Abstract
Background
Breast cancer (BC) brain metastases (BM) can have discordant hormonal or human epidermal growth factor receptor 2 (HER2) expression compared with corresponding primary tumors. This study aimed to describe incidence, predictors, and survival outcomes of discordant receptors and associated subtype switching in BM.
Methods
BCBM patients seen at 4 tertiary institutions who had undergone BM resection or biopsy were included. Surgical pathology reports were retrospectively assessed to determine discordance between the primary tumor and the BCBM. In discordant cases, expression in extracranial metastases was also assessed.
Results
In BM from 219 patients, prevalence of any discordance was 36.3%; receptor-specific discordance was 16.7% for estrogen, 25.2% for progesterone, and 10.4% for HER2. Because estrogen and progesterone were considered together for hormonal status, 50 (22.8%) patients switched subtype as a result; 20 of these switches were HER2 based. Baseline subtype predicted switching, which occurred in up to 37.5% of primary HR+ patients. Moreover, 14.8% of initially HER2-negative patients gained HER2 in the BM. Most (63.6%) discordant patients with extracranial metastases also had discordance between BM and extracranial subtype. Loss of receptor expression was generally associated with worse survival, which appeared to be driven by estrogen loss (hazard ratio = 1.80, P = 0.03). Patients gaining HER2 status (n = 8) showed a nonsignificant tendency toward improved survival (hazard ratio = 0.64, P = 0.17).
Conclusions
In this multicenter study, we report incidence and predictors of subtype switching, the risk of which varies considerably by baseline subtype. Switches can have clinical implications for prognosis and treatment choice.
Keywords: brain metastases, breast cancer, receptor discordance, subtype
Key Points.
Breast cancer switches subtype in up to 37.5% of brain metastases, depending on baseline subtype.
HER2 gains occur in 14.8% of HER2-negative patients developing brain metastases.
Subtype can differ between primary, intracranial, and extracranial distant metastases.
Importance of the Study.
BM differ from their primary tumors on a genetic and phenotypic level. While it is known that BCBM can have discordant estrogen, progesterone, or HER2 expression, previous studies have been limited by sample size, and it is currently unknown in which patients a clinically relevant subtype switch could be anticipated. This large, multi-institutional study sheds light on previously unanswered aspects of this question, including the primary subtype-specific risk of crossover between breast and brain and the relation between intra- and extracranial metastatic profiles in discordant patients. Using our data and recent literature, we discuss different clinical scenarios in which receptor discordance/subtype switching could have a relevant impact on a patient’s prognosis and treatment. These results could inform clinicians when deciding whether obtaining BM tissue could have additional clinical value—for example, in patients who could be considered for either upfront radiotherapy or surgical resection.
Brain metastases (BM) are the most common intracranial tumors and are common sequelae of metastatic breast cancer (BC).1 Clinical subtype—as determined by estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status—is central to decision making in BC management and influences incidence, prognosis, and treatment of BCBM.2–4
Expression of ER, PR, and HER2 can differ between primary breast cancer and distant metastases. This discordance seems to occur in all metastatic sites and may influence subtype-directed management strategies.5 Current breast cancer guidelines advise re-biopsy and reassessment of ER, PR, and HER2 status in distant metastases.6–8 However, because of the invasive nature of neurosurgical procedures and the fact that BM resection or biopsy is not the standard of care for the majority of BCBM patients, knowledge on discordance in the brain is limited. Although minimally invasive central nervous system–targeted diagnostic approaches such as liquid biopsy and blood-based assays are emerging, these modalities are largely limited to the research setting.9 A few studies have investigated BM discordance,10–18 but most have been relatively small in size, which limited their ability to perform meaningful subtype-specific analysis. Moreover, the relation between receptor expression in intra- and extracranial distant metastases has not been investigated. As a result, the value of obtaining additional tissue in BCBM patients is unclear.
In this study, we aimed to compare ER, PR, and HER2 expression between primary breast cancer and BM in a large, multicenter cohort of BCBM patients. The primary objective was to describe the incidence of receptor discordance and resultant subtype switches. Secondary objectives were to identify predictors of discordance, compare intra- and extracranial receptor expression within patients, and explore survival outcomes of concordant and discordant patients.
Methods
Study Design and Data Collection
Data were collected from patient records in 4 tertiary academic hospitals: Brigham and Women’s Hospital/Dana-Farber Cancer Institute and Massachusetts General Hospital in Boston, Massachusetts; Haaglanden Medical Center in The Hague, Netherlands; and the University Medical Center Utrecht in Utrecht, Netherlands. Inclusion criteria were (i) female patients with BCBM who (ii) had a pathology report of BCBM that reported on ER, PR, or HER2 expression and (iii) had available data on ER, PR, or HER2 expression in the primary tumor. Additionally, (iv) in order to be included for survival analysis, patients had to have had their BM diagnosis before April 1, 2018, in order to ensure sufficient follow-up.
Data on receptor expression were collected from surgical pathology reports of primary breast cancers and BM. If available, receptor expression in extracranial metastases was also collected. ER and PR expressions were considered positive if there was >10% staining of cells in immunohistochemistry testing; this cutoff was used because it was the most common cutoff clinically used in all treatment sites over the duration of the study period. HER2 was considered positive if the pathology report described strong (3+) overexpression on immunohistochemical staining or moderate (2+) overexpression combined with HER2 amplification in fluorescent in situ hybridization (HER2/centromere 17 ratio >2.0).
Patients whose primary tumor pathology report was not available for direct review were included as long as their initial receptor status was well reported in clinical notes and apparent in treatment decisions. For example, if a patient’s primary breast cancer was reported in her oncology notes to be HER2+ and the patient received trastuzumab through another health care facility, she was considered breast tumor HER2+ even in the absence of an on-site pathology report of the primary tumor.
Based on receptor status, tumor subtypes were classified as follows: HR+/HER2+ (hormone receptor positive [ie, ER or PR positive] and HER2 positive]), HR+/HER2− (ER or PR positive and HER2 negative), HR−/HER2+ (ER and PR negative and HER2 positive), or HR−/HER2− (triple negative). Beside receptor expression, the following variables were collected: age, race/ethnicity, patterns and intervals of metastatic spread, hormonal and HER2-targeted therapy prior to the appearance of BM, surgical and radiation treatment modalities, and overall survival from the time of BM diagnosis.
Statistical Analysis
Data were analyzed using R version 3.4.3. Categorical variables were described using counts and percentages. Continuous variables were reported with mean and standard deviation if they followed a normal distribution; otherwise, median and interquartile range (IQR) were reported. Univariate and multivariate logistic regression were performed to determine predictors of receptor discordance.
Cox regression and the log-rank test were used to conduct survival analyses. Overall survival was defined as the interval between BM and death or loss to follow-up. Patients who did not die within the study period were censored at their date of last encounter or at the study cutoff date of April 1, 2019, whichever occurred first. When analyzing the survival impact of discordance, we used a multivariate Cox proportional hazards model, adjusting for age at BM diagnosis, hospital of treatment, receptor status (ie, effect of HER2 discordance was adjusted for hormonal receptor [HR] status, and vice versa), and additional potential confounders identified in univariate analysis (P < 0.10). Kaplan–Meier curves were created to visualize cumulative survival differences. P-values <0.05 were considered statistically significant.
Ethics Statement
Data in the United States institutions were collected under Partners and Dana-Farber institutional review board approval. Data in the Dutch institutions were collected after approval by the medical-ethical research committee. The need for informed consent was waived for this study.
Results
Descriptive Statistics
A total of 219 patients met inclusion criteria. Of these, 216 patients had complete data on ER expression, 210 on PR expression, and 201 on HER2 expression. In total, 193 patients had complete data on the expression of all 3 receptors in both breast and brain.
Table 1 presents baseline characteristics. The median age at BM resection was 52 years. In primary breast cancer, 117 (53.4%) patients were ER positive, 79 (36.9%) were PR positive, and 96 (43.8%) were HER2 positive. Ninety patients (41.1%) had no extracranial metastases at BM resection; median time between the primary tumor and the onset of BM was 36 months. Resection was performed in all but 3 patients, who underwent biopsy. Adjuvant BM treatment consisted of stereotactic radiosurgery (n = 128; 59.3%) and/or whole-brain radiotherapy (n = 115; 53.5%).
Table 1.
Baseline characteristics (N = 219)
| n (%) | |
| Race/ethnicity | |
| White | 185 (84.5) |
| Asian | 5 (2.3) |
| Black | 13 (5.9) |
| Hispanic | 3 (1.4) |
| Native American | 1 (.5) |
| Other/unknown | 12 (5.5) |
| Mean age, y, at diagnosis (SD) | 51.85 (10.61) |
| ER status breast | |
| Positive | 117 (53.4) |
| Negative | 102 (46.6) |
| Not determined | 0 (0.0) |
| PR status breast | |
| Positive | 79 (36.9) |
| Negative | 135 (61.6) |
| Not determined | 5 (2.3) |
| HER2 status breast | |
| Positive | 96 (43.8) |
| Negative | 114 (52.1) |
| Not determined | 9 (4.1) |
| History of hormonal treatment before BM onset | 126 (58.6) |
| History of HER2-targeted treatment before BM onset | 101 (47.4) |
| Extracranial metastases at the time of BM diagnosis | |
| Yes | 129 (58.9) |
| No | 90 (41.1) |
| Brain metastasis free interval, mo, median [IQR] | 35.7 [18.2, 72.7] |
| Treatment | |
| Neurosurgical resection | 216 (99.1) |
| Stereotactic radiosurgery | 128 (59.3) |
| Whole-brain radiotherapy | 115 (53.5) |
| Year of BM diagnosis/treatment | |
| 2001–2005 | 13 (5.9) |
| 2006–2010 | 48 (21.9) |
| 2011–2018 | 158 (72.1) |
Brain metastasis free interval is the time period between the primary breast cancer diagnosis and the first brain metastasis.
Incidence of Discordance
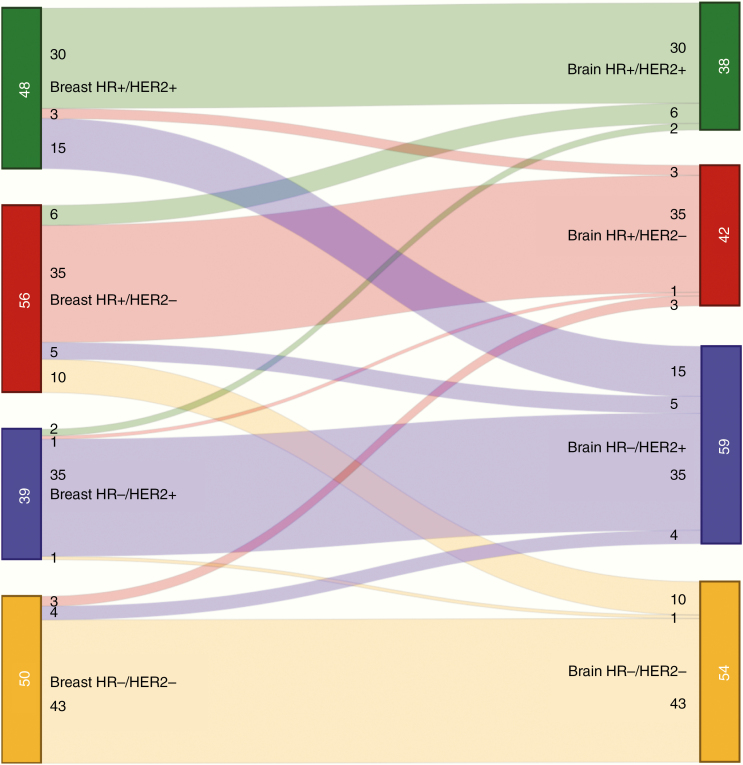
Detailed information regarding subtypes in the primary tumor and the BM is outlined in Table 2 and methodology used for the determination of receptor status is presented in Supplementary Tables 1 and 2. Out of 193 complete cases, 70 (36.3%) were discordant for ER, PR, or HER2. In 50 cases (22.8%), this resulted in a switch of tumor subtype (Table 2B). The remaining 20 cases featured a loss of ER or PR which did not lead to a loss of overall HR status. For individual receptors, discordance rates were as follows: ER, 36/216 cases (16.7%, of which 14.8% losses and 1.9% gains); PR, 53/210 cases (25.2%; 22.4% losses, 2.9% gains); and HER2, 21/201 cases (10.4%; 2.5% losses, 8.0% gains; Table 2C). Notably, in the group of patients who were HER2− at baseline (n = 108), 16 experienced a gain of HER2 status (14.8%). Figure 1 visualizes subtype crossover for all complete cases. The risk of switching varied depending on subtype of the primary tumor. A subtype switch occurred in 37.5% of both primary HR+/HER2− (21/56) and HR+/HER2+ (18/48) breast cancers. Primary triple negative cases switched in 14.0% (7/50), while primary HR−/HER2+ had the lowest incidence of switching at 10.3% (4/39).
Table 2.
Tumor subtypes and discordances
| Subtypes in Primary Tumor and Brain Metastasis | N | (%) | ||||
|---|---|---|---|---|---|---|
| Total number of cases | 219 | (100) | ||||
| Primary tumor subtype | ||||||
| HR+/HER2+ | 53 | (24.2) | ||||
| HR+/HER2− | 61 | (27.9) | ||||
| HR−/HER2+ | 42 | (19.2) | ||||
| HR−/HER2− | 53 | (24.2) | ||||
| Not determined | 10 | (4.6) | ||||
| Brain metastasis subtype | ||||||
| HR+/HER2+ | 40 | (18.3) | ||||
| HR+/HER2− | 47 | (21.5) | ||||
| HR−/HER2+ | 64 | (29.2) | ||||
| HR−/HER2− | 55 | (25.1) | ||||
| Not determined | 13 | (5.9) | ||||
| Overall discordance | N | (%) | ||||
| Total number of complete cases | 193 | (100) | ||||
| Breast—brain discordance (for any receptor) |
Discordant: 70
Concordant: 123 |
(36.3) (63.7) |
||||
| Breast—brain discordance (leading to a different subtype) |
Discordant: 50
Concordant: 143 |
(22.8) (78.2) |
||||
| Receptor-specific discordance | ||||||
| Receptor | ER | (%) | PR | (%) | HER2 | (%) |
| Total number of cases with determined receptor status | 216 | (100) | 210 | (100) | 201 | (100) |
| Primary tumor receptor status | + 117 | (54.2) | + 79 | (37.6) | + 93 | (46.3) |
| −99 | (45.8) | −131 | (62.4) | −108 | (53.7) | |
| Brain metastasis receptor status | + 89 | (41.2) | + 38 | (18.1) | + 104 | (51.7) |
| −127 | (58.8) | −172 | (81.9) | −97 | (48.3) | |
| Discordant | 36 | (16.7) | 53 | (25.2) | 21 | (10.4) |
| Gain of expression (% of receptor negative primary tumors) | 4 | (4.1) | 6 | (4.6) | 16 | (14.8) |
| Loss of expression (% of receptor positive primary tumors) | 32 | (27.4) | 47 | (59.5) | 5 | (5.4) |
+ = positive; − = negative; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.
Fig. 1.
Subtype switching.
Legend: Width of the bands indicates number of patients crossing over. + = positive; − = negative; HR = hormonal receptor; HER2 = human epidermal growth factor receptor 2.
For 98 cases from the Dana-Farber Cancer Institute, granular data on the percentage of ER/PR staining were available in both the primary tumor and the BM. This group was used to conduct a sensitivity analysis of ER/PR discordance using different staining thresholds. In the sample, discordance rates were similar when using the 10% versus 1% threshold for ER (16.3% vs 13.3%) or PR (33.3 vs 35.4%). Granular data are presented in Supplementary Table 3.
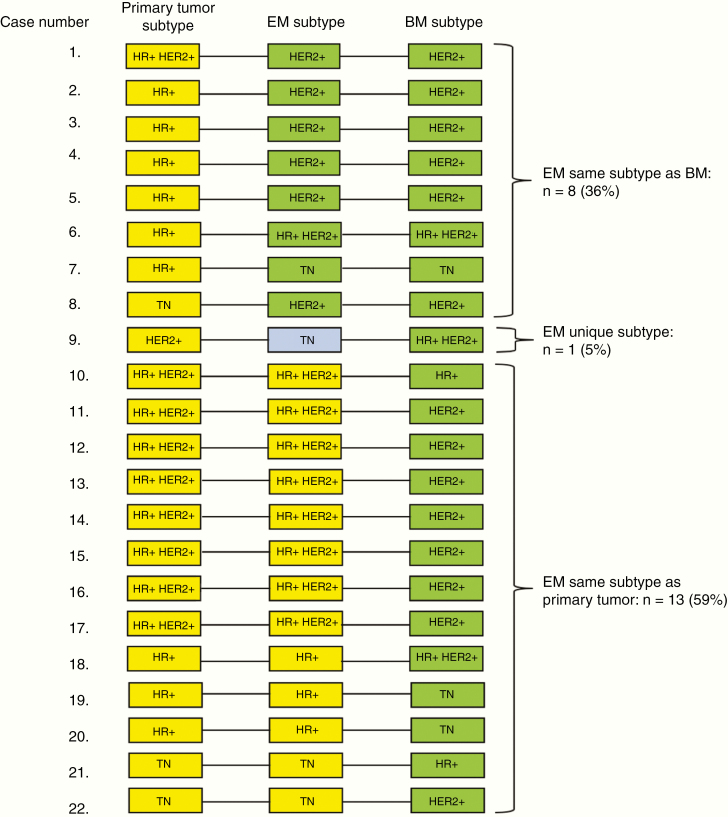
For 22 discordant cases, receptor expression in extracranial metastases was known. Here, subtype of the extracranial metastasis was concordant with the BM in 8 cases (36.4%). In the remaining 14 (63.6%) discordant cases, BM subtype was also discordant with extracranial subtype. In 13 cases (59.1%), the primary tumor and extracranial metastasis had the same subtype. In the remaining case (4.5%), 3 different subtypes were found in primary tumor, extracranial metastasis, and BM (Fig. 2).
Fig. 2.
Relation between intra- and extracranial subtype expression in discordant cases.
Legend: + = positive; − = negative; EM = extracranial metastasis; TN = triple negative.
Predictors of Discordance
In univariate logistic regression, ER+ and PR+ status in the primary tumor were associated with future receptor discordance (P < 0.001). Age at primary breast cancer, race/ethnicity, HER2 expression in the primary tumor, the presence of extracranial metastases at BM onset, or the interval between primary tumor and brain metastasis showed no association (all P-values >0.05). In multivariate analysis, both ER+ status (odds ratio [OR] = 3.07, 95% CI = 1.32–7.24; P = 0.009) and PR+ status (OR = 3.41; 95% CI = 1.54–7.78; P = 0.003) independently predicted discordance. These results remained significant after P-value adjustment for 7 degrees of freedom (ER: P = 0.03, PR: P = 0.02). Hormonal and HER2-targeted therapies prior to the onset of BM were administered in >90% of patients with HR+ and HER2+ tumors, respectively. Therefore, we were not able to meaningfully analyze the impact of systemic therapy on receptor switching.
Survival Analyses
Out of 193 complete cases, 144 were diagnosed before April 1, 2018, fitting the minimum follow-up criterion. Median survival since BCBM surgery in this cohort was 22.4 months (IQR: 6.5–51.1). All BM subtypes had significantly longer survival when compared with triple negative, while in the primary subtypes, only the HR−/HER2+ subtype had significantly improved survival over triple negative (Supplementary Table 4).
When looking at subtype discordance, patients were discretized as those who gained either HR+ or HER2+ expression, those who only lost an expression, and those who did not change subtype. After adjusting for age and treatment institution, patients who gained expression (n = 14) lived longer than those who did not switch (n = 94), although this did not reach statistical significance (55.6 vs 23.3 mo, P = 0.23; Supplementary Table 6 and Supplementary Figure 1). Patients who lost expression (n = 36) experienced a trend toward worse survival (16.6 vs 23.3 mo, P = 0.08; Supplementary Table 5 and Supplementary Figure 1).
To explore what drove these trends, we looked at receptor-specific discordance. In multivariate regression, loss of ER status predicted worse survival (hazard ratio = 1.80; P = 0.03), while gain of HER2 status showed a nonsignificant tendency toward longer survival (hazard ratio = 0.41, P = 0.17). No other discordances were associated with deviations in survival outcomes (Supplementary Table 5 and Supplementary Figure 1).
Systemic Therapy After Neurosurgery
Data on systemic therapy after craniotomy for BM were available for 181 patients. Of these, 157 received any type of postoperative systemic therapy. Forty-seven patients received endocrine therapy, 83 received HER2-targeted therapy, and 104 received chemotherapy. A granular breakdown of post-craniotomy systemic therapy stratified by subtype of primary tumor and brain metastases is presented in Supplementary Table 6. Within the group with sufficient follow-up, 13/15 patients with a HER2 gain received HER2-targeted therapy after craniotomy. Moreover, 29/31 patients with an HR loss received no further postoperative endocrine therapy.
Receipt of subtype-specific (ie, endocrine or HER2-targeted) therapy after BM resection was significantly associated with better overall survival, after adjusting for BM subtype, number of previous treatment lines, and institution of treatment (hazard ratio = 0.42, P = 0.001); this was especially pronounced for HER2-targeted therapy in HER2+ patients (hazard ratio = 0.18, P < 0.001). Because of increasing subgroup heterogeneity and small sample size, we were not able to meaningfully analyze the interactions between different subtype switches and postoperative therapies.
Discussion
In this large, multicenter cohort of 219 BCBM patients, we describe subtype-specific incidence, predictors, and clinical implications of subtype switching between BCBM and primary tumors as well as extracranial metastases.
A recent meta-analysis by Schrijver et al5 pooled previous series on receptor discordance in metastatic BC. A subset analysis for metastases to the brain included one retrospective cohort of 120 patients15 and a number of smaller series10–14,16–19 reporting between 20 and 50 patients, totaling 344 and 399 patients pooled for hormonal and HER2 discordance respectively. Results in the present study fall in line with this meta-analysis (ER discordance 16.7% in this study vs 20.8% in pooled analysis; PR discordance 25.2% vs 23.3%; HER2 discordance 10.4% vs 12.5%).5 Several genetic studies have corroborated these results; Brastianos et al20 found potentially clinically informative primary/BM discordances in >50% of analyzed BCBMs. Priedigkeit et al21 reported expression changes in clinically actionable genes in the majority of patients, with around 20% HER2 gain in baseline HER2− tumors.21
Baseline ER+ and PR+ status predicted discordance, resulting in a 37.5% switching rate in HR+ primary tumor patients. HR−/HER2+ primaries, on the other hand, had the lowest chance of switching subtype (10.2%). One explanation could be intratumoral heterogeneity.13,15,17,19 If a primary tumor contains a mixture of cell populations with varying receptor expression, subpopulations of ER− or HER2+ cells may be more neurotropic than others and be selected in brain metastases.22–25 This could also explain the relative preponderance of HER2 gain in our data, as opposed to the general breast cancer population, where HER2 loss occurs twice as often5; patients with HER2 loss would be less likely to develop BCBM. There may also be biological differences at baseline in tumors which eventually switch subtype that are also associated with BCBM. Garrido-Castro et al have reported that HR+ tumors that eventually lose HR tend to exhibit more basal features (eg, TP53 mutations) and fewer luminal features (eg, PIK3CA mutations) in the original tumor.26 Finally, heterogeneity in tissue fixation or interpathologist variation of tissue interpretation may account for a percentage of discordances.27–29 However, discordance rates were not lower in a large, blinded, single-pathologist series15 compared with other studies.5
The majority of patients with primary/BM discordance and data on extracranial distant sites also had intracranial/extracranial metastasis discordance. These findings lie in line with the emerging paradigm that brain metastases undergo branched evolution away from the genomic profile of their primary tumors or extracranial metastases,20 suggesting that it may be hard to infer BM profile from previously available extracranial metastases.
While we found some variation in survival based on receptor discordance, especially ER loss, these results should be interpreted with caution given the small sample size of discordant subgroups. While ER loss has been identified as a negative prognostic factor in breast cancer,13,30,31 previous studies in BCBM have not reported this effect.15,18
Strengths and Limitations
The main strengths of the present study are its large size and multi-institutional design. The study’s retrospective nature warrants caution when interpreting the results, in particular those related to survival outcomes. It also limited our ability to reliably reconstruct the treating oncologists’ rationales for choice of post-craniotomy systemic therapy and thereby to determine when subtype switches played a role in changing management. By presenting a breakdown of post-craniotomy systemic therapies in concordant and discordant cases, we hope to have provided a general overview of the clinical course in different patient groups. However, future studies should aim to evaluate treatment decisions in a prospective setting. Our analysis only included patients with resected or biopsied BM. Most BM patients do not undergo neurosurgery and it is unknown whether discordance rates would differ in these patients. Lastly, we used electronic pathology reports and did not perform central review and retesting of receptor expression due to logistical limitations. The international character of our study presented the main logistical obstacle to central tissue collection. Studies performing central review15 and those that did not5 have generally reported similar discordance rates; still, this limitation underlines the importance of interpreting our results in light of Schrijver’s meta-analysis.5 To address potential temporal variation in the use of a 10% versus 1% threshold for ER/PR positivity, we performed sensitivity analysis which showed similar discordance rates for both cutoff values; this is consistent with a previous sensitivity analysis in Schrijver’s meta-analysis.5
Implications
Subtype discordance may influence prognosis and treatment choice in BCBM patients. In the current series, HER2-targeted therapy after BM was strongly associated with longer survival. Previous studies have shown similar effects, even in patients without extracranial metastases.32–36 As clinical data accumulate for the efficacy of subtype-specific systemic therapies for BCBM (eg, lapatinib, tucatinib, or neratinib for HER2+ BCBM, endocrine therapy and cyclin-dependent kinase 4/6 inhibitors for ER+ BCBM), it is likely that knowledge of the BM receptor status will become increasingly important.34,37–44
In patients considered for either upfront radiation or resection with or without adjuvant radiation, our results would lean in favor of resection. Given the invasive nature of neurosurgery, however, our data also highlight the importance of developing less invasive ways to assess the receptor status of BM. While there has been active research in blood-based assays, including circulating tumor cells or cell-free tumor DNA, at the current time the concordance between BCBM receptor status and receptor status as determined using these technologies is largely unknown.9
Conclusion
In this multicenter, retrospective cohort of 219 patients, discordance between primary BC and BM was observed in 36.3% of patients, leading to a subtype switch in 22.8%. The risk of discordance in BM varied according to primary receptor status, with 37.5% of primary HR+ patients switching subtype. The majority of primary/BM discordant patients were also discordant between intra- and extracranial metastases. Given the potential clinical impact of a subtype switch, obtaining BM tissue could be considered in selected patients with a high risk of discordance.
Supplementary Material
Conflict of interest statement. Priscilla K. Brastianos has received funding from Merck, BMS. and Pfizer. She has received speaker’s honoraria from Merck and Genentech-Roche and is a consultant for Tesaro, Angiochem, Genentech-Roche, and Lilly. Nancy U. Lin has received funding for clinical trials from Genentech, Seattle Genetics, Novartis, Merck, and Pfizer, and has received compensation from Puma and Daiichi for service as a consultant. The other authors report no conflicts of interest.
Authorship statement. All authors contributed to the study conception and/or design. Data collection was performed by AFCH, AC, VK, AA, MH, CN, and MLDB. Institutional supervision for participating institutions was performed by NUL, PKB, MLDB, and JJCV. Statistical analysis was performed by AFCH. The first draft of the manuscript was written by AFCH. All authors critically revised subsequent versions of the manuscript. AFCH was responsible for compiling the final draft. All authors read and approved the final draft.
Previous presentations. Preliminary findings of the work as described in this manuscript have been presented at the 2019 Annual Meeting of the American Association of Neurological Surgeons (AANS) on April 13–17 in San Diego, CA, USA. This work has also been accepted for presentation at the 9th Annual Brain Metastases Research and Emerging Therapy Conference on October 4 and 5 in Marseille, France.
Funding
This work was supported in part by a grant from the Breast Cancer Research Foundation (to N.U.L.).
References
- 1. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. [DOI] [PubMed] [Google Scholar]
- 2. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin NU. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 2013;7:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schrijver WAME, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–580. [DOI] [PubMed] [Google Scholar]
- 6. Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–252. [DOI] [PubMed] [Google Scholar]
- 7. Carlson RW, Allred DC, Anderson BO, et al. ; National Comprehensive Cancer Network Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(7):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33(24):2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boire A, Brandsma D, Brastianos PK, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. 2019;21(5):571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaedcke J, Traub F, Milde S, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20(8):864–870. [DOI] [PubMed] [Google Scholar]
- 11. Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol. 2008;90(2):223–228. [DOI] [PubMed] [Google Scholar]
- 12. Omoto Y, Kurosumi M, Hozumi Y, et al. Immunohistochemical assessment of primary breast tumors and metachronous brain metastases, with particular regard to differences in the expression of biological markers and prognosis. Exp Ther Med. 2010;1(4):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brogi E, Murphy CG, Johnson ML, et al. Breast carcinoma with brain metastases: clinical analysis and immunoprofile on tissue microarrays. Ann Oncol. 2011;22(12):2597–2603. [DOI] [PubMed] [Google Scholar]
- 15. Duchnowska R, Dziadziuszko R, Trojanowski T, et al. ; Polish Brain Metastasis Consortium Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swain SM, Baselga J, Miles D, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomson AH, McGrane J, Mathew J, et al. Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br J Cancer. 2016;114(7):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jung J, Lee SH, Park M, et al. Discordances in ER, PR, and HER2 between primary breast cancer and brain metastasis. J Neurooncol. 2018;137(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Priedigkeit N, Hartmaier RJ, Chen Y, et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 2017;3(5):666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allott EH, Geradts J, Sun X, et al. Intratumoral heterogeneity as a source of discordance in breast cancer biomarker classification. Breast Cancer Res. 2016;18(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beca F, Polyak K. Intratumor heterogeneity in breast cancer. Adv Exp Med Biol. 2016;882:169–189. [DOI] [PubMed] [Google Scholar]
- 24. Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–4198. [DOI] [PubMed] [Google Scholar]
- 26. Garrido-Castro A, Hughes M, Cherniack A, et al. Abstract PD9-01: genomic alterations associated with loss of HR expression in metastatic breast cancer. Cancer Res. 2019;79(4 Supplement):PD09-01. [Google Scholar]
- 27. Altundag K. Is there a role of breast pathologist in diagnostic challenges of discordances in ER, PR, and HER2 between primary breast cancer and brain metastasis? J Neurooncol. 2018;138(1):219. [DOI] [PubMed] [Google Scholar]
- 28. Price JA, Grunfeld E, Barnes PJ, Rheaume DE, Rayson D. Inter-institutional pathology consultations for breast cancer: impact on clinical oncology therapy recommendations. Curr Oncol. 2010;17(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–3038. [DOI] [PubMed] [Google Scholar]
- 30. Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20(12):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karlsson E, Lindström LS, Wilking U, Skoog L, Johansson U, Bergh J. Discordance in hormone receptor status in breast cancer during tumor progression. J Clin Oncol. 2010;28(15_suppl):1009–1009. [Google Scholar]
- 32. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 33. Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol. 2007;85(3):311–317. [DOI] [PubMed] [Google Scholar]
- 34. Niwińska A. Brain metastases as site of first and isolated recurrence of breast cancer: the role of systemic therapy after local treatment. Clin Exp Metastasis. 2016;33(7):677–685. [DOI] [PubMed] [Google Scholar]
- 35. Yap YS, Cornelio GH, Devi BC, et al. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br J Cancer. 2012;107(7):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freedman RA, Gelman RS, Anders CK, et al. ; Translational Breast Cancer Research Consortium TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 39. Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22(3):625–630. [DOI] [PubMed] [Google Scholar]
- 40. Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105(3):613–620. [DOI] [PubMed] [Google Scholar]
- 41. Bergen ES, Berghoff AS, Medjedovic M, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res. 2019;25(9):2737–2744. [DOI] [PubMed] [Google Scholar]
- 42. Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010;21(5):942–948. [DOI] [PubMed] [Google Scholar]
- 43. Anders CK, Rhun EL, Bachelot TD, et al. A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR+, HER2- metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):1017–1017. [Google Scholar]
- 44. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.