Abstract
The growing epidemic of obesity and diabetes represents a growing health emergency, exemplified by a marked increase in cardiovascular and renal disease. As such, healthcare systems are increasingly focussing on therapeutic approaches to address these challenges. Cardiovascular outcome trials (CVOTs) evaluating glucagon-like peptide-1 (GLP-1) analogues have previously observed significant improvements in major adverse cardiac events in people with type 2 diabetes (T2D). However, their impact in obese people without T2D is unknown. The SELECT study is the first pharmacotherapy study in obesity powered for cardiovascular superiority and investigates the impact of semaglutide on cardiovascular disease outcomes in overweight and obese people without T2D. The results of this study will potentially redefine obesity management, especially as secondary outcomes of the study will include evaluation of health-related quality of life and incident diabetes rates. In another potentially evolutionary therapeutic step for the incretin class of therapeutic agents, the FLOW study is the first dedicated study to investigate the effects of GLP-1 receptor analogues on renal and cardiovascular outcomes in people with renal impairment and T2D. Post-hoc analyses of GLP-1 analogue CVOTs have demonstrated reduced adverse renal outcomes associated with their use. In this review we discuss the known impact of GLP-1 analogues on cardiovascular, weight and renal outcomes in previous CVOTs. We further discuss the importance of the ongoing SELECT and FLOW studies on shifting the paradigm of obesity pharmacotherapy and in adding to our understanding of renal disease management in people with T2D.
Keyword: Chronic kidney disease, FLOW study, GLP-1 analogue, Obesity, SELECT study, Semaglutide, Type 2 diabetes
Key Summary Points
| Glucagon-like peptide-1 (GLP-1) analogues are known to reduce major adverse cardiac events (MACE) in people with type 2 diabetes (T2D). |
| Secondary outcomes from previous cardiovascular outcome trials (CVOTs) support a reduction in renal events in people with T2D associated with GLP-1 analogue use, although no dedicated renal outcome study has yet been undertaken. |
| The SELECT study is the first GLP-1 analogue CVOT in people without diabetes, with the aim to determine whether semaglutide reduces MACE in overweight and obese people. |
| The FLOW study is the first dedicated GLP-1 analogue renal outcome trial in people with T2D, with the aim to determine whether semaglutide reduces adverse renal events in people with T2D and impaired renal function. |
| Semaglutide has the potential to have a major clinical impact in people with obesity, T2D and/or renal impairment, which will be addressed by the SELECT and FLOW studies discussed in this review. |
Background
The syndrome of obesity, diabetes, hypertension and dyslipidaemia is the major risk factor for many co-morbidities, such as cardiovascular disease (CVD) and chronic kidney disease (CKD) [1]. Despite some progress in the pharmacotherapy for people with such metabolic diseases, there remains an unmet need in many people with these conditions as morbidity, mortality and quality of life outcomes typically remain very poor [2, 3], contributing to a substantial economic burden to healthcare systems [4]. Therefore, treatments which improve cardiovascular and/or renal outcomes in people with risk factors such as obesity or diabetes would prove to have substantial impact on both clinical and economic outcomes in a patient population with an ever-increasing burden of these complex medical problems [5, 6].
Over the last decade, cardiovascular outcome trials (CVOTs) to evaluate the cardiovascular safety of diabetes medications in people with type 2 diabetes (T2D) have been conducted, with clinically important cardiovascular benefits observed in association with the use of both sodium-glucose co-transporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) analogues in people with T2D [7, 8]. However, whilst some trials evaluating SGLT-2 analogues have primarily studied renal outcomes in people with renal impairment and diabetes [9], dedicated GLP-1 analogue trials have not yet been taken in this area, with the renal safety and benefits of these medications being determined as secondary endpoints. There is also a growing focus on the potential benefits of SGLT-2 inhibitors in people without diabetes, particularly from the perspective of heart failure with both preserved and reduced ejection fraction. There is also a growing interest in the role of GLP-1 agonists outside the glucose-lowering indication of T2D, particularly in people with obesity.
Here we review previous GLP-1 analogue CVOTs, focussing on cardiovascular, renal and body weight outcomes. Furthermore, we focus on both the SELECT and FLOW studies that aim to determine the impact of the GLP-1 analogue semaglutide on cardiovascular outcomes in people with obesity without diabetes and renal outcomes in people with T2D and CKD, respectively.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Previous Cardiovascular and Renal Outcome Studies for GLP-1 Analogues
Albiglutide
Albiglutide was evaluated in the Harmony Outcomes trial in 9463 people with T2D and established CVD over a median follow-up period of 1.6 years. At baseline, the mean estimated glomerular filtration rate (eGFR) was 79.0 mL/min/1.73 m2. The primary outcome of 3-point major adverse cardiac events (MACE) occurred in fewer participants receiving albiglutide than placebo (hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.68–0.90). Secondary outcome analysis revealed no significant changes in renal function associated with albiglutide use. Additional weight loss in those receiving albiglutide compared with those administered placebo was 0.66 kg at 8 months and 0.83 kg at 16 months [10]. This CVOT has the shortest duration of follow-up of all GLP-1 analogue CVOTs published to date, which may have limited the potential to observe the effects of albiglutide on cardiovascular and renal events and changes in risk factors in the trial.
Dulaglutide
The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) study investigated the cardiovascular safety of dulaglutide in 9901 participants with T2D and previous cardiovascular event or cardiovascular risk factors over a median follow-up period of of 5.4 years. At baseline, the mean baseline eGFR was 76.9 mL/min/1.73 m2. The primary outcome of 3-point MACE occurred in significantly fewer people using dulaglutide than placebo (HR 0.88, CI 0.79–0.99). A subsequent analysis of renal outcomes associated with dulaglutide found a significant reduction in the number of participants attaining the renal composite outcome (HR 0.85, CI 0.77–0.93), largely driven by a reduction in new macroalbuminuria (HR 0.77, 0.68–0.87) [11, 12]. Participants using dulaglutide had a 1.46 kg greater weight loss than those in the placebo group, resulting in a greater body mass index (BMI) reduction of 0.53 kg/m2. In contrast to the Harmony Outcomes study evaluating albiglutide, this CVOT had the longest follow-up of all such studies investigating GLP-1 analogues.
Exenatide
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) study evaluated the cardiovascular safety of exenatide in 14,752 participants with T2D with or without CVD over a median follow-up period of 3.2 years. At baseline, mean eGFR was 76.3 mL/min/1.73 m2. The primary outcome of 3-point MACE occurred in fewer participants receiving exenatide than placebo (HR 0.91, CI 0.83–1.00). As part of a secondary analysis, the composite renal outcome occurred in fewer people receiving exenatide (HR 0.85, CI 0.73–0.98), with a lower rate of incident macroalbuminuria and worsening eGFR. Participants receiving exenatide were observed to have an additional mean 1.27 kg weight loss compared with the placebo group [13, 14]. Despite having the largest study population of all the GLP-1 analogue CVOTs, this study did not meet statistical significance for superiority with respect to major cardiovascular outcomes against placebo.
Liraglutide
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial evaluated the cardiovascular safety of liraglutide in 9340 people with T2D and either a history of CVD or cardiovascular risk factors over a median follow-up period of 3.8 years. At baseline, 76.9% of participants had an eGFR > 60 mL/min/1.73 m2. The primary outcome of 3-point MACE occurred in fewer people receiving liraglutide (HR 0.87, CI 0.78–0.97). Renal outcomes were evaluated as an exploratory secondary outcome, revealing that the renal composite outcome occurred in significantly fewer participants receiving liraglutide than in those receiving placebo (HR 0.78, CI 0.67–0.92). Whilst obesity was not a prespecified inclusion criterion, participants receiving liraglutide had 2.3 kg greater weight loss than those receiving placebo [15, 16]. The study was the first to demonstrate superiority with GLP-1 analogue use versus placebo with respect to 3-point MACE in people with T2D. However, the participants had a relatively high cardiovascular risk compared with the participants in other CVOTs evaluating GLP-1 analogues.
Lixisenatide
The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) study evaluated the cardiovascular safety of lixisenatide in 6068 people with T2D and a recent history of either myocardial infarction (MI) or hospitalisation for unstable angina over a median follow-up of 25 months. At baseline, the mean eGFR for participants in the study was 75.9 mL/min/1.73 m2. There was no significant difference in the number of participants reaching the primary endpoint (HR 1.02, CI 0.89–1.17). Renal outcomes were explored in a secondary analysis, observing a reduced mean percentage change in urinary albumin secretion and no differences in adverse renal events. Participants receiving lixisenatide were observed to have an additional weight loss of just 0.7 kg compared to placebo over the study period [17, 18].
Semaglutide
The first CVOT to explore the cardiovascular safety of semaglutide was the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes-6 (SUSTAIN-6) study, in which 3297 participants with T2D and a history of CVD or cardiovascular risk factors were observed over a median period of 2.1 years. At baseline, 71.5% of participants had an eGFR > 60 mL/min/1.73 m2. The primary composite outcome of 3-point MACE occurred in significantly fewer participants receiving semaglutide than in those receiving placebo (HR 0.74, CI 0.58–0.95). As a secondary outcome, reduced incident of new or worsening nephropathy was associated with semaglutide (HR 0.64, CI 0.46–0.88), largely driven by reduced macroalbuminuria (HR 0.54, CI 0.37–0.77). Participants receiving semaglutide lost more body weight than participants in the placebo group, with those on semaglutide 0.5 mg losing 2.9 kg more and those receiving semaglutide 1.0 mg losing 4.3 kg more than those in the placebo group [19].
The subsequent Peptide Innovation for Early Diabetes Treatment-6 (PIONEER-6) study evaluated oral semaglutide in 3183 participants with T2D and CVD or cardiovascular risk factors over a median follow-up period of 15.9 months. At baseline, 72.5% of participants had an eGFR > 60 mL/min/1.73 m2. The primary outcome of 3-point MACE occurred in significantly fewer participants receiving Semaglutide resulted in numerically, albeit not statistically significantly, fewer CV events (HR 0.79, CI 0.57–1.11). There were no reports of significant renal outcomes associated with semaglutide use in this study. In terms of body weight loss, participants receiving semaglutide lost a mean 3.4 kg more than did those in the placebo group [20]. Interestingly, the 3-point MACE was similar to the SUSTAIN-6 study in evaluating injectable semaglutide, although over a shorter study duration.
There is little debate that GLP-1 analogues reduce MACE in people with T2D. Indeed, a meta-analysis undertaken by Kristensen and colleagues [8] of GLP-1 analogue CVOTs demonstrated a 12% risk reduction (HR 0.88, CI 0.82–0.94) in 3-point MACE associated with GLP-1 analogue use. There was a reduction in the risk of cardiovascular death (HR 0.88. CI 0.81–0.96), fatal or non-fatal stroke (HR 0.84, CI 0.76–0.93), fatal or non-fatal MI (HR 0.91, CI 0.84–1.00), broad renal composite outcome (HR 0.83, CI 0.78–0.89) and all-cause mortality (HR 0.88, CI 0.88–0.95). However, previously completed CVOTs evaluating GLP-1 analogues include participants with T2D and varying cardiovascular risk, renal dysfunction and BMI, as summarised in Table 1. The results of completed CVOTs are presented in Table 2. Whilst GLP-1 analogues are associated with an increased risk of developing gastrointestinal side-effects, including nausea, vomiting and diarrhoea, there is little evidence to suggest a significantly increased risk of severe hypoglycaemia, pancreatitis, thyroid carcinoma or pancreatic cancer [8]. The ongoing SELECT and FLOW studies evaluating semaglutide in people without T2D and with CKD, respectively, have different study populations and outcomes than previous GLP-1 analogue trials, as discussed in detail in the following sections.
Table 2.
Trial outcomes from previously completed cardiovascular outcome trials evaluating the use of glucagon-like peptide-1 analogues
| Drug (study) | Cardiovascular disease | Renal disease | Weight |
|---|---|---|---|
| Albiglutide (HARMONY) n = 9463 [10] |
CV composite: HR 0.78 (CI 0.68–0.90) CV death: HR 0.93 (CI 0.73–1.19) All-cause mortality: 0.95 (CI 0.79–1.16) |
Change in eGFR (mL/min/1.73 m2): 8 months: − 1.1 (CI − 1.84 to − 0.39) 16 months: − 0·43 (CI − 1.26–0.41) |
Weight change (kg): 8 months: − 0.6 (CI − 0.83 to − 0.49) 16 months − 0.3 (CI − 1.06 to − 0.60) |
| Dulaglutide (REWIND) n = 9901 [11, 12] |
CV composite: HR 0.88 (CI 0.79–0.99) CV death: HR 0.91 (CI 0.78–1.06) All-cause mortality: HR 0·90 (0.80–1.01) |
Renal composite: HR 0.85 (CI 0.77–0.93) New macroalbuminuria: HR 0.77 (CI 0.68–0·87) Change in eGFR over study: + 0·.2 mL/min/1.73 m2 (CI − 0.11–0.96) |
Weight change (kg): − 1.46 (CI 1.25–1.67) Change in BMI (kg/m2): 0.53 (CI 0.46–0.61) |
| Exenatide (EXSCEL) n = 14,752 [13, 14] |
CV composite: HR 0.91 (CI 0.83–1.00) CV death: HR 0.88 (CI 0.76–1.02) All-cause mortality: HR 0.86 (CI 0.77–0.97) |
Renal composite: HR 0.85, CI 0.73–0.98 New macroalbuminuria: 2.2% (exenatide) vs. 2.5% (placebo) Change in eGFR over study: + 0.21 mL/min.1.73 m2 (CI − 0.27–0.70) |
Weight change (kg): − 1.27 (CI − 1.40 to − 1.13) |
| Liraglutide (LEADER) n = 9340 [15, 16] |
CV composite: HR 0.87; (CI 0.78–0.97) CV death: HR 0.78 (CI 0.66–0.93) All-cause mortality: HR 0.85 (HR 0.74–0.97) |
Renal composite: HR 0.78; (CI 0.67–0.92) New macroalbuminuria: HR 0.74 (CI 0.60–0.91) Change in eGFR over study: + 0.38 mL/min.1.73 m2 |
Weight change (kg): − 2.3 (CI−2.5–2.0) |
| Lixisenatide (ELIXA) n = 6068 [17, 18] |
CV composite: HR 1.02 (CI 0.89–1.17) CV death: HR 0.98 (CI 0.78–1.22) All-cause mortality: HR 0.94 (CI 0.78–1.13) |
New macroalbuminuria: HR 0.81 (CI 0.66–0.99) | Weight change (kg): − 0.7 (CI −0.9 to − 0.5) |
| Semaglutide (SUSTAIN-6) n = 3297 [19] |
CV composite: HR 0.74 (CI 0.58–0.95) CV death: 0.98 (CI 0.65–1.48) All-cause mortality: 1.05 (0.74–1.50) |
Renal composite: HR 0.64 (CI 0.46–0.88) New macroalbuminuria: HR 0.54 (CI 0.37–0.77) |
Weight change (kg): Semaglutide 0.5 mg: 2.9 (CI−3.5 to −2.3) Semaglutide 1.0 mg: 4.3 (CI−4.9 to −3.8) |
| Semaglutide (PIONEER-6) n = 3183 [20] |
CV composite: HR 0.79 (CI 0.57–1.11) CV death: HR 0.49 (CI 0.27–0.92) All-cause mortality: HR 0.51 (CI 0.31–0.84) |
eGFR ratio baseline to end of study: 0.98 | Weight change: − 3.4 kg |
Table 2 compares the cardiovascular, renal and weight change results from previously completed CVOTs
CI 95% Confidence interval, HR hazard ratio
Table 1.
Baseline participant characteristics in previously completed cardiovascular outcome trials evaluating the use of glucagon-like peptide-1 analogues
| Drug (study) | Cardiovascular disease | Renal disease (eGFR [mL/min/1.73m2]) | Obesity (BMI [kg/m2])a | Primary outcome |
|---|---|---|---|---|
| Albiglutide (HARMONY) n = 9463 [10] |
T2D (HbA1c > 7.0% (> 53 mmol/mol)) Established CVD |
Mean: 79.0 > 90: 29.8% 60–89: 46.7% < 60: 23.5% |
Mean: 32.3b < 30: 38.2% > 30: 61.3% |
Time to occurrence of: - death from CVD, - nonfatal MI or - nonfatal stroke |
| Dulaglutide (REWIND) n = 9,901 [11, 12] |
T2D (HbA1c < 9.5% (< 80 mmol/mol)) Age > 50 years with CVD or CV RF |
Mean: 76.9a > 90: 25.6% 60–89: 49.5% 30–59: 21.2% < 30: 1.1% |
Mean: 32.3 < 32: 53.7% > 32: 46.3% |
Time to occurrence of: - death from CVD, - nonfatal MI or - nonfatal stroke |
| Exenatide (EXSCEL) n = 14,752 [13] |
T2D (HbA1c 48–86 mmol/mol (6.5–10.0%) Previous CVD (73.1%) or no CVD (26.9%) |
Mean: 76.3 > 90: 29.0% 60–89: 49.3% 30–59: 21.6% < 30: 0.1% |
Mean: 31.8 < 30: 36.7% > 30: 63.3% |
Time to occurrence of: - death from CVD, - nonfatal MI or - nonfatal stroke |
| Liraglutide (LEADER) n = 9340 [15, 16] |
T2D (HbA1c > 7.0% (> 53 mmol/mol)) Age > 50 years + CVD, or age > 60 years + CV RF |
> 90: 35.1% 60–89: 41.8% 30–59: 20.7% < 30: 2.4% |
Mean: 32.5 < 30: 38.3% > 30: 61.7% |
Time to occurrence of: - death from CVD, - nonfatal MI or - nonfatal stroke |
| Lixisenatide (ELIXA) n = 6068 [17, 18] |
T2D (HbA1c 5.5–11.0% (36–96 mmol/mol)) MI or hospitalised for UA in last 180 days |
Mean: 75.9 > 90: 23.3% 60–89: 53.4% 30–59: 23.1% < 30: 0.2% |
Mean: 30.2 |
Time to occurrence of: - death from CVD, - nonfatal MI, - nonfatal stroke or - hospitalisation for UA |
| Semaglutide (SUSTAIN-6) n = 3297 [19] |
T2D (HbA1c > 7.0% (> 53 mmol/mol)) Age > 50 years + CVD, or > 60 years + CV RF |
> 90: 30.0% 60–89: 41.5% 30–59: 25.2% < 30: 3.3% |
Mean 32.8 < 30: 35.9% > 30: 61.7% |
Time to occurrence of: - death from CVD, - nonfatal MI or - nonfatal stroke |
| Semaglutide (PIONEER-6) n = 3183 [20] |
T2D (HbA1c > 7.0% (> 53 mmol/mol)) Age > 50 years + CVD, or > 60 years + CV RF |
> 90: 28.9%a 60–89: 43.6% 30–59: 26.0% < 30: 0.9% |
Mean 32.3 < 30: 40.2% > 30: 59.8% |
Time to occurrence of: - death from CVD, - nonfatal MI or - nonfatal stroke |
Table 1 summarises the baseline characteristics of study participants in previously completed cardiovascular outcome trialsCVOTs, comparing prevalence (as percentage of participants) of cardiovascular disease, renal disease and BMI in participants in each study
BMI Body mass index, CV RF cardiovascular risk factors, CVD cardiovascular disease, eGFR estimated glomerular filtration rate, HbA1c glycated haemoglobin, MI myocardial infarction, T2D type 2 diabetes, UA unstable angina
aSome eGFR data are missing
bSome BMI data are missing
Table 3.
Inclusion and exclusion criteria for the SELECT study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adults aged ≥ 45 years at the time of signing informed consent | CV related |
| BMI ≥ 27 kg/m2 | - MI, stroke, hospitalisation for unstable angina pectoris or TIA < 60 days prior to screening |
| Established CV disease | - Planned revascularisation known on the day of screening |
| - Prior MI | - NYHA Class IV heart failure |
| - Prior stroke | Glycaemia-related |
| - Symptomatic PAD | - HbA1c ≥ 48 mmol/mol (6.5%) |
| - History of T1D or T2D | |
| - Treatment with any GLP-1 analogue < 90 days before screening | |
| Other | |
| - History of pancreatitis | |
| - History of MEN type 2 or MTC | |
| - ESRD or dialysis | |
| - History of malignancy < 5 years prior to screening | |
| - Severe psychiatric disorder | |
| - Pregnancy, breast-feeding or intention to become pregnant | |
| - Any disorder or unwillingness which might jeopardise the patient’s safety or protocol compliance |
The Select Study: Expanding Our Understanding of Obesity Management
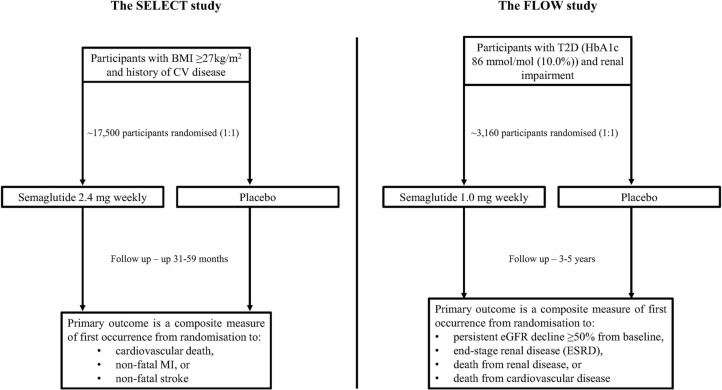
The SELECT study is a randomised, double-blind, parallel-group, placebo-controlled trial with the aim to determine the impact of subcutaneous semaglutide 2.4 mg once weekly on cardiovascular outcomes in overweight or obese participants with CVD who do not have diabetes. The primary endpoint is a 3-point MACE composite measure of cardiovascular death, nonfatal MI or nonfatal stroke. Secondary measures include cardiovascular outcomes, renal outcomes, changes in body weight and glycaemic control. The study commenced in October 2018 and is due to complete in September 2023, with an estimated 17,500 participants due to enrol [21, 22]. However, given the current health climate amidst the coronavirus pandemic we anticipate there may be some delays in study recruitment which may affect the study completion date. Inclusion and exclusion criteria are presented in Table 3, and the study design is summarised in Fig. 1.
Fig. 1.
Summary of the design of the SELECT and FLOW studies. BMI Body mass index, CV cardiovascular, eGFR estimated glomerular filtration rate, HbA1c glycated haemoglobin, MI myocardial infarction, T2D type 2 diabetes. Figure is adapted from Ryan et al. [21], ClinicalTrials.gov [22] and ClinicalTrials.gov [32]
The SELECT study is the first pharmacotherapy study in obesity designed to evaluate for cardiovascular outcome superiority. The results of this study have the potential to extend our understanding of both the clinical and economic value of obesity pharmacotherapy and as such may lead to a paradigm shift in the way in which obesity medications are reimbursed. Currently, the health benefits supporting the economic strategy of obesity pharmacotherapy focus on weight loss as a surrogate of endpoint benefits and health-related quality of life (HRQOL) gains [23]. However, weight loss per se may not beget improved cardiovascular health outcomes, as previously illustrated by the LOOK-AHEAD study. In that study, whilst significant weight loss in people with T2D was associated with improved glycated haemoglobin (HbA1c) and multiple cardiovascular risk factors, it did not translate into reduced cardiovascular events over a median follow-up of 9.6 years [24]. However, the cardiovascular event rate in the control arm of the study was low, thereby limiting the power of the study. Nevertheless, weight loss associated with liraglutide 3.0 mg in people with obesity not diagnosed with T2D was associated with several improved cardiovascular risk factors, including glycaemic control, blood pressure and lipid profile compared with placebo [25]. However, the study did not report on MACE associated with this weight loss, and follow-up was relatively limited at 56 weeks. In contrast, the SELECT study has the potential to demonstrate that GLP-1 analogue-mediated weight loss in obese people, without diabetes, can result in improved cardiovascular outcomes. As such, this study could be considered as a potential landmark trial in our understanding of obesity management strategies.
Previous CVOTs evaluating GLP-1 analogues have used populations that include subjects with T2D and high-risk or established CVD, as shown in Table 1 [10–13, 15, 17, 19]. In the SELECT study, the population is unique compared with previous CVOTs because no participant has underlying diabetes, all have a BMI ≥ 27 kg/m2 and CVD. Therefore, any cardiovascular benefit observed in the SELECT study would imply that these benefits are unlikely due to improved glycaemic control and that other mechanisms are implicated, such as changes in weight, blood pressure, dyslipidaemia, endothelial function and markers of inflammation [26]. A subgroup analysis of the LEADER study found a greater reduction in 3-point MACE in people with BMI > 30 kg/m2 (HR 0.82, CI 0.71–0.94) than in people with BMI < 30 kg/m2 (HR 0.96, CI 0.81–1.15), which translates to a 14% greater relative risk reduction in obese participants. Interestingly, when participants were compared according to baseline HbA1c, the difference in cardiovascular outcomes was less apparent. Participants with a baseline HbA1c > 8.3% (67 mmol/mol) had a HR 0.84 (95% CI 0.72–0.98) for 3-point MACE, versus a HR 0.89 (95% CI 0.76–1.05) in participants with a baseline HbA1c ≤ 8.3% (67 mmol/mol), which is a 5% reduction in the relative risk [15]. This result implies that the impact of GLP-1 analogues on MACE risk reduction is influenced more by weight than by glycaemic control.
Trials investigating weight loss outcomes associated with GLP-1 analogues have observed a clinically important mean additional weight loss, with a greater weight loss associated with semaglutide than with other GLP-1 analogues, such as dulaglutide and liraglutide [27–29]. However, the cardiovascular benefit of this additional weight loss and of other improved cardiometabolic measures associated with GLP-1 analogues is unknown, particularly in people without diabetes. Previous studies evaluating semaglutide in mixed cohorts including people with or without diabetes have found significant weight loss in participant subgroups with and without diabetes [30]. Thus, the SELECT study will evaluate semaglutide in the treatment for obesity and prevention of CVD in addition to its benefits on cardiovascular risk factors, including glycaemic control and other metabolic measures [21].
Two key secondary outcome measures from the SELECT study are changes in glycaemic control and HRQOL measures. Data on changes in glycaemic control will determine the potential impact of semaglutide on incident diabetes in this high-risk cohort. Previous studies found that liraglutide use in people with prediabetes and BMI > 30.0 kg/m2 was associated with an almost 80% relative risk reduction in incident T2D over 3 years [31]. Determining the impact of semaglutide on HRQOL may further support semaglutide use in this group and back changes in health economic policy, such as the reimbursement of obesity medications. Other secondary measures evaluating changes in body weight and renal function will also help inform approaches to optimal weight management.
The Flow Study: Informing the Role GLP-1 Receptor Agonists on Diabetic Kidney Disease
The FLOW study is a randomised, double-blind, parallel-group, placebo-controlled trial evaluating the safety and impact of semaglutide 1 mg once weekly in people with renal impairment and T2D on major adverse renal events. The primary endpoint is a renal composite measure of a persistent eGFR decline ≥ 50% from the start of trial, end-stage renal disease, death from renal disease or death from CVD. Secondary outcome measures include renal outcomes, cardiovascular outcomes, changes in body weight, glycaemic control and blood pressure. The study aims to recruit 3160 participants and commenced in June 2019, with an estimated completion date of August 2024 [32]. Similar to the SELECT study, we expect delays in completion of the study given the current health climate during the coronavirus pandemic. Criteria for participant inclusion are presented in Table 4, and the study design is summarised in Fig. 1.
Table 4.
Inclusion and exclusion criteria for the FLOW study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Aged ≥ 18 years at time of consent | Congenital or hereditary renal disease including PKD or congenital urinary tract malformations |
| Diagnosed with T2D | Use of any GLP-1 analogue < 30 days prior to screening |
| HbA1c ≤ 86 mmol/mol (10.0%) | MI, stroke, hospitalisation for unstable angina or TIA < 60 days prior to screening |
| Renal impairment defined as either: | NYHA Class IV heart failure |
| - eGFR 50–75 mL/min/1.73 m2 and UACR 300–5000 mg/g, | Planned coronary, carotid or peripheral artery revascularisation |
| - eGFR 25–50 mL/min/1.73 m2 and UACR 100–5000 mg/g | Haemodialysis or peritoneal dialysis < 90 days prior to screening |
| Uncontrolled or potentially unstable diabetic retinopathy or maculopathy. Verified by examination < 90 days prior to screening | |
| Treatment with maximum tolerated dose of an ACE inhibitor or ARB, unless contraindicated and stable for 4 weeks prior to screening |
The FLOW study is the first dedicated renal outcomes trial evaluating GLP-1 analogues in people with T2D. Previously, the only diabetes medication to have a similar dedicated renal outcomes study is the SGLT-2 inhibitor canagliflozin, in the CREDENCE trial [33]. Therefore, the FLOW study has the potential to have a major impact on improving our understanding of how to better manage people with T2D and renal disease in addition to our current management which often focusses on cardiovascular measures. We recently reviewed renal outcomes from CREDENCE and CVOTs which assessed renal outcomes, usually as secondary measures in SGLT-2 inhibitor CVOTs, and observed significant benefits associated with their use [34]. As discussed earlier and presented in Table 1, some benefits in renal outcomes have been observed as secondary measures in GLP-1 analogue CVOTs in people with T2D [34], largely mediated through effects on albuminuria. However, there is also variability between the CVOTs in the number of participants with differing degrees of renal disease; as such, the true effect of GLP-1 analogue therapy on renal outcomes is not completely understood. Thus, this dedicated renal outcome trial has the potential to support major progress in the management of people with T2D and kidney disease.
The FLOW study would also help further define the economic value of using GLP-1 analogues in people with T2D, particularly from the perspective of kidney disease, by establishing any potential reductions in the number of patients attaining major adverse renal outcomes, as discussed in the trial design. Whilst the number of people with T2D requiring major treatments such as dialysis or renal transplant therapy is low, the event cost is very high. In the UK alone in 2012, the cost of treating renal disease in people with diabetes was estimated at 10% of total health service spending in diabetes (£0.8 billion), projected to increase to a total £1.2 billion by 2035/2036 [35]. Indeed, the annual cost per patient undergoing renal dialysis is estimated at over £31,000 [36]. As such, the results of the FLOW study would help granulate our appreciation of the health economic impact of semaglutide. Additionally, the secondary outcome measures in the FLOW study exploring changes in CVD outcomes, body weight, blood pressure, lipid and glycaemic control will add substantially to the growing body of evidence which supports semaglutide use in routine diabetes care.
Conclusions
The personal and economic burden of obesity, diabetes and their cardiometabolic complications remains substantial despite some progress in their management. To date, CVOTs evaluating GLP-1 analogues have used broadly similar study populations, including people with T2D and established or high-risk of CVD. Ongoing studies to evaluate the cardiovascular and renal impact of semaglutide are using different study populations compared with previous CVOTs. The SELECT study includes overweight and obese people with established CVD without diabetes. The results of this trial will help us understand whether GLP-1 analogues can reduce MACE independent of diabetes diagnosis. This may result in a shift in not only the way in which we view outcomes in obesity trials, but also in the economic strategy used to reimburse obesity pharmacotherapy. The FLOW study is the first dedicated renal outcomes study evaluating a GLP-1 analogue in people with renal impairment and T2D, and will add substantially to the evidence available from secondary analyses of previous CVOTs. Similarly, further analyses of the FLOW study and previous CVOTs evaluating semaglutide will help us better understand the health economic benefits associated with its use beyond simple measures of metabolic control.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
David M Williams has no conflicts of interest to declare. Marc Evans received financial support for consultancy from Novartis, Merck Sharp & Dohme Corp. and Novo Nordisk and has served on the speaker’s bureau for Novartis, Lilly, Boehringer lngelheim, Merck Sharp & Dohme Corp., Novo Nordisk, Janssen and Takeda. Marc Evans is also the Editor-in-Chief of Diabetes Therapy.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12826736.
The original online version of this article was revised due to scientific error in the text.
Change history
1/8/2021
The original article can be found online.
References
- 1.Lee SJ, Lee HJ, Oh HJ, et al. Metabolic syndrome status over 2 years predicts incident chronic kidney disease in mid-life adults: a 10-year prospective cohort study. Sci Rep. 2018;8(1):12237. doi: 10.1038/s41598-018-29958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11:74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120–129. doi: 10.4239/wjd.v8.i4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14(4):435. doi: 10.3390/ijerph14040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magliano DJ, Islam RM, Barr ELM, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. 2019;366:l5003. doi: 10.1136/bmj.l5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7(4):276–288. doi: 10.1007/s13679-018-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e014908. doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 9.Lo KB, Gul F, Ram P, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020;10(1):1–10. doi: 10.1159/000503919. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 13.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bethel MA, Metnz RJ, Merrill P, et al. Renal outcomes in the exenatide study of cardiovascular event lowering (EXSCEL) Diabetes. 2018;67:1. doi: 10.2337/db18-ti01. [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann JFE, Ørsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 18.Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):859–869. doi: 10.1016/S2213-8587(18)30268-7. [DOI] [PubMed] [Google Scholar]
- 19.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 20.Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 21.Ryan DH, Lingvay I, Colhoun HM, et al. SELECT (Semaglutide effects on cardiovascular outcomes in people with overweight or obesity) rationale and design. Am Heart J. 2020 doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT). 2020. https://clinicaltrials.gov/ct2/show/NCT03574597. Accessed 28 July 2020
- 23.Baum C, Andino K, Wittbrodt E, et al. The challenges and opportunities associated with reimbursement for obesity pharmacotherapy in the USA. Pharmaco Econo. 2015;33(7):643–653. doi: 10.1007/s40273-015-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Look AHEAD Research Group. Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2014;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 30 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 26.Del Olmo-Garcia MI, Merino-Torres JF. GLP-1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. J Diabetes Res. 2018;8:4020492. doi: 10.1155/2018/4020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astrup A, Carraro R, Fine N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36(6):843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 29.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 30.O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 31.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov (2020) A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). https://clinicaltrials.gov/ct2/show/NCT03819153. Accessed 28 July 2020.
- 33.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 34.Williams DM, Nawaz A, Evans M. Renal outcomes in type 2 diabetes: a review of cardiovascular and renal outcome trials. Diabetes Ther. 2020;11(2):369–386. doi: 10.1007/s13300-019-00747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–862. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 36.Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356(9241):1543–1550. doi: 10.1016/S0140-6736(00)03123-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.