Abstract
Purpose
Physical activity (PA) represents the first line of defence against diseases characterised by increased inflammation status, such as metabolic and infectious diseases. Conversely, a sedentary lifestyle—associated with obesity, type 2 diabetes and cardiovascular disorders—negatively impacts on general health status, including susceptibility to infections. At a time of a pandemic SARS-CoV2 infection, and in the context of the multiorgan crosstalk (widely accepted as a mechanism participating in the pathophysiology of all organs and systems), we examine the complex interplay mediated by skeletal muscle contraction involving the immune system and how this contributes to control health status and to counteract viral infections. In so doing, we review the molecular mechanisms and expression of molecules modulated by PA, able to provide the proper molecular equipment against viral infections such as the current SARS-CoV2.
Methods
A critical review of the literature was performed to elucidate the molecular mechanisms and mediators induced by PA that potentially impact on viral infections such as SARS-CoV2.
Results
We showed the effects mediated by regular moderate PA on viral adverse effects through the regulation of biological processes involving the crosstalk between skeletal muscle, the immune system and adipose tissue. Evidence was provided of the effects mediated by modulation of the expression of inflammation markers.
Conclusion
A tigth association between PA and reduction in inflammation status allows effective counteracting of SARS-CoV2 infection. It is therefore essential to persuade people to keep active.
Keywords: Physical activity, Inflammation, Cytokines, COVID-19, Healthy lifestyle, Metabolic disorders, Immune system
Introduction
Since January 2020, the pandemic wave of the novel severe acute respiratory syndrome coronavirus (SARS-CoV2) has hit almost all countries. Data updated July 27 2020, report 16,481,022 cases and 653,296 deaths worldwide for the coronavirus disease 2019 (COVID-19) (https://www.worldometers.info/coronavirus/). COVID-19 has a high transmission rate, and the current lack of a specific vaccine means that we cannot rule out the possibility of a second wave.
To prevent and limit the spread of the virus, almost all national health surveillance departments have adopted “lockdown” policies, including social distancing, frequent hand washing, use of face masks, travel restrictions, and suspension of physical and recreational activities. Furthermore, in the event of a very high reproduction number (R > 2), many governments have imposed quarantine. Confinement at home, lasting from 4 to 8 weeks, has negatively impacted the population in several ways. The consequences of imposed lockdown include a substantial change in lifestyle characterised by an increase in sedentary routines and weight gain (Martinez-Ferran et al. 2020; King et al. 2020; Narici et al. 2020). Such a long period of restricted movement has a negative impact on everybody, regardless of age, sex and ethnicity, and particularly on sedentary and elderly subjects. Older people, who generally have reduced physical activity (PA), have proven to be the preferential target of the SARS-CoV2 infection, while children often have a milder form of the disease and their deaths have been extremely rare (Ludvigsson 2020). The reasons why the outcomes are highly heterogeneous (asymptomatic, mild symptoms or severe respiratory syndrome resulting in death) are largely unknown (Lai et al. 2020), though several comorbidities have been associated with an increased rate of contagiousness, as well as with a worse prognosis for the disease (Rodriguez-Morales et al. 2020). It is noteworthy that many of the comorbidities are metabolic-related disorders and/or immune dysregulations, both associated with increased inflammation status and always affected by regular PA. On the other hand, a sedentary lifestyle represents a clear risk factor for many diseases, including viral infections (Knight 2012).
In this scenario, regular PA is instrumental in providing the correct molecular equipment to counteract a new putative pandemic wave. The present manuscript reviews the current scientific knowledge concerning the molecular effects mediated by regular PA on the immune system, skeletal muscle and adipose tissues focusing specifically on the crosstalk mediated by inflammatory cytokines. To our knowledge, specific evidence regarding the influence of PA on the prevention of severe symptoms of patients suffering from SARS-CoV2 are not available. Therefore, we examined, for the first time, the molecular effects induced by PA that potentially could mitigate deleterious effects induced by SARS-CoV2 infection.
General features of SARS-CoV2 infection and comorbidities
Coronaviruses (CoVs) belong to the subfamily of coronavirinae, classified into four genera, namely α-coronavirus, β-coronavirus, γ-coronavirus and δ-coronavirus, (de Groot et al. 2013; Li et al. 2005). Of these four subfamilies, only α and β CoVs are able to infect humans, causing respiratory disease with a wide range of clinical phenotypes, from a mild influenza to severe respiratory disease and, in the worst case, death (Hashem et al. 2020). CoVs are large enveloped viruses with a positive large single-stranded RNA, ranging from 26 to 32 kb (Perlman and Netland 2009). Two βCoVs, namely SARS-CoV and MERS-CoV, caused a severe epidemic respiratory syndrome in 2002 and 2012, respectively (Ahn et al. 2020), while SARS-CoV2 emerged in 2019, reaching pandemic proportion within a few months of its appearance. The reservoirs of CoVs are bats and the intermediate host has been identified for SARS and MERS in masked palm civet cats and camels, respectively; the intermediate host of SARS-CoV2 has not yet been identified, although pangolins and mink are possible candidates (Xu et al. 2020).
SARS-CoV2 infects human epithelial cells through its surface glycoprotein, named Spike, which binds the angiotensin-converting enzyme 2 (ACE2) transmembrane protein (Spinelli et al. 2020); ACE2 mediates the virus’s entry into the cells. Although ACE2 is expressed in vascular endothelia, renal and cardiovascular tissues, skeletal muscle and epithelia of the small intestine and testes, the high expression in alveolar epithelial cells accounts for the specificity of the lung infection and the respiratory symptoms (Jia et al. 2005). Recently, the modelling structure of SARS-CoV-2 Spike predicts that this glycoprotein can also interact with human dipeptidyl peptidase 4 (DPP4) (Bassendine et al. 2020). Spike proteins form homotrimers protruding from the viral surface, thus contacting host cells. Spike monomer comprises two domains, S1 and S2: the first mediates receptor association and stabilisation, while the latter promotes membrane fusion (Perrotta et al. 2020).
Physiologically, ACE2 converts Angiotensin II (Ang II) into Angiotensin 1–7 (Ang 1–7), which activates, via Mas receptor, the so-called “counter-regulatory” or “vasodilator” Renin Angiotensin System (RAS) pathway: it provides a natural protection against acute lung injury, promoting multi-organ beneficial effects including vasodilatory, anti-proliferation, cardioprotective, anti-inflammatory and anti-fibrotic effects (Gaddam et al. 2014; Nunes-silva et al. 2017). The activation of this vasodilator RAS pathway opposes the molecular and cellular effects of the “classical” RAS pathway, involving ACE, Ang II and Angiotensin type 1 (AT1) receptor, which is associated to vasoconstriction, cell proliferation, organ hypertrophy and aldosterone release (Nunes-silva et al. 2017).
During SARS-CoV2 infection, the virus induces a downexpression of the ACE2 proteins as a consequence of the entry into the cells, thus reducing/compromising the beneficial vasodilator effects of the ACE2-Mas receptor pathway, thus contributing to the onset of the respiratory syndrome (Cheng et al. 2020). Virus replication itself causes an acute inflammatory response due to the activation of the innate immune system and the induction of cytokine expression by virus components such as the single-strand RNA. Accordingly, COVID-19 patients have high levels of circulating cytokines, termed “hypercytokinaemia”, which is directly correlated to disease severity (Wu et al. 2020). In very severe cases, high levels of circulating cytokines are referred to as a “cytokine storm”. Interestingly, clinical worsening is associated with a pronounced increase in the inflammatory state (Jamilloux et al. 2020).
As mentioned above, although highly infectious, SARS-CoV2 has a higher incidence among older people and/or those with comorbidities associated with ageing. Literature data have shown that metabolic diseases increase morbidity and mortality in patients with COVID-19, though the prevalence rate varied in different studies as well as in country-specific data (Guan et al. 2020; Wang et al. 2020; Pecoraro et al. 2017; Li et al. 2020). Singhal (2020) reported a prevalence of hypertension, type 2 diabetes and cardiovascular disorders in 21%, 11% and 7% of patients, respectively (Singhal 2020). Similarly, in a study involving 46,248 patients, Yang et al. (2020) described a prevalence of hypertension, type 2 diabetes and CVD in 17%, 8% and 5% of patients, respectively. The Epidemiology Working Group of the Chinese Center for Disease Control and Prevention investigated 20,982 patients affected by SARS-CoV2 and found that hypertension, type 2 diabetes and CVD were associated in 13%, 5% and 4% of patients, respectively [“The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China” 2020]. An Italian study by Onder et al. (2020) found type 2 diabetes in 36% and CVD in 43% of 355 Italian patients with SARS-CoV2. Similar evidence of risk among patients with type 2 diabetes has been reported for the two earlier CoV infections, SARS in 2002 and MERS in 2012 (Yang et al. 2006; Yu et al. 2006 Badawi and Ryoo 2016). In addition, plasma glucose levels and type 2 diabetes are independent predictors for mortality and morbidity in patients with SARS. Even for H1N1 influenza, metabolic disorders have been associated with symptom severity and mortality (Papp et al. 2002; Sun et al. 2016). Obesity has been added to the comorbidities able to exacerbate risk factors for poor outcome in coronavirus disease 2019 (COVID-19) (Vaduganathan et al. 2020).
Impact of physical exercise on organs’ response to viral infections (including SARS-CoV2)
Immune system/PA interplay under viral infection
PA includes all activities performed daily, including work, transportation and structured exercise training, and, in association with equilibrated diet, represents the main component of a healthy lifestyle. Regular moderate PA increases cardiorespiratory fitness, reduces risk of cardiovascular mortality and improves psychosocial wellbeing (Sigal et al. 2006).
Innate immunity, the first-line host defence system, is able to recognise general patterns associated with viral, bacterial and fungal infections, thus eliminating pathogen-damaged cells (Amano et al. 2014). Specifically, after exposure to a pathogen, the cells that present antigens block its replication via the phagocytosis of infected host cells; in particular, the defence system acts through pattern recognition receptors (PRRs), such as Toll-like (TLRs) and nucleotide-binding oligomerisation domain (NOD)-like receptors (NLRs), which bind the pathogen’s components (Amano et al. 2014). Through this mechanism, a long-lasting and highly specific adaptive immune response can also be triggered. Moreover, the rapid PRR activation of signalling cascades leads to release of different cytokines and chemokines, which initiate an inflammatory response.
Clinical evidence shows that many chronic inflammatory diseases contribute to a very poor outcome of infectious diseases; on the other hand, infectious diseases worsen the prognosis of metabolic chronic disorders (Fezeu et al. 2011). Immunocompromised subjects therefore have a high susceptibility to human pathogen infections, including COVID-19, due to an impaired innate immune system, including neutrophil and monocyte dysfunctions (Guan and Zhong 2020). The underlying mechanisms probably involve protein kinase C activation and TLR overexpression, with consequent inhibition of the neutrophil function and decreased phagocytosis (Gupta et al. 2007).
There is growing evidence focused on particular populations (i.e., obese and/or diabetic patients and HIV patients) that immune system activity is positively affected by type, intensity and duration of exercise (Nieman and Wentz 2019; Bermon et al. 2017). PA performed regularly and at moderate intensity (~ 60% VO2max to 60 min) stimulates the immune system by improving the function and action of tissue macrophages and promoting the activation and recirculation of key immune system factors, such as immunoglobulins, anti-inflammatory cytokines, neutrophils, NK cells, cytotoxic T cells and immature B cells. At molecular level, moderate PA induces downregulation of TLR expression and/or inhibition of TLR activation, in particular reduction of monocyte TLR4 expression (Collao et al. 2020).
It is noteworthy that moderate-intensity exercise and high cardiorespiratory fitness levels positively affect the expression of different immune markers in obesity, diabetes, cancer, cardiovascular disease and cognitive dysfunction (Zbinden-Foncea et al. 2020).
Moreover, PA elicits potent effects on the immune system by reducing the risk, duration and severity of different viral infections, presumably including COVID-19 (Zbinden-Foncea et al. 2020). In a recent study conducted in obese mice with H1N1 viral infection, the authors demonstrated that PA reversed the immune system alterations associated with obesity in the host’s immune defence (Warren et al. 2015). These results supports the positive effects of exercise on stimulation of the impaired immune system response in obese mice, thus promoting better recovery from viral infection (Luzi and Radaelli 2020).
Over time, these favourable changes can improve immunosurveillance against infectious pathogens and protect or mitigate the symptoms of infectious diseases (Nieman and Wentz 2019; Davison et al. 2016; Zheng et al. 2015); conversely, high-intensity training (> 70–75% VO2max), competitive sport and related physiological, metabolic and psychological stress are strongly associated with temporary negative changes in the immune response, inflammation, oxidative stress and increased risk of disease (Laddu et al. 2020). In this regard, it is important to highlight that there is a growing body of evidence indicating the positive effects of chronic exercise on immune competency in healthy young and/or elderly subjects, and even more so on immunocompromised patients (Sellami et al. 2018). On the other hand, the high risk of opportunistic infections and impairment of immune functions after high-intensity training continue to be debated due to contradictory data: studies reporting that acute exercise increases infections need to be cautiously considered (Campbell and Turner 2018). This susceptibility to infections, particularly respiratory infections, may be ascribed to other limiting factors (such as prolonged stress conditions, nutritional deficiency and exposure to an unhealthy environment) rather than to acute exercise.
Despite the controversial association between the immune system’s susceptibility to infections and exercise (regular-moderate to high-intensity), PA is a valid immunotherapeutic preventive strategy capable of improving immune response and therefore human quality of life (Rada et al. 2018).
Muscle/PA interplay under viral infection
Several studies dealing with the association between exercise and inflammation affirm that regular moderate PA (65–85%HRmax) (Hammami et al. 2020) reduces inflammation status, counteracts the insurgence of metabolic diseases, such as obesity and diabetes, and improves health status in subjects with increased inflammatory status, such as older people, subjects with CVD etc. (Nicklas et al. 2008; Park et al. 2014; Lancaster and Febbraio 2014; Allen et al. 2015; Pedersen 2017).
The anti-inflammatory response induced by regular PA is mediated by skeletal muscle contraction through the release of muscle-derived cytokines (myokines). Moderate PA induces a marked increase in serum levels of cytokines involved in the regulation of inflammation, such as IL-10, IL-1 receptor antagonist (IL-1ra), and IL-37 (Pedersen and Febbraio 2008; Fernandes et al. 2019; Abbasi et al. 2014; Nold et al. 2010). On the other hand, another myokine, IL-6, acts in suppressing the secretion of pro-inflammatory cytokines in several tissues, contributing to the creation of an anti-inflammatory environment for several hours after exercise.
In support of the anti-inflammatory effects of PA in the general population, exercise-induced IL-6 production has been shown to be regulated by the interaction of two pathways, that of activated nuclear T cell factor (NFAT) and that of mitogenic protein kinase activated by glycogen-p38 (MAPK) (Muñoz-Cánoves et al. 2013); as a consequence, the expression of TNFα or NF-kB increase during prolonged inflammatory responses (Pedersen and Febbraio 2008; Pedersen 2017). Similarly, in healthy exercising individuals there is a decrease in the pro-inflammatory macrophages of subtype 1 (M1) present in the muscles and an increase in the anti-inflammatory macrophages of subtype 2 (M2). PGC1α expression, which increases rapidly after one bout of exercise, has also been shown to generate polarisation of macrophages from M1 pro-inflammatory to M2 anti-inflammatory (Dinulovic et al. 2016). Moreover, PGC1α is also able to suppress the expression of inflammatory and increase the expression of anti-inflammatory cytokines, respectively (Eisele et al. 2015).
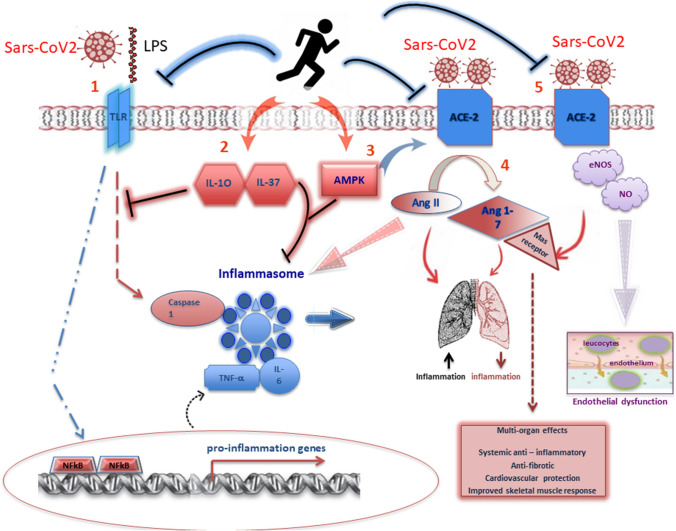
Another mechanism supporting the anti-inflammatory effects of regular PA could be ascribed to the modulation of TLR expression on monocytes and macrophages (Rada et al. 2018; Flynn and McFarlin 2006). In the past decade, the association between increased expression of TLRs, sedentary lifestyle, inflammation status and disease has been consolidated. McFarlin and colleagues also demonstrated, in physically active young and elderly subjects, a significant reduction in lipopolysaccharide-stimulated systemic production of IL-6, IL1β, TNF-α, high-sensitive C-reactive protein (hsCRP) and TLR4 expression (McFarlin et al. 2006). However, the molecule that could play a key role in mediating all the positive anti-inflammatory effects induced by PA in skeletal muscle is the Activated Mitogen Protein Kinase (AMPK). This fuel-sensing enzyme, activated in contracting skeletal muscles, stimulates energy-generating pathways such as glycolysis and fatty acid oxidation, and decreases energy-consuming processes such as protein and lipid bio-synthesis (Richter and Ruderman 2009). PA-mediated AMPK signaling accomplishes a dual purpose: it activates energy metabolism and can indirectly inhibit the inflammatory response induced by the NF-κB. This mechanism is associated with chronic stress, likewise occurring in metabolic syndrome as well as in type 2 diabetes and obesity (Liu and Chang 2018). Figure 1 shows the inflammatory and immune events regulated by PA, through the release of cytokines and the activation of elements of innate and adaptive immunity in muscle and adipose tissue.
Fig. 1.
Physical activity and anti-inflammatory response in SARS-CoV2 infection. Regular physical activity may reduce the acute inflammatory response through at least five mechanisms: (1) by reducing the inflammatory signalling pathway mediated by TLRs; (2) by increasing anti-inflammatory cytokines such as IL-10 and IL-37 which could inhibit the TLR-inflammatory signalling cascade and mitigate the inflammatory action produced by the inflammasome; (3) by reducing lung inflammation through the activation of AMPK and promoting the conversion from Ang II to Ang 1–7; (4) presumably, by the activation of ACE2-Mas receptor vasodilator pathway, reducing lung inflammation and promoting some beneficial multi-organ effects; (5) most probably, by restoring nitric oxide (NO) levels, in order to counteract the endothelial dysfunction, thus contributing to pulmonary vasodilation and antithrombotic activity
Lastly, the positive effects of regular PA on the heart and cardiovascular system should also be considered; it is well established that PA decreases the risk of CV disease and slows the progression of endothelial dysfunction, improving the blood flow and the organ perfusion (Broderick et al. 2019).
However, despite the robust evidence of the beneficial effects of PA on cardiovascular health, the mechanisms that promote cardiorespiratory fitness and decreases CVD risk are far from fully elucidated.
PA induces positive adaptations to both the heart and vascular system, leading to reductions in blood pressure, resting heart rate and atherogenic markers expression (Nystoriak and Bhatnagar 2018; Platt et al. 2015; Vega et al. 2017). In particular, the cell signalling molecular pathways involved in the exercise-induced cardiac beneficial response include—the phosphoinositide 3-kinase (PI3K)/Akt pathway, a crucial mediator of cardiac physiologic hypertrophic growth (Fukazawa et al. 2003; McMullen et al. 2003); the cellular signaling, through IGF-1R, and/or the insulin receptor (IR), that can induce cardiac metabolic adaptations. Furthermore, the activation of the ErbB2/ErbB4 tyrosine kinase receptors by the growth factor neuregulin-1 can stimulate PI3K signaling (D’Uva et al. 2015; Fukazawa et al. 2003), thus, promoting cardiac regeneration in the adult heart (Bersell et al. 2009; D’Uva et al. 2015). Neuregulin-1 expression is up-regulated in the heart after exercise (Cai et al. 2016; Waring et al. 2014); however, the exact role of this molecule in the cardiac adaptation to exercise, is still unclear.
Nitric oxide (NO) is known to be an important mediator of the beneficial effects of exercise both in heart and vascular system (Nystoriak and Bhatnagar 2018; Platt et al. 2015; Vega et al. 2017). NO generated by endothelial nitric oxide synthase (eNOS) during exercise, activates soluble guanylate cyclase (sGC) leading to cGMP increase and the activation of protein kinase G (PKG): the activation of this pathway has been shown to be cardioprotective (Rainer and Kass 2016). Furthermore, exercise-induced cardiac and circulating NO increase, protects against ischemia/reperfusion injury (Calvert et al. 2011). Finally, there is evidence that eNOS contributes to the cardiac metabolic adaptation to exercise, by increasing both mitochondrial biogenesis as well as PGC-1a expression (Vettor et al. 2014).
Lastly, regular PA could induce angiogenesis, even if the mechanisms underlying this process are still far from being elucidated; most probably, the increased expression of NO after exercise induces upregulation of pro-angiogenic factors, particularly Vascular Endothelial Growth Factor (VEGF), resulting in increased angiogenesis, with positive effects on endothelial function (Prior et al. 2004; Leosco et al. 2008).
SARS-CoV-2 can cause a cytokine storm that leads to the activation and the way out of inflammatory cytokines in a positive feedback round of inflammation; IL-6, C-reactive protein (CRP), D-dimer and ferritin, are the main cytokines to be used as predictors of poor prognosis for SARS-CoV2 (Taguchi and Mukai 2019; Donath et al. 2019; Quirch et al. 2020). On top of that, during disease worsening, a further gradual increase in IL-6 has been observed, and extremely high levels have been observed in dead patients (Ye et al. 2020; Zheng et al. 2015).
Furthermore, viral infections, including Sars-Cov2, are also characterised by endothelial dysfunction, with both eNOS and nitric oxide (NO) reduced expression and abnormally rapid blood clotting. Regular PA could counteract the pro-inflammatory effects caused by the virus; indeed, it has been hypothesised that the restoration of NO, regardless of eNOS, may counteract endothelial dysfunction and contribute to pulmonary vasodilation and antithrombotic activity (Green 2020). NO is also reported to compromise the binding between coronavirus S-protein and its host receptor, ACE-2. NO seems to regulate the S-nitrosylation of both viral cysteine proteases and host serine protease, TMPRSS2, a critical event for viral cellular entry (Hoffmann et al. 2020; Shulla et al. 2011).
Furthermore, ACE-2 prevents VEGF effects on vascular permeability during acute lung injury; in SARS-Cov-2 infection, where higher VEGF concentrations are reported, ACE-2 is downregulated and thus cannot counteract the VEGF-A effects, leading to the increase in vascular permeability and worsening of endothelial damage (Turkia M. COVID-19, Vascular Endothelial Growth Factor (VEGF) and Iodide (June 3, 2020). Available at SSRN: https://ssrn.com/abstract=3604987 or https://dx.doi.org/10.2139/ssrn.3604987).
Although, to date, the effects of PA on the ACE2 /Mas receptor vasodilator RAS pathway in humans has not been investigated, several experimental studies supported the idea that exercise can stimulate the vasodilator, counter-regulatory RAS pathway, inhibiting, at the same time, the action of the classic RAS pathway, thus promoting the beneficial multi-organ effects of ACE2 described above (Nunes-Silva et al. 2017).
Furthermore, many studies have recently demonstrated that the phosphorylation of ACE2 (Yan et al. 2020) improves Ang1-7, via AMPK, in pulmonary endothelial cells, thus reducing pulmonary hypertension (Zhang et al. 2018). Prata and colleagues demonstrated that moderate PA performed by mice with bleomycin-induced pulmonary fibrosis increased Ang1-7 via ACE2 in lung lesions, making these mice less susceptible to the disease (Prata et al. 2017).
Lastly, LPS, the pathogenic component of the cell wall of Gram-negative bacteria, and probably of SARS-CoV2, through LTR, could trigger a cascade of inflammation. This TLR-mediated intracellular pro-inflammatory signalling involves several proteins able to stimulate caspase 1 and induce activation of inflammasomes and transcription of pro-inflammatory genes through the nuclear factor-kappa B (NF-κB) (Zbinden-Foncea et al. 2020).
Figure 2 summarises the above-mentioned PA-mediated molecular mechanisms, providing evidence for the positive effects of PA in counteracting inflammation in SARS-COV2 infection.
Fig. 2.
Physical activity and anti-inflammatory response in muscle and adipose tissue. Physical activity induces an anti-inflammatory response in muscle and adipose tissue through the involvement of cells (e.g., macrophages), cytokines [e.g., interleukins (ILs)] and adipokines (e.g., adiponectin)
Adipose tissue/PA interplay under viral infection
Regular PA plays a critical role in maintaining adipose tissue physiology by controlling adiposity deposition, inflammatory state, immune responses and endocrine activity (Boa et al. 2017). As a result, PA reduces the inflammatory state of adipose tissue depending on the type, duration and intensity of the exercise (Golbidi and Laher 2014; Nigro et al. 2014). Conversely, inactivity together with an increase in energy intake leads to adipocyte hypertrophy, recruitment of immunological cells and release of pro-inflammatory adipokines (Kirk et al. 2020). The number of weekly bouts of exercise and their intensity are additional factors to take into account when looking at the positive effects of PA on adipose tissue health, above all if aimed at counteracting viral infection. In influenza virus epidemics, exercising at low to moderate intensity has been associated with lower risk of influenza-associated mortality (compared with exercising never or seldomly), while exercising frequently and at high intensity does not show any benefit (Wong et al. 2008).
On the other hand, the health of adipose tissue directly impacts on both non-communicable diseases, such as obesity and type 2 diabetes and communicable diseases, such as viral infections. Obesity has been added to the major comorbidities able to exacerbate risk factors for poor outcome also in COVID-19 (Vaduganathan et al. 2020; Klang et al. 2020) and identified as a risk factor for increased severity and mortality in non-pandemic and pandemic influenza (Honce and Schultz-Cherry 2019).
The mechanisms that underlie the connection between adipose tissue physiology and viral infections such as SARS-CoV2 are only partially known; one might be the involvement of ACE2, which is expressed in adipocytes and overexpressed in adipocytes of obese and/or type 2 diabetes patients compared to lean subjects (Kruglikov and Scherer 2020). ACE2-expressing adipocytes have also been reported as one of the entry points for some viruses, such as H1N1, type A influenza and SARS-CoV, though to the best of our knowledge there is no evidence for SARS-CoV2 entry through adipocytes (Gu and Korteweg 2007; Maier et al. 2018; Ryan and Caplice 2020). On top of this, adipose tissue supports sustained viral infection (Ryan and Caplice 2020), since it has been reported that it may act as a viral reservoir, as already proven for H5N1 virus infection (Nishimura et al. 2000).
Another mechanism linking adipose tissue health to viral infections is the endocrine function. Known as adipokines, several hormones secreted by adipose tissue are involved in the regulation of a number of biological processes, including energy storage, immune functions and inflammatory responses (Mancuso 2016). Aside from the production of adipokines, adipose tissue can also synthesise many cytokines, including IL-6, MCP1 and TNFα, which are collectively referred to as adipomyokines (Görgens et al. 2015). Collectively, these mediators can participate in the fight against viral infections by modulating the systemic inflammatory and immune state (Bourgeois et al. 2019). In obese and diabetic subjects, the increase in active immune cells is also responsible for the release of pro-inflammatory factors that in turn further promote macrophage (M1 phenotype) infiltration and mediate T cell recruitment and activation (Saltiel and Olefsky 2017). Some of these factors, like IL-6, are systemically released in the circulation, while others, like TNFα, are mainly retained in adipose tissue, where they act in the local hormonal milieu, leading to dysregulation in adipokine production (Desruisseaux et al. 2007; Corbi et al. 2019).
In addition, limited data suggest that obesity-induced systemic inflammation (described above) primes the immune system to generate a very intense cytokine storm when elicited by an infection (Ramos Muniz et al. 2018). It is essential to stress that a cytokine storm has been identified in the most severe COVID-19 cases, and the even more intense storm in obese COVID-19 patients may partially explain the elevated poor prognosis and mortality in these patients (Coperchini et al. 2020). In light of the above, unhealthy adipose tissue may, at least in part, contribute to a worse disposition towards and prognosis for viral infections—such as COVID-19—in the context of an already dysfunctional immune system (Desruisseaux et al. 2007).
The most powerful tool in maintaining or restoring the physiological state of adipose tissue is PA. The anti-inflammatory effects of regular PA on adipose tissue can be summarised in some fundamental molecular mechanisms (Pedersen and Febbraio 2008; Mathur and Pedersen 2008; Flynn and McFarlin 2006). The first mechanism can be ascribed to the reduction in fat mass and improvement in body composition, which result in decreased circulating levels of pro-inflammatory cytokines and adipokines, such as TNFα, retinol binding protein 4, resistin and leptin, and in increased levels of anti-inflammatory cytokines and adipokines, such as adiponectin and IL-10 (Gonzalez-Gil et al. 2019; Mujumdar et al. 2011; Ben Ounis et al. 2009; Lim et al. 2008; Metsios et al. 2020; Nigro et al. 2014; Orrù et al. 2017). Importantly, this regulation of adipokine levels depends on PA intensity and duration: chronic and moderate-intensity PA works better that high-intensity exercise in favouring the right balance of adipokine secretion (Görgens et al. 2015; Lehnig and Stanford 2018).
Secondly, the regulatory effects of PA on the endocrine function of adipose tissue could be either direct (as described above) or indirect, passing through the action of myokines from muscle tissue that in turn affect adipokine release as well as lipid and glucose metabolism (Laurens et al. 2020). Among the other myokines, IL-6, IL-8, and FGF seem to be most involved not only in favouring adipokine production, but also in controlling immune cell infiltration into adipose tissue, avoiding an imbalance in adipose vs non-adipose cells (Laurens et al. 2020).
In addition, in obesity PA is involved in the inhibition of inflammation through the reduction of plasma FFA levels, which suppresses TLR activation, one of the main triggers of the obesity-induced inflammatory response (Flynn and McFarlin 2006; Gleeson et al. 2011; Ringseis et al. 2015; Rada et al. 2018). Specifically, saturated fatty acids can activate TLR4, TLR2, which forms heterodimers in the plasma membrane, along with TLR1 or TLR6, inducing the synthesis of many cytokines (Rogero and Calder 2018).
Lastly, previous studies have reported that beneficial PA can induce the release and mobilisation of FAs from adipocytes to deliver them to working muscles, contributing to changes in the amount and composition of adipose tissue lipids (Mika et al. 2019).
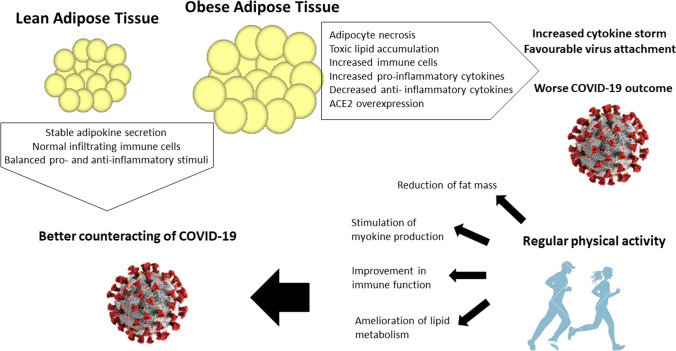
Figure 3 schematically reports the crosstalk between adipose tissue and PA under COVID-19.
Fig. 3.
Impact of physical activity on the response of adipose tissue to viral infections. Obese adipose tissue, contrary to lean adipose tissue, is characterised by several alterations that impair the anti-viral response (i.e., increased immune cells, increased pro-inflammatory cytokines, ACE2 overexpression). Regular physical activity improves most of these mechanisms (i.e., reduction in fat mass, improvement in immune function, amelioration of lipid metabolism), helping virus clearance
Conclusion
At a time of pandemic infection due to SARS-CoV2 infection, given the negative impact of metabolic disorders on susceptibility to infections it is essential to persuade people to keep active. Indeed, a promising approach to limiting fatal outcomes of COVID-19 and preventing serious symptoms is the adoption of lifestyle practices consistent with good immune health. In this regard, PA represents the first line of defence against metabolic disorders that negatively impact on susceptibility to infections. Nonetheless, lessons from previous influenza pandemics suggest that a sedentary lifestyle contributes to the creation of a positive environment for viral infections. Although to date specific data about the influence of PA on the prevention of severe symptoms of patients suffering from SARS-CoV2 are not available, this review provides strong evidence that regular PA represents a non-pharmacological tool that could improve the prognosis in the survivors.
As we wait for an anti-COVID-19 vaccine or the most promising treatment for the new CoV, regular and structured PA could be a complementary preventive intervention aimed at positively modulating immune response, thus reducing the negative impact of comorbidities in COVID-19 infection. Identifying intervention measures would be of major importance in containing the spread of the virus given that it does not seem that the pandemic will be brought under control in the near future.
Acknowledgements
This study was funded by “PRIN 2017” (code 2017RS5M44) to PB, the University of Naples “Parthenope”.
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- AMPK 5′
Adenosine monophosphate-activated protein kinase
- ARDS
Acute respiratory distress syndrome
- CoV
Coronavirus
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- DPP4
Dipeptidyl peptidase 4
- FoxO
Forkhead box O
- hsCRP
High-sensitive C-reactive Protein
- H1N1
Hemagglutinin Type 1 and Neuraminidase Type 1
- IL
Interleukin
- IL-1ra
Interleukin-1 receptor antagonist
- IL-6R
Interleukin-6 receptor
- LPS
Lipopolysaccharide
- M1
Macrophages of subtype 1
- M2
Macrophages of subtype 2
- MERS-CoV
Middle East respiratory syndrome coronavirus
- NFAT
Activated nuclear T cell factor
- NF-kB
Nuclear factor-kappa B
- NLRs
Nucleotide-binding oligomerisation domain (NOD)-like receptors
- PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator
- PRRs
Pattern recognition receptors
- SARS-CoV2
Severe acute respiratory syndrome coronavirus 2
- SIRT1
Sirtuin 1
- SNS
Sympathetic nervous system
- TLRs
Toll-like receptors
- TNF-α
Tumor necrosis factor-alpha
Author contributions
EN and AD contributed to the conception of the study. EN, RP, AA, AM, EI, and AE performed library searches and relevant data assembly. EN, RP, AA, AM, EI, AE, wrote the manuscript. EN, SO, PB, PK and AD critically revised the manuscript. All authors approved the final version.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Compliance with ethical standards
Conflicts of interest
The authors have no conflicts of interest that are directly relevant to the content of this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pasqualina Buono, Email: pasqualina.buono@uniparthenope.it.
Aurora Daniele, Email: aurora.daniele@unicampania.it.
References
- Abbasi A, Hauth M, Walter M, Hudemann J, Wank V, Niess AM, Northoff H. Exhaustive exercise modifies different gene expression profiles and pathways in LPS-stimulated and un-stimulated whole blood cultures. Brain Behav Immun. 2014;39:130–141. doi: 10.1016/j.bbi.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Ahn D-G, Shin H-J, Kim M-H, Lee S, Kim H-S, Myoung J, Kim B-T, Kim S-J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Sun Y, Woods JA. Exercise and the regulation of inflammatory responses. Prog Mol Biol Transl Sci. 2015;135:337–354. doi: 10.1016/bs.pmbts.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A, Ryoo SG. Prevalence of diabetes in the 2009 Influenza A (H1N1) and the middle east respiratory syndromec Coronavirus: a systematic review and meta-analysis. J Public Health Res. 2016;5(3):733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassendine MF, Bridge SH, McCaughan GW, Gorrell MD. Covid-19 and co-morbidities: a role for Dipeptidyl Peptidase 4 (DPP4) in disease severity? J Diabetes. 2020 doi: 10.1111/1753-0407.13052. [DOI] [PubMed] [Google Scholar]
- Ben Ounis O, Elloumi M, Lac G, Makni E, Van Praagh E, Zouhal H, Tabka Z, Amri M. Two-month effects of individualized exercise training with or without caloric restriction on plasma adipocytokine levels in obese female adolescents. Ann Endocrinol. 2009;70(4):235–241. doi: 10.1016/j.ando.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bermon S, Castell LM, Calder PC, Bishop NC, Blomstrand E, Mooren FC, Krüger K, Kavazis AN, Quindry JC, Senchina DS, Nieman DC, Gleeson M, Pyne DB, Kitic CM, Close GL, Larson-Meyer DE, Marcos A, Meydani SN, Wu D, Nagatomi R, et al. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Boa BCS, Yudkin JS, van Hinsbergh VWM, Bouskela E, Eringa EC. Exercise effects on perivascular adipose tissue: endocrine and paracrine determinants of vascular function. Br J Pharmacol. 2017;174(20):3466–3481. doi: 10.1111/bph.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, Le Grand R, Damouche A, Béréziat V, Lambotte O. Specific biological features of adipose tissue, and their impact on HIV persistence. Front Microbiol. 2019;10:2837. doi: 10.3389/fmicb.2019.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick TL, Sennott JM, Gutkowska J, Jankowski M. Anti-inflammatory and angiogenic effects of exercise training in cardiac muscle of diabetic mice. Diabetes Metab Syndr Obes. 2019;12:565–573. doi: 10.2147/DMSO.S197127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M-X, Shi X-C, Chen T, Tan Z-N, Lin Q-Q, Du S-J, Tian Z-J. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 2016;149:1–9. doi: 10.1016/j.lfs.2016.02.055. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Condit ME, Aragón JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108(12):1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Wang Y, Wang G-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collao N, Rada I, Francaux M, Deldicque L, Zbinden-Foncea H. Anti-inflammatory effect of exercise mediated by toll-like receptor regulation in innate immune cells—a review. Int Rev Immunol. 2020;39(2):39–52. doi: 10.1080/08830185.2019.1682569. [DOI] [PubMed] [Google Scholar]
- Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbi G, Polito R, Monaco ML, Cacciatore F, Scioli M, Ferrara N, Daniele A, Nigro E. Adiponectin expression and genotypes in italian people with severe obesity undergone a hypocaloric diet and physical exercise program. Nutrients. 2019 doi: 10.3390/nu11092195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- Davison G, Kehaya C, Wyn Jones A. Nutritional and physical activity interventions to improve immunity. Am J Lifestyle Med. 2016;10(3):152–169. doi: 10.1177/1559827614557773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RAM, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LLM, Snijder EJ, Stephens GM, Woo PCY, Zaki AM, Zambon M, Ziebuhr J. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desruisseaux MS, Nagajyothi T, M. E., Tanowitz, H. B., & Scherer, P. E. Adipocyte, adipose tissue, and infectious disease. Infect Immun. 2007;75(3):1066–1078. doi: 10.1128/IAI.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinulovic I, Furrer R, Di Fulvio S, Ferry A, Beer M, Handschin C. PGC-1α modulates necrosis, inflammatory response, and fibrotic tissue formation in injured skeletal muscle. Skeletal Muscle. 2016;6:38. doi: 10.1186/s13395-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Meier DT, Böni-Schnetzler M. Inflammation in the pathophysiology and therapy of cardiometabolic disease. Endocr Rev. 2019;40(4):1080–1091. doi: 10.1210/er.2019-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele PS, Furrer R, Beer M, Handschin C. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem Biophys Res Commun. 2015;464(3):692–697. doi: 10.1016/j.bbrc.2015.06.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Fernandes P, de Mendonça Oliveira L, Brüggemann TR, Sato MN, Olivo CR, Arantes-Costa FM. Physical exercise induces immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front Immunol. 2019;10:854. doi: 10.3389/fimmu.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, Kengne A-P, Hercberg S, Czernichow S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12(8):653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev. 2006;34(4):176–181. doi: 10.1249/01.jes.0000240027.22749.14. [DOI] [PubMed] [Google Scholar]
- Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35(12):1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014;13(4):224–234. doi: 10.2174/1871528113666140713164506. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res. 2014;2014:726861. doi: 10.1155/2014/726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil AM, Peschard-Franco M, Castillo EC, Gutierrez-DelBosque G, Treviño V, Silva-Platas C, Perez-Villarreal L, Garcia-Rivas G, Elizondo-Montemayor L. Myokine-adipokine cross-talk: potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome. Diabetol Metab Syndr. 2019;11:63. doi: 10.1186/s13098-019-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J. Exercise and regulation of adipokine and myokine production. Progr Mol Biol Transl Sci. 2015;135:313–336. doi: 10.1016/bs.pmbts.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Green SJ. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4–5):149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W-J, Zhong N-S. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med. 2020 doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- Guan W-J, Liang W-H, Zhao Y, Liang H-R, Chen Z-S, Li Y-M, Liu X-Q, Chen R-C, Tang C-L, Wang T, Ou C-Q, Li L, Chen P-Y, Sang L, Wang W, Li J-F, Li C-C, Ou L-M, Cheng B, He J-X, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin N Am. 2007;21(3):617–638, vii. doi: 10.1016/j.idc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hammami A, Harrabi B, Mohr M, Krustrup P. Physical activity and coronavirus disease 2019 (COVID-19): specific recommendations for home-based physical training. Manag Sport Leis. 2020 doi: 10.1080/23750472.2020.1757494. [DOI] [Google Scholar]
- Hashem AM, Alghamdi BS, Algaissi AA, Alshehri FS, Bukhari A, Alfaleh MA, Memish ZA. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: a narrative review. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honce R, Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, Walzer T, François B, Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PBJ. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Burke LM, Halson SL, Hawley JA. The challenge of maintaining metabolic health during a global pandemic. Sports Med. 2020;50(7):1233–1241. doi: 10.1007/s40279-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk B, Feehan J, Lombardi G, Duque G. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep. 2020 doi: 10.1007/s11914-020-00599-y. [DOI] [PubMed] [Google Scholar]
- Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JA. Physical inactivity: associated diseases and disorders. Ann Clin Lab Sci. 2012;42(3):320–337. [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddu DR, Lavie CJ, Phillips SA, Arena R. Physical activity for immunity protection: inoculating populations with healthy living medicine in preparation for the next pandemic. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35(6):262–269. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Laurens C, Bergouignan A, Moro C. Exercise-released myokines in the control of energy metabolism. Front Physiol. 2020;11:91. doi: 10.3389/fphys.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exp Biol. 2018 doi: 10.1242/jeb.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leosco D, Rengo G, Iaccarino G, Golino L, Marchese M, Fortunato F, Zincarelli C, Sanzari E, Ciccarelli M, Galasso G, Altobelli GG, Conti V, Matrone G, Cimini V, Ferrara N, Filippelli A, Koch WJ, Rengo F. Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res. 2008;78(2):385–394. doi: 10.1093/cvr/cvm109. [DOI] [PubMed] [Google Scholar]
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang L-F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Choi SH, Jeong I-K, Kim JH, Moon MK, Park KS, Lee HK, Kim Y-B, Jang HC. Insulin-sensitizing effects of exercise on adiponectin and retinol-binding protein-4 concentrations in young and middle-aged women. J Clin Endocrinol Metab. 2008;93(6):2263–2268. doi: 10.1210/jc.2007-2028. [DOI] [PubMed] [Google Scholar]
- Liu H-W, Chang S-J. Moderate exercise suppresses NF-κB signaling and activates the SIRT1-AMPK-PGC1α axis to attenuate muscle loss in diabetic db/db mice. Front Physiol. 2018;9:636. doi: 10.3389/fphys.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020 doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020 doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S, Burger-Calderon R, Kuan G, Harris E, Balmaseda A, Gordon A. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis. 2018;218(9):1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P. The role of adipokines in chronic inflammation. Immuno Targets Ther. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ferran M, de la Guía-Galipienso F, Sanchis-Gomar F, Pareja-Galeano H. metabolic impacts of confinement during the COVID-19 pandemic due to modified diet and physical activity habits. Nutrients. 2020 doi: 10.3390/nu12061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, Timmerman KL, Coen PM. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol Ser A Biol Sci Med Sci. 2006;61(4):388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100(21):12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. 2020 doi: 10.1016/j.berh.2020.101504. [DOI] [PubMed] [Google Scholar]
- Mika A, Macaluso F, Barone R, Di Felice V, Sledzinski T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front Physiol. 2019;10:26. doi: 10.3389/fphys.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar PP, Duerksen-Hughes PJ, Firek AF, Hessinger DA. Long-term, progressive, aerobic training increases adiponectin in middle-aged, overweight, untrained males and females. Scand J Clin Lab Invest. 2011;71(2):101–107. doi: 10.3109/00365513.2011.554995. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici M, De Vito G, Franchi M, Paoli A, Moro T, Marcolin G, Grassi B, Baldassarre G, Zuccarelli L, Biolo G, di Girolamo FG, Fiotti N, Dela F, Greenhaff P, Maganaris C. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur J Sport Sci. 2020 doi: 10.1080/17461391.2020.1761076. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu F-C, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Itamura S, Iwasaki T, Kurata T, Tashiro M. Characterization of human influenza A (H5N1) virus infection in mice: neuro-, pneumo- and adipotropic infection. J Gen Virol. 2000;81(Pt 10):2503–2510. doi: 10.1099/0022-1317-81-10-2503. [DOI] [PubMed] [Google Scholar]
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Silva A, Rocha GC, Magalhaes DM, Vaz LN, Salviano de Faria MH, Simoes E Silva AC. Physical exercise and ACE2-angiotensin-(1–7)-mas receptor axis of the renin angiotensin system. Protein Pept Lett. 2017;24(9):809–816. doi: 10.2174/0929866524666170728151401. [DOI] [PubMed] [Google Scholar]
- Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135. doi: 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Orrù S, Nigro E, Mandola A, Alfieri A, Buono P, Daniele A, Mancini A, Imperlini E. A functional interplay between IGF-1 and adiponectin. Int J Mol Sci. 2017 doi: 10.3390/ijms18102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Li X, Zhuang J, Wang R, Uhal BD. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L713–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- Park Y-M, Myers M, Vieira-Potter VJ. Adipose tissue inflammation and metabolic dysfunction: role of exercise. Mo Med. 2014;111(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- Pecoraro A, Nigro E, Polito R, Monaco ML, Scudiero O, Mormile I, Cesoni Marcelli A, Capasso M, Habetswallner F, Genovese A, Daniele A, Spadaro G. Total and high molecular weight adiponectin expression is decreased in patients with common variable immunodeficiency: correlation with Ig replacement therapy. Front Immunol. 2017;8:895. doi: 10.3389/fimmu.2017.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47(8):600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta F, Matera MG, Cazzola M, Bianco A. Severe respiratory SARS-CoV2 infection: does ACE2 receptor matter? Respir Med. 2020;168(April):105996. doi: 10.1016/j.rmed.2020.105996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt C, Houstis N, Rosenzweig A. Using exercise to measure and modify cardiac function. Cell Metab. 2015;21(2):227–236. doi: 10.1016/j.cmet.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata LO, Rodrigues CR, Martins JM, Vasconcelos PC, Oliveira FMS, Ferreira AJ, da Rodrigues-Machado MG, Caliari MV. Original research: ACE2 activator associated with physical exercise potentiates the reduction of pulmonary fibrosis. Exp Biol Med. 2017;242(1):8–21. doi: 10.1177/1535370216665174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol (1985) 2004;97(3):1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- Quirch M, Lee J, Rehman S. Hazards of the cytokine storm and cytokine-targeted therapy in COVID-19 patients: a review. J Med Int Res. 2020 doi: 10.2196/20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada I, Deldicque L, Francaux M, Zbinden-Foncea H. Toll like receptor expression induced by exercise in obesity and metabolic syndrome: a systematic review. Exerc Immunol Rev. 2018;24:60–71. [PubMed] [Google Scholar]
- Rainer PP, Kass DA. Old dog, new tricks: novel cardiac targets and stress regulation by protein kinase G. Cardiovasc Res. 2016;111(2):154–162. doi: 10.1093/cvr/cvw107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Muniz MG, Palfreeman M, Setzu N, Sanchez MA, Saenz Portillo P, Garza KM, Gosselink KL, Spencer CT. Obesity exacerbates the cytokine storm elicited by francisella tularensis infection of females and is associated with increased mortality. Biomed Res Int. 2018;2018:3412732. doi: 10.1155/2018/3412732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringseis R, Eder K, Mooren FC, Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21:58–68. [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK, Franco-Paredes C, Henao-Martinez AF, Paniz-Mondolfi A, Lagos-Grisales GJ, Ramírez-Vallejo E, Suárez JA, Zambrano LI, Villamil-Gómez WE, Balbin-Ramon GJ, Rabaan AA, Harapan H, Sah R, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018 doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation and cytokine amplification in COVID-19. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Investig. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, Bragazzi N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187. doi: 10.3389/fimmu.2018.02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85(2):873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli FR, Conti F, Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol. 2020 doi: 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Q, Yang G, Lin C, Zhang Y, Yang P. Weight and prognosis for influenza A(H1N1)pdm09 infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta-analysis. Infect Dis. 2016;48(11–12):813–822. doi: 10.1080/23744235.2016.1201721. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Mukai K. Innate immunity signalling and membrane trafficking. Curr Opin Cell Biol. 2019;59:1–7. doi: 10.1016/j.ceb.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 2017;25(5):1012–1026. doi: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettor R, Valerio A, Ragni M, Trevellin E, Granzotto M, Olivieri M, Tedesco L, Ruocco C, Fossati A, Fabris R, Serra R, Carruba MO, Nisoli E. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab. 2014;306(5):E519–E528. doi: 10.1152/ajpendo.00617.2013. [DOI] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2014;35(39):2722–2731. doi: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KJ, Olson MM, Thompson NJ, Cahill ML, Wyatt TA, Yoon KJ, Loiacono CM, Kohut ML. Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PLoS ONE. 2015;10(6):e0129713. doi: 10.1371/journal.pone.0129713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C-M, Lai H-K, Ou C-Q, Ho S-Y, Chan K-P, Thach T-Q, Yang L, Chau Y-K, Lam T-H, Hedley AJ, Peiris JSM. Is exercise protective against influenza-associated mortality? PLoS ONE. 2008;3(5):e2108. doi: 10.1371/journal.pone.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhao S, Teng T, Abdalla AE, Zhu W, Xie L, Wang Y, Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020 doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, Sun GZ, Yang GR, Zhang XL, Wang L, Xu X, Xu XP, Chan JCN. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-M, Wong RS-M, Wu EB, Kong S-L, Wong J, Yip GW-K, Soo YOY, Chiu MLS, Chan Y-S, Hui D, Lee N, Wu A, Leung C-B, Sung JJ-Y. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82(964):140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbinden-Foncea H, Francaux M, Deldicque L, Hawley JA. Does high cardiorespiratory fitness confer some protection against pro-inflammatory responses after infection by SARS-CoV-2? Obesity (Silver Spring) 2020 doi: 10.1002/oby.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dong J, Martin M, He M, Gongol B, Marin TL, Chen L, Shi X, Yin Y, Shang F, Wu Y, Huang H-Y, Zhang J, Zhang Y, Kang J, Moya EA, Huang H-D, Powell FL, Chen Z, Shyy JY-J, et al. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med. 2018;198(4):509–520. doi: 10.1164/rccm.201712-2570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Cui G, Chen J, Gao H, Wei Y, Uede T, Chen Z, Diao H. Regular exercise enhances the immune response against microbial antigens through up-regulation of toll-like receptor signaling pathways. Cell Physiol Biochem. 2015;37(2):735–746. doi: 10.1159/000430391. [DOI] [PubMed] [Google Scholar]