM) for 24 h. Group size is n = 3 per dose. b LDH release after increasing Zn dose (50, 100, 200 µM) for different durations (24; 48; 72 h). Group size is n = 3 per time point. c Effect of Zn (50 µM) and/or Cd (20 µM) on ECT MT protein content. Group size is n = 3 per group. d Effect of Zn (50 µM) and/or Cd (20 µM) on MT-1 gene expression (qPCR). Group size is n = 5 per group. *P < 0.05 and **P < 0.01 vs. corresponding Ctrl ECT.
Histology
ECTs were harvested from the Tissue TrainTM plate on day 7 and the mid-portion of the ECT was washed 3 times in PBS then placed in a Tissue Tek® Cryomold (Torrance, CA) with OCT compound for embedding followed by snap freezing in liquid nitrogen. Individual 5 µm cryostat sections were mounted, fixed in acetone for 5–10 min, washed in 1% Triton-X-100/PBS for 1 h at room temperature, then blocked with 1% Triton-X-100/PBS + 10% FBS for 1 h. ECT sections were stained with rabbit anti-Nrf2 (1:500 dilution; Abcam, Cambridge, MA) as previously published [34, 35]. Other sections were mounted using SlowFade™ Gold Antifade Mountant to image DAPI (Thermo Fisher Scientific, Eugene, OR). Nuclear Nrf2 translocation was detected by fluorescence microscopy.
Protein extraction and Western blotting
Protocols for ECT Western blot assays have previous described [4]. Briefly, ECTs were washed thoroughly in ice-cold PBS the rapidly homogenized in lysis buffer (100 µL/ECT) at 4 °C for 4 h. After measuring protein concentration, equal protein aliquots (20 μg) were loaded for analysis. Primary antibodies included Cleaved Caspase 3 [36], HO-1 (1:1,000 dilution; Cell Signaling Technology, Danvers, MA), and Nrf2 (1:1,000 dilution; Abcam, Cambridge, MA) using anti-rabbit secondary antibodies (1:5,000 dilution; Cell Signal, Danvers, MA). We report cleaved-caspase 3 rather than total caspase 3 because of the inability to detect both cleaved and total caspase 3 in the same Western (Supplemental Fig. 1). MT expression was detected by a modified Western blot protocol as previously described using an MT antibody (1:1,000 dilution; Dako, Carpinteria, CA) [37]. Protein expression was normalized to GAPDH (1:3,000 dilution; Abcam, Cambridge, MA).
RNA extraction and real-time PCR quantification
RNA extraction and quantitative real-time PCR was performed as previously described [4, 33].
TdT-mediated dUTP nick-end labeling assay (TUNEL)
We used the DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI) to determine the apoptotic cell proportion following the Promega protocol [4]. TUNEL positive cells were counted in 15 fields per ECT
Lactate dehydrogenase release in culture medium
We used Pierce™ LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific, Rockford, IL) to determine the LDH release into the culture medium [38] as an index of necrotic cell death [39]. Briefly, 50 µL of each sample medium was transferred to a 96-well flat-bottom plate in duplicate wells, mixed with 50 µL of Reaction Mixture, then incubated room temperature for 30 min in the dark followed by the addition of 50 µL of Stop Solution to each sample well. Light absorbance at 490 and 680 nm was measured using a Molecular Devices SprectraMax (Molecular Devices, Sunnyvale, CA) to quantify signal (490 nm) and noise (680 nm) absorbance.
Measurement of metal accumulation in ECT and ECT supernatant
To measure ECT Cd and Zn, ECTs were removed, washed with Milli-Q water, then heated to dryness. ECT supernatant was also collected on day 7 during ECT harvest. All samples were digested by 1 mL of 70% nitric acid at 65 °C for 4 h, diluted into 34 mL deionized water containing 2% nitric acid, then analyzed inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Scientific, Waltham, USA). Concentrations are reported as nanograms of metal per ECT or per 50 µL medium (ng/ECT or ng/50 µL, [40]).
Statistical analyses
Data are presented as mean ± standard error of the mean (SEM). Statistical differences were determined using two-sided, unpaired Student’s t tests or two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. P value < 0.05 was considered statistically significant.
Results
Dose and time response of Cd toxicity
We noted a dose-dependent increase of cleaved caspase3, a surrogate for cell death, after ECT Cd treatment for 24 h, as well as a time-dependent effect of Cd toxicity measured by increasing induced cleaved caspase 3 expression (Fig. 1a). As has been noted in previous ECT studies, there is a low level of time-dependent cell death in the ECT model [4, 41] so that treatment effects need to be determined using groups of similar culture duration. Cleaved caspase 3 expression increased at 24 h after 15 µM and 20 µM Cd dosing, however, lower dose Cd, 10 µM, induced increased cleaved caspase 3 expression only after 48 and 72 h of exposure (Fig. 1a). Cumulative LDH release into the culture medium, an additional indicator of cell injury, also showed a dose- and time-dependent response to Cd treatment. Because a 20 µM Cd dose was required to induce significantly increased cleaved caspase 3 and LDH at 24 h, we used this dose and duration for all subsequent experiments (Fig. 1b). The decrease of caspase 3 cleavage after 72 h incubation with 15 µM and 20 µM Cd may be due to progressive cell loss resulting in fewer cells able to produce cleaved caspase 3 (most of ECTs died at 72 h time point, see Table 1). ECT function also showed a dose- and time-dependent responses to Cd toxicity with a progressive inhibition of ECT beating with increased Cd dose and duration (Table 1). We defined Cd-induced changes in ECT function a global classification scheme: normal function (Nl); increased ECT deformation during shortening with regional dyssynchrony (A); increased ECT deformation during shortening with regional dyssynchrony (B); decreased beat rate with global dyssynchrony (C); and arrested beating (D). Representative videos of each classification are available in the Supplemental Data.
Cd treatment had a negative effect on ECT function starting at 8 h after treatment (Table 2) with a progressive shift from normal, synchronous beating to increased deformation with regional dyssynchrony followed by global dyssynchrony and sensation of beating by 72 h after treatment in WT ECT, similar to our previously published data [4]. TUNEL assay positive (green) cells increased after Cd treatment compared with Ctrl ECTs (Fig. 1c).
Table 2.
Zn prevents Cd-induced abnormal ECT beating pattern
| 8 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| Ctl (n = 5) | Nl | Nl | Nl | Nl |
| Cd 20 μM (n = 10) | 90% A, 10% B | 20% A, 80% B | 20% C, 80% D | 100% D |
| Zn 50 μM (n = 4) | Nl | Nl | Nl | Nl |
| Cd 20 + Zn 50 μM (n = 8) | Nl | Nl | Nl | Nl |
ECT function following Cd exposure was digitally recorded for 15 s at 8, 24, 48, and 72 h starting at the time of Cd treatment on day 6. ECT function was then qualitatively classified as: normal function (Nl); increased ECT deformation during shortening with regional dyssynchrony (A); increased ECT deformation during shortening with regional dyssynchrony (B); decreased beat rate with global dyssynchrony (C); and arrested beating (D). Group sizes ranged from n = 4–10. WT ECTs displayed dysfunction 8 h after 20 μM Cd exposure with progressive dysfunction resulting in absent contractions by 72 h after Cd exposure. Zn cotreatment with Cd prevented Cd induced ECT dysfunction. n = 4–10 ECTs per group.
Zn and Cd effect on MT expression
Both Zn and Cd increase MT expression in multiple cell types including CM [37]. Therefore, we determined the impact of Zn and Cd treatment on MT expression in murine ECTs. Zn induced a significant dose-dependent increase in MT expression (Fig. 2a) and noted increased LDH release as evidence for Zn toxicity only at higher doses (Fig. 2b). We found that 200 µM Zn induced a significant increase of LDH release by 24 h and no increase in LDH release was observed up to 72 h after a single 50 µM Zn dose. Therefore, we chose 50 µM Zn dosing for subsequent experiments. Both Cd (20 µM) and Zn (50 µM) induced increased MT expression in murine ECT, and cotreatment with Cd and Zn further increased MT expression (Fig. 2). Increased MT-1 gene expression paralleled the increase in MT protein expression noted after Zn and/or Cd ECT treatment (Fig. 2d).
Zn impact on Cd toxicity
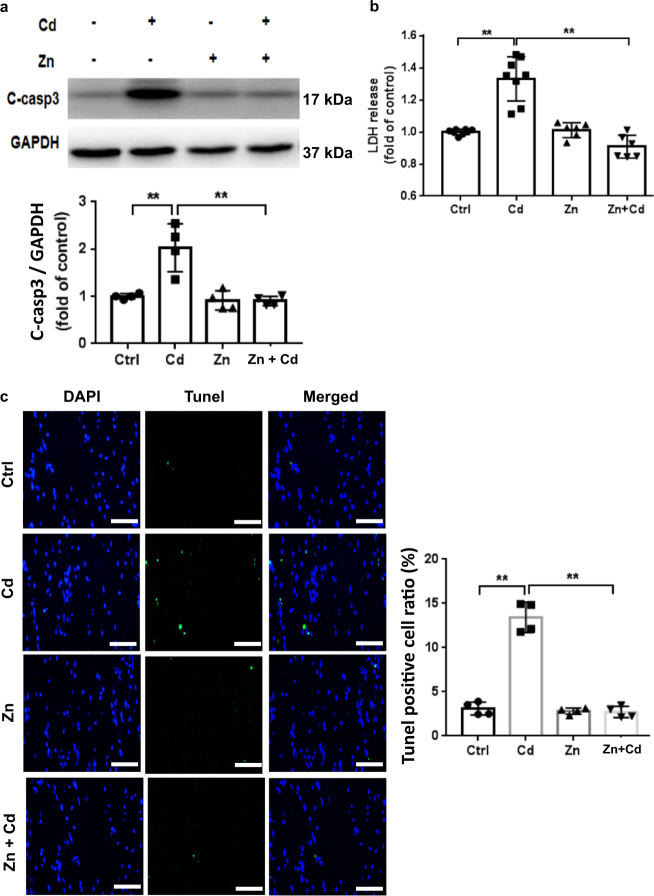
Several studies have reported a therapeutic role of Zn in reducing Cd toxicity in vivo and in vitro [14, 15]. We therefore tested the ability of Zn to reduce Cd-induced ECT toxicity. As noted above, Cd treatment increased CM apoptosis in ECTs indicated by increased cleaved caspase 3 and Zn reduced Cd-induced cleaved caspase 3 (Fig. 3a). Similar results were detected by LDH assay in culture medium (Fig. 3b). Cd-induced ECT toxicity increased the ECT TUNEL positive cell ratio (Fig. 3c) as well as some nonnuclear TUNEL staining consistent with cell death and nuclear rupture. Consistent with the ability of Zn to block Cd-induced cell injury, we noted that Zn prevented Cd-induced ECT functional impairment (Table 2).
Fig. 3. Zn reduces ECT Cd toxicity.
a Cleaved caspase 3 expression following Zn (50 µM) and/or Cd (20 µM) for 24 h. Group size is n = 4 per group. b LDH release following Zn (50 µM) and/or Cd (20 µM) for 24 h. Group size ranged from n = 6–8 per group. c Representative ECT immunofluorescent staining for terminal transferase dUTP nick end labeling assay (Tunel, green) and nuclei (DAPI, blue) and summary Tunel data for each group. Group size is n = 4 per group. ×40 magnification, Scale bar = 50 μm, **P < 0.01 vs. corresponding groups.
The role of MT in Zn protection from Cd toxicity
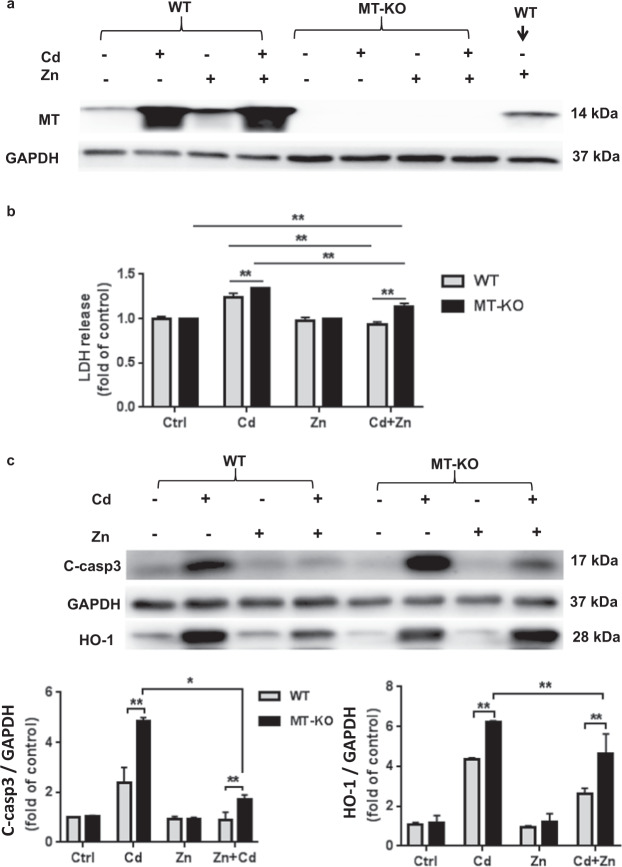
Zn-induced MT overexpression has been reported to be partially responsible for the Zn protective effect in several models [42–44]. Therefore, to further evaluate Zn-dependent and independent roles for MT in ECT protection from Cd toxicity we generated ECTs from MT-KO cardiac cells in comparison with ECTs composed of control 129s cardiac cells. We confirmed that MT-KO derived ECTs lacked MT expression and that MT was not induced by Zn in MT-KO ECTs (Fig. 4a). We noted no differences (gel compaction, beat rate, cellularity) in ECT formulated from FVB versus 129S WT cells. We treated WT-ECT and MT-KO ECT with Cd, Zn, or Zn + Cd for 24 h. Cd-induced apoptosis was increased in MT-KO ECT based on increased LDH release in culture medium (Fig. 4b) and increased cleaved caspase 3 expression (Fig. 4c). Zn treatment (50 µM × 24 h) blocked increased cleaved caspase 3 and LDH release in the Zn + Cd WT ECT group. It is important to note that Zn treatment partially suppressed Cd-induced increased cleaved caspase 3 and LDH release in MT-KO ECT (Fig. 4b, c) consistent with an MT independent and as well as an MT requirement for Zn protective effects.
Fig. 4. MT plays a role in Zn protection from ECT Cd toxicity.
a MT expression in WT and in MT-KO ECTs following Zn (50 µM) and/or Cd (20 µM) for 24 h. b LDH release into media following Zn (50 µM) and/or Cd (20 µM) for 24 h. Group size ranged from n = 6–8 per group. c Cleaved caspase 3 and HO-1 expression in WT and MT-KO ECT in each treatment group (Ctrl; Cd; Zn; Zn + Cd). Group size is n = 4 per group. *P < 0.05 and **P < 0.01 vs. corresponding Ctrl ECT.
Effect of Cd and Zn on Nrf2 and downstream gene expression
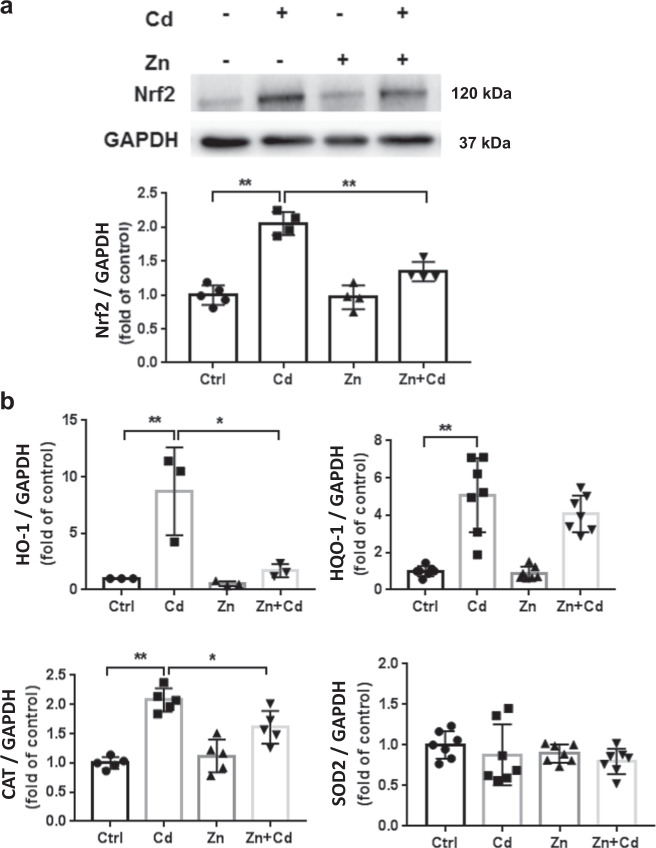
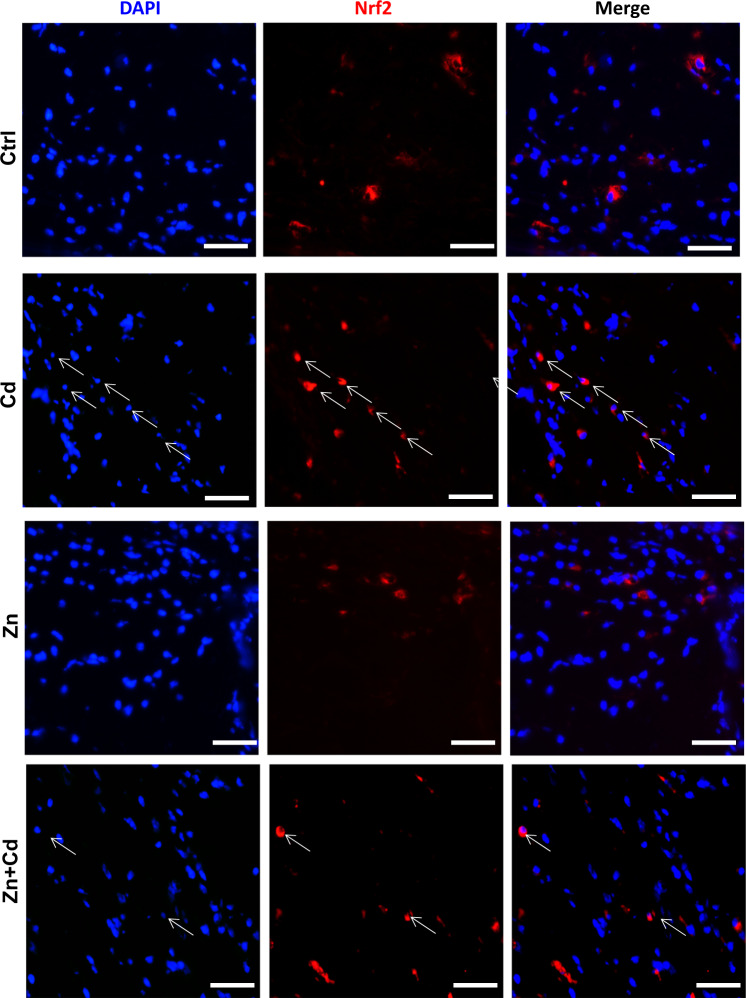
The Nrf2 pathway has been shown to be upregulated during the antioxidative response to cellular stresses, including Nrf2 induction following Cd exposure [45] and Zn-mediated Nrf2 induction [34, 46]. Following ECT Cd treatment, Nrf2 was significantly activated (Fig. 5a) as were Nrf2 downstream genes HO-1, NOQ-1, and CAT (Fig. 5b). This Cd-mediated increase in Nrf2 and downstream genes was reduced by Zn cotreatment (Fig. 5). Of note, ECT treatment with Cd, Zn, and Zn + Cd had no effect on SOD2 (Fig. 5b). Cd treatment increased Nrf2 nuclear translocation that was partially reduced by Zn cotreatment (Fig. 6).
Fig. 5. Cd and Zn induce ECT Nrf2 and downstream genes.
a ECT Nrf2 following Zn (50 µM) and/or Cd (20 µM) for 24 h. Group size is n = 4 per group. b qPCR results of Nrf2 downstream genes (HO-1, NQO-1, CAT, SOD2) following Zn (50 µM) and/or Cd (20 µM) for 24 h. Group size ranged from n = 3–7 per group. *P < 0.05 vs. corresponding groups. **P < 0.01 vs. corresponding groups.
Fig. 6. Evidence of ECT Nrf2 activation after Cd and/or Zn treatment.
Representative ECT stained for Nrf2 (Red) and nuclei (DAPI, blue) following Zn (50 µM) and/or Cd (20 µM) for 24 h. Arrows indicate representative positive cells in which Nrf2 translocated into nucleus, ×40 magnification, scale bar = 50 μm.
Involvement of HO-1 in Cd toxicity and in Zn protection from Cd toxicity in ECTs
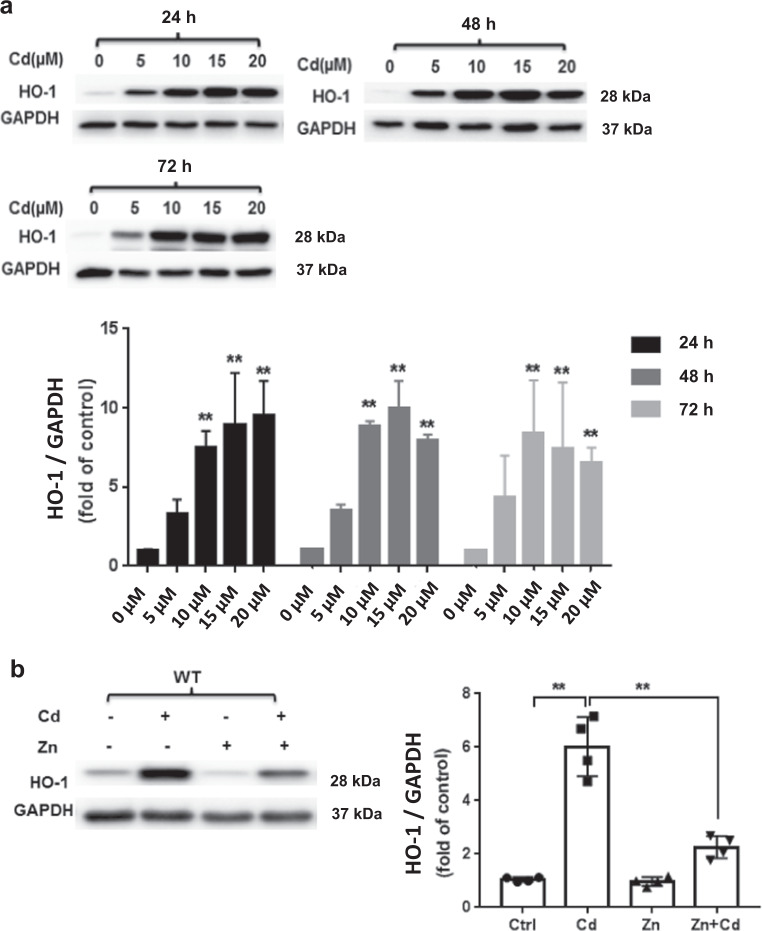
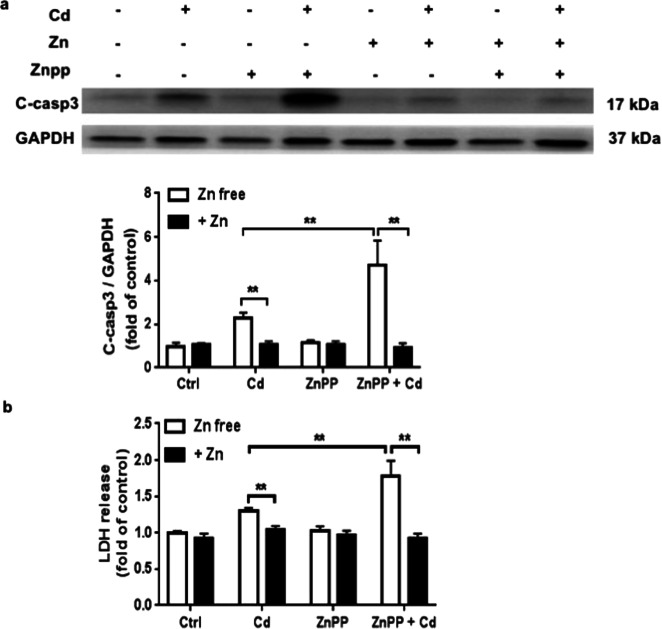
Heme oxygenase-1 (HO-1), a stress-inducible enzyme that mediates antioxidative and cytoprotective effects to maintain cellular redox homeostasis and protects cells from oxidative stress. We noted a dose- and time-dependent increase in HO-1 expression after ECT Cd treatment (Fig. 7a) that was reduced by Zn cotreatment (Fig. 7b). We also found that ECT pretreatment with the HO-1 inhibitor, ZnPP (10 µM) enhanced Cd toxicity indicated by increased cleaved caspase3 expression and increased LDH release (Fig. 8a, b). Interestingly, HO-1-inhibition had no impact on Zn protection from Cd toxicity (Fig. 8a, b) suggesting that Zn and HO-1 are both involved in the ECT response to Cd toxicity, but are not co-dependent.
Fig. 7. Cd and/or Zn alter ECT HO-1 expression.
a ECT HO-1 expression after increasing Cd concentration (0, 5, 10, 15, 20 µM) for increasing duration (24, 48, 72 h). Group size ranged from n = 3–8 per time point. **P < 0.01 vs. corresponding Ctrl group. b ECT HO-1 expression following Zn (50 µM) and/or Cd (20 µM) for 24 h. (Ctrl, Cd, Zn, Zn + Cd). Group size is n = 4 per group. **P < 0.01 vs. corresponding group.
Fig. 8. The HO-1 inhibitor ZnPP increases ECT Cd toxicity.
a Cleaved caspase 3 following treatment with Zn (50 µM) and/or Cd (20 µM) and/or ZnPP (10 µM) for 24 h by WB. Group sizes ranged from n = 3–4 per group. **P < 0.01 vs. corresponding group. b LDH release following treatment with Zn (50 µM) and/or Cd (20 µM) and/or ZnPP (10 µM) for 24 h. Group sizes ranged from n = 3–4 per group. **P < 0.01 vs. corresponding group.
Zn reduces ECT Cd uptake as an MT-independent protection from Cd toxicity
Cd and Zn are known to compete for cellular uptake via metal transporters and therefore Zn inhibition of Cd uptake could function as an MT-independent mechanism to reduce Cd toxicity. We measured ECT Cd and Zn uptake and found that Zn cotreatment with Cd significantly reduced ECT Cd accumulation with a complementary increase in culture medium Cd content (Table 3). As would be expected, Zn treatment increased ECT Zn content, though somewhat surprisingly, Cd and Zn cotreatment further increased ECT Zn content (Table 3).
Table 3.
Cd and Zn content in ECT tissue and in ECT culture media
| Cd (ng/ECT) | Zn (ng/ECT) | Cd (ng/40 μL media) | Zn (ng/40 μL media) | |
|---|---|---|---|---|
| Ctl (n = 3) | 0 | 37.56 ± 0.70 | 0.05 ± 0.01 | 13.28 ± 0.78 |
| Cd 20 μM (n = 3) | 66.70 ± 2.09 | 36.05 ± 3.20 | 76.35 ± 1.15 | 9.98 ± 1.19 |
| Zn 50 μM (n = 3) | 0 | 67.78 ± 0.87 | 0.06 ± 0.01 | 196.68 ± 2.18 |
| Cd 20 + Zn 50 μM (n = 3) | 56.44 ± 1.3** | 75.10 ± 2.66* | 88.37 ± 1.56** | 191.84 ± 1.57 |
As expected, Cd content was only noted in treated ECTs. Zn was present in Ctrl ECTs and increased after Zn treatment. ECT Cd content decreased and media Cd content increased following Zn and Cd cotreatment. *P < 0.05 vs. corresponding 50 µM Zn group, **P < 0.01 vs. corresponding 20 µM Cd group. n = 3 ECTs per group
Discussion
Our previous study established the basic paradigm of our murine ECT model to study Cd toxicity and noted that CM specific MT overexpression increased ECT tolerance to Cd toxicity. In the current study, we found that Zn could induce MT overexpression in a dose-dependent manner (Fig. 2a), and Zn cotreatment with Cd could totally block the cytotoxicity of Cd (Fig. 3). We also defined that MT play a partial role in Zn protection on Cd toxicity by using MT-KO-derived ECTs (Fig. 4b, c). In addition, we found that Nrf2/HO-1 signaling pathway was involved in Cd toxicity (Figs. 5–7, 8a, b), but it was not related to Zn protection on Cd toxicity (Fig. 8a, b).
Cd is a toxic heavy metal. In vitro and in vivo studies have proven the toxic effects of Cd on a variety of tissues, including the liver [47], kidney [48], heart [49], lung [50], etc. Upon uptake into the body, Cd can complex with MT, a known heavy metal chelator, for detoxification [51]. In wild-type and MT-null mice given increasing doses of Cd, a remarkable acquired tolerance to Cd lethality is evident in wild-type mice, with a sevenfold difference in LD50 values, however, such tolerance does not happen in MT-null mice [52]. Moreover, Cd exposure in MT-null mice results in nephrotoxicity at one-tenth the nephrotoxic dose for control mice [21]. Conversely, MT overexpression mice are protected against acute Cd lethality and hepatotoxicity [53]. Our current study used an established neonatal murine ECT in vitro model to determine dose- and time-dependent Cd toxicity with adverse effects noted on ECT function and cell death and a protective effect of MT overexpression, as we have previously noted [4]. We now show that Cd exposure in MT-KO ECTs increased cell death with increased cleaved Caspase-3 and LDH release and ECT dysfunction confirming a critical role for MT in the response to Cd exposure.
The metal ion Zn has been shown to be essential for normal biologic function in most living cells as a cofactor for many enzymes including the important Zn-finger proteins. In addition to its structural and catalytic properties [54] during normal homeostasis, Zn has been also shown to support cellular antioxidant responses via several pathways including the inhibition of NADPH oxidase, activation of superoxide dismutase (SOD), induction of MT, and the upregulation of Nrf2 and Nrf2 downstream genes [55].
It has been reported that the mechanism for Zn-induced tolerance to Cd toxicity results primarily from the induction of MT synthesis [22, 23]. In renal cell lines from WT and MT-1/MT-2 knockout (MT-KO) mice, pretreatment with Zn increases MT expression and enhanced Cd resistance in WT cells; however, Zn pretreatment of MT-KO does not increase tolerance to Cd [20]. Similar to previous in vivo studies, we noted that Zn prevented Cd induced-cell death in WT ECTs [20], however, Zn treatment was only partially protective in MT-KO ECTs, likely via MT-independent inhibition of Cd uptake. Further studies are needed to determine the dose–responses of individual ECT lineages (CM, EC, F) to Cd toxicity and Zn prevention of toxicity.
Many studies have investigated the role of oxidative stress and the balance between ROS/reactive nitrogen species (RNS) production in Cd-mediated cellular toxicity [56]. The level of cellular oxidative stress is dynamically determined by a biological system’s ability to detoxify these reactive intermediates. NF-E2-related nuclear factor 2 (Nrf2) is rapidly upregulated early in the response to oxidative stress and then translocates to the nucleus to increase the transcription of oxidative stress response genes as an adaptive mechanism. Under normal conditions, Nrf2 is sequestered by kelch-like ECH associating protein 1 (Keap1) in the cytosol and degraded by proteasomes. In response to oxidative stimuli, Nrf2 is released from Keap1, translocates into the nucleus, and induces its target genes by binding to antioxidant response elements (ARE) to regulate antioxidant-mediated gene expression [57]. We found that murine ECT Cd exposure increased Nrf2 expression and enhanced the translocation of Nrf2 into the nucleus, with an increase in Nrf2 downstream gene expression, similar to the adaptive Nrf2 response that has been noted in vitro [1, 58]. Zn treatment also facilitated the Nrf2 adaptive response. We noted a reduced Nrf2 induction following Cd treatment in Zn treated ECTs consistent with reduced injury and reduced injury response. Additional studies using confocal imaging and lineage-specific staining may provide an additional refinement of the ECT lineages (CM, EC, F, etc.) that show increased Nrf2 expression and nuclear translocation in response to Cd toxicity and electron microscopic imaging may provide additional insights into subcellular organelle injury following Cd exposure.
ECTs have multiple advantages over monolayer 2D culture methods including the formation of isotropic and aligned in vitro 3D myocardial tissues that electrically and mechanically function similar to in vivo myocardium. ECTs rapidly mature in vitro, begin to beat synchronously from culture day 3, and display altered beating rate and pattern in response to environmental stress (Cd, [4]. In the current study we noted that ECT dysfunction within 8 h of Cd treatment and dose-dependent suspension of beating (Tables 1, 2). Further studies are required to define the roles of altered calcium signaling [21, 59], mitochondrial function [60, 61], and changes in oxidative stress and ROS as potential mechanisms for Cd-mediated altered ECT electrical and mechanical function. ECTs have one additional, essential advantage over in vivo models which is the ability to customize cell composition to include multiple mesoderm lineages and/or genetically altered CM [30, 62]. Our current study uses ventricular myocardial cells from normal and transgenic newborn pups. This approach is highly adaptable to use transgenic models of interest to explore specific signaling pathways and to human stem-cell-derived mesodermal cells including CM, myofibroblasts, endothelial cells, and mural cells [33, 62]. Further studies are required to determine ECT lineage-specific sensitivities to Cd injury and potential differential effectiveness of Zn to prevent lineage-specific Cd related injury.
HO-1 is one of the key downstream genes induced by Nrf2 signaling pathway with important antioxidant and cytoprotective effects via a reduction in intracellular levels of the prooxidant Heme and by the increased production of cytoprotective levels of carbon monoxide (CO) and biliverdin (BV) [63]. Not surprisingly, we noted a significant dose- and time-dependent induction of HO-1 expression in response to ECT Cd exposure. We confirmed the adaptive role of HO-1 related to ECT Cd toxicity using an HO-1 inhibitor (ZnPP), which increased ECT sensitivity to Cd toxicity. However, HO-1 inhibition by ZnPP did not reduce Zn-mediated protection from Cd toxicity, confirming multiple cellular adaptive responses to Cd toxicity.
Cd and Zn possess similar chemical properties and have been shown to compete for uptake in a variety of tissues [13, 64–66] and our results are consistent that one mechanism for Zn-mediated reduction in Cd toxicity is reduced Cd accumulation in ECTs. Further studies are required to identify the specific transporters and transporter efficiencies by which Cd and Zn compete for cellular uptake. Candidate transporters for both Cd and Zn include Zip8 [67], Zip14 [68], DMT1 [69], and several calcium channels [59]. Specific transporter knockout mice may be helpful as novel sources for cardiac cells to further explore Zn and Cd transport and Cd toxicity in myocardial tissues. Since ROS play an important role in Cd toxicity and in Zn protection, further studies are also required to quantify and explore modulation of ROS in Zn-mediated protection for Cd toxicity.
Supplementary information
Acknowledgements
This work was supported by the Kosair Charities Pediatric Heart Research Fund to BK, the U.S-China Pediatric Research Exchange Training Program to LC and BBK, and the National Natural Science Foundation of China to HLJ (81470495). All personnel expenses and partial research-related expenses for HTY and JZ were provided by Jilin University through a collaborative research agreement between the University of Louisville and Jilin University, Changchun, China. Basic science experiments were completed at the University of Louisville, Louisville, KY, USA.
Author contributions
HTY designed and performed experiments, analyzed data, prepared and reviewed manuscript for submission. JZ designed and performed experiments, analyzed data, prepared and reviewed manuscript for submission. JXX prepared and reviewed manuscript for submission. LC designed experiments, prepared and reviewed manuscript for submission. JYL prepared and reviewed manuscript for submission. HLJ designed experiments, prepared and reviewed manuscript for submission. BBK designed and performed experiments, analyzed data, prepared and reviewed manuscript for submission.
Footnotes
These authors contributed equally: Hai-tao Yu, Juan Zhen
Contributor Information
Hong-lei Ji, Email: jihonglei@aliyun.com.
Bradley B Keller, Email: brad.keller@louisville.edu.
Supplementary information
The online version of this article (10.1038/s41401-019-0320-y) contains supplementary material, which is available to authorized users.
References
- 1.Thevenod F, Lee WK. Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch Toxicol. 2013;87:1743–86. doi: 10.1007/s00204-013-1110-9. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami T, Nishiyama K, Kadota Y, Sato M, Inoue M, Suzuki S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol Appl Pharmacol. 2013;272:625–36. doi: 10.1016/j.taap.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Wang X, Zhou D, Li T, Tang L, Gong T, et al. Modelling cadmium-induced cardiotoxicity using human pluripotent stem cell-derived cardiomyocytes. J Cell Mol Med. 2018;22:4221–35. doi: 10.1111/jcmm.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Ye F, Yuan F, Cai L, Ji H, Keller BB. Neonatal murine engineered cardiac tissue toxicology model: Impact of metallothionein overexpression on cadmium-Induced Injury. Toxicol Sci. 2018;165:499–511. doi: 10.1093/toxsci/kfy177. [DOI] [PubMed] [Google Scholar]
- 5.Caciari T, Sancini A, Fioravanti M, Capozzella A, Casale T, Montuori L, et al. Cadmium and hypertension in exposed workers: a meta-analysis. Int J Occup Med Environ Health. 2013;26:440–56. doi: 10.2478/s13382-013-0111-5. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Liao Q, Chillrud SN, Yang Q, Huang L, Bi J, et al. Environmental exposure to cadmium: health risk assessment and its associations with hypertension and impaired kidney function. Sci Rep. 2016;6:29989. doi: 10.1038/srep29989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res. 2008;106:284–6. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Hecht EM, Arheart KL, Lee DJ, Hennekens CH, Hlaing WM. Interrelation of cadmium, smoking, and cardiovascular disease (from the National Health and Nutrition Examination Survey) Am J Cardiol. 2016;118:204–9. doi: 10.1016/j.amjcard.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Smetana RH, Glogar DH. Role of cadmium and magnesium in pathogenesis of idiopathic dilated cardiomyopathy. Am J Cardiol. 1986;58:364–6. doi: 10.1016/0002-9149(86)90082-2. [DOI] [PubMed] [Google Scholar]
- 10.Borne Y, Barregard L, Persson M, Hedblad B, Fagerberg B, Engstrom G. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort study. BMJ Open. 2015;5:e007366. doi: 10.1136/bmjopen-2014-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiol (Camb, Mass) 2013;24:421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limaye DA, Shaikh ZA. Cytotoxicity of cadmium and characteristics of its transport in cardiomyocytes. Toxicol Appl Pharmacol. 1999;154:59–66. doi: 10.1006/taap.1998.8575. [DOI] [PubMed] [Google Scholar]
- 14.Szuster-Ciesielska A, Stachura A, Słotwińska M, Kamińska T, Śnieżko R, Paduch R, et al. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145:159–71. doi: 10.1016/s0300-483x(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Wu C, Tu J, Ling Y, Hu N, Zhang Y, et al. Assessment of cadmium-induced hepatotoxicity and protective effects of zinc against it using an improved cell-based biosensor. Sens Actuators A Phys. 2013;199:156–64. [Google Scholar]
- 16.Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 1994;13:2870–5. doi: 10.1002/j.1460-2075.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275:34803–9. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 18.Jackson KA, Valentine RA, Coneyworth LJ, Mathers JC, Ford D. Mechanisms of mammalian zinc-regulated gene expression. Biochem Soc Trans. 2008;36(Pt 6):1262–6. doi: 10.1042/BST0361262. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari S, Melchiorre C, Dostie K, Laukens D, Devisscher L, Louwrier A, et al. Detection and manipulation of the stress response protein metallothionein. Curr Protoc Toxicol. 2017;71:17.19.1–17.19.28. doi: 10.1002/cptx.17. [DOI] [PubMed] [Google Scholar]
- 20.Kennette W, Collins OM, Zalups RK, Koropatnick J. Basal and zinc-induced metallothionein in resistance to cadmium, cisplatin, zinc, and tertbutyl hydroperoxide: studies using MT knockout and antisense-downregulated MT in mammalian cells. Toxicol Sci. 2005;88:602–13. doi: 10.1093/toxsci/kfi318. [DOI] [PubMed] [Google Scholar]
- 21.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–94. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Liu Y, Michalska AE, Choo KH, Klaassen CD. Metallothionein plays less of a protective role in cadmium-metallothionein-induced nephrotoxicity than in cadmium chloride-induced hepatotoxicity. J Pharmacol Exp Ther. 1996;276:1216–23. [PubMed] [Google Scholar]
- 23.Urani C, Melchioretto P, Canevali C, Crosta GF. Cytotoxicity and induction of protective mechanisms in HepG2 cells exposed to cadmium. Toxicol Vitr. 2005;19:887–92. doi: 10.1016/j.tiv.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Waalkes MP. Cadmium carcinogenesis. Mutat Res. 2003;533:107–20. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Souza V, Escobar Mdel C, Bucio L, Hernandez E, Gutierrez-Ruiz MC. Zinc pretreatment prevents hepatic stellate cells from cadmium-produced oxidative damage. Cell Biol Toxicol. 2004;20:241–51. doi: 10.1023/b:cbto.0000038462.39859.2f. [DOI] [PubMed] [Google Scholar]
- 26.Brzoska MM, Galazyn-Sidorczuk M, Rogalska J, Roszczenko A, Jurczuk M, Majewska K, et al. Beneficial effect of zinc supplementation on biomechanical properties of femoral distal end and femoral diaphysis of male rats chronically exposed to cadmium. Chem Biol Interact. 2008;171:312–24. doi: 10.1016/j.cbi.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Tang W, Sadovic S, Shaikh ZA. Nephrotoxicity of cadmium-metallothionein: protection by zinc and role of glutathione. Toxicol Appl Pharmacol. 1998;151:276–82. doi: 10.1006/taap.1998.8465. [DOI] [PubMed] [Google Scholar]
- 28.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, et al. Three-dimensional reconstitution of embryonic CMs in a collagen matrix: a new heart muscle model system. FASEB J Off Publ Federation Am Societies Exp Biol. 1997;11:683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 29.Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, et al. Development of a drug screening platform based on engineered heart tissue. Circ Res. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 30.de Lange WJ, Hegge LF, Grimes AC, Tong CW, Brost TM, Moss RL, et al. Neonatal mouse-derived engineered cardiac tissue: a novel model system for studying genetic heart disease. Circ Res. 2011;109:8–19. doi: 10.1161/CIRCRESAHA.111.242354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H, Zandstra PW, Radisic M. Engineered heart tissue model of diabetic myocardium. Tissue Eng Part A. 2011;17:1869–78. doi: 10.1089/ten.tea.2010.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiburcy M, Didie M, Boy O, Christalla P, Doker S, Naito H, et al. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res. 2011;109:1105–14. doi: 10.1161/CIRCRESAHA.111.251843. [DOI] [PubMed] [Google Scholar]
- 33.Nakane T, Masumoto H, Tinney JP, Yuan F, Kowalski WJ, Ye F, et al. Impact of cell composition and geometry on human induced pluripotent stem cells-derived engineered cardiac tissue. Sci Rep. 2017;7:45641. doi: 10.1038/srep45641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Cui W, Tan Y, Luo P, Chen Q, Zhang C, et al. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J Cell Mol Med. 2014;18:895–906. doi: 10.1111/jcmm.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu J, Cheng Y, Wu H, Kong L, Wang S, Xu Z, et al. Metallothionein Is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66:529–42. doi: 10.2337/db15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segatto M, Fico E, Gharbiya M, Rosso P, Carito V, Tirassa P, et al. VEGF inhibition alters neurotrophin signalling pathways and induces caspase-3 activation and autophagy in rabbit retina. J Cell Physiol. 2019; 234: 18297–307. [DOI] [PubMed]
- 37.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–54. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 38.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–46. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 39.Chan FK, Moriwaki K, De Rosa MJ. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Toxicological profile for cadmium. Atlanta, GA: Agency for Toxic Substances and Disease Registry (US); 2012. PMID: 24049863. [PubMed]
- 41.Vantler M, Karikkineth BC, Naito H, Tiburcy M, Didié M, Nose M, et al. PDGF-BB protects cardiomyocytes from apoptosis and improves contractile function of engineered heart tissue. J Mol Cell Cardiol. 2010;48:1316–23. doi: 10.1016/j.yjmcc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Eibl JK, Abdallah Z, Ross GM. Zinc-metallothionein: a potential mediator of antioxidant defence mechanisms in response to dopamine-induced stress. Can J Physiol Pharmacol. 2010;88:305–12. doi: 10.1139/Y10-022. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Menendez S, Garcia M. The zinc-metallothionein redox system reduces oxidative stress in retinal pigment epithelial cells. Nutrients. 2018;10:1874. doi: 10.3390/nu10121874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun W, Wang Y, Miao X, Wang Y, Zhang L, Xin Y, et al. Renal improvement by zinc in diabetic mice is associated with glucose metabolism signaling mediated by metallothionein and Akt, but not Akt2. Free Redic Biol Med. 2014;68:22–34. doi: 10.1016/j.freeradbiomed.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Mandal AK, Son YO, Pratheeshkumar P, Wise JTF, Wang L, et al. Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol Appl Pharmacol. 2018;353:23–30. doi: 10.1016/j.taap.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F, Li Y, Cao Y, Li C. Zinc might prevent heat-induced hepatic injury by activating the Nrf2-antioxidant in mice. Biol Trace Elem Res. 2015;165:86–95. doi: 10.1007/s12011-015-0228-4. [DOI] [PubMed] [Google Scholar]
- 47.Go YM, Sutliff RL, Chandler JD, Khalidur R, Kang BY, Anania FA, et al. Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicol Sci. 2015;147:524–34. doi: 10.1093/toxsci/kfv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23:783–92. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- 49.Markiewicz-Gorka I, Januszewska L, Michalak A, Prokopowicz A, Januszewska E, Pawlas N, et al. Effects of chronic exposure to lead, cadmium, and manganese mixtures on oxidative stress in rat liver and heart. Arh za Hig Rada i Toksikologiju. 2015;66:51–62. doi: 10.1515/aiht-2015-66-2515. [DOI] [PubMed] [Google Scholar]
- 50.Kiran Kumar KM, Naveen Kumar M, Patil RH, Nagesh R, Hegde SM, Kavya K, et al. Cadmium induces oxidative stress and apoptosis in lung epithelial cells. Toxicol Mech Methods. 2016;26:658–66. doi: 10.1080/15376516.2016.1223240. [DOI] [PubMed] [Google Scholar]
- 51.Thirumoorthy N, Shyam Sunder A, Manisenthil Kumar K, Senthil Kumar M, Ganesh G, Chatterjee M. A review of metallothionein isoforms and their role in pathophysiology. World J Surgical Oncol. 2011;9:54. doi: 10.1186/1477-7819-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JD, Liu Y, Klaassen CD. Protective effect of metallothionein against the toxicity of cadmium and other metals. Toxicology. 2001;163:93–100. doi: 10.1016/s0300-483x(01)00375-4. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Liu J, Iszard MB, Andrews GK, Palmiter RD, Klaassen CD. Transgenic mice that overexpress metallothionein-I are protected from cadmium lethality and hepatotoxicity. Toxicol Appl Pharmacol. 1995;135:222–8. doi: 10.1006/taap.1995.1227. [DOI] [PubMed] [Google Scholar]
- 54.Turan B, Tuncay E. Impact of labile zinc on heart function: From physiology to pathophysiology. Int J Mol Sci. 2017;18:pii: E2395. [DOI] [PMC free article] [PubMed]
- 55.Prasad AS. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol. 2014;28:364–71. doi: 10.1016/j.jtemb.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Nemmiche S. Oxidative signaling response to cadmium exposure. Toxicol Sci. 2017;156:4–10. doi: 10.1093/toxsci/kfw222. [DOI] [PubMed] [Google Scholar]
- 57.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–86. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278:2396–402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 59.Kovacs G, Montalbetti N, Franz MC, Graeter S, Simonin A, Hediger MA. Human TRPV5 and TRPV6: key players in cadmium and zinc toxicity. Cell Calcium. 2013;54:276–86. doi: 10.1016/j.ceca.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–43. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Finsterer J, Ohnsorge P. Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol. 2013;67:434–45. doi: 10.1016/j.yrtph.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Masumoto H, Nakane T, Tinney JP, Yuan F, Ye F, Kowalski WJ, et al. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci Rep. 2016;6:29933. doi: 10.1038/srep29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waza AA, Hamid Z, Ali S, Bhat SA, Bhat MA. A review on heme oxygenase-1 induction: is it a necessary evil. Inflamm Res. 2018;67:1–10. doi: 10.1007/s00011-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 64.Kaji T, Takata M, Hoshino T, Miyahara T, Kozuka H, Kurashige Y, et al. Role of zinc in protection against cadmium-induced toxicity in formation of embryonic chick bone in tissue culture. Toxicol Lett. 1988;44:219–27. doi: 10.1016/0378-4274(88)90149-x. [DOI] [PubMed] [Google Scholar]
- 65.Sterenborg I, Vork NA, Verkade SK, van Gestel CA, van Straalen NM. Dietary zinc reduces uptake but not metallothionein binding and elimination of cadmium in the springtail, Orchesella cincta. Environ Toxicol Chem. 2003;22:1167–71. [PubMed] [Google Scholar]
- 66.Zhang D, Liu J, Gao J, Shahzad M, Han Z, Wang Z, et al. Zinc supplementation protects against cadmium accumulation and cytotoxicity in Madin-Darby bovine kidney cells. PLoS One. 2014;9:e103427. doi: 10.1371/journal.pone.0103427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Li H, Soleimani M, Girijashanker K, Reed JM, He L, et al. Cd2+ versus Zn2+ uptake by the ZIP8 HCO3-dependent symporter: kinetics, electrogenicity and trafficking. Biochem Biophys Res Commun. 2008;365:814–20. doi: 10.1016/j.bbrc.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharm. 2008;73:1413–23. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knopfel M, et al. DMT1: which metals does it transport? Biol Res. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.