Abstract
Purpose
To investigate the dynamic evolution of image features of COVID-19 patients appearing as a solitary lesion at initial chest CT scan.
Materials and methods
Twenty-two COVID-19 patients with solitary pulmonary lesion from three hospitals in China were enrolled from January 18, 2020 to March 18, 2020. The clinical feature and laboratory findings at first visit, as well as characteristics and dynamic evolution of chest CT images were analyzed. Among them, the CT score evaluation was the sum of the lung involvement in five lobes (0–5 points for each lobe, with a total score ranging from 0 to 25).
Results
22 COVID-19 patients (11 males and 11 females, with an average age of 40.7 ± 10.3) developed a solitary pulmonary lesion within 4 days after the onset of symptoms, the peak time of CT score was about 11 days (with a median CT score of 6), and was discharged about 19 days. The peak of CT score was positively correlated with the peak time and the discharge time (p < 0.001, r = 0.793; p < 0.001, r = 0.715). Scan-1 (first visit): 22 cases (100%) showed GGO and one lobe was involved, CT score was 1.0/1.0 (median/IQR). Scan-2 (peak): 15 cases (68%) showed crazy-paving pattern, 19 cases (86%) showed consolidation, and 2.5 lobes were involved, CT score was 6.0/12.0. Scan-3 (before discharge): ten cases (45%) showed linear opacities, none had crazy-paving pattern, and 2.5 lobes were involved, CT score was 6.0/11.0. Scan-4 (after discharge): three cases (19%) showed linear opacities and one lobe was involved, CT score was 2.0/5.0.
Conclusion
The chest CT features are related to the course of COVID-19 disease, and dynamic chest CT scan are helpful to monitor disease progress and patients’ condition. In recovered patients with COVID-19, the positive CT manifestations were found within 4 days, lung involvement peaking at approximately 11 days, and discharged at about 19 days. The patients with more severe the lung injury was, the later the peak time appeared and the longer the recovery time was. Although the lesion was resolved over time, isolation and reexamination were required after discharge.
Keywords: COVID-19, SARS-CoV-2, Pneumonia, Computed tomography
Introduction
In December 2019, an unknown viral pneumonia was first reported in Wuhan, Hubei province, China, and spread rapidly [1]. Chinese Center for Disease Control and Prevention (CDC) analyzed the patients’ lower respiratory tract samples and found a novel coronavirus that has not been reported. On February 11, 2020, the International Committee on the Taxonomy of Viruses (ICTV) classified and named it severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On the same day, the WHO officially named the disease caused by the virus Corona Virus Disease 2019 (COVID-19). COVID-19 is spreading rapidly, which had been recognized as a global pandemic. As of March 30, it has caused 693,224 confirmed cases and 33,106 deaths in more than 200 countries and regions worldwide [2].
The typical chest CT findings of COVID-19 showed multiple ground-glass opacity, consolidation and interstitial inflammation [3–5]. However, there were few studies focused on the dynamic changes of chest CT images of patients with COVID-19 at present. A recent study analyzed the changes of chest CT findings in 21 COVID-19 patients during hospitalization by CT score [6]. But some of the cases included in their study were not in early stages, and the study excluded those who developed hypoxemia or respiratory distress. So their studies may not show the overall evolution of the disease. As lung solitary lesions were rare and early manifestations of COVID-19 [6–8], this study collected 22 COVID-19 patients with lung solitary lesions on the first CT scan in three hospitals, and aims to describe dynamic changes of chest CT manifestations and clarifies the value of chest CT in predicting the course of COVID-19.
Materials and methods
Patients
From January 18, 2020 to March 18,2020, 22 COVID-19 patients diagnosed by China Resources & WISCO General Hospital (Wuhan, Hubei Province), the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, Zhejiang Province) and Yueqing People's Hospital (Wenzhou, Zhejiang Province) were retrospectively analyzed. The inclusion criteria were as follows:(1) Patients with positive detection of SARS-CoV-2 in respiratory or blood specimens; (2) initial chest CT scan appearing as solitary lesion; (3) meeting the discharge criteria in the diagnosis and treatment of COVID-19 (trial 7th edition) published by the National Health Commission of the People’s Republic of China [9]; (4) patients underwent ≥ 3 chest CT scans before discharge, and CT imaging data were complete and accessible. All patients were recorded with initial clinical symptoms, peripheral blood routine examination, hypersensitive c-reactive protein examination, the interval between the onset of initial symptoms and first CT scan and the period between the onset of initial symptoms and discharge. “Severe pneumonia” was defined as any of the followings: (1) respiratory rate ≥ 30 breaths/min; (2) oxygen saturation ≤ 93% at rest; (3) PaO2/FiO2 ≤ 300 mmHg: (4) patients with chest radiography showing significant progression of lesions greater than 50% within 24–48 h managed are being treated for severe pneumonia [9]. This retrospective study was consistent with the principles of the Helsinki Declaration and was approved by the ethics committee. Given that this is a retrospective study, the need for patients’ written informed consent was waived.
Chest CT protocols and infection control protocols
All CT scans were performed with one of the five CT systems: SOMATOM Definition AS and SOMATOM Scope (Siemens, Germany), HiSpeed NX/i and LightSpeed VCT (GE, America), Brilliance 16 CT (Philips, America). The scanning parameters were as follow: tube voltage: 120 kV(p), tube current–exposure time product: range 50–200 mAs, slice thickness: 5 mm, reconstruction thickness: 0.625–1.5 mm, matrix:512 × 512, field of view 350 × 350 mm. Scanning position: all patients were supine with arms extended and held their breath during the CT scan.
To reduce nosocomial infection, there were 1–2 designated CT scanners in every hospital which were only used to scan COVID-19 patients. And channels were established between the isolation wards and the designated CT scanners for COVID-19 patients only. After the CT examinations of the patients, disinfected the examination bed surface, floor, air and equipment [10].
Evaluation of the HRCT features
The CT images were independently evaluated by two radiologists with rich chest radiology diagnostic experience (with 13 and 21 years of experience, separately), when differences arose, they reached a consensus through consultation. All images were showed in lung windows (window width, 1500 HU; window level: − 750 HU) and mediastinal windows (window width, 450 HU; window level, 45 HU). The CT findings assessed according to the following CT characteristics: (1) number of affected lobes; (2) zones of involved lung: left upper lobe, left lower lobe, right upper lobe, right middle lobe, right lower lobe; (3) maximum diameter of lesions (4) predominant distribution of lesions: subpleural distribution, central distribution or both; (5) features of lesions: presence of ground-glass opacities (GGO), crazy-paving pattern, consolidation and linear opacities; (6) pleural effusion. (7) CT score: the semi-quantitative score was used to evaluate the extent of disease at CT [11]. The abnormal manifestations included GGO, consolidation and interstitial involvement (ignoring the pre-existing pulmonary disease). Each lobe was rated based on the following: score 0, 0% involvement; score 1, less than 6% involvement; score 2, 6–25% involvement; score 3, 26–50% involvement; score 4, 51–75% involvement; and score 5, more than 75% involvement. The total score is obtained by adding up the partial scores of five lobes (theoretically ranging from 0 to 25). Based on the course of disease and CT score, chest CT examinations were chosen and grouped as: (1) Scan-1: the first chest CT examination during the course of the disease; (2) Scan-2: the CT examination with the peak of CT score during the hospitalization; (3) Scan-3: the last chest CT examination before hospital discharge; and (4) Scan-4: the first chest CT after discharge.
Statistical analysis
SPSS Statistics Software (version 22; IBM, New York, USA) was used to statistically analyzed all data. The categorical variables were expressed as counts and percentages, and the continuous variables were expressed by mean and standard deviation (SD) if it fitted the normal distribution, or median and interquartile range (IQR) if did not. Spearman rank correlation coefficient was used to analyze the correlation between two groups of continuous variables. The analyses were considered significant when p value less than 0.05.
Results
Clinical characteristics of the patients
Among all patients, there were 11 men and 11 women, they were 40.7 (SD 10.3; range 23–54) years old. Patient characteristics and laboratory results were summarized in Table 1. The most common initial symptom was fever (11/22, 50%), and 4 (18%) patients were asymptomatic at their first visit, but sought medical care due to exposure to confirmed cases. On admission, laboratory examinations of 14 (64%) patients were normal. 2 (9%) and three (14%) patients had decreased white blood cell count and lymphocyte count, respectively. 5 (23%) patients showed elevated levels of C-reactive protein. All patients were discharged between 11 and 44 days (median, 19 days) after the onset of symptoms (Table 1).
Table 1.
Patient characteristics and laboratory results
| Patient (n = 22) | |
|---|---|
| Age (years) | 40.7 ± 10.3 |
| Sex | |
| Men | 11 (50%) |
| Women | 11 (50%) |
| Initial symptoms | |
| Fever | 11 (50%) |
| Cough | 9 (41%) |
| Expectoration | 3 (14%) |
| Pharyngalgia | 2 (9%) |
| Chest pain | 2 (9%) |
| Chest tightness | 4 (18%) |
| Nausea and vomiting | 1 (5%) |
| Abdominal pain | 1 (5%) |
| Myalgia | 7 (32%) |
| Fatigue | 5 (23%) |
| Headache | 1 (5%) |
| Laboratory examination | |
| White blood cell count | |
| Normal | 20 (91%) |
| Descend | 2 (9%) |
| Lymphocyte count | |
| Normal | 19 (86%) |
| Descend | 3 (14%) |
| CRP | |
| Normal | 17 (77%) |
| Increased | 5 (23%) |
| The interval between the onset of initial symptoms and discharge (days) | 19.0 (10.25) |
The categorical variables were expressed as count (percentage of the total). The continuous variables were expressed as mean ± SD or median (IQR)
Dynamic changes of chest CT imaging
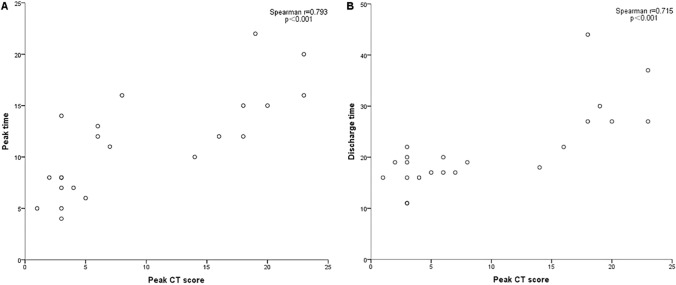
A total of 88 chest CT examinations was performed in 22 patients in the period of hospitalization, and 16 of them underwent chest CT scan after discharge. Based on the course of disease and CT score, Scan-1 to Scan-4 were shown (Table 2, Figs. 1, 2, 3): Scan-1 (first visit, n = 22) (Figs. 1a, 2a, 3a); Scan-2 (peak, n = 22) (Figs. 1b, 2b, 3b); Scan-3 (before discharge, n = 22) (Figs. 1c, 2c, 3c); and Scan-4 (after discharge, n = 16) (Fig. 1d). Maximum lung involvement with CT score of 6 points at about 11 days after the initial onset of symptoms. The interval between onset of symptoms and discharge ranged from 11 to 44 days, with the median interval 19 days. And the peak value of CT score was positively correlated with the peak time and discharge time (Fig. 4).
Table 2.
CT features of the patients with COVID-19 at different stages
| Scan-1 (n = 22) | Scan-2 (n = 22) | Scan-3 (n = 22) | Scan-4 (n = 16) | |
|---|---|---|---|---|
| The period between the onset of initial symptoms and the CT scan (days) | 1.0 (1.25) | 11.2 ± 4.9 | 18.3 ± 7.9 | 27.0 (12.5) |
| CT score | 1.0 (1.0) | 6.0 (12.0) | 4.5 (11.0) | 2.0 (5.0) |
| Number of affected lobes | 1.0 | 2.5 (4.0) | 2.5 (4.0) | 1.0 (3.0) |
| 0 | 0 | 0 | 1 (5%) | 5 (31%) |
| 1 | 22 (100%) | 8 (36%) | 8 (36%) | 5 (31%) |
| 2–4 | 0 | 6 (27%) | 5 (23%) | 3 (19%) |
| 5 | 0 | 8 (36%) | 8 (36%) | 3 (19%) |
| Zones of involved lung | ||||
| Left upper lobe | 4 (18%) | 12 (55%) | 9 (41%) | 2 (13%) |
| Left lower lobe | 7 (32%) | 18 (82%) | 10 (45%) | 4 (25%) |
| Right upper lobe | 2 (9%) | 9 (41%) | 16 (73%) | 9 (56%) |
| Right middle lobe | 2 (9%) | 10 (45%) | 11 (50%) | 5 (31%) |
| Right lower lobe | 7 (32%) | 16 (73%) | 16 (73%) | 8 (50%) |
| Maximum diameter of lesion* | 25.1 ± 12.7 | |||
| Predominant distribution of lesions | ||||
| No lesion | 0 | 0 | 1 (5%) | 5 (31%) |
| Subpleural distribution | 16 (73%) | 9 (41%) | 8 (36%) | 6 (38%) |
| Central distribution | 6 (27%) | 5 (23%) | 3 (14%) | 1 (6%) |
| Both | 0 | 8 (36%) | 10 (45%) | 4 (25%) |
| Ground-glass opacities | ||||
| Yes | 22 (100%) | 22 (100%) | 16 (73%) | 9 (56%) |
| No | 0 | 0 | 6 (27%) | 7 (44%) |
| Crazy-paving pattern | ||||
| Yes | 8 (36%) | 15 (68%) | 0 | 0 |
| No | 14 (64%) | 7 (32%) | 22 (100%) | 16 (100%) |
| Consolidation | ||||
| Yes | 9 (41%) | 19 (86%) | 15 (68%) | 4 (25%) |
| No | 13 (59%) | 3 (14%) | 7 (32%) | 12 (75%) |
| Linear opacities | ||||
| Yes | 0 | 7 (32%) | 10 (45%) | 3 (19%) |
| No | 22 (100%) | 15 (68%) | 12 (55%) | 13 (81%) |
| Pleural effusion | 0 | 5 (23%) | 0 | 0 |
The categorical variables were expressed as count (percentage of the total). The continuous variables were expressed as mean ± SD or median (IQR)
*With the development of the disease, some lesions were fused and diffused, and the maximum diameter could not be obtained. Therefore, only the maximum diameter at the first CT scan was provided
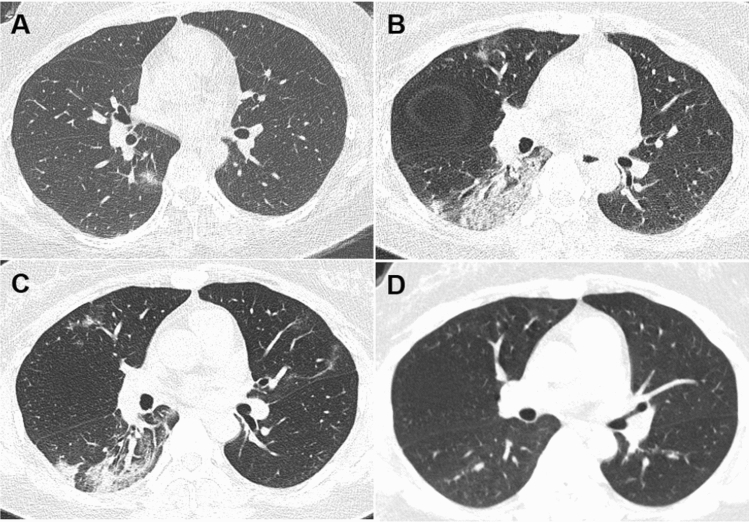
Fig. 1.
Dynamic changes of CT imaging in a 35-year-old woman with fever (37.8 °C) for 1 day. a At the first chest CT scan (day 1), a ground-glass lesion with partial consolidation was shown in the subpleural area of the right lower lobe; b at peak time (day 10), the lesion in the right lower lobe expanded with interlobular septal thickening and consolidation, and new lesions appeared in the upper lobes; c before discharge (day 17), the lesions were gradually absorbed and linear opacities were observed in the bilateral upper lobes; d after discharge (day 51), chest CT scan showed few GGO in the right lung, and the consolidation and linear opacities disappeared
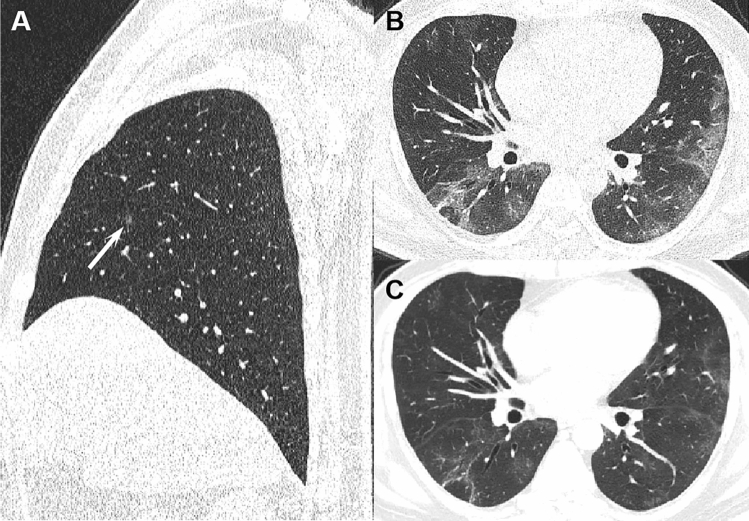
Fig. 2.
A 48-year-old man with COVID-19 presented no obvious symptoms initially. a At the first chest CT scan (day 0), a sub-centimeter lesion was found in the right middle lobe; b at peak time (day 16), it developed to diffuse lesions in bilateral lung with GGO, consolidation and interlobular septal thickening; c before discharge (day 33), it showed remarkable absorption on CT image, GGO and linear opacities were observed
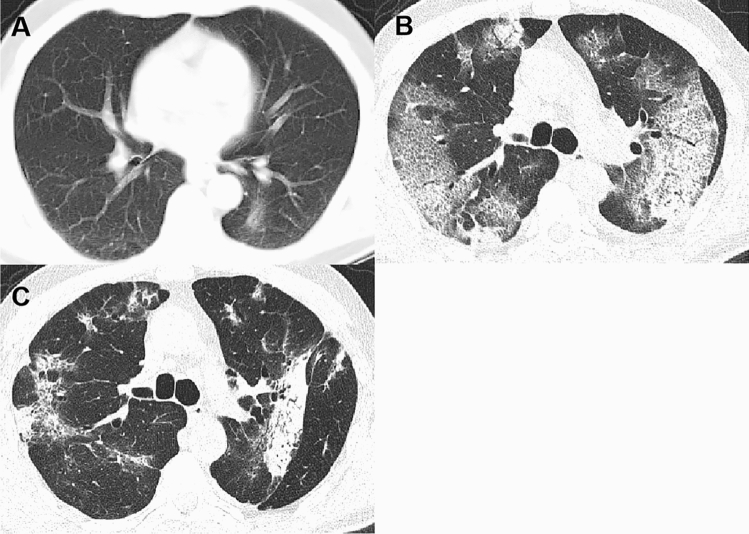
Fig. 3.
A 51-year-old man was hospitalized for polyps and the pulmonary lesion was accidentally detected by chest CT. a At the first chest CT scan (day 0), it showed a patchy opacity in the left lower lobe; b at peak time (day 20), it developed to bilateral lung with GGO, consolidation and crazy-paving pattern; c before discharge (day 26), the CT scan demonstrated GGO, consolidation, reticulation and traction bronchiectasis
Fig. 4.
The peak CT score is positively correlated with peak time (a) and discharge time (b) in 22 COVID-19 patients. Spearman rank correlation test was performed, because the discharge time and the peak CT score did not coincide with normal distribution and Spearman r and p values were provided in graphs
In Scan-1, the interval between the first CT examination and the onset of disease was 0–4 days, and the median interval was 1 day. Of the 22 patients with unifocal involvement, the lesions tended to be distributed in the lower lobe [14/22 (64%)] and subpleural area [16/22 (73%)]. The maximum diameter of the lesion was 6-48 mm, and four cases presented as sub-centimeter nodules. The CT findings of this stage were GGO [22/22 (100%)], crazy-paving pattern [8/22 (36%)] and consolidation [9/22 (41%)]. CT score was 1–2 points. No patients had pleural effusion (Table 2).
In Scan-2, 22 patients showed varying degrees of progression in lung lesions. The interval between the onset of the symptoms and Scan-2 was 4–22 days. The number of affected lobes were 1–5 and median was 2.5. Eight (36%) patients had only one affected lobe, while another eight (36%) patients had five involved lobes. 5 of these eight patients had deteriorated into severe pneumonia. In the stage, predominant distribution of lesions was subpleural [9/22 (41%)] and both [8/22 (36%)]; more lesions showed crazy-paving pattern [15/22 (68%)] and consolidation [19/22 (86%)], and five (23%) patients presented a small amount of pleural effusion. The peak value of CT score was 1–23 points.
In Scan-3, the lesions were gradually absorbed and tended to be stable, and patients recovered in accordance with clinical cure standard. The period between the onset of disease and the CT scan was 6–41 days. The number of involved lobes was ranged 0–5, with a median of 2.5. 1 patient had no lesion in chest CT scan. GGO lesion [16 (73%)] and consolidation [15 (68%)] were significantly absorbed, and 10 (45%) patients had linear opacities, while no lesion presenting as crazy-paving pattern. CT score was 0–20 points. None of the patients had pleural effusion.
In Scan-4, all 16 patients had further absorption of the lung lesions compared with the Scan-3, and the interval from the onset of initial symptoms was 22–51 days. The number of affected lung lobes was 0–5 with a median of 1 lobe. In this stage, negative CT findings were revealed in 5 (31%) patients. 9 (56%) patients showed GGO, 4 (25%) patients presented consolidation, and 3 (19%) patients had linear opacities. CT score was 0–12 points.
Discussion
Coronavirus, an RNA virus widely found in nature, has identified six subtypes in the past half century. Similar to the other two highly pathogenic coronaviruses in human, severe acute respiratory syndrome coronavirus (SARS-CoV) and middle eastern respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2 can cause severe respiratory disease [3, 12]. And there was 82% homology between SARS-CoV-2 and SARS-CoV at the nucleotide level [13]. The most common symptom of COVID-19 is fever, with an incidence of about 83–98.6% [14, 15]. Although fever was the most common initial symptom in our study, only 50% of the patients presented with fever, and four (18%) patients were asymptomatic initially. The proportion of patients with leukopenia and increased C-reactive protein was 14% (3/22) and 23% (5/22), respectively, which was also lower than previous studies [15]. In our study, initial symptoms of the patients were mild and the positive rate of laboratory examination was low. That revealed the patients were in the early stage of the disease at first visit [6], and in this stage, the immune response was not fully activated [16, 17].
Most studies had shown that, similar to other viral pneumonia, COVID-19 tended to involve in bilateral lower lobes and distribute in the subpleural region, which might be related to viral invasion of the bronchioles and alveoli, as well as higher perfusion in the lower lung lobes [4, 6, 18]. In this study, most of the lesions were subpleural (64%) on the first CT examination. With the progression of disease, the size of lesions increased, the number of lesions and involved lung lobes increased, and the lesions spread from the subpleural region to the central area of the lung, which was consistent with previous studies [6, 8, 19]. Pleural effusion was an uncommon imaging finding in COVID-19 patients [5, 6, 20]. A recent study found that pleural effusion was more common in severe and critical COVID-19 patients [18], another study pointed that pleural effusion generally occurred in the advanced phase [21]. In our study, all patients had no pleural effusion on the first CT scan, but a small pleural effusion was found in 5 (23%) patients at the time of maximum lung lesions during hospitalization, among whom four were patients with severe COVID 19. So we proposed that pleural effusion might correspond to clinical worsening.
We studied 22 patients with COVID-19 and found that lung involvement peaking at approximately 11 days and the median discharge time was about 19 days, which was similar to study results of Pan et al. [6] and Zhou et al. [22]. In addition, we further found that the peak value of CT score was positively correlated with the peak time and discharge time. This meant that patients with more severe the lung injury was, the later the peak time appeared and the longer the recovery time was. In 16 patients who underwent CT examination after discharge, compared with the previous CT scan, all the lung lesions showed different degrees of absorption. It is noteworthy that only 19% (3/16) of the patients presented linear opacities in CT, which was significantly lower than the last CT scan before discharge [10 (45%)]. This indicates that the linear opacities showed on CT was not equivalent to actual pathologic fibrosis, although no relevant pathological studies have been conducted. A similar view was proposed in a previous study on SARS [11]. At the same time, it should also be noted that some patients who had reached the discharge standards still had lesions in the lung despite their clinical symptoms were obviously improved and their nucleic acid test is negative. A small number of recovered patients were re-hospitalized because of the re-positive nucleic acid detection [23]. Therefore, the diagnosis and treatment of COVID-19 (trial 7th edition) suggested that follow-up visits should be conducted after discharge and CT reexamination should be performed if necessary [9]. Our study showed the continuous absorption of lesions in some patients after discharge, which proves that this measure is reasonable. However, it has to be admitted that this increased the risk of radiation exposure to some degree. Although low-dose CT scan was not used in this study, it should be considered for follow-up examinations.
During dynamic observation, we found that eight patients in our study developed to bilateral lung diffuse lesions. And according to the diagnosis and treatment of COVID-19 (trial 7th edition) [9], five of eight patients were classified as severe pneumonia, while the other three were in a stable condition. This suggested that the severity of disease was consistent with the imaging findings to some degree, but not exactly match. It is consistent with a recent research by Guan et al. [24]. The study enrolled 1099 patients with COVID-19 found that the area of lung injury in severe patients was usually larger than non-severe patients in CT examination, but there were also 26.3% of non-severe patients and 8.7% of severe patients with no abnormalities in chest CT scan.
Notably, we found four patients appearing as a solitary sub-centimeter lesion on the first CT scan, two of them involved only one lobe throughout the course of disease, while the other two developed to multilobe distribution. On the basis of a previous study on SARS by Chang et al. [11], we speculated that the differing severity of lung injury in patients with COVID-19 could be related to viral loads and variable immune response of the individuals, and had little relation with the initial CT manifestations. Liu et al. [25] also proposed a similar view, suggesting that viral load was the key to determining the severity of the disease. Therefore, even if the lesions of some patients are limited and small at the time of the first chest CT, they should never be ignored, and chest CT examinations can help to monitor patients’ condition.
There are some limitations in this study. First, since it was a retrospective study, the patients did not perform CT scan at regular intervals and the therapeutic measures of the patients were different, all of which made certain influence on the course of observation of the disease. Second, because solitary pulmonary lesions is the rare CT findings of COVID-19, a relatively small number of patients were included in the study. Finally, when calculating the CT score, we counted GGO, consolidation and linear opacities as 1 point, and did not distinguish them by different points, which might be the reason why the CT score before discharge in some patients was still close to the peak CT score.
To sum up, the chest CT features are related to the course of COVID-19 disease, with certain characteristics and dynamic changes, and CT examinations are helpful to monitor the patients’ condition. Dynamic changes of CT images in the chest: the positive CT manifestations were found in patients with COVID-19 within 0–4 days after the onset of the initial symptoms, lung involvement peaking at approximately 11 days, then the pulmonary lesions improved significantly and reached the discharge standard at about 19 days after the onset of the initial symptoms. Moreover, the patients with more severe the lung injury was, the later the peak time appeared and the longer the recovery time was. Although the lesions were resolved over time in most patients, isolation and follow-up visits were required after discharge and CT reexamination should be performed if necessary.
Acknowledgements
The authors would like to thank all hospital staff for their efforts to combat COVID-19.
Funding
The Science and Technology Planning Projects of Wenzhou (Grant no. Y20180769). Health Foundation for Creative Talents in Zhejiang Province, China. Project Foundation for the College Young and Middle-aged Academic Leader of Zhejang Province, China.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This retrospective study was consistent with the principles of the Helsinki Declaration and was approved by the ethics committee (Grant no. 2020-008). Given that this is a retrospective study, the need for patients’ written informed consent was waived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Coronavirus disease 2019 (COVID-19) situation report—70. Geneva: World Health Organization; 2020. [Google Scholar]
- 3.Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:230. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of the People's Republic of China. Diagnosis and treatment of COVID-19 (trial 7th edition). 2020 [DOI] [PMC free article] [PubMed]
- 10.Deng M. The prevention and management of the coronavirus disease 2019 (COVID-19) outbreak in radiology departments in epidemic areas. Jpn J Radiol. 2020;38(6):483–488. doi: 10.1007/s11604-020-00974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YC, Yu CJ, Chang SC, Galvin JR, Liu HM, Hsiao CH, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 12.Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. medRxiv. 2020:2020.02.10.20021584.
- 17.Liu W, Zhao M, Liu K, Xu K, Wong G, Tan W, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Han R, Yu P, Wang S, Xia L. A correlation study of CT and clinical features of different clinical types of 2019 novel coronavirus pneumonia. Chin J Radiol. 2020;54:E003. [Google Scholar]
- 19.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1–7. doi: 10.2214/AJR.19.22415. [DOI] [PubMed] [Google Scholar]
- 20.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295:16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H, Fu L, Jin Y, Shao J, Zhang S, Zheng N, et al. Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J Clin Lab Anal. 2020;34:e23392. doi: 10.1002/jcla.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020:2020.02.06.20020974.
- 25.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]