Abstract
Triple-negative breast cancer (TNBC) has a high degree of malignancy. The endothelin B receptor (EDNRB) serves an important role in the occurrence and development of cancer. The present study aimed to investigate the prognostic value of EDNRB in TNBC. A total of 99 cases of TNBC were collected from the Henan Cancer Hospital database and 159 cases of TNBC were collected from The Cancer Genome Atlas database. A χ2 test was used to analyze the association between EDNRB and clinicopathological data. Kaplan-Meier analysis and multivariate Cox regression analysis were used to analyze the association between EDNRB and prognosis, and to establish two models. The discrimination degree of the models was evaluated using time-dependent receiver operating characteristic curves and concordance index (C-index), whereas the accuracy and net benefit of the models were evaluated using integrated discriminant improvement (IDI) and decision curves. EDNRB expression was low in TNBC samples (P<0.01). Age (P=0.01), tumor size (P=0.04) and N stage (P=0.01) were associated with EDNRB expression. EDNRB expression was positively associated with stromal score (P<0.01), but not immune score. High expression levels of EDNRB indicated favorable disease-free survival time (hazard ratio, 0.38; 95% CI, 0.15–0.98; P=0.04). The integrated area under the curve and C-index of the new model were increased compared with the old model following the addition of EDNRB expression as a parameter. The IDI values for prediction of the 3- and 5-year survival rates were 0.04 (P=0.02) and 0.05 (P=0.01), respectively. The results of decision curve analysis showed that the new model had higher clinical net benefit than the old model in the range of 3-year survival rate <0.52. In conclusion, EDNRB was associated with a favorable prognosis in patients with TNBC, and may be used as a novel prognostic biomarker.
Keywords: endothelin B receptor, triple-negative breast cancer, prognosis, biological markers
Introduction
Breast cancer is one of the most common malignant tumors in females (1). In the Asian population, triple-negative breast cancer (TNBC) accounts for 10–17% of all breast cancer cases (2). TNBC refers to a type of breast cancer lacking estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 expression (3). TNBC is associated with a poor prognosis, a unique metastasis pattern and highly malignant biological behavior (2). At present, there is no effective clinical treatment for TNBC, and effective targets and biomarkers for prognosis are urgently required. The endothelin family consists of three isoforms (ET-1, ET-2 and ET-3), which can bind G-coupled protein endothelin A receptor and endothelin B receptor (EDNRB) via autocrine or paracrine signaling pathways (4,5). EDNRB is located on chromosome 13 and is mainly expressed in endothelial cells, macrophages and vascular smooth muscle cells (6,7). EDNRB may activate numerous cancer-associated signaling pathways, including the mitogen-activated protein kinase/Erk 2 and PI3K/AKT signaling pathways (8,9). In addition, previous studies have noted that EDNRB, when combined with ET-1, affects cell proliferation and migration, and is associated with lymph angiogenesis and lymphatic metastasis (10–12). EDNRB expression exhibits tissue specificity in cancer. It is highly expressed in glioma, but is expressed at low levels in prostate and liver cancer, and its high expression is associated with a favorable prognosis (13–15). However, the expression and clinical significance of EDNRB in TNBC remain unclear, and there is a lack of large-scale clinical studies. The purpose of the present study was to investigate the association with clinicopathological characteristics and the prognostic value of EDNRB in TNBC.
Materials and methods
Patient cohorts
The cancer genome atlas (TCGA) cohort
The present study included tissues from patients with primary TNBC (n=159) and para-cancerous tissues from patients with breast cancer (n=112) obtained from TCGA (16). These included 14 pairs of matched cancerous and para-cancerous tissues. Data regarding the gene expression levels of EDNRB in each tissue were obtained from the database, and the expression levels were Log2 transformed to analyze the difference in EDNRB gene expression between tumor and normal tissues. Subsequently, 142 patients with primary TNBC with complete clinical data were included in the analysis of the association between EDNRB expression and clinicopathological data. The median value of EDNRB expression was set as the boundary (exact value, 8.348); expression below the median value was considered negative; and expression above the median value was considered positive. In this cohort, the age ranged between 29 and 90 years (median, 55); 69.0% (n=98) of the patients were postmenopausal; 73.9% (n=105) of the patients had a T stage >2; 86.6% (n=123) of the patients had an N stage of 0 or 1; and the positive rate of EDNRB was 52.8%.
Henan cancer hospital (HNCH) cohort
The present study retrospectively consecutively collected 99 cases of TNBC between January 2013 and February 2018 at the Department of Breast Surgery of Henan Cancer Hospital (Zhengzhou, China). The inclusion criteria were as follows: i) Patients with primary TNBC; ii) no distant metastasis at first diagnosis; and iii) direct surgical resection or no pathological complete response was achieved following neoadjuvant chemotherapy. Patients were followed up using the outpatient registration system and disease-free survival (DFS) was determined. DFS was defined as no local or regional recurrence, no distant recurrence and no contralateral invasive breast cancer. In this cohort, the age ranged between 27 and 69 years (median, 48); 60.6% (n=60) of patients were not menopausal; 66.7% (n=66) of patients had a T stage >2; 72.7% (n=72) of patients had an N stage of 0 or 1; and the positive rate of EDNRB was 23.2%. The present study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University.
Immunohistochemistry
Tissues were fixed with 10% neutral buffered formalin at room temperature for 12 h, before being embedded in paraffin. Rabbit anti-human EDNRB monoclonal antibody (cat. no. 31191; Signalway Antibody LLC, College Park, MD, USA), secondary antibody (cat. no. sp-9001; OriGene Technologies, Inc., Rockville, MD, USA) and diaminobenzidine (DAB) chromogenic solution (cat. no. sp-9001; OriGene Technologies, Inc.) were purchased. Immunohistochemical staining was performed according to the streptavidin-peroxidase method. Paraffin-embedded specimens were cut into 5-µm sections. Following conventional xylene dewaxing and alcohol gradient dehydration, the specimens were placed in citric acid buffer (pH 6.0) for antigen repair at 100°C and rinsed in PBS three times for 5 min each. Sections were incubated with 3% hydrogen peroxide at room temperature for 25 min (avoiding light) to block endogenous peroxidase activity. The sections were rinsed in PBS three times for 5 min each, and normal 10% bovine calf serum (cat. no. B7446; Sigma-Aldrich; Merck KGaA) was added for 20 min at room temperature. Subsequently, the serum was dried. EDNRB primary antibody (dilution, 1:500) was added dropwise to the sections, and the sections were laid flat in a wet box at 4°C overnight. PBS was used for three washes of 5 min each. Horseradish peroxidase-labeled anti-rabbit secondary antibody (dilution, 1:1,000) was added, and incubated at room temperature for 2 h, followed by DAB color development, hematoxylin re-dyeing at room temperature for 90 min, conventional dehydration, drying and sealing. The results were interpreted as previously described (17,18). Briefly, a score was assigned according to the staining degree: 0, basic non-staining (0); 1, light yellow (+); 2, brown (++); and 3, dark brown (+++). Sections were observed under a bright-field upright microscope (Olympus Corporation) at 10×40 high magnification, three fields of view were observed for each section, the percentage of positively stained tumor cells in each field of view was calculated and the average value was used for scoring as follows: 0 points, no positive staining of tumor cells; 1 point, 1–25%; 2 points, 26–50%; 3 points, 51–75%; and 4 points, >75%. Finally, the percentage was multiplied by the dyeing intensity score to obtain the total score. A score of 0 was considered negative, a score of 1–4 was considered weakly positive, a score of 5–8 was considered moderately positive and a score of 9–12 was considered strongly positive. Finally, in the present study, a total score £4 was considered negative and a total score >4 was considered positive. The immunohistochemical results were confirmed by two blinded pathologists.
Statistical analysis
GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA), SPSS v23.0 software (IBM Corp., Armonk, NY, USA) and R software 3.6.1 (Lucent Technologies) were used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference. Independent sample correction t-test was used to compare the difference in EDNRB gene expression between tumor and normal tissues in the TCGA cohort, and a paired t-test was used to compare the differences in expression levels between paired tissues. The stromal, immune and ESTIMATE scores were calculated using the ‘ESTIMATE’ package in R software (19). The correlation between EDNRB expression and the score was analyzed using Spearman's correlation analysis. A two-sided χ2 test was used to analyze the association between EDNRB expression and clinicopathological data. Binary logistics regression analysis was used for multivariate analysis. Survival analysis was performed using the Kaplan-Meier method and a log-rank test. Based on the results of the multivariate Cox regression analysis, the present study established two prediction models to further illustrate the predictive value of EDNRB expression for prognosis. Model 1 consisted of N stage and NAC, while model 2 also included EDNRB expression as a parameter. Integrated area under the curve (iAUC) of time-dependent receiver operating characteristic (ROC) curves, concordance index (C-index), integrated discriminant improvement (IDI) and decision curve analyses were performed using the model to determine the model discrimination and the prediction accuracy for survival, and to determine the value of EDNRB for clinical net benefit.
Results
Expression levels of EDNRB in TNBC are lower than those in normal tissues
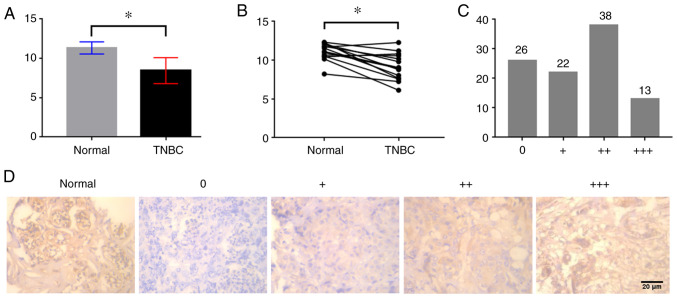
The present study investigated the difference in EDNRB expression between TNBC and normal breast tissues in the TCGA cohort. As shown in Fig. 1A, the expression levels of EDNRB in TNBC tissues were lower than those in normal breast tissues (P<0.01). Similarly, analysis of the difference between 14 cases of TNBC and adjacent normal tissues in the TCGA cohort indicated that the expression levels of EDNRB in TNBC were relatively low (P<0.01; Fig. 1B). Additionally, in immunohistochemical analysis of 99 cases, 26 cases were uncolored, and 13 cases exhibited dark brown staining (Fig. 1C). EDNRB was mainly expressed in the cytoplasm and was highly expressed in normal breast tissues (Fig. 1D).
Figure 1.
Expression of EDNRB in TNBC and normal breast tissue. (A) Analysis of differential EDNRB expression between TNBC and normal breast tissues in the TCGA cohort. (B) Analysis of differential EDNRB expression between TNBC and adjacent normal tissues in the TCGA cohort. (C) Immunohistochemical staining intensity distribution of 99 cases of TNBC. (D) Immunohistochemical results of normal breast tissues and TNBC tissues at 10×40 high magnification. *P<0.01. EDNRB, endothelin B receptor; TNBC, triple-negative breast cancer.
Association between EDNRB expression and clinicopathological data of patients with TNBC
In order to study the association between EDNRB and clinicopathological data of patients with TNBC, the present study collected clinicopathological data of the TCGA and HNCH cohorts (Table I). The TCGA cohort included 142 patients with TNBC with complete clinicopathological data. Univariate analysis revealed that EDNRB expression was associated with T stage and N stage (P=0.04 and P=0.01, respectively). Furthermore, multivariate analysis demonstrated that T stage [odds ratio (OR), 0.40; P=0.03] and N stage (OR, 4.3; P=0.02) were independent predictors of EDNRB expression. The lower the T stage and the higher the N stage, the more positive EDNRB expression was. The present study collected 99 samples from patients with TNBC in the HNCH cohort. Univariate analysis revealed that age and menopausal status were associated with EDNRB expression (P=0.01 and P=0.02, respectively). Multivariate analysis demonstrated that age was an independent predictor of EDNRB expression (OR, 3.37; P=0.01), and higher age was associated with positive EDNRB expression.
Table I.
Correlation between EDNRB and clinicopathological data.
| EDNRB | EDNRB | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HNCH number | Negative | Positive | P-value | OR | 95% CI | P | TCGA number | Negative | Positive | P-value | OR | 95% CI | P-value |
| Age | 0.01 | 3.37 | 1.28–8.87 | 0.01 | 0.62 | |||||||||
| <50 | 61 (61.6) | 52 (85.2) | 9 (14.8) | 50 (35.2) | 25 (50.0) | 25 (50.0) | ||||||||
| ≥50 | 38 (38.4) | 24 (63.2) | 14 (36.8) | 92 (64.8) | 42 (45.7) | 50 (54.3) | ||||||||
| Menopausal status | 0.02 | 0.78 | ||||||||||||
| Premenopausal | 60 (60.6) | 51 (85.0) | 9 (15.0) | 44 (31.0) | 20 (45.5) | 24 (54.5) | ||||||||
| Postmenopausal | 39 (39.4) | 25 (64.1) | 14 (35.9) | 98 (69.0) | 47 (48.0) | 51 (52.0) | ||||||||
| Tumor size, cm | 0.50 | 0.04 | 0.40 | 0.18–0.90 | 0.03 | |||||||||
| ≤2 | 33 (33.3) | 24 (72.7) | 9 (27.3) | 37 (26.1) | 12 (32.4) | 25 (67.6) | ||||||||
| >2 | 66 (66.7) | 52 (78.8) | 14 (21.2) | 105 (73.9) | 55 (52.4) | 50 (47.6) | ||||||||
| Pathological N stage | 0.23 | 0.01 | 4.30 | 1.33–13.89 | 0.02 | |||||||||
| 0-1 | 72 (72.7) | 53 (73.6) | 19 (26.4) | 123 (86.6) | 63 (51.2) | 60 (48.8) | ||||||||
| 2-3 | 27 (27.3) | 23 (85.2) | 4 (14.8) | 19 (13.4) | 4 (21.1) | 15 (78.9) | ||||||||
| NAC | 0.95 | |||||||||||||
| Yes | 35 (35.4) | 27 (77.1) | 8 (22.9) | |||||||||||
| No | 64 (64.6) | 49 (76.6) | 15 (23.4) | |||||||||||
HNCH, Henan Cancer Hospital; TCGA, The Cancer Genome Atlas; OR odds ratio; CI, confidence interval; N, lymph node; NAC, neoadjuvant chemotherapy; EDNRB, endothelin receptor B.
EDNRB expression is positively correlated with stromal score
In order to analyze the correlation between EDNRB expression and non-tumor components in the tumor microenvironment, the immune, stromal and ESTIMATE scores of TNBC tissues from the TCGA cohort (n=159) were calculated. Each patient had a stromal score, an immune score and an ESTIMATE score. Stromal scores ranged between-1,525.21 and 1,571.25 (mean, 145.89), immune scores ranged between-1,684.49 and 2,529.03 (mean, 546.82) and ESTIMATE scores ranged between-3,068.11 and 3,258.92 (mean, 692.72). EDNRB expression was correlated with stromal score (rs=0.44; P<0.01), immune score (rs=0.19; P=0.02) and ESTIMATE score (rs=0.35; P<0.01; Table II).
Table II.
Correlation between EDNRB and tumor microenvironment.
| Variable | Score | rs | 95% CI | P-value |
|---|---|---|---|---|
| Stromal score | 145.89±693.84 | 0.44 | 0.30~0.56 | <0.01 |
| Immune score | 546.82±901.60 | 0.19 | 0.03~0.34 | 0.02 |
| ESTIMATE score | 692.72±1411.76 | 0.35 | 0.20~0.49 | <0.01 |
ESTIMATE score was calculated by stromal score plus immune score. CI, confidence interval.
EDNRB expression is associated with a favorable prognosis
Using the outpatient registration system, the HNCH cohort was followed up (median follow-up time, 36 months). Among them, 78.3% (n=18) of the patients in the EDNRB-positive group had DFS events, while 47.4% (n=36) of the patients in the EDNRB-negative group had DFS events. Univariate Cox regression analysis revealed that N stage (P=0.01), neoadjuvant chemotherapy (NAC; P=0.01) and EDNRB expression (P=0.03) may be associated with the prognosis of patients with TNBC (Table III). Furthermore, when adjusted for confounding factors, multivariate Cox regression analysis demonstrated that EDNRB expression (P=0.04), N stage (P=0.01) and NAC (P=0.02) were independent predictors of prognosis in patients with TNBC (Table III). Kaplan-Meier analysis demonstrated that negative EDNRB expression was associated with an adverse prognosis in patients with TNBC (P=0.02; Fig. 2).
Table III.
Univariate and multivariate Cox regression analysis of disease-free survival.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | 0.54 | 0.28–1.04 | 0.06 | |||
| Menopausal status | 0.54 | 0.28–1.03 | 0.06 | |||
| Tumor size | 1.13 | 0.81–1.58 | 0.46 | |||
| Pathological N stage | 4.04 | 2.23–7.31 | 0.01 | 2.45 | 1.23–4.92 | 0.01 |
| NAC | 3.46 | 1.87–6.40 | 0.01 | 2.45 | 1.19–5.02 | 0.02 |
| EDNRB | 0.36 | 0.14–0.91 | 0.03 | 0.38 | 0.15–0.98 | 0.04 |
HR, hazard ratio; CI, confidence interval; NAC, neoadjuvant chemotherapy; EDNRB Endothelin receptor B.
Figure 2.
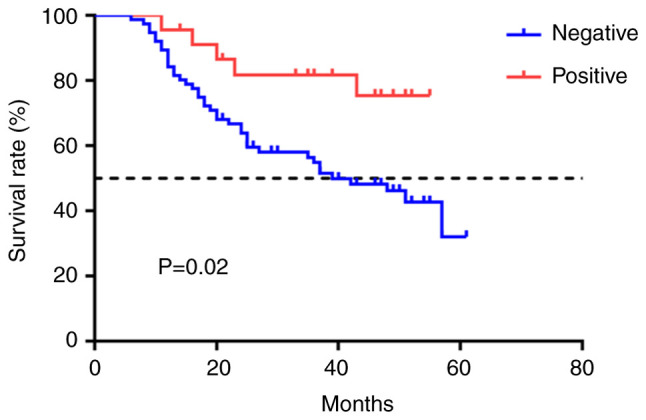
Kaplan-Meier survival curve of EDNRB expression of patients in HNCH cohort. EDNRB, endothelin B receptor; HNCH, Henan cancer hospital.
Addition of EDNRB expression improves the predictive ability of model 1 for prognosis
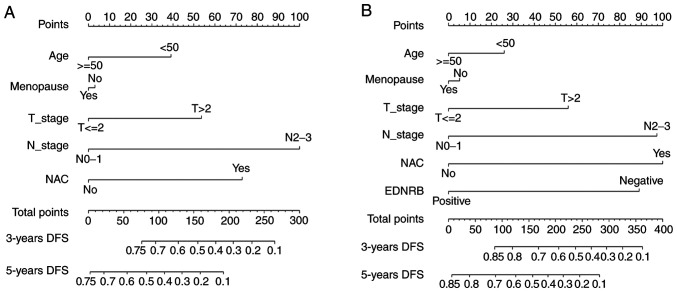
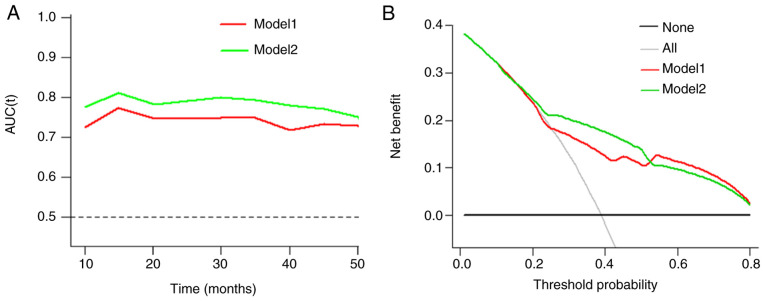
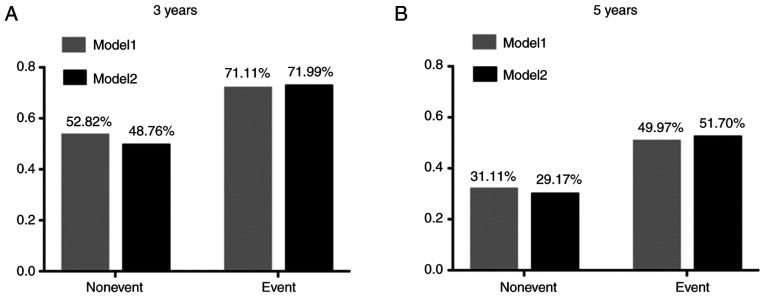
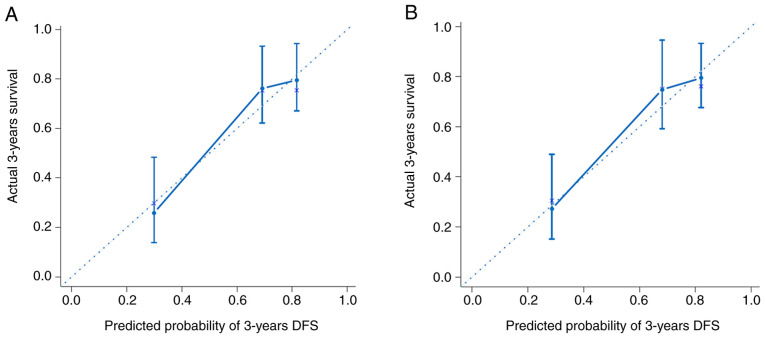
These models were presented as a nomogram (Fig. 3). When calculating the iAUC value of the ROC curve between 5 and 50 months to evaluate the model discrimination, the results revealed that the iAUC value of model 2 was larger than that of model 1 after adding EDNRB expression as a parameter (0.78 vs. 0.74; Fig. 4A; Table IV). Similarly, the C-index of model 2 was greater than that of model 1 (0.73 vs. 0.69; Table IV), which indicated that the model discrimination degree was improved after the EDNRB expression parameter was added. Furthermore, the clinical significance of EDNRB was analyzed by comparing the net benefits of model 1 and model 2 using decision curve analysis. As shown in Fig. 4B, compared with model 1, in the interval where the 3-year survival rate threshold was <0.52, the net benefit of model 2 was higher according to the decision curve analysis. IDI was calculated to judge the improvement of the model. The results demonstrated that the IDI values of the model to predict the 3- and 5-year survival rates were 0.04 (P=0.02) and 0.05 (P=0.01; Fig. 5; Table IV), respectively. These results demonstrated that adding EDNRB expression as a parameter may increase the accuracy of prognosis prediction. Additionally, the calibration plot for the prediction of 3-year DFS in patients with TNBC exhibited good agreement between nomogram predictions and actual observation (Fig. 6).
Figure 3.
Nomogram to predict 3-year and 5-year DFS of patients with TNBC. (A) Nomogram of Model 1 for 3-year and 5-year DFS. (B) Nomogram of Model 2 for 3-year and 5-year DFS. DFS, disease-free survival; TNBC, triple-negative breast cancer.
Figure 4.
Clinical value analysis of Models 1 and 2. (A) Time-dependent receiver operating characteristic curve analysis of prediction model. (B) Decision curve analysis for Model 1 and Model 2 in prediction of DFS of TNBC at the 3-year point. Model 1 consisted of N stage and NAC. Model 2 consisted of N stage, NAC and EDNRB expression. DFS, disease-free survival; TNBC, triple-negative breast cancer; N, lymph node; NAC, neoadjuvant chemotherapy.
Table IV.
Validation of the prognostic value of EDNRB.
| Variable | iAUC | C-index (95% CI) | IDI for 3 years | P1 | IDI for 5 years | P2 |
|---|---|---|---|---|---|---|
| Model 1 | 0.74 | 0.69 (0.61–0.76) | 0.04 | 0.02 | 0.05 | 0.01 |
| Model 2 | 0.78 | 0.73 (0.65–0.81) |
iAUC, integrated area under the curve of time-dependent ROC; C-index, concordance index; CI, confidence interval; IDI, integrated discrimination improvement. The P-value reflects the IDI test results of 3 and 5 years. Model 1 consists of N stage and NAC; Model 2 consists of N stage, NAC and EDNRB expression.
Figure 5.
Prediction model IDI analysis. (A) IDI analysis of prediction model predicting 3-year survival rate. (B) IDI analysis of prediction model predicting 5-year survival rate. The Y axis is the average value of the disease occurrence probability predicted by the model for each individual. IDI, integrated discriminant improvement.
Figure 6.
Calibration plots of the nomogram for 3-year DFS. (A) Prediction of Model 1 for 3-year DFS. (B) prediction of Model 2 for 3-year DFS. The X-axis represents that the nomogram-predicted DFS probability; the Y-axis represents that actual DFS probability. The plot along the 45-degree line indicates a prefect calibration model in which the predicted probabilities are identical to the actual outcomes. DFS, disease-free survival.
Discussion
TNBC is highly heterogeneous, with a high risk of local recurrence and distant metastasis. At present, chemotherapy is the main clinical treatment method (20,21). EDNRB serves an important role in cancer development and lymphatic metastasis (8–11,22). At present, the expression and clinical significance of EDNRB in TNBC remain unclear. The results of the present study demonstrated that EDNRB was expressed at low levels in TNBC, and is associated with favorable prognostic and predictive value.
The positive expression rate of EDNRB in the present study was 23.2%, which was close to the positive expression rate of 22.2% in all breast cancer types in a previous study (23). In line with the results of studies on prostate and liver cancer (14,15), the present study revealed that EDNRB expression was low in TNBC samples, and this was also observed in the TCGA cohort. This indicated that EDNRB may serve an anticancer role in TNBC.
The tumor microenvironment is a complex milieu, which includes endothelial cells, fibroblasts, immune cells and mesenchymal stem cells (24). Immune cells and stromal cells are the main non-tumor components of the tumor microenvironment (24). Stromal cells are considered to serve an important role in tumor growth, progression and spread (25,26). The present study demonstrated that EDNRB expression was moderately positively correlated with the matrix score (rs=0.44; P<0.01), but weakly correlated with the immune score (rs=0.19; P=0.02). This suggested that the role of EDNRB in the occurrence and development of TNBC may be associated with stromal cells rather than immune cells, and this should be validated in future studies.
Several studies have demonstrated that EDNRB may be associated with the occurrence and development of tumors (13–15). In the HNCH cohort, multivariate analysis revealed that age was an independent predictor of EDNRB expression (OR, 3.37; 95% CI, 1.28–8.87; P=0.01). This result was verified in the TCGA cohort. The average age in the TCGA cohort was higher than that in the HNCH cohort (55.79 years vs. 48.76 years), and the positive rate of EDNRB was higher (52.8% vs. 23.2%). Wülfing et al (12), revealed that EDNRB expression is associated with tumor size in breast cancer. The present study reported that EDNRB expression was also associated with T stage in TNBC. A previous study has demonstrated that EDNRB is involved in lymph angiogenesis following activation by ET-1 (11). In line with this, in the present study, multivariate analysis in the TCGA cohort revealed that N stage was an independent predictor of EDNRB expression (OR, 4.30; 95% CI, 1.33–13.89; P=0.02). Lymph node involvement is usually associated with the prognosis of patients, and the worse the involvement, the poorer the prognosis (27). In the present study, EDNRB expression was positively associated with lymph node stage, while the DFS time was longer in patients with high EDNRB expression. This indicated that EDNRB may serve different roles in different stages of tumor occurrence and development, which requires further research and verification at the molecular level.
EDNRB is associated with the prognosis of various types of cancer (13–15). A previous study had demonstrated that high expression levels of EDNRB were associated with a poor prognosis in breast cancer (18), and this conclusion was based on prognosis analysis of the difference in expression of EDNRB in all types of breast cancer, of which only 25.1% were TNBC. In the present studym with a median follow-up time of 36 months, Kaplan-Meier survival analysis revealed that the relapse risk of EDNRB-positive patients was 0.36 times that of EDNRB-negative patients [hazard ratio (HR), 0.36; 95% CI, 0.14–0.91; P=0.03]. In contrast to a study by Gu et al (18), all cases in the HNCH cohort in the present study were classified as TNBC, with 61.6% of patients being younger than 50 years. The difference between the two conclusions was due to the difference in the characteristics of the patients involved. Multivariate Cox regression analysis was used to further correct for confounding factors. The results revealed that EDNRB expression was an independent predictor of prognosis (HR, 0.38; 95% CI, 0.15–0.98; P=0.04). Subsequently, two models were established using the multivariate Cox regression analysis results, and the accuracy of EDNRB for the prediction of prognosis was analyzed using multiple indicators and angles. IDI indicated the extent to which a new marker reclassifies subjects, and it is a method to evaluate the ability of a new marker to predict a binary outcome of interest (28). The results suggested that the IDI values of the model for predicting the 3- and 5-year survival rates were 0.04 and 0.05, respectively, following the addition of the EDNRB parameter, and the difference was statistically significant. This indicated that EDNRB may improve the prediction accuracy of the model for prognosis. Decision curve analysis is a method for the evaluation of clinical net benefit (29). In the present study, the decision curves of models 1 and 2 intersected at the 3-year survival rate threshold of 0.52, below which model 2 had a higher net benefit. Additionally, the analysis revealed that the result was of clinical significance. The model predicted a 3-year survival rate of 0.52 as a high-risk threshold in clinical decision-making. Intervention measures should be implemented within the range where the threshold is <0.52, and there was no clinical value in the range >0.52.
The present study had certain advantages and disadvantages. To the best of our knowledge, the present study was the first large-scale study to analyze the association between EDNRB and clinicopathological data, and the prognosis of patients with TNBC. However, the disadvantages were as follows: This was a retrospective clinical study with low evidence level; the end point of the present study was DFS, but no overall survival analysis was performed; the follow-up time of this study was short, so the accuracy of predicting 5-year DFS was low, meaning that the calibration plot and decision curve analysis results for predicting 5-year DFS could not be obtained; and the present study lacked molecular mechanism research at the cellular level.
In conclusion, the present study investigated EDNRB expression in TNBC and its association with prognosis. EDNRB expression was correlated with stromal scores. Patients with TNBC with low EDNRB expression had a shorter DFS time, and EDNRB may improve the ability to predict prognosis. This suggests that EDNRB may be used as a novel biomarker for the prognosis of TNBC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TNBC
triple-negative breast cancer
- ER
estrogen receptor
- PR
progesterone receptor
- HER2
human epidermal growth factor receptor
- EDNRA
endothelin A receptor
- EDNRB
endothelin B receptor
- MAPK
mitogen-activated protein kinase
- Erk
extracellular signal-regulated kinase 2
- PI3K/AKT
phosphatidylinositol 3-kinase/protein kinase B
- DFS
disease-free survival
- iAUC
Integrated area under the curve
- C-index
concordance index
- IDI
integrated discriminant improvement
- OR
odds ratio
- HNCH
Henan Cancer Hospital
- NAC
neoadjuvant chemotherapy
- CI
confidence interval
Funding
The present study was supported by a grant from Henan Province Medical Science and Technology Research Project (SBGJ2018088).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SL performed the immunohistochemical experiment, analyzed the experimental results and wrote the manuscript; JYZ assisted in the immunohistochemical experiment; JJZ and DJ assisted in analyzing the experimental results and revising the manuscript; ZL designed the experiment and corrected the final manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University. Written informed consent was obtained from the patients when their samples were first collected.
Patient consent for publication
All patients provided informed consent for publication of data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Kar S, Lai X, Cai W, Arfuso F, Sethi G, Lobie PE, Goh BC, Lim LHK, Hartman M, et al. Triple negative breast cancer in Asia: An insider's view. Cancer Treat Rev. 2018;62:29–38. doi: 10.1016/j.ctrv.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagnato A, Natali PG. Endothelin receptors as novel targets in tumor therapy. J Transl Med. 2004;2:16. doi: 10.1186/1479-5876-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Namiki A, Hirata Y, Fukazawa M, Ishikawa M, Moroi M, Aikawa J, Yabuki S, Machii K. Endothelin-1- and endothelin-3-induced vasorelaxation via endothelium-derived nitric oxide. Jpn J Pharmacol. 1992;58(Suppl 2):326P. [PubMed] [Google Scholar]
- 8.Vacca F, Bagnato A, Catt KJ, Tecce R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000;60:5310–5317. [PubMed] [Google Scholar]
- 9.Green DS, Rupasinghe C, Warburton R, Wilson JL, Sallum CO, Taylor L, Yatawara A, Mierke D, Polgar P, Hill N. A cell permeable peptide targeting the intracellular loop 2 of endothelin B receptor reduces pulmonary hypertension in a hypoxic rat model. PLoS One. 2013;8:e81309. doi: 10.1371/journal.pone.0081309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morbidelli L, Orlando C, Maggi CA, Ledda F, Ziche M. Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. Am J Physiol. 1995;269:H686–H695. doi: 10.1152/ajpheart.1995.269.2.H686. [DOI] [PubMed] [Google Scholar]
- 11.Spinella F, Caprara V, Garrafa E, Castro V, Rosanò L, Natali PG, Bagnato A. Endothelin axis induces metalloproteinase activation and invasiveness in human lymphatic endothelial cells. Can J Physiol Pharmacol. 2010;88:782–787. doi: 10.1139/Y10-050. [DOI] [PubMed] [Google Scholar]
- 12.Wülfing P, Diallo R, Kersting C, Wülfing C, Poremba C, Rody A, Greb RR, Böcker W, Kiesel L. Expression of endothelin-1, endothelin-A, and endothelin-B receptor in human breast cancer and correlation with long-term follow-up. Clin Cancer Res. 2003;9:4125–4131. [PubMed] [Google Scholar]
- 13.Vasaikar S, Tsipras G, Landázuri N, Costa H, Wilhelmi V, Scicluna P, Cui HL, Mohammad AA, Davoudi B, Shang M, et al. Overexpression of endothelin B receptor in glioblastoma: A prognostic marker and therapeutic target? BMC Cancer. 2018;18:154. doi: 10.1186/s12885-018-4012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastian PJ, Ellinger J, Heukamp LC, Kahl P, Müller SC, von Rücker A. Prognostic value of CpG island hypermethylation at PTGS2, RAR-beta, EDNRB, and other gene loci in patients undergoing radical prostatectomy. Eur Urol. 2007;51:665–674. doi: 10.1016/j.eururo.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Luo B, Dang YW, He RQ, Chen G, Peng ZG, Feng ZB. The clinical significance of endothelin receptor type B in hepatocellular carcinoma and its potential molecular mechanism. Exp Mol Pathol. 2019;107:141–157. doi: 10.1016/j.yexmp.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Network, corp-author. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren G, Tian Q, An Y, Feng B, Lu Y, Liang J, Li K, Shang Y, Nie Y, Wang X, Fan D. Coronin 3 promotes gastric cancer metastasis via the up-regulation of MMP-9 and cathepsin K. Mol Cancer. 2012;11:67. doi: 10.1186/1476-4598-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, Han S, Cui M, Xue J, Ai L, Sun L, Zhu X, Wang Y, Liu C. Knockdown of endothelin receptor B inhibits the progression of triple-negative breast cancer. Ann N Y Acad Sci. 2019;1448:5–18. doi: 10.1111/nyas.14039. [DOI] [PubMed] [Google Scholar]
- 19.Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belkacemi Y, Hanna NE, Besnard C, Majdoul S, Gligorov J. Local and regional breast cancer recurrences: Salvage therapy options in the new era of molecular subtypes. Front Oncol. 2018;8:112. doi: 10.3389/fonc.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger Y, Bernasconi CC, Juillerat-Jeanneret L. Targeting the endothelin axis in human melanoma: Combination of endothelin receptor antagonism and alkylating agents. Exp Biol Med (Maywood) 2006;231:1111–1119. [PubMed] [Google Scholar]
- 23.Wülfing P, Kersting C, Tio J, Fischer RJ, Wülfing C, Poremba C, Diallo R, Böcker W, Kiesel L. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10:2393–2400. doi: 10.1158/1078-0432.CCR-03-0115. [DOI] [PubMed] [Google Scholar]
- 24.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 27.Beenken SW, Urist MM, Zhang Y, Desmond R, Krontiras H, Medina H, Bland KI. Axillary lymph node status, but not tumor size, predicts locoregional recurrence and overall survival after mastectomy for breast cancer. Ann Surg. 2003;237:732–739. doi: 10.1097/01.SLA.0000065289.06765.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. doi: 10.1093/aje/kwr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.